Pharmaceutical composition for amelioration of lower urinary tract symptom associated with prostatomegaly
A composition and drug technology, applied in the direction of drug combination, pharmaceutical formula, urinary system diseases, etc., can solve the problem of no reported improvement of urination symptoms
Inactive Publication Date: 2010-06-23
ASTELLAS PHARMA INC
View PDF2 Cites 3 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
However, the literature does not report its improvement effect on voiding symptoms
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
Embodiment
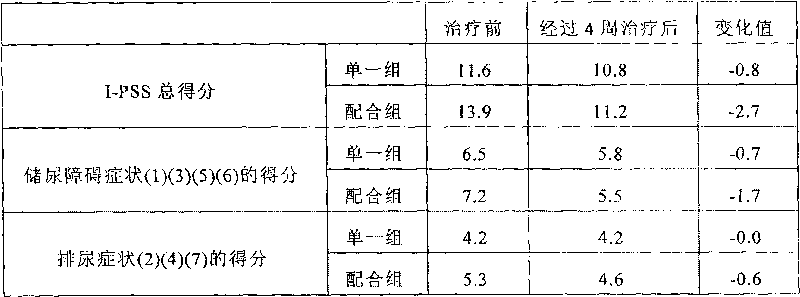
[0081] The combined therapeutic effect of the active ingredients in the pharmaceutical composition of the present invention was confirmed by the following tests.
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
 Login to View More
Login to View More Abstract
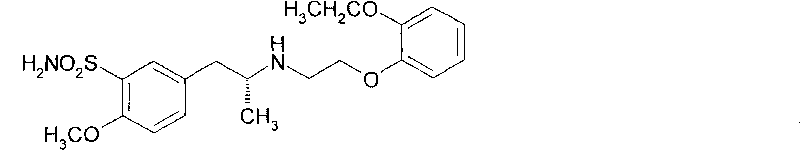
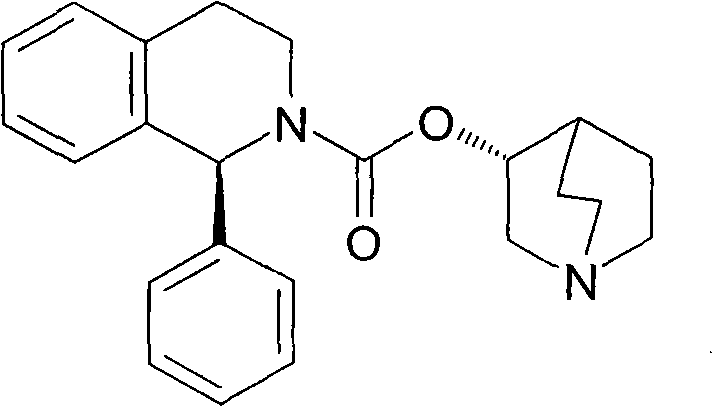
Disclosed is a pharmaceutical composition comprising (R)-5-(2-¢¢2-(2-ethoxyphenoxy)ethyl!amino!propyl)-2-methoxybenzene-1-sulfonamide (tamsulosin) or a pharmaceutically acceptable salt thereof and (1S)-1-phenyl-1,2,3,4-tetrahydroisoquinoline-2-carboxylate (3R)-quinuclidin-3-yl ester (solifenacin) or a pharmaceutically acceptable salt thereof as active ingredients, particularly a pharmaceutical composition for ameliorating a lower urinary tract symptom associated with prostatomegaly. Also disclosed is use of a combination of tamsulosin or a pharmaceutically acceptable salt thereof and solifenacin or a pharmaceutically acceptable salt thereof for the amelioration of a lower urinary tract symptom associated with prostatomegaly. Further disclosed is a combination therapy.
Description
technical field [0001] The present invention relates to a medicine, in particular to a pharmaceutical composition for improving lower urinary tract symptoms accompanied by prostatic hypertrophy, especially containing (R)-5-(2-{[2-(2-ethoxyphenoxy Base) ethyl] amino} propyl) -2-methoxybenzene-1-sulfonamide (hereinafter referred to as tamsulosin (tamsulosin)) or its pharmaceutically acceptable salt, and (1S)-1-benzene Base-1,2,3,4-tetrahydroisoquinoline-2-carboxylic acid (3R)-quinuclidin-3-yl ester (hereinafter referred to as solifenacin (solifenacin)) or its pharmaceutically acceptable Pharmaceutical composition with salt as active ingredient. The present invention also relates to the combined use of tamsulosin or a pharmaceutically acceptable salt thereof and solifenacin or a pharmaceutically acceptable salt thereof for improving lower urinary tract symptoms accompanied by prostatic hypertrophy, and a combined treatment method. Background technique [0002] The prostate is...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More Patent Type & Authority Applications(China)
IPC IPC(8): A61K31/4725A61K31/18A61P13/02A61P13/08
CPCA61K31/18A61K31/4725A61P13/02A61P13/08A61K2300/00
Inventor 柿崎秀宏吉田正贵内田武
Owner ASTELLAS PHARMA INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com