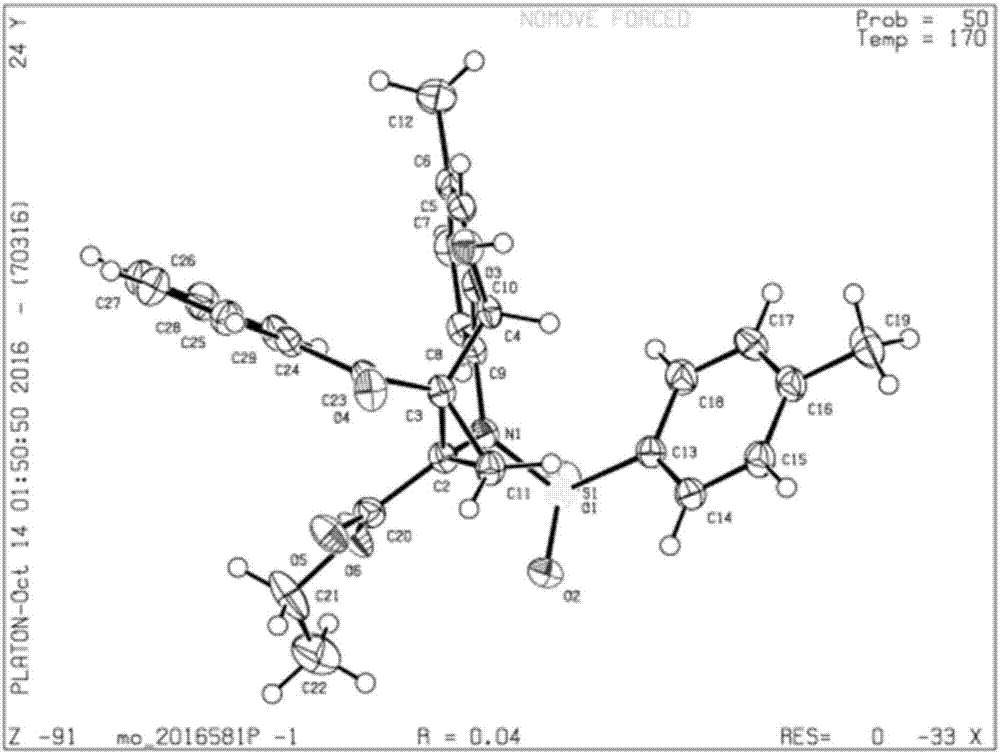
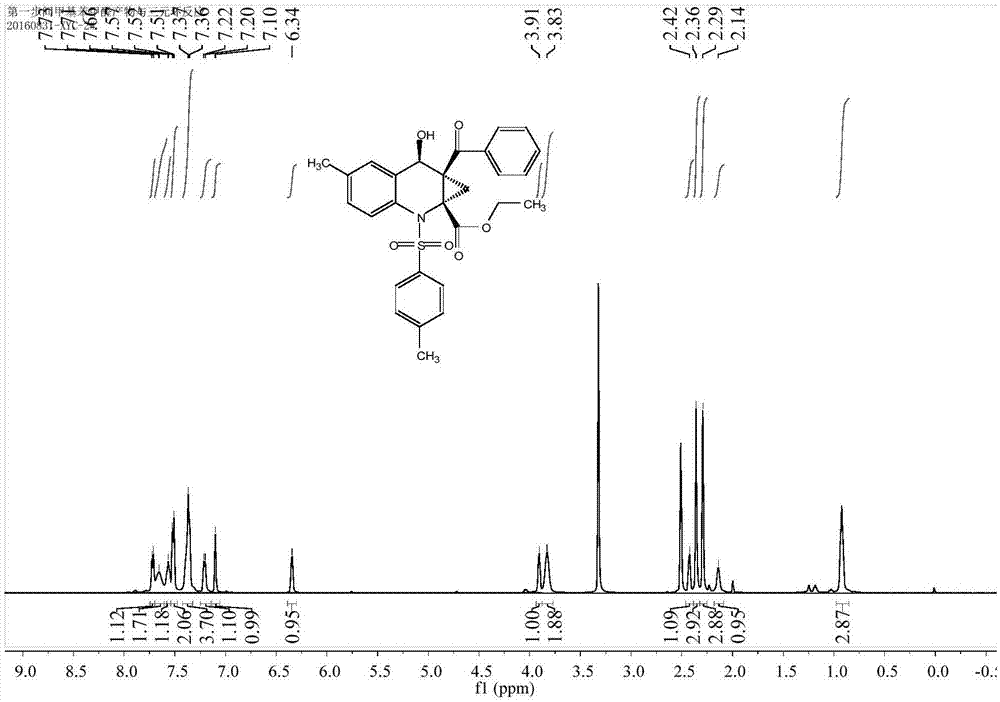
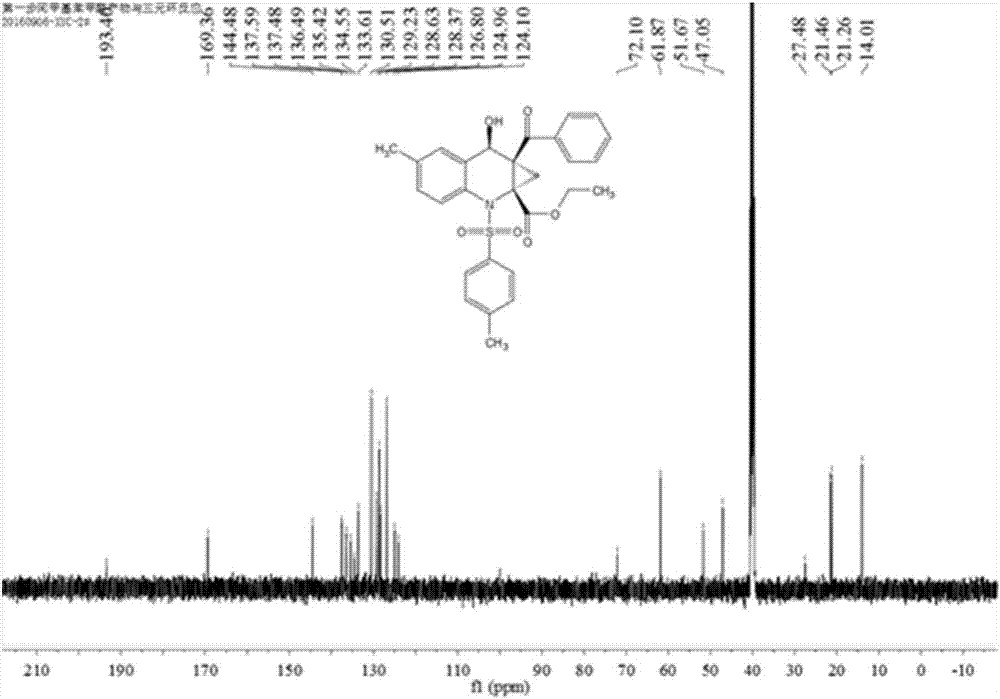
Preparation method of chiral cyclopropyl amino acid
A cyclopropyl and amino acid technology, which is applied in the field of simple preparation of chiral cyclopropyl amino acids, can solve the problems of affecting the reaction yield, numerous reaction steps, cumbersome operation, etc. the effect of pressure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] A total of 30.4 mg (0.1 mmol) of N-(2-formyl-4-methylphenyl)-4-toluenesulfonamide, 25.3 mg (0.1 mmol) of ethyl-2-benzoyl-1-chlorocyclopropanecarboxylic acid ) and 65.2 mg (0.2 mmol) of cesium carbonate were added to 2 mL of N, N-dimethylformamide solvent, and the reaction solution was placed on a magnetic stirrer and stirred at room temperature. Whether the reaction is complete, or a reactant has all reacted, stop the reaction. Add water and dichloromethane to the reaction solution for extraction, add anhydrous sodium sulfate to dry and remove water, filter and take the filtrate, finally add silica gel powder and spin dry dichloromethane, purify the product by column chromatography, and spin dry the solvent again , vacuumized to obtain 41.3mg of cyclopropyl amino acid, yield 82%.
Embodiment 2
[0040] A total of 30.4 mg (0.1 mmol) of N-(2-formyl-4-methylphenyl)-4-toluenesulfonamide, 25.3 mg (0.1 mmol) of ethyl-2-benzoyl-1-chlorocyclopropanecarboxylic acid ) and 65.2 mg (0.2 mmol) of cesium carbonate were added to 2 mL of dimethyl sulfoxide solvent, and the reaction solution was placed on a magnetic stirrer and stirred at room temperature. , or a reactant has all reacted, stop the reaction. Add water and dichloromethane to the reaction solution for extraction, add anhydrous sodium sulfate to dry and remove water, filter and take the filtrate, finally add silica gel powder and spin dry dichloromethane, purify the product by column chromatography, and spin dry the solvent again , vacuumized to obtain 40.2 mg of cyclopropyl amino acid, yield 80%.
Embodiment 3
[0042] A total of 30.4 mg (0.1 mmol) of N-(2-formyl-4-methylphenyl)-4-toluenesulfonamide, 25.3 mg (0.1 mmol) of ethyl-2-benzoyl-1-chlorocyclopropanecarboxylic acid ) and 65.2 mg (0.2 mmol) of cesium carbonate were added to 2 mL of acetonitrile solvent, and the reaction solution was placed on a magnetic stirrer and stirred at room temperature. The reactants have all reacted, stop the reaction. Add water and dichloromethane to the reaction solution for extraction, add anhydrous sodium sulfate to dry and remove water, filter and take the filtrate, finally add silica gel powder and spin dry dichloromethane, purify the product by column chromatography, and spin dry the solvent again , vacuumized to obtain 34.3 mg of cyclopropyl amino acid, yield 68%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com