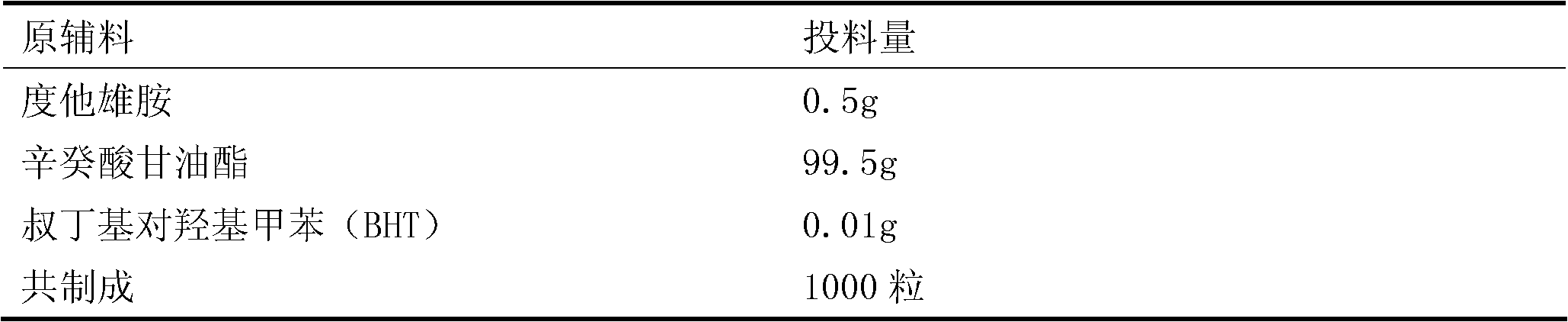
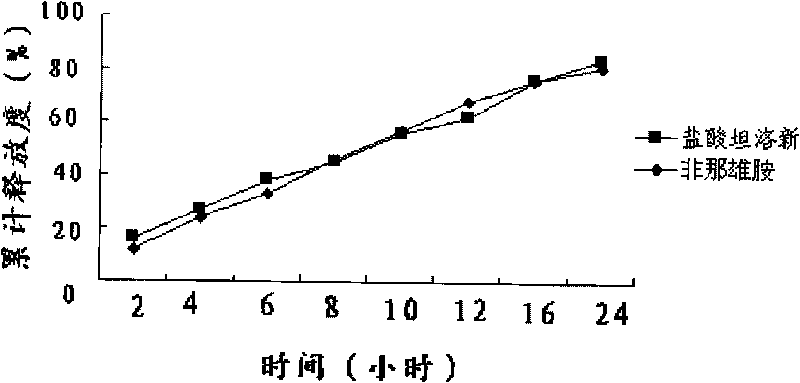
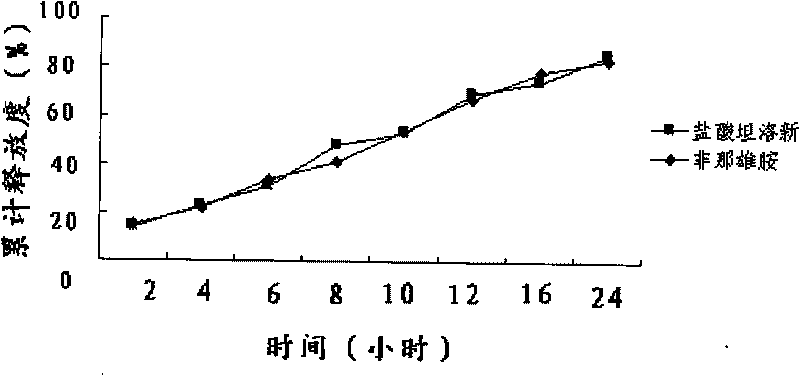
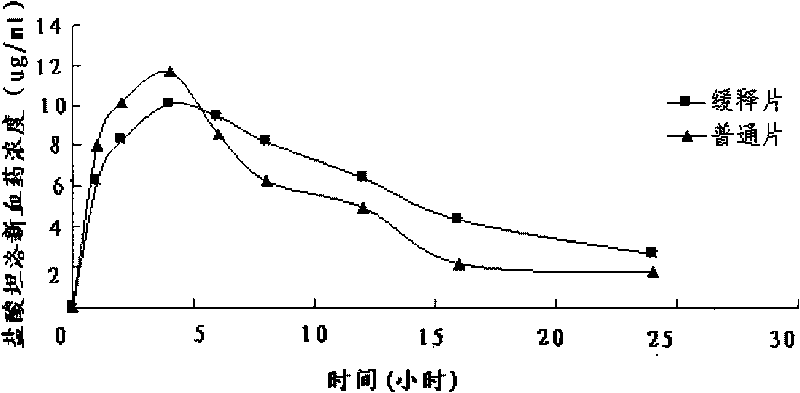
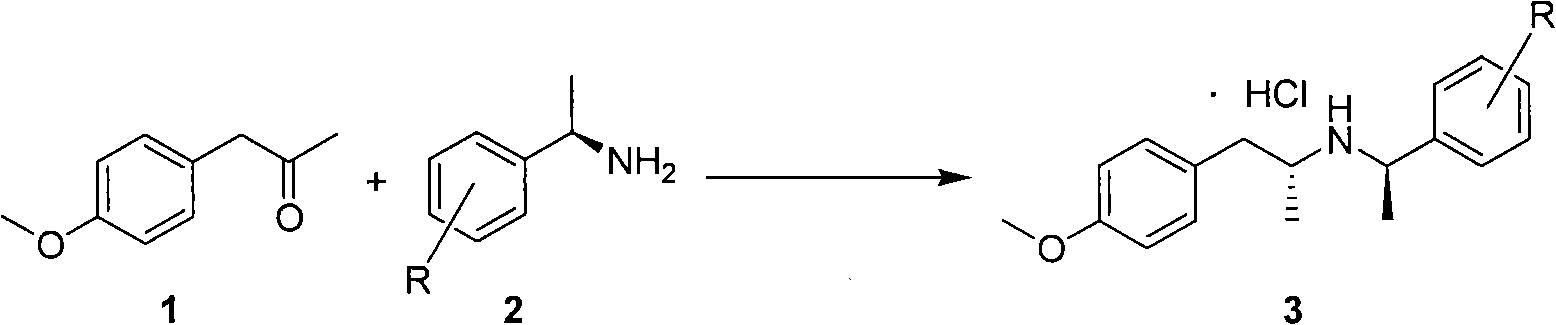Patents
Literature
66 results about "Tamsulosin" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
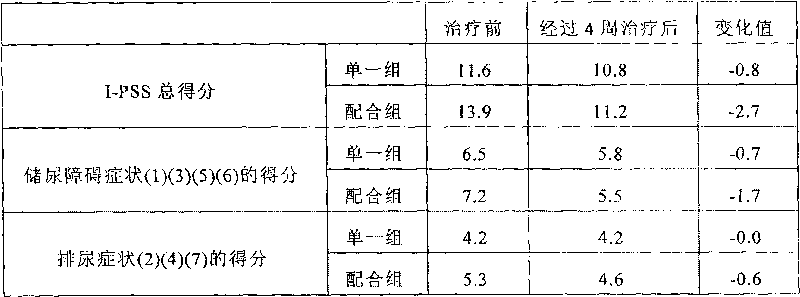
Tamsulosin is used by men to treat the symptoms of an enlarged prostate (benign prostatic hyperplasia-BPH).
Overactive bladder treating drug
A therapeutic agent for overactive bladder containing tamsulosin or a pharmaceutically acceptable salt thereof as an effective ingredient.
Owner:ASTELLAS PHARMA INC
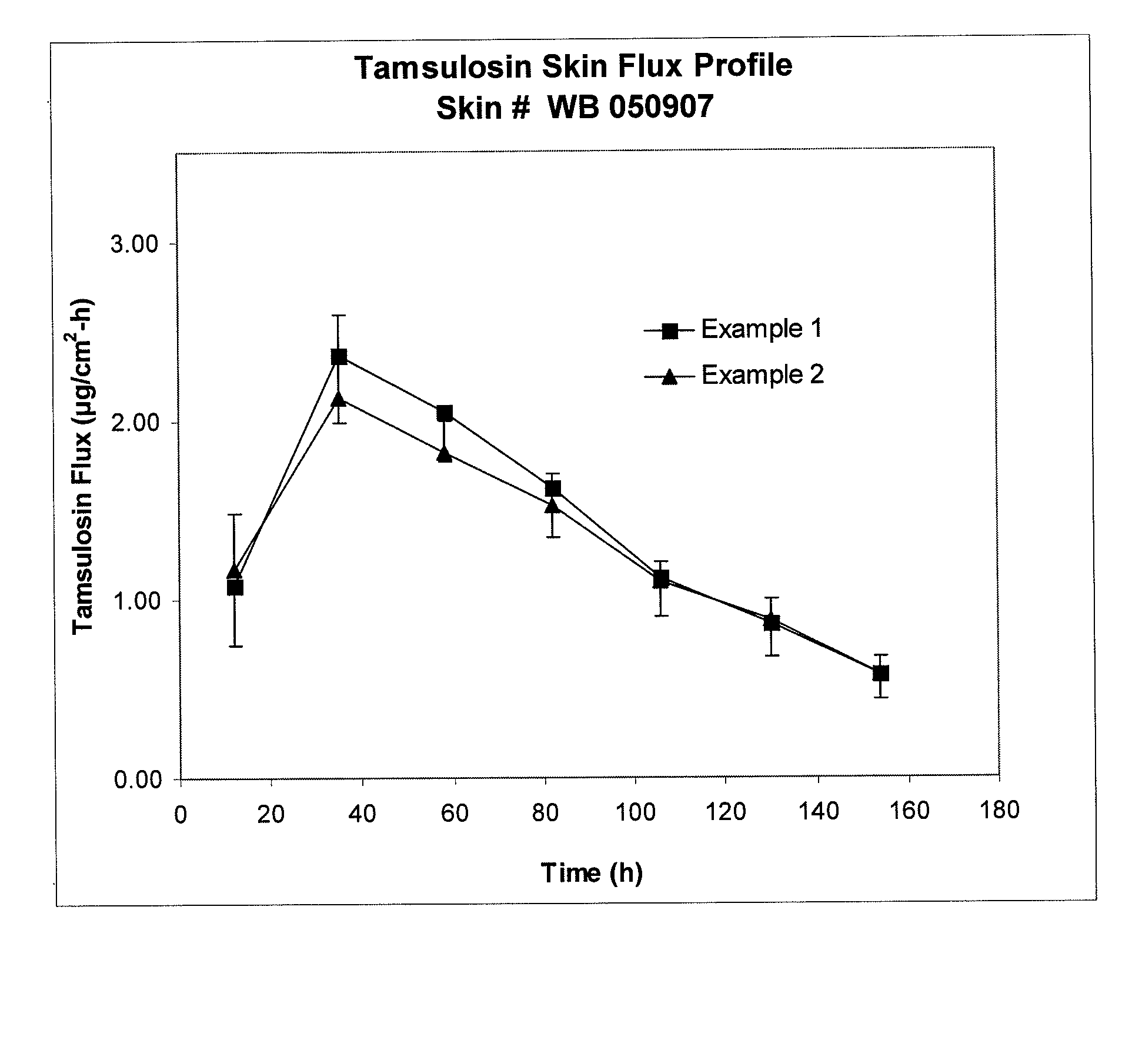
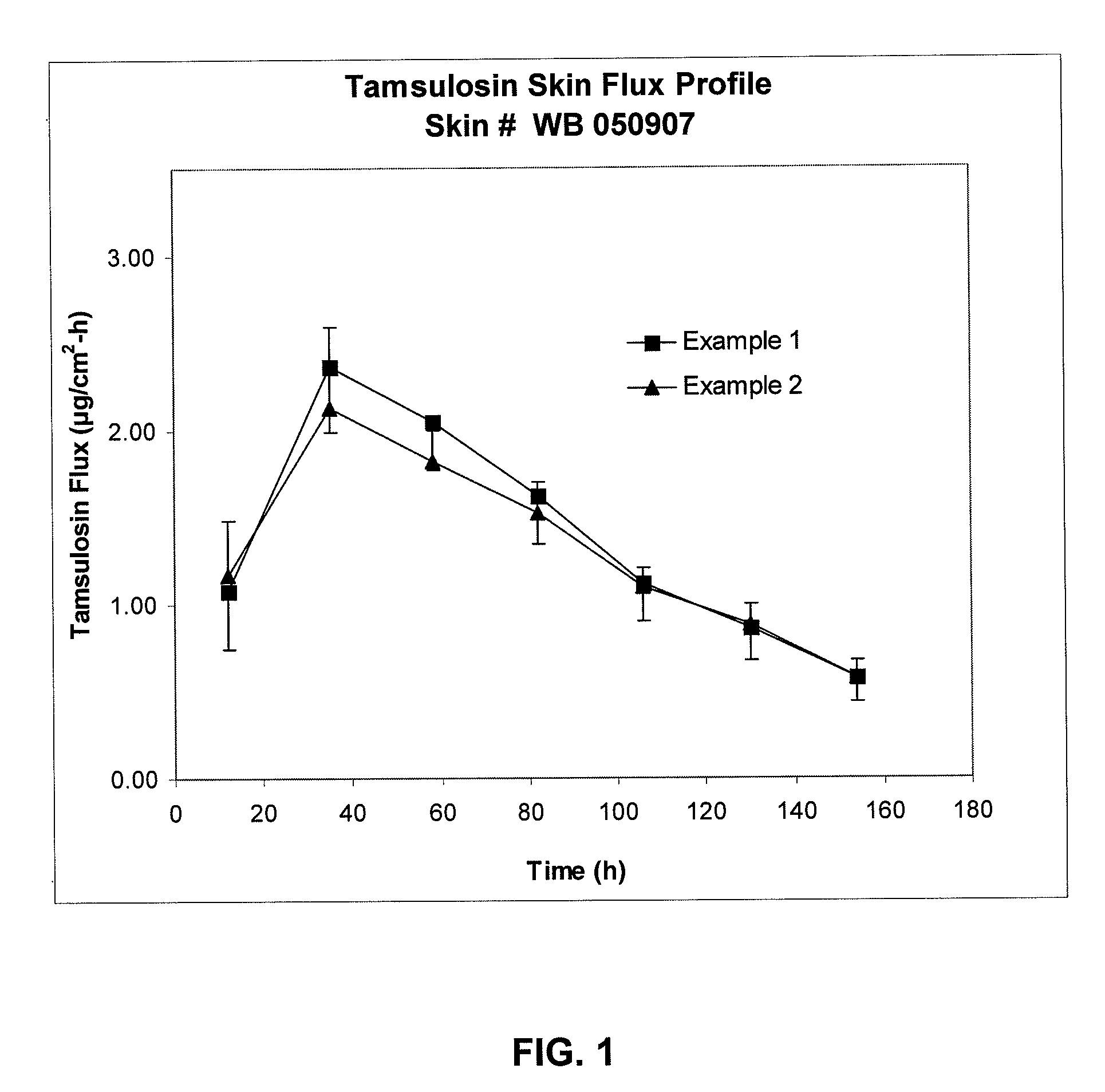
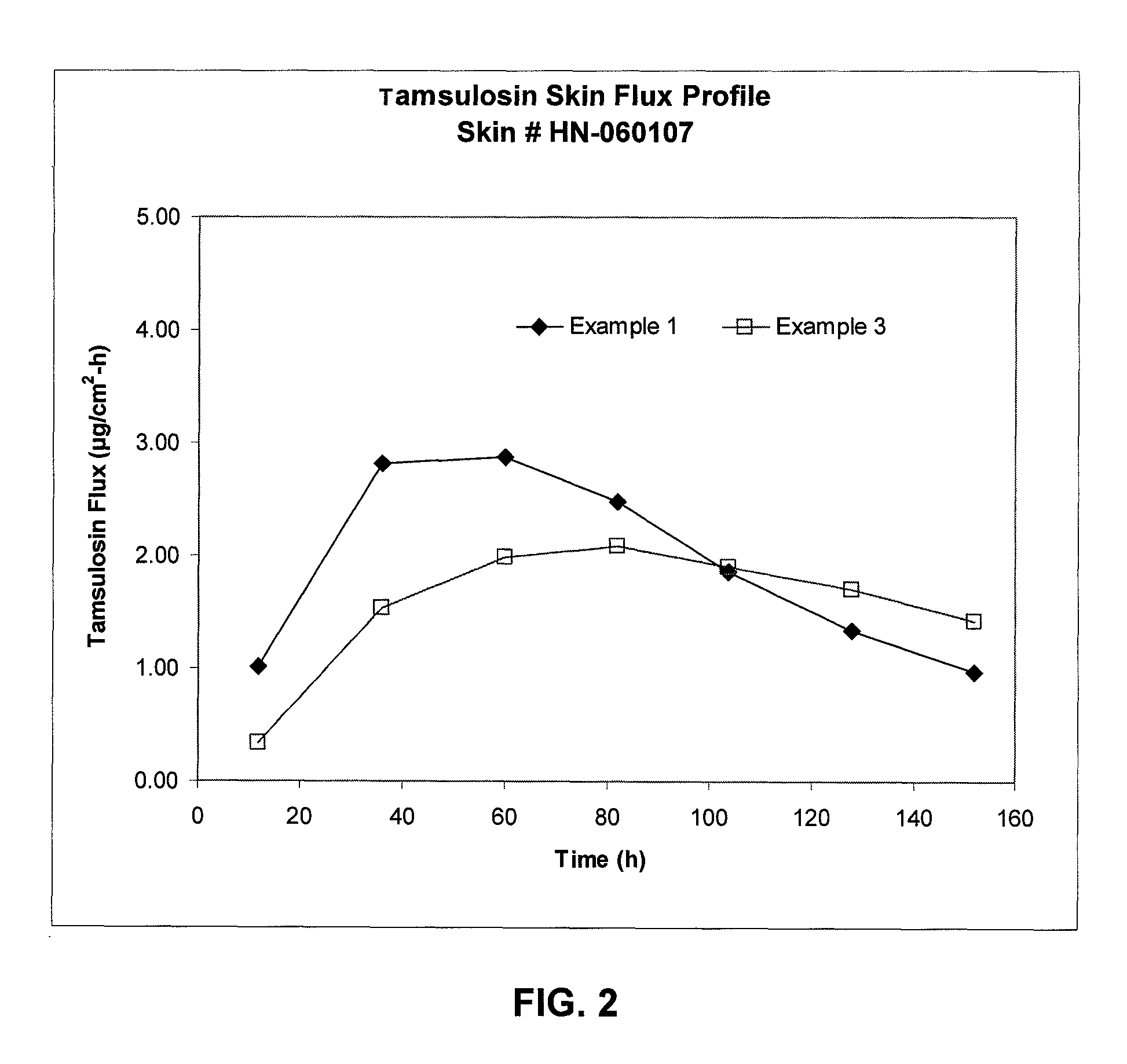
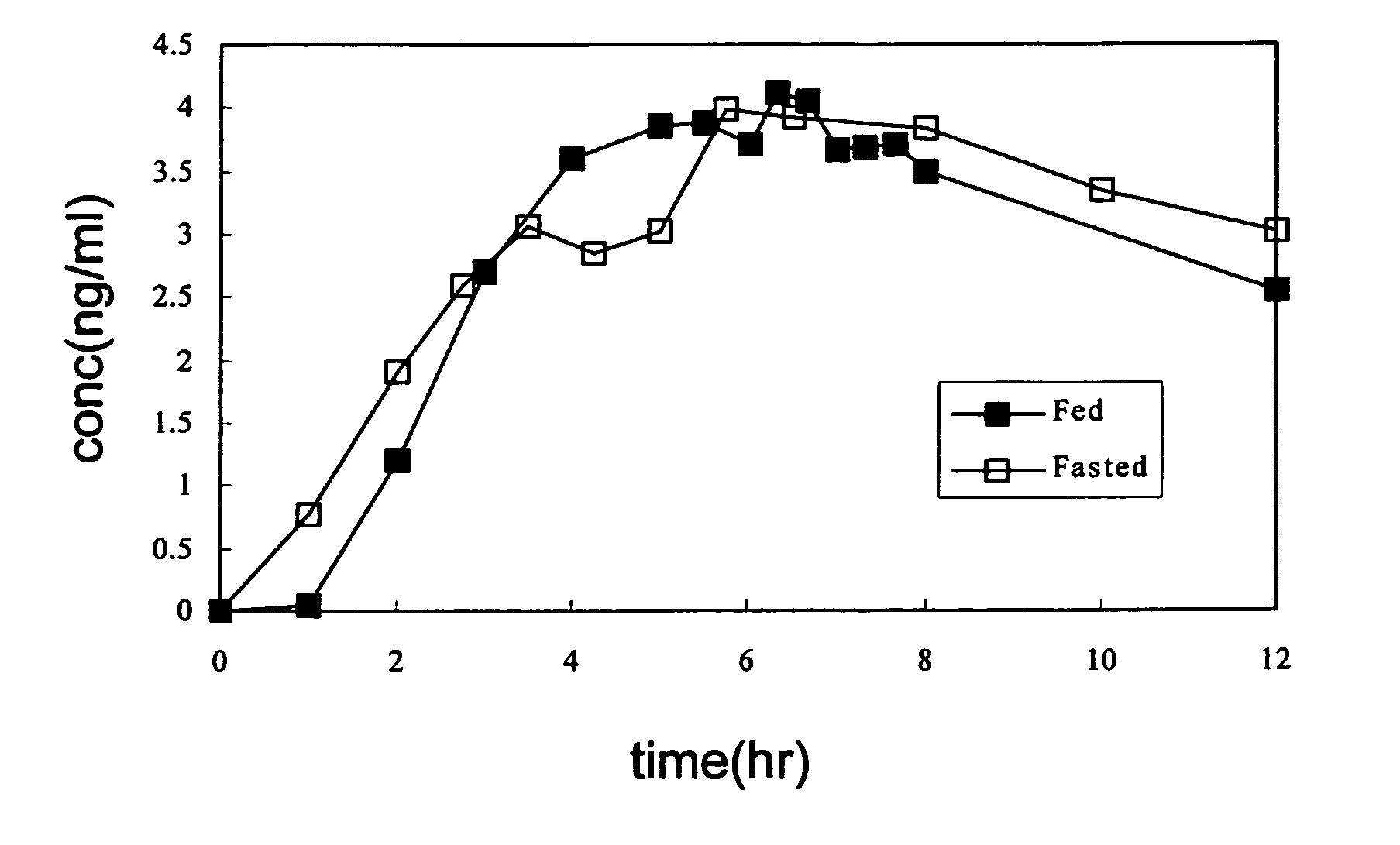
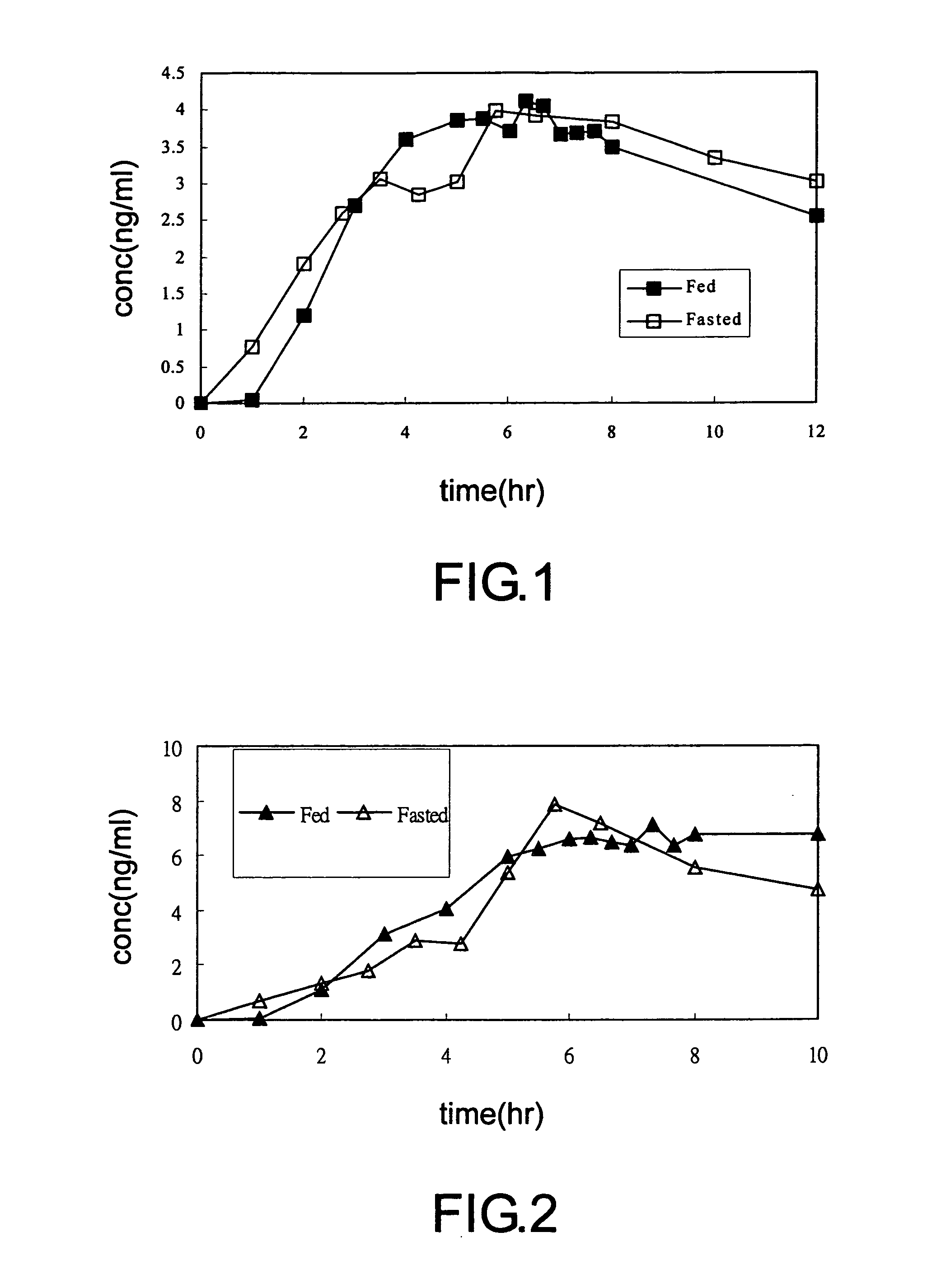
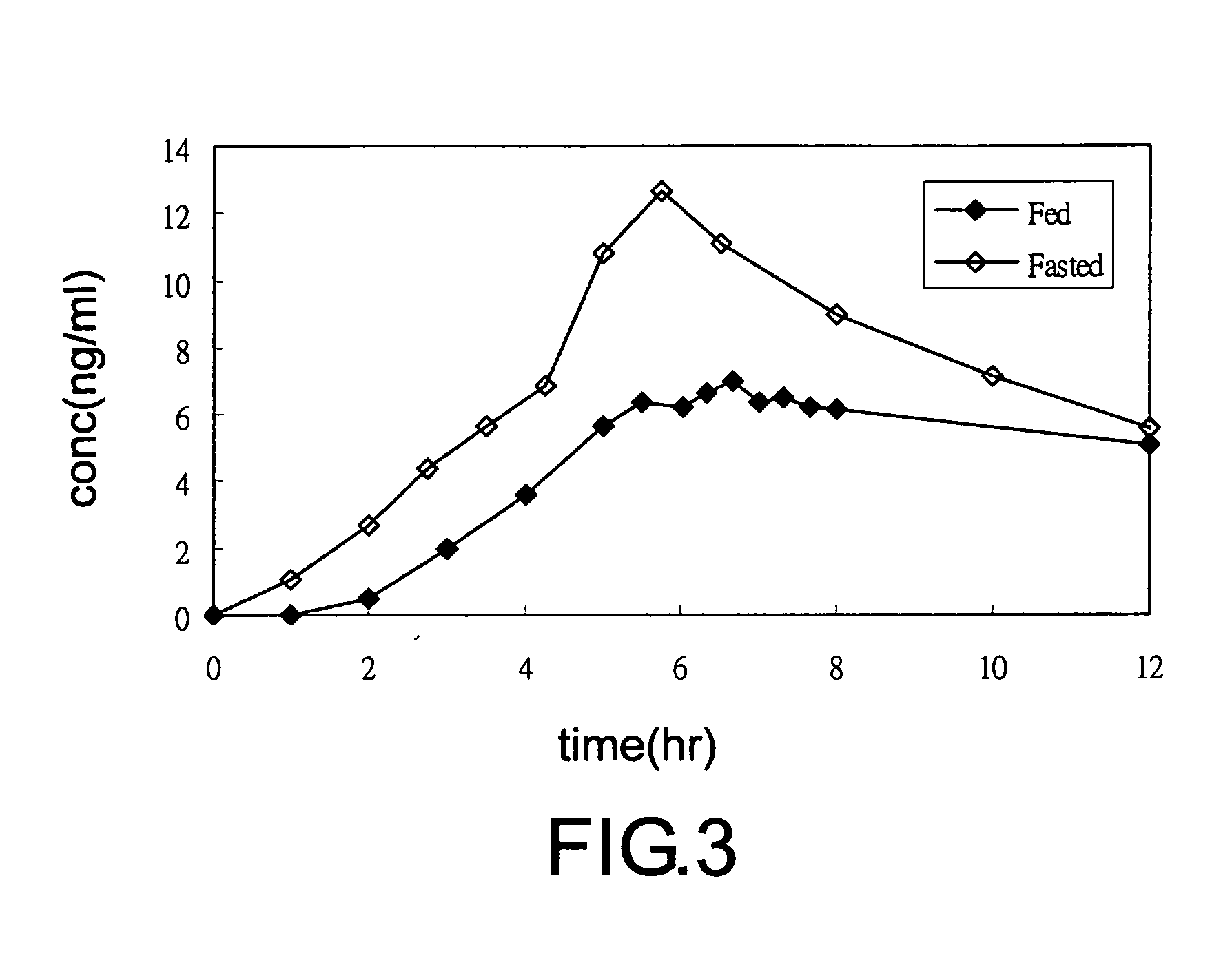
Transdermal administration of tamsulosin
In an aspect of the invention, a composition for making a patch for the transdermal delivery of tamsulosin is provided. The composition comprises (a) at least about 1 wt % tamsulosin or a pharmaceutically acceptable salt of tamsulosin, (b) at least about 40 wt % polyisobutylene adhesive or hydrophobic synthetic rubber adhesive, (c) about 1-20 wt % of an aprotic solvent in which tamsulosin dissolves readily, (d) about 1-20 wt % of an unsaturated fatty acid or an alpha-hydroxy acid or a mixture of unsaturated fatty acids or alpha-hydroxy acids or of both unsaturated fatty acids and alpha-hydroxy acids, (e) a lipophilic permeation enhancer, and (f) a matrix modifier.
Owner:CORIUM PHARMA SOLUTIONS INC
Pharmaceutical pellets comprising tamsulosin
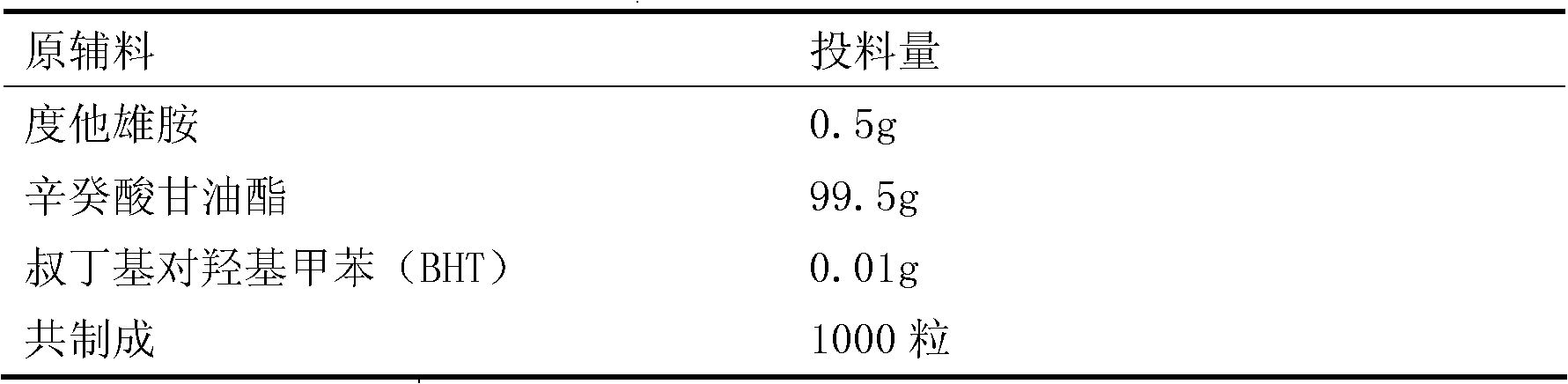
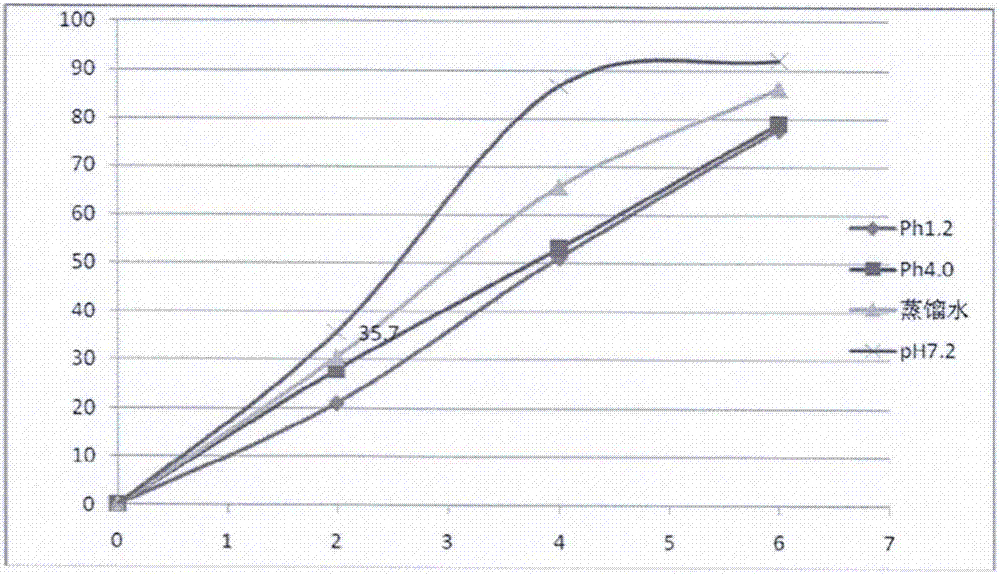
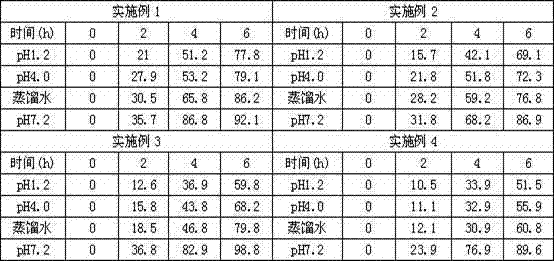
ActiveUS7018658B2Sure easyUrinary disorderPharmaceutical non-active ingredientsTamsulosinEnteric coating
Tamsulosin pellets having an advantageous release profile are formed. The pellets have an enteric coating and release less than 10% of the tamsulosin in two hours in SGF.
Owner:SYNTHON PHARMA
Pharmaceutical pellets comprising tamsulosin
Tamsulosin pellets having an advantageous release profile are formed. The pellets have an enteric coating and release less than 25% of the tamsulosin in two hours in SGF.
Owner:SYNTHON BV
Overactive bladder treating drug
A therapeutic agent for overactive bladder containing tamsulosin or a pharmaceutically acceptable salt thereof as an effective ingredient.
Owner:ASTELLAS PHARMA INC
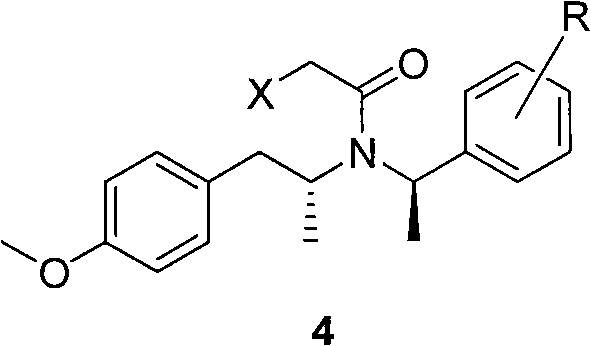
Preparation method of tamsulosin
InactiveCN101284807ALow costRaw materials are easy to getSulfonic acid amide preparationChemical productsSubstituted phenethylamine
The invention discloses a method for making tamsulosin, which takes methoxyphenylacetone as the initial raw material and (R)-1-phenethylamine or substituted phenethylamine as chiral auxiliary reagent, and obtains the final raw material drug, namely tamsulosin through diastereoselective reductive amination, salifying, haloacetylization, halosulfonation, amination, alkylation, acylamide reduction and debenzylation. The method for making the tamsulosin has the advantages that: the method has low cost and easy-obtaining raw materials, each reaction is suitable for industrial production, and the obtained chemical product has high purity.
Owner:2Y CHEM
Sustained release tamsulosin formulations
A sustained release tamsulosin formulation contains tamsulosin, a hydrophobic polymer, a microsphere forming agent and a diluent. The hydrophobic polymers include pH-dependent and pH-independent polymers are used as the release-modulating agent to control the dissolution profile of tamsulosin formulation so that the formulation releases tamsulosin slowly and continuously as the formulation passed through the stomach and gastrointestinal tract.
Owner:STANDARD CHEM PHARM
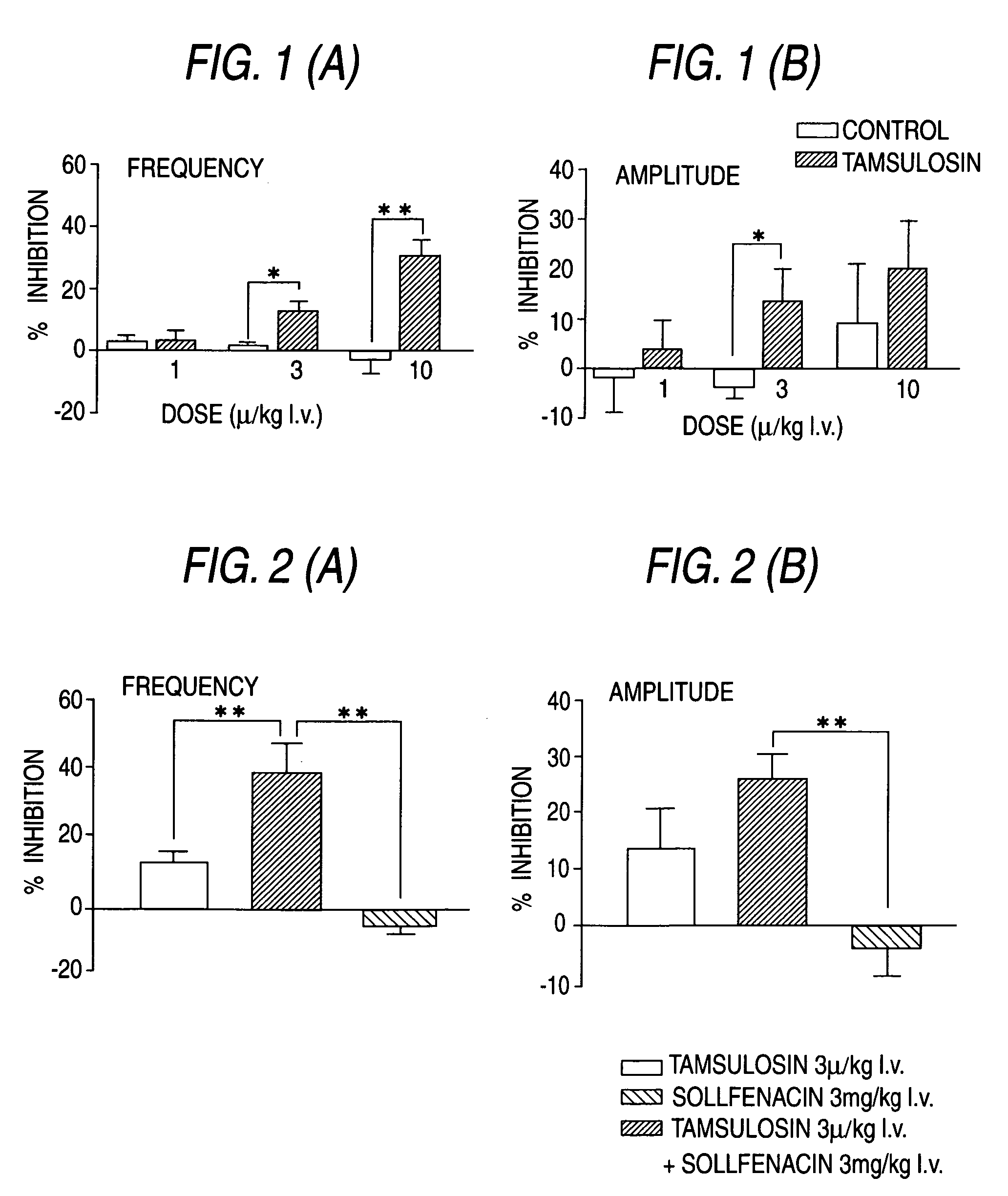
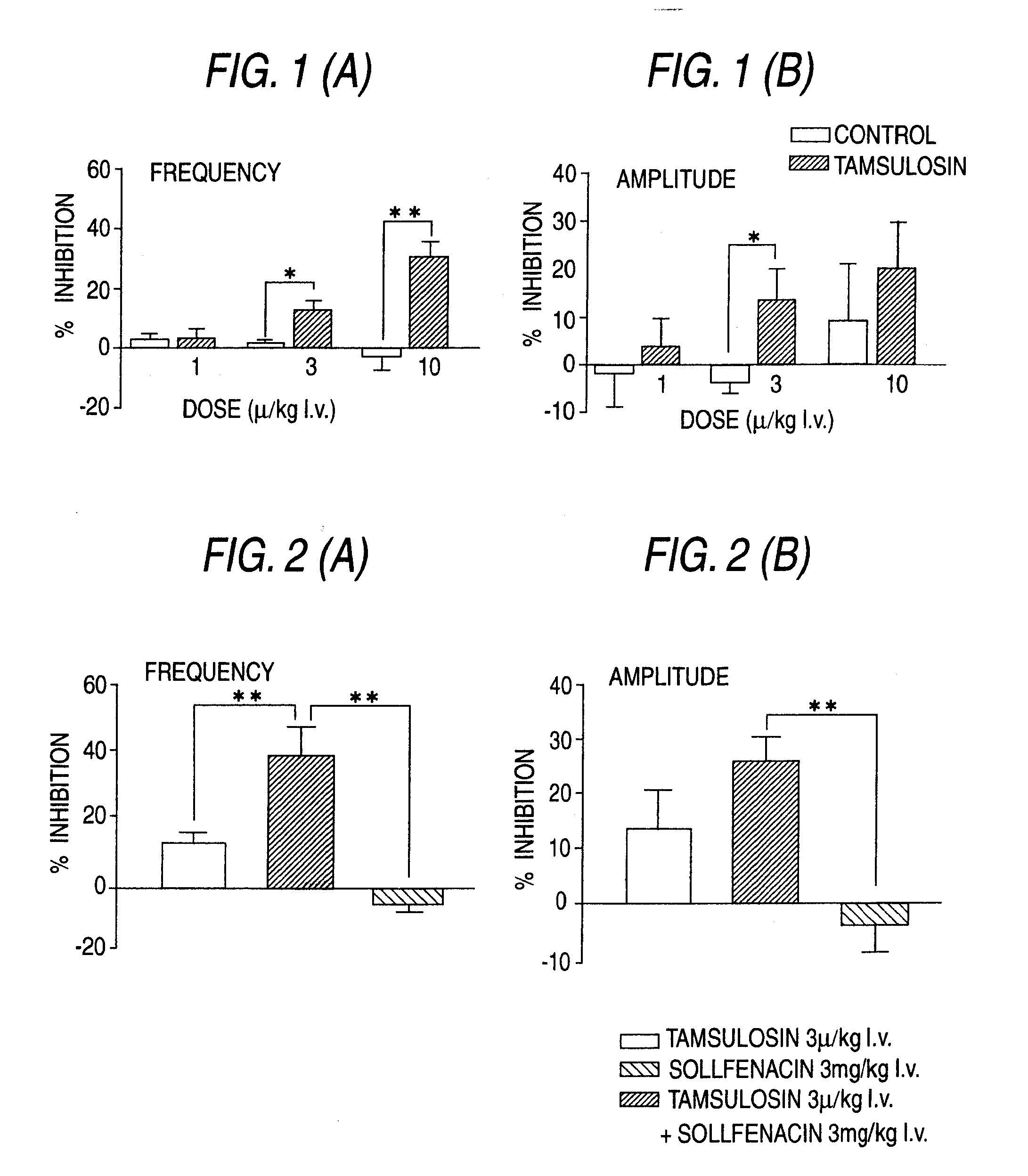
Combined Use of an Alpha-Adrenergic Receptor Antagonist and an Anti-Muscarinic Agent
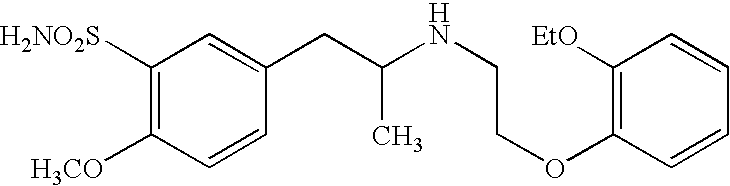
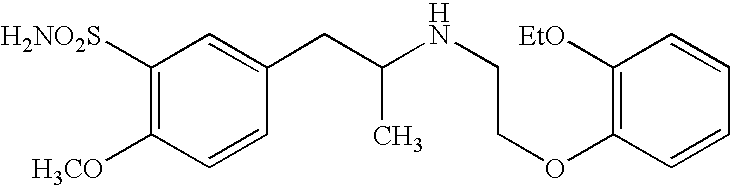
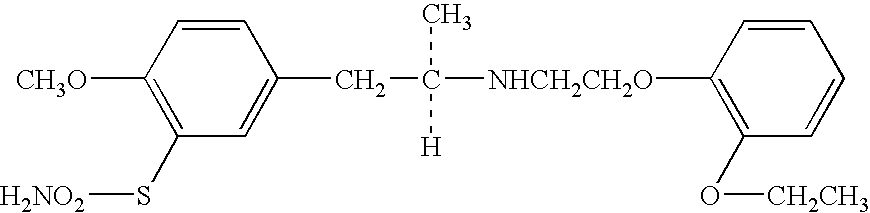
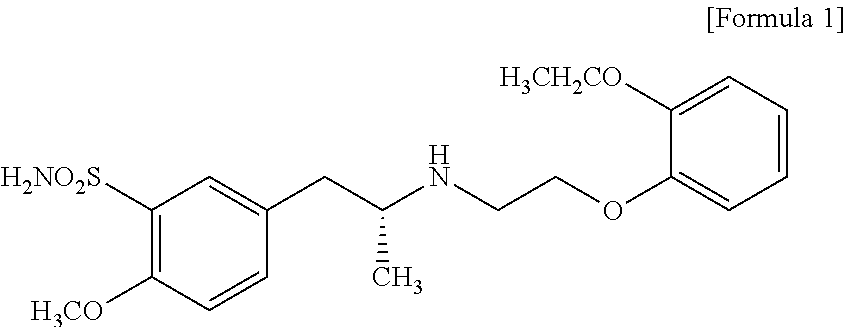
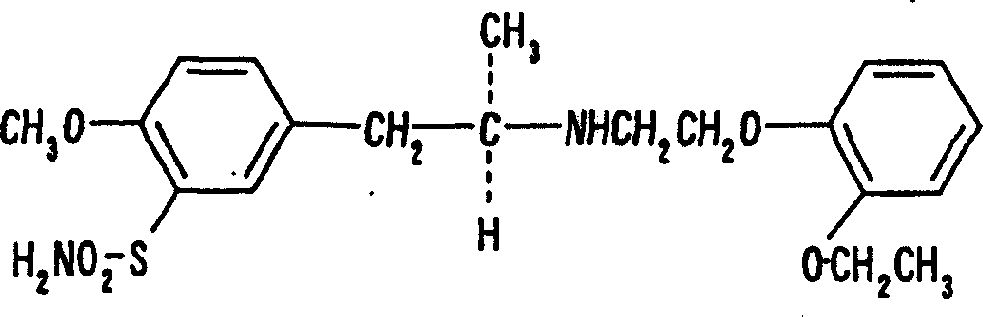
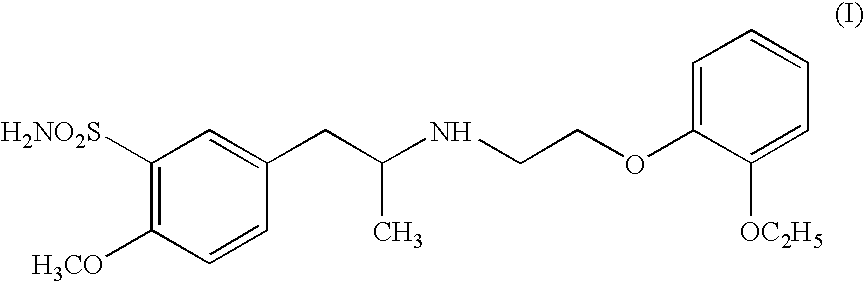
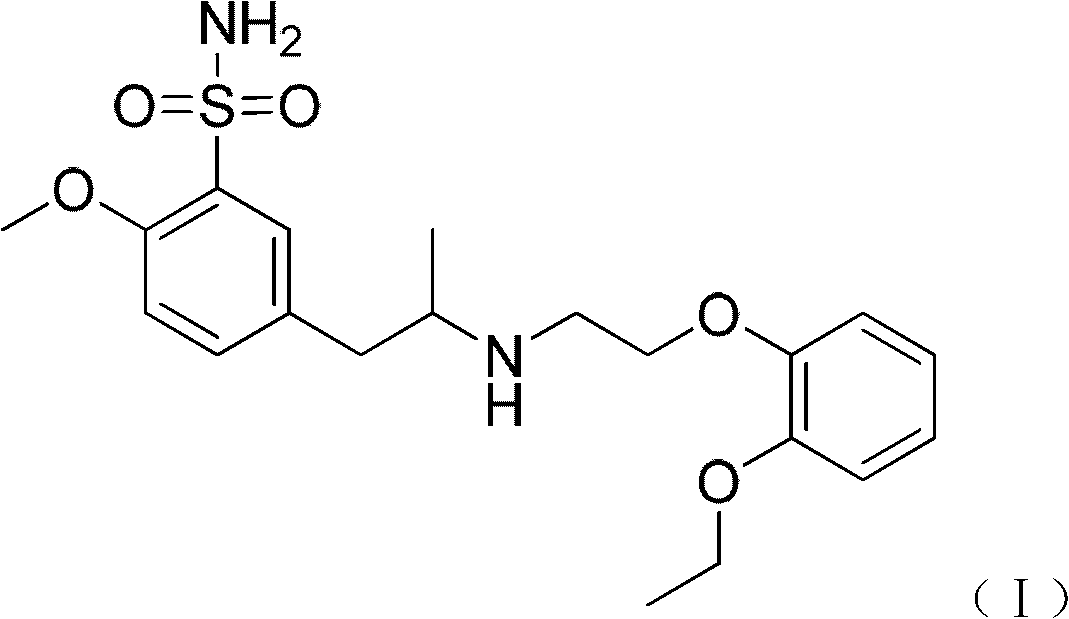
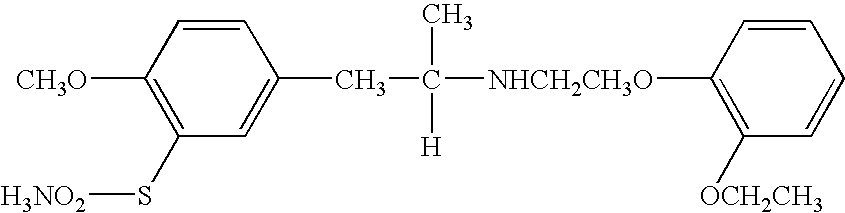
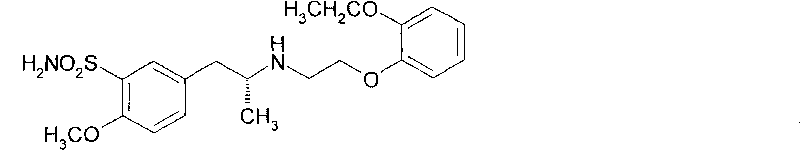
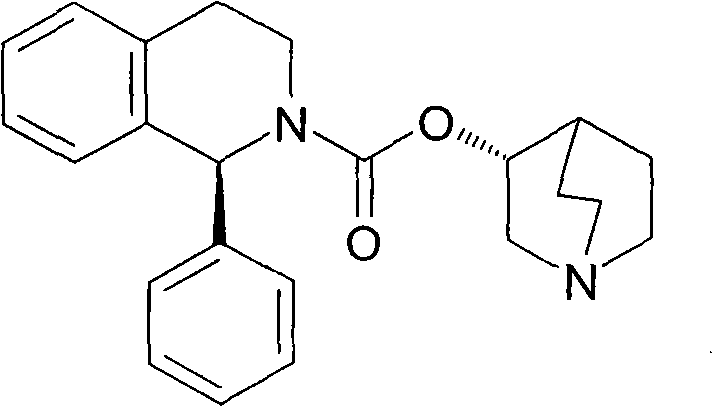
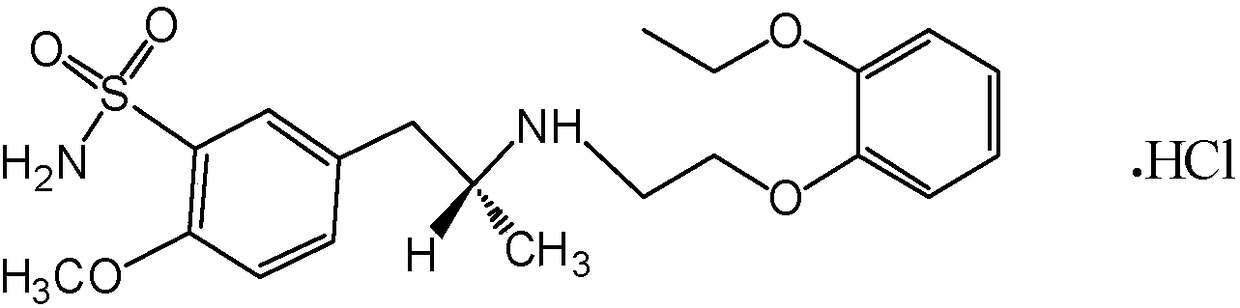
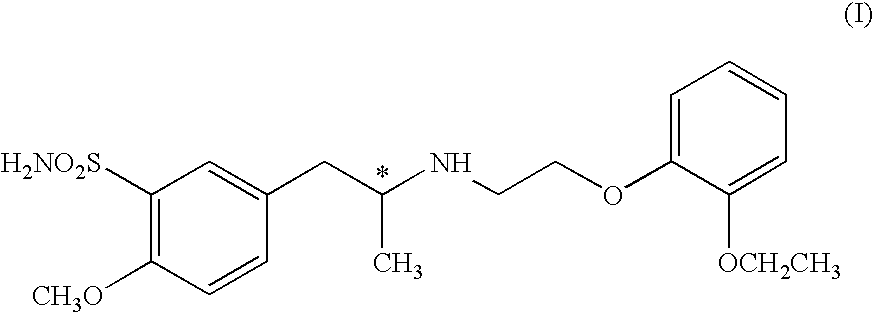
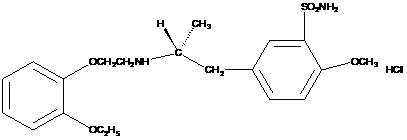
The combined use of (R)-5-(2-{[2-(2-ethoxyphenoxy)ethyl]amino}propyl)-2-methoxybenzene-1-sulfonamide (tamsulosin), or its pharmaceutically acceptable salt, and (1S)-1-phenyl-1,2,3,4-tetrahydroisoquinoline-2-carboxylic acid (3R)-quinuclidin-3-yl ester (solifenacin), or its pharmaceutically acceptable salt, for the preparation Of a medicament for the improvement of lower urinary tract symptoms associated with benign prostatic hyperplasia (LUTS / BPH) with a substantial storage component is provided.
Owner:ASTELLAS IRELAND
Slow-release composition containing tamsulosin and preparation thereof
The invention provides a slow-release composition containing tamsulosin and preparation thereof; and a slow-release micro-pill preparation which is prepared by adding a certain thinning agent and retarding agent has the release rate being consistent with the need.
Owner:BEIJING TRADE STAR MEDICAL TECH
Therapeutic agent for overactive bladder
A medicinal composition for treatments for overactive bladder which contains tamsulosin or a pharmaceutically acceptable salt thereof as an active ingredient.
Owner:ASTELLAS PHARMA INC
Sustained release tamsulosin formulations
A sustained release tamsulosin formulation contains tamsulosin, a hydrophobic polymer, a microsphere forming agent and a diluent. The hydrophobic polymers include pH-dependent and pH-independent polymers are used as the release-modulating agent to control the dissolution profile of tamsulosin formulation so that the formulation releases tamsulosin slowly and continuously as the formulation passed through the stomach and gastrointestinal tract. The present invention further relates to a method for preparing the sustained release tamsulosin formulation.
Owner:STANDARD CHEM & PHARMA
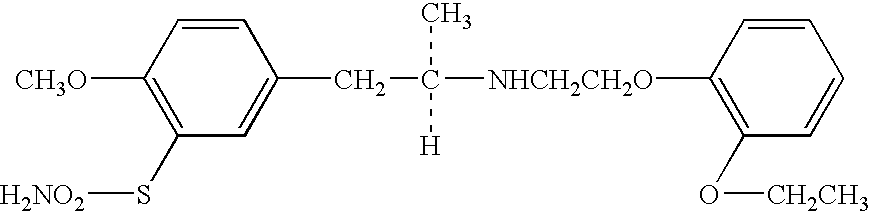
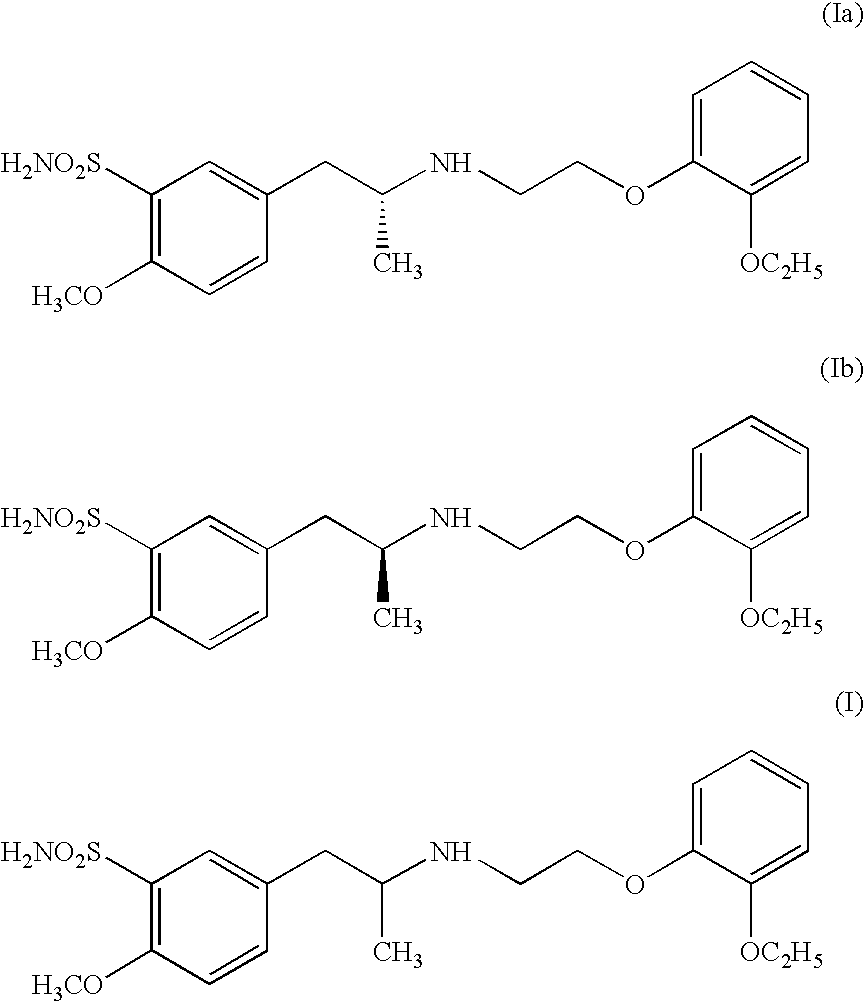
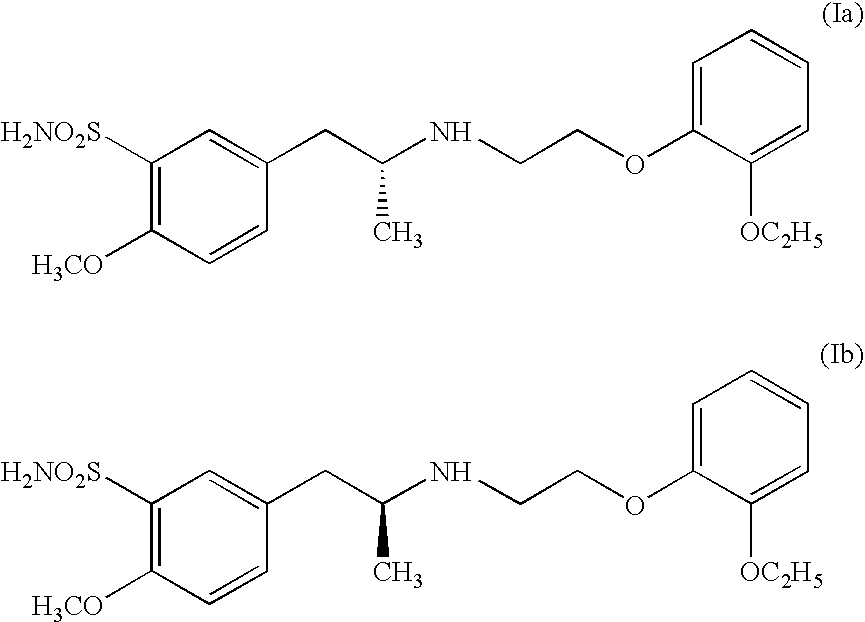
Process for preparing R- and S-isomers of (R)-5-(2-( (2-(2-ethoxyphenoxy) ethyl) amino) propyl) -2-methoxybenzenesulfonamide
InactiveUS20050079589A1Organic compound preparationOrganic chemistry methodsOrganic solventEnantiomer
A process for preparing optically pure enantiomers of R-(−)tamsulosin of formula Ia and S-(+)tamsulosin of formula Ib by resolving racemic tamsulosin of formula I by means of (1R)-(−)-camphor-10-sulfonic acid and (1S)-(+)-camphor-10-sulfonic acid, resp., in an environment of organic solvents, water or mixtures thereof.
Owner:FARMAK
Compound tamsulosin and finasteride sustained release capsule and preparation method thereof
InactiveCN101703511AStable blood concentrationProlong the action timePharmaceutical delivery mechanismUrinary disorderSustained release pelletsSide effect
The invention relates to compound tamsulosin and finasteride sustained release capsules and a preparation method thereof. The compound sustained release capsule is prepared by taking tamsulosin and finasteride as medical bioactive ingredients to prepare medicine-containing pellets, coating the medicine-containing pellets by coating materials to prepare sustained release pellets, and placing the sustained release pellets into hard capsule shells. The compound tamsulosin and finasteride sustained release capsules are used for treating benign prostatic hyperplasis (BPH), can be very slowly released to maintain stable plasma concentration and longer action time, and have the advantages of less toxic and side-effects and convenient application.
Owner:SHENYANG PHARMA UNIVERSITY
Compound preparation and preparation method thereof
InactiveCN102247379AReduced risk of concurrent acute urinary retentionUrinary disorderAmide active ingredientsActive componentHard Capsule
The invention discloses a compound preparation, and a preparation method thereof. The compound preparation comprises active components of tamsulosin and dutasteride, wherein tamsulosin is enteric pellets, particles or tablets, and dutasteride is soft capsules. tamsulosin and dutasteride are filled in one hard capsule. The invention also discloses the preparation method of the compound preparation.
Owner:BEIJING RUNDEKANG MEDICAL TECH CO LTD
Combination of polyethylene glycol and tamsulosin and pharmaceutical compound comprising same
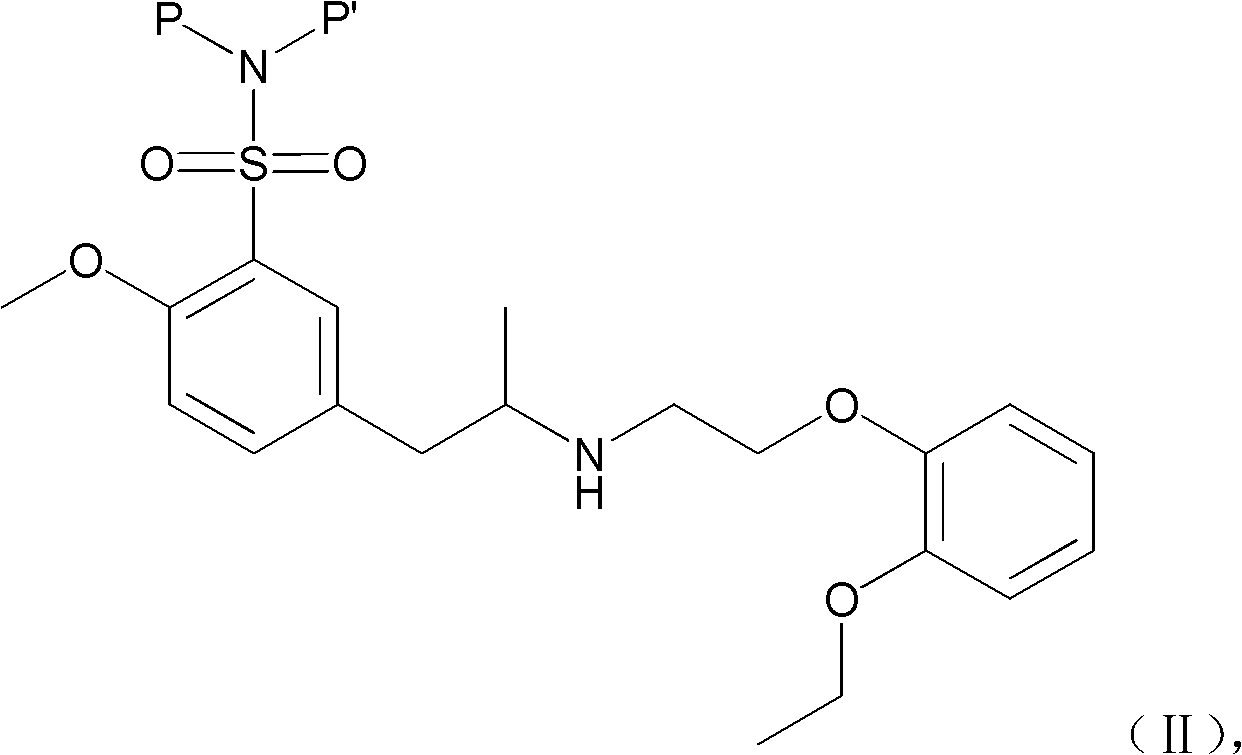
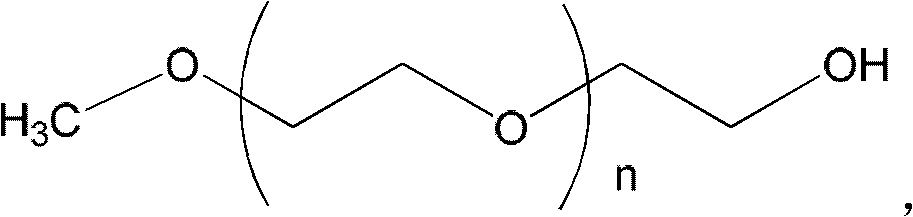
ActiveCN103127520APromote absorptionReduce transmittanceUrinary disorderPharmaceutical non-active ingredientsAlkyl transferTamsulosin
The invention provides a combination of polyethylene glycol and tamsulosin and a pharmaceutical compound comprising the combination which is as shown in a general formula (II). In the combination, a P and a P' are H or polyethylene glycol, and the P and the P' cannot be the H at the same time. The tamsulosin structurally contains a sulfamide group, the low-molecular-weight polyethylene glycol is introduced through an alkylation reaction, and therefore hydrophobicity of the tamsulosin is lowered, hydrophilism of the tamsulosin is increased, and the blood-brain barrier permeability of the tamsulosin is lowered. Therefore, the toxicity of the tamsulosin is lowered.
Owner:JENKEM TECH CO LTD TIANJIN
Medicinal compositions for treating evacuatory insufficiency
InactiveUS6861070B1Effective therapyReduce prostatic pressurePowder deliveryNervous disorderNeurogenic Urinary BladderTamsulosin
A therapeutic agent for voiding dysfunction associated with neurogenic bladder where said agent contains tamsulosin or a pharmaceutically acceptable salt thereof.
Owner:ASTELLAS PHARMA INC
Use of alpha-adrenergic blockers for the treatment of dysmenorrhea
A method of treating primary dysmenorrhea which comprises administering to a human female suffering from the same a therapeutically effective amount of alpha-adrenergic blocker. Exemplary alpha-adrenergic blockers are phenoxybenzamine, alfuzosin, doxazosin, terazosin, prazosin, and tamsulosin, or a pharmaceutically acceptable salt or ester thereof. Tamsulosin HCl is preferred.
Owner:BOEHRINGER INGELHEIM PHARMA INC
Composite preparation and preparing method thereof
InactiveCN102309495AReduced risk of concurrent acute urinary retentionUrinary disorderAmide active ingredientsHard CapsuleTamsulosin
The invention relates to a composite preparation, and preparing method and use thereof. The composite preparation uses Tamsulosin and Dutasteride as active ingredients, wherein Tamsulosin is in the form of enteric pellet, granule or tablet, Dutasteride is in the form of soft capsule, and both of them are filled in a hard capsule. The invention discloses a preparing method of the composite preparation.
Owner:BEIJING RUNDEKANG MEDICAL TECH CO LTD
Tamsulosin and finasteride compound sustained release tablets and preparation method thereof
InactiveCN101703510AGood synergyPromote apoptosisPharmaceutical delivery mechanismUrinary disorderSustained Release TabletSide effect
The invention relates to compound sustained release tablets taking tamsulosin and finasteride as medicinal active ingredients, wherein the tamsulosin can also exist in the form of other pharmaceutically acceptable salts. The preparation also comprises one or more pharmaceutically acceptable sustained release carriers and auxiliary materials consisting of conventional preparations besides main components. The compound sustained release tablets have the advantages of good therapeutic effect, fewer side effects, and safe and reliable use, can maintain steadier blood drug concentration and longer action time due to the sustained release of the medicament, and can be used for the treatment of benign prostatic hyperplasia (BPH).
Owner:SHENYANG PHARMA UNIVERSITY
Combination therapy for male sexual dysfunction
InactiveUS20170326139A1Reliable and effective and convenient on demand treatmentImproves overall sexual well-beingPill deliveryAmide active ingredientsTadalafilEscitalopram
Pharmaceutical formulations containing a serotonin reuptake inhibitor and a smooth muscle relaxant are provided for the treatment of premature ejaculation and the increase in intravaginal ejaculatory latency time. Specific formulations contain tadalafil (1-30 mg per dose) or tamsulosin (0.05-2 mg) and escitalopram (1-30 mg) in daily dose and on-demand formulations.
Owner:ATP THERAPEUTICS
Tamsulosin sustained release pellet and preparation method thereof
ActiveCN102579359APharmaceutical non-active ingredientsUrinary disorderSustained release pelletsSolubility
The invention provides a tamsulosin sustained release pellet. The tamsulosin sustained release pellet comprises a medicine-containing pellet core and a sustained release coating layer, wherein the sustained release coating layer comprises a hydrophobic matrix and a hydrophilic polymer which is sensitive to pH values. The pH values of a high molecular polymer in the dissolving process are 1 to 14, so that medicines are released at different pH values; and simultaneously, the high molecular polymer can be used as a carrier for controlling tamsulosin to be released, so that a prescription is simplified, preparation difficulties are reduced, and the tamsulosin sustained release pellet is easy to industrially produce in batches. Surprisingly, the preparation can be used for preparing various tamsulosin sustained release formulations without dependence on the physicochemical property of the tamsulosin.
Owner:COSCI MED TECH CO LTD
Tamsulosin sustained-release pellet preparation and preparation method thereof
InactiveCN103315962ADrug release rate is difficult to controlUrinary disorderPharmaceutical non-active ingredientsSustained release pelletsTamsulosin
The invention provides a tamsulosin sustained-release pellet, and the drug release of the sustained-release pellet is controlled through a blank pellet core coated with a drug-loaded sustained-release layer and then coated with an enteric coating, and the drug can be both released in an acidic or weak alkaline environment and is absorbed well in vivo. Also, release homogeneity is good, preparation technology is simple and repeatability is good.
Owner:COSCI MED TECH CO LTD
Pharmaceutical composition for amelioration of lower urinary tract symptom associated with prostatomegaly
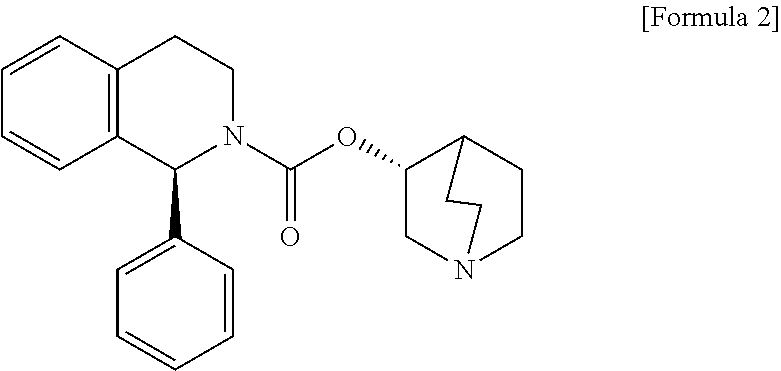
Disclosed is a pharmaceutical composition comprising (R)-5-(2-¢¢2-(2-ethoxyphenoxy)ethyl!amino!propyl)-2-methoxybenzene-1-sulfonamide (tamsulosin) or a pharmaceutically acceptable salt thereof and (1S)-1-phenyl-1,2,3,4-tetrahydroisoquinoline-2-carboxylate (3R)-quinuclidin-3-yl ester (solifenacin) or a pharmaceutically acceptable salt thereof as active ingredients, particularly a pharmaceutical composition for ameliorating a lower urinary tract symptom associated with prostatomegaly. Also disclosed is use of a combination of tamsulosin or a pharmaceutically acceptable salt thereof and solifenacin or a pharmaceutically acceptable salt thereof for the amelioration of a lower urinary tract symptom associated with prostatomegaly. Further disclosed is a combination therapy.
Owner:ASTELLAS PHARMA INC
Tamsulosin orally disintegrating tablet composition with slow release performance
ActiveCN108066304AHigh hardnessGood disintegrationInorganic non-active ingredientsUrinary disorderOrally disintegrating tabletTamsulosin
The invention relates to a tamsulosin orally disintegrating tablet composition with slow release performance. Concretely, the invention relates to an orally disintegrating tablet, which is a tablet obtained through pressing by using a tableting process. The tablet comprises a tablet base body and a plurality of coated micropills, wherein the tablet base body is prepared from a plurality of auxiliary materials; the coated micropills are basically uniformly dispersed in the tablet base body; the coated micropills comprise pill cores containing active ingredients and at least one layer of coatingcovering the surfaces of the pill cores. The invention further relates to a method for preparing the orally disintegrating tablet. The invention further relates to a method for improving the hardnessof the orally disintegrating tablet and improving the disintegrating performance. According to the method, the orally disintegrating tablet is pressed through a tableting process. The tablet comprises the tablet base body prepared from a plurality of auxiliary materials and a plurality of coated micropills basically uniformly dispersed in the tablet base bodies. The orally disintegrating tablet provided by the invention has excellent effects shown in the description in each aspect.
Owner:SHENZHEN WANHE PHARMA
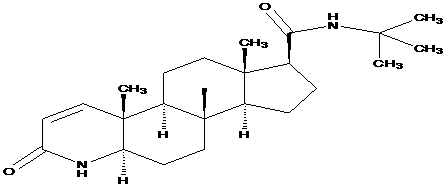
Process for the Preparation of Tamsulosin
InactiveUS20080262089A1Reduce amountEfficient separationBiocideOrganic compound preparationTriethylphosphiteTrimethyl phosphite
The invention includes an improved process for producing tamsulosin comprising reacting 5-(2-aminopropyl)-2-methoxybenzenesulfonamide with 2-(o-ethoxyphenoxy)ethyl bromide in an organic phosphite solvent to obtain tamsulosin. Optically pure (R)-5-(2-aminopropyl)-2-methoxybenzenesulfonamide can be employed to produce optically pure (R)-tamsulosin product. The organic phosphite solvent utilized in the reaction can include tri-alkyl phosphites such as triethyl phosphite, trimethyl phosphite, and tributyl phosphite. Additionally, processes for producing tamsulosin having a low concentration of by-product contaminants, such as 5-((R)-2-{Bis-[2-(2-ethoxyphenoxy)ethyl]amino}-propyl)-2-methoxybenzenesulfonamide, and the use of such by-products to monitor the chemical purity of tamsulosin, are provided.
Owner:MEDICHEM
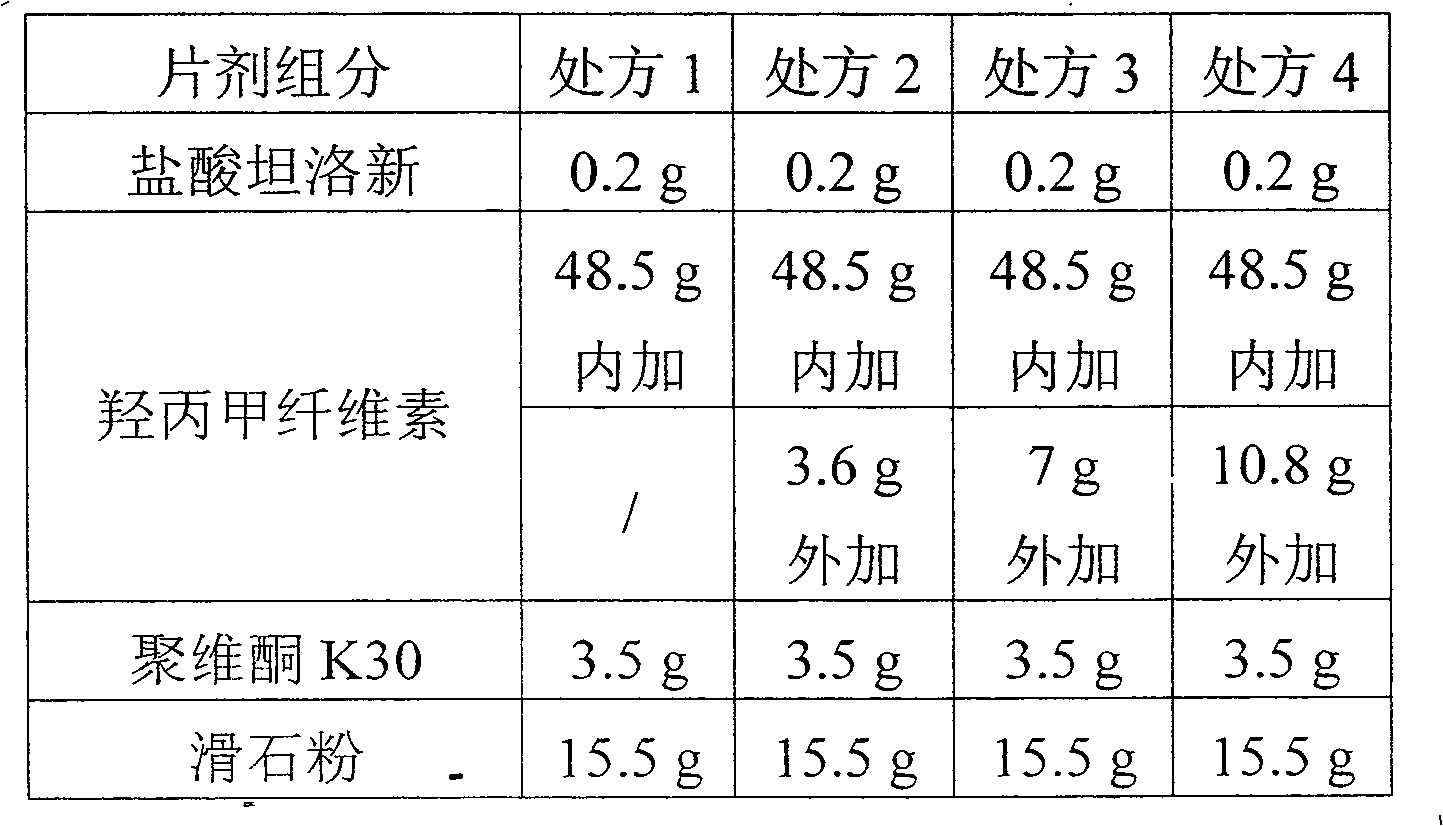
Tamsulosin sustained release tablet and preparation method thereof
ActiveCN101596171ASimple production processGood sustained release effectPharmaceutical delivery mechanismPharmaceutical non-active ingredientsSustained Release TabletMedicine
The invention provides a Tamsulosin tablet, which is prepared by the following steps: taking Tamsulosin, and dissolving in ethanol; adding a part of hydroxypropyl methyl cellulose and other proper excipients, evenly mixing, and pelletizing; and then adding the rest hydroxypropyl methyl cellulose, fully mixing, and pressing into the sustained release tablet. The release of the Tamsulosin tablet in two hours is effectively controlled within 40 percent, and the sustained release performance of the medicament is greatly improved. The preparation method has simple adopted equipment and concise production process.
Owner:KUNMING JIDA PHARMA
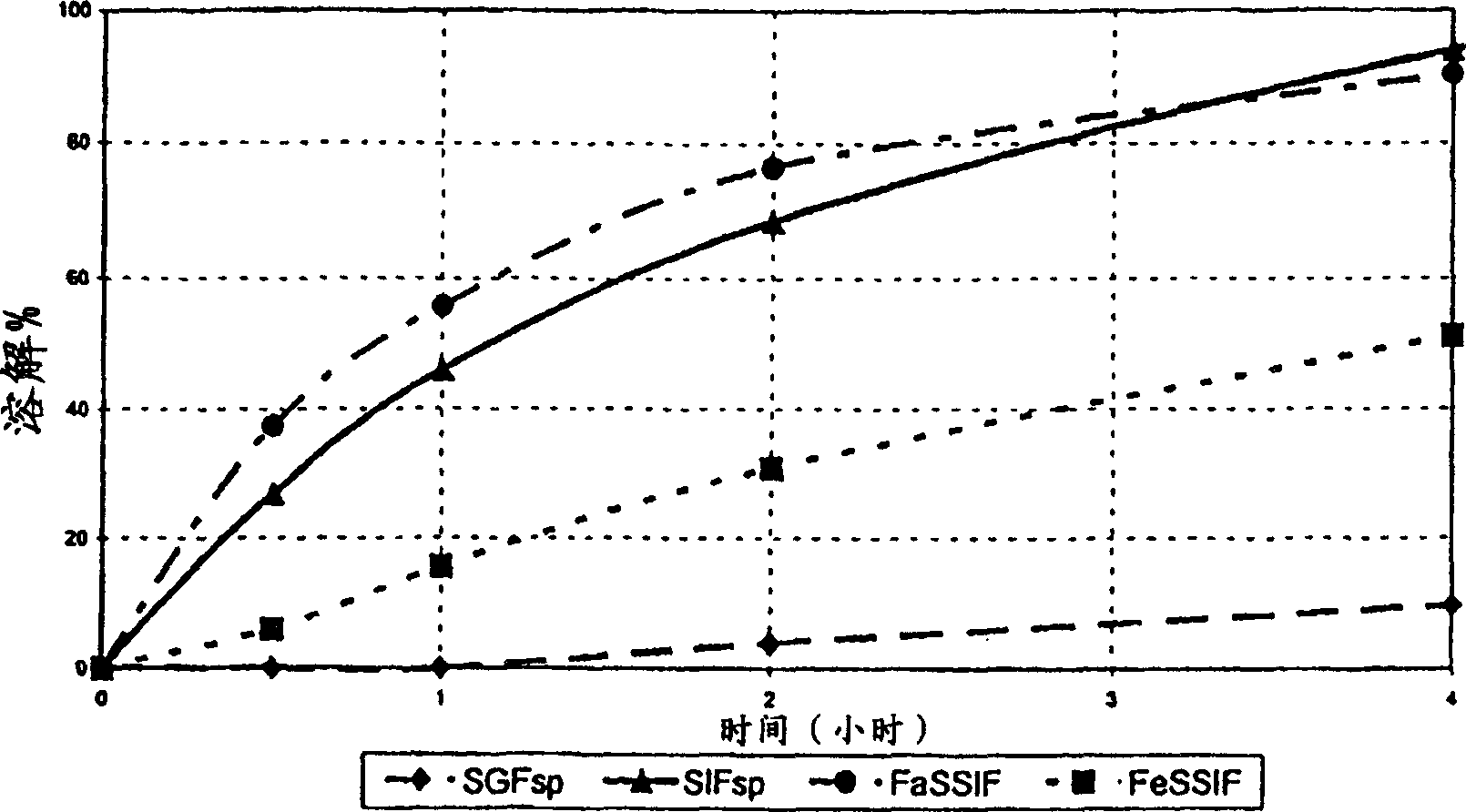
Modified release tamsulosin tablets
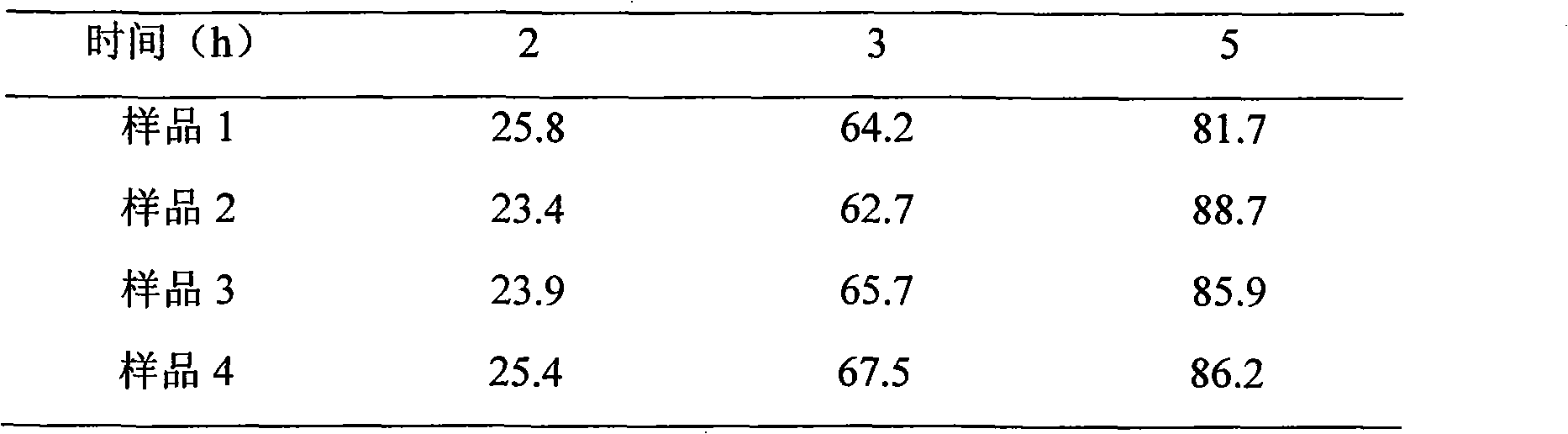
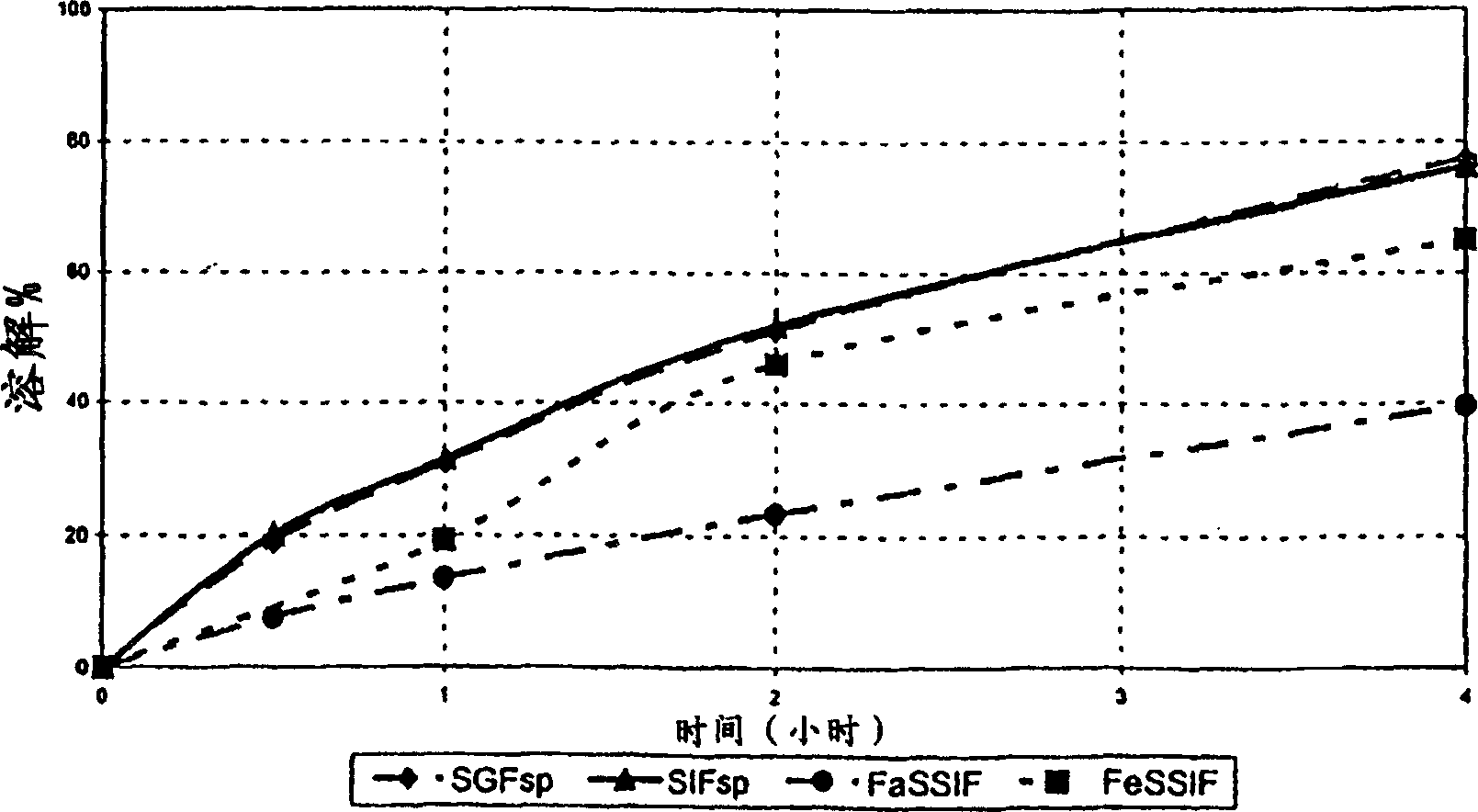
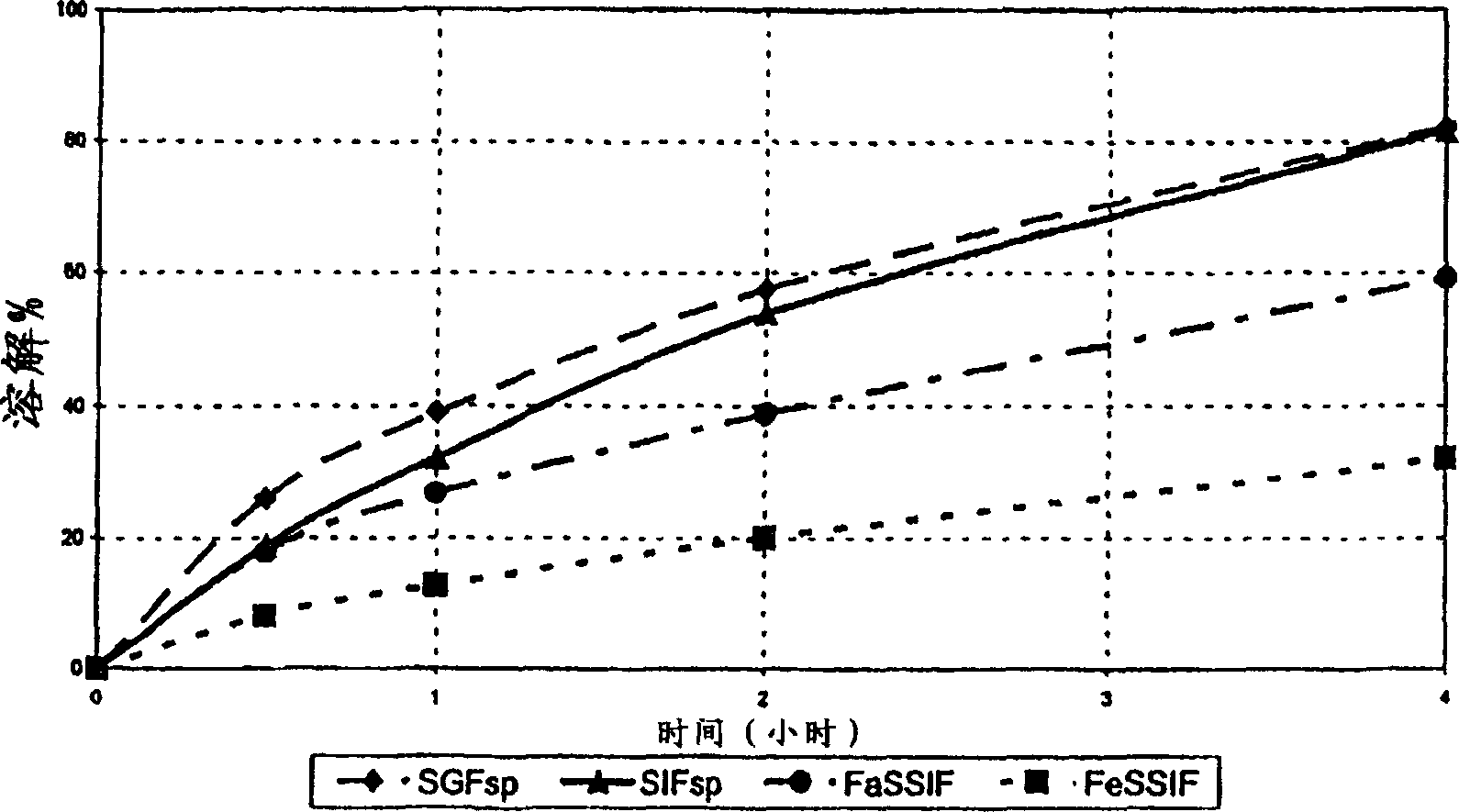
The invention relates to an adjustment release tablet comprising a tablet matrix having dispersed tamsulosin or a pharmaceutically acceptable salt thereof, and optionally having an enteric coating over said matrix, wherein said tablet has a dissolution profile such that in each of the media SIF, FaSSIF, and FeSSIF, said tablet releases not more than 60% of said tamsulosin at 2 hours elapsed time in USP 2 apparatus using 500 ml of said media at 50-100 rpm paddle speed.
Owner:SYNTHON BV
Compound tablet containing tamsulosin (slow-release) and finasteride (quick-release) and preparation method thereof
The invention relates to a compound tablet containing tamsulosin (slow-release) and finasteride (quick-release) and a preparation method of the compound tablet, and belongs to the field of pharmaceutical formulations.
Owner:江苏开元医药有限公司 +2
Hypogastric and/or perineal pain-relieving agent
A pharmaceutical composition for improving chronic pelvic cavity pain syndrome due to urinary dysfunction where said composition contains tamsulosin or pharmaceutically acceptable salts thereof.
Owner:YAMANOUCHI PHARMA CO LTD
Application of periplaneta Americana to preparation of medicine for treating prostatitis
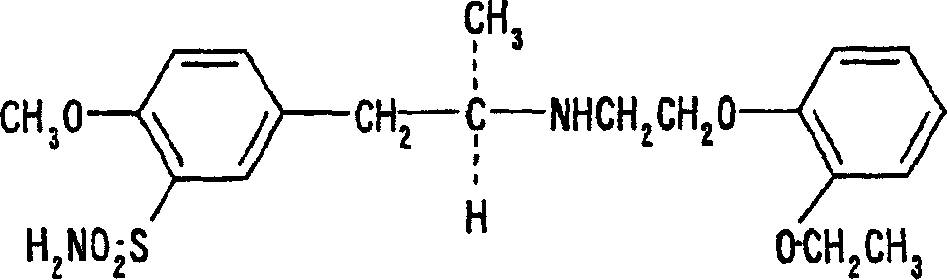
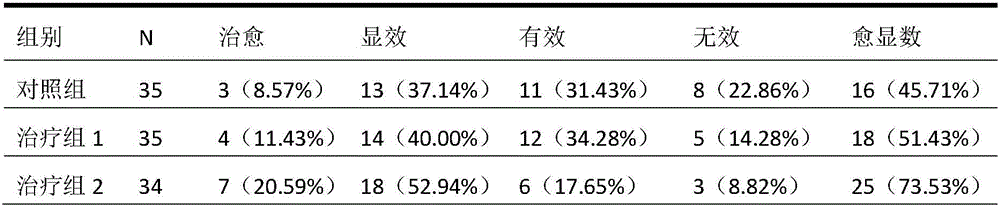
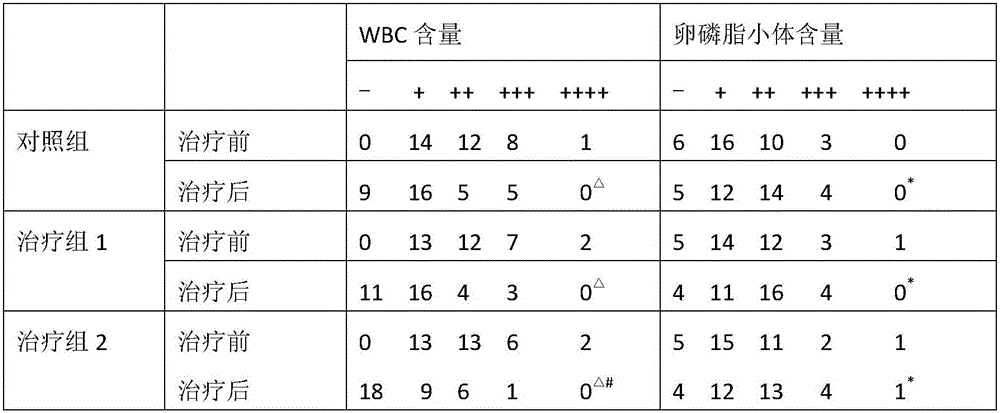
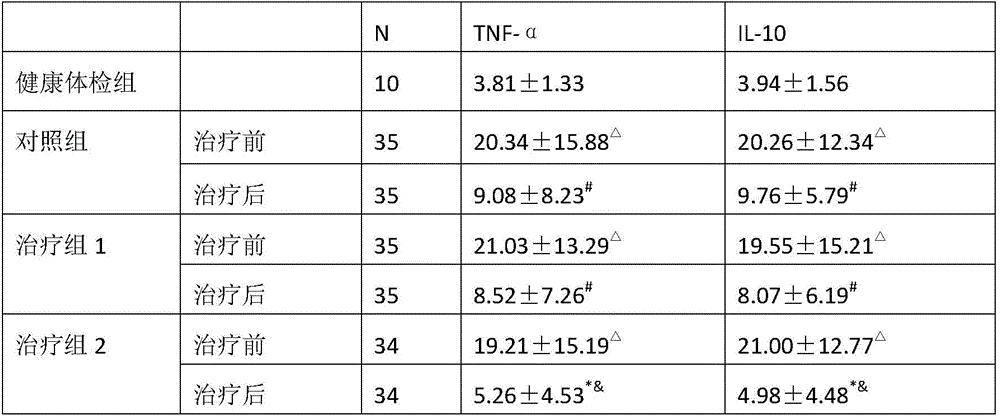
ActiveCN106074614AReduce contentGood effectAnthropod material medical ingredientsPharmaceutical delivery mechanismInflammatory factorsMedicine
The invention provides application of periplaneta Americana to preparation of a medicine for treating prostatitis. The medicine can reduce the WBC content in prostatic fluid of prostatitis patients and decrease inflammatory factors in prostatic fluid of the prostatitis patients, and prostatitis refers to IIIA prostatitis. The medicine contains a periplaneta Americana extract, levofloxacin and tamsulosin which are simultaneously or separately fed. Clinical test results show that the medicine has the clinical effective rate of up to 73.53% on IIIA prostatitis patients, and can remarkably reduce contents of WBC, TNF-alpha and IL-10 in prostatic fluid of the IIIA prostatitis patients.
Owner:SICHUAN GOODDOCTOR PANXI PHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com