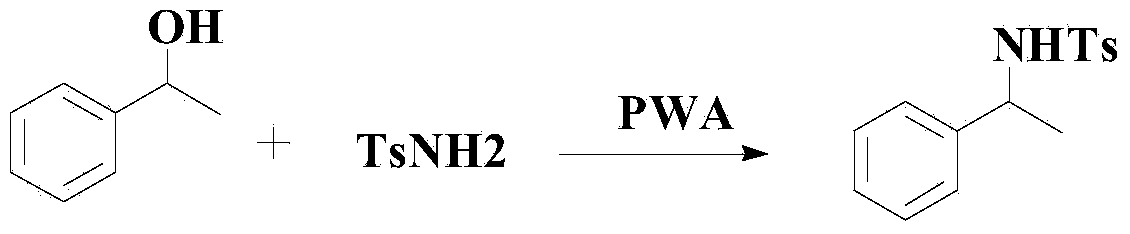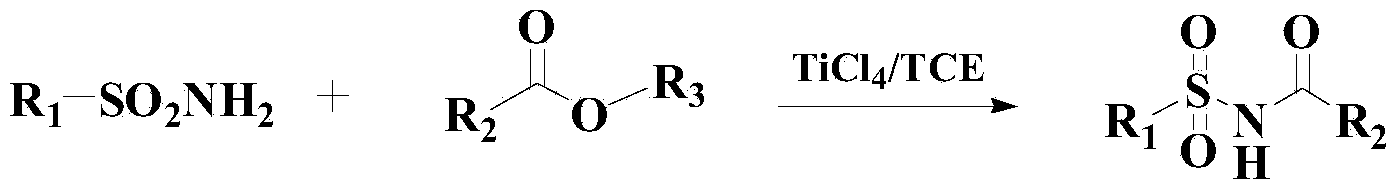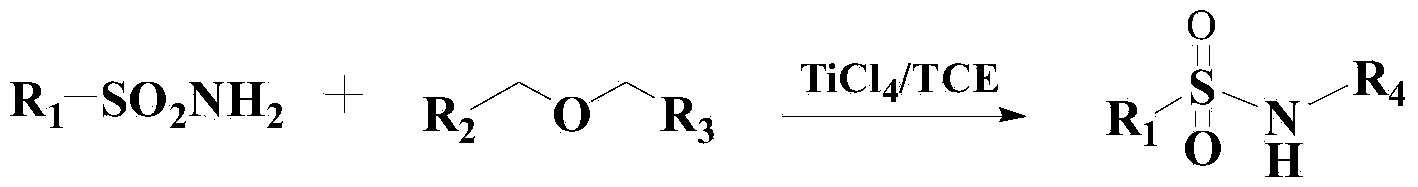Method for synthesizing benzene sulfonamide compounds
A technology of benzenesulfonamide and compound, which is applied in the preparation of sulfonic acid amide, chemical instruments and methods, organic compound/hydride/coordination complex catalyst, etc. The problem of poor reactivity of base ethers, etc., achieves broad-scale industrial application prospects and market potential, high-efficiency reaction, and mild reaction effects.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
[0037] Weigh the mixture of ethyltriphenylphosphonium bromide, AgCl and porphyrin according to the mass ratio of 0.7:1.2:1, grind and mix evenly to obtain the catalyst of the present invention, which is used in the following specific examples according to the dosage.
Embodiment 1
[0039]
[0040] Add 300ml of toluene to the reactor, then add 1mol of methyl tert-butyl ether and 1.5mol of benzenesulfonamide, heat up to 60°C, then add 0.3g of catalyst, and stir the reaction for 8 hours under the thermometer. After the reaction, N-tert-butylbenzenesulfonamide was obtained by vacuum concentration, column chromatography separation and drying, with a yield of 96.3% and a purity of 98.2% (HPLC).
[0041] 1 H-NMR (CDCl 3 , 270MHz) δ: 7.92 (m, 2H), 7.49, (m, 3H), 4.82 (br, 1H), 1.21 (s, 9H).
[0042] MS m / z: 213.08 (M+1,100).
Embodiment 2
[0044]
[0045] Add 300ml of toluene to the reactor, then add 1mol of methyl tert-butyl ether and 1.4mol of p-toluenesulfonamide, heat up to 50°C, then add 0.5g of catalyst to it and stir for 7 hours. Chromatographic separation and drying gave N-tert-butyl p-toluenesulfonamide with a yield of 96.5% and a purity of 98.4% (HPLC).
[0046] 1 H-NMR (CDCl 3 ,270MHz) δ: 7.78(d,2H), 7.29(d,2H), 4.85(br,1H), 2.44(s,3H), 1.22(s,9H).
[0047] MS m / z: 227.06 (M+1,100).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com