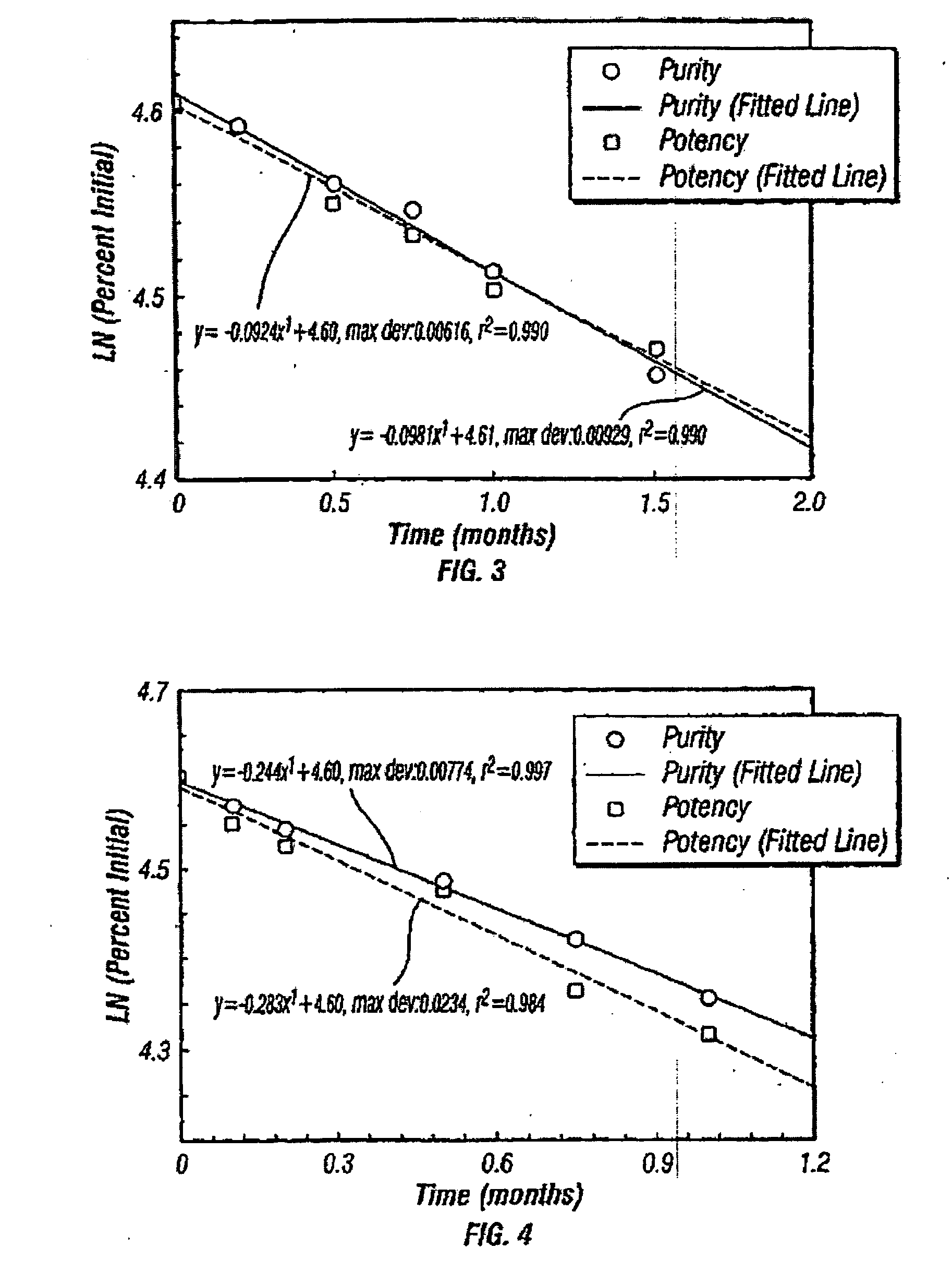
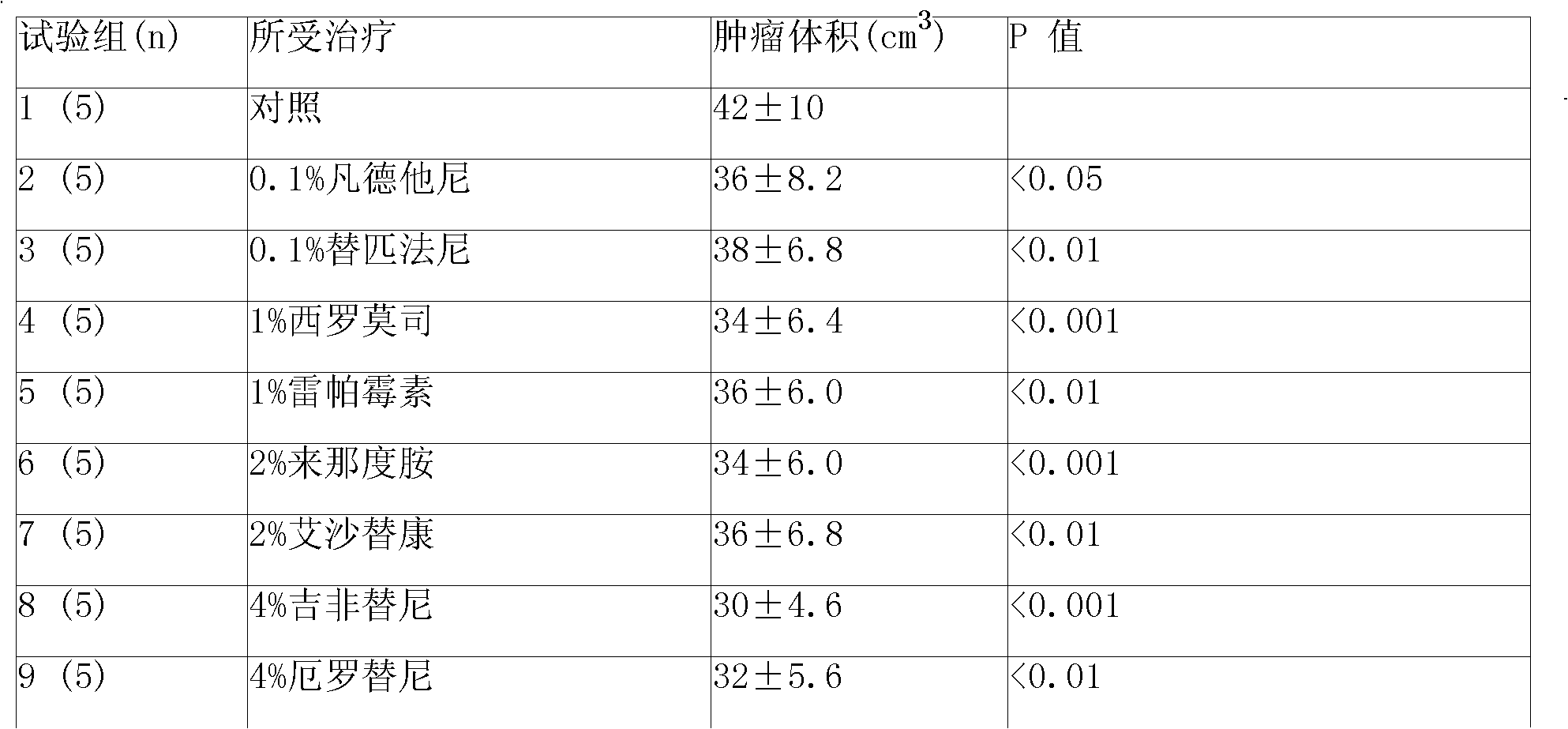
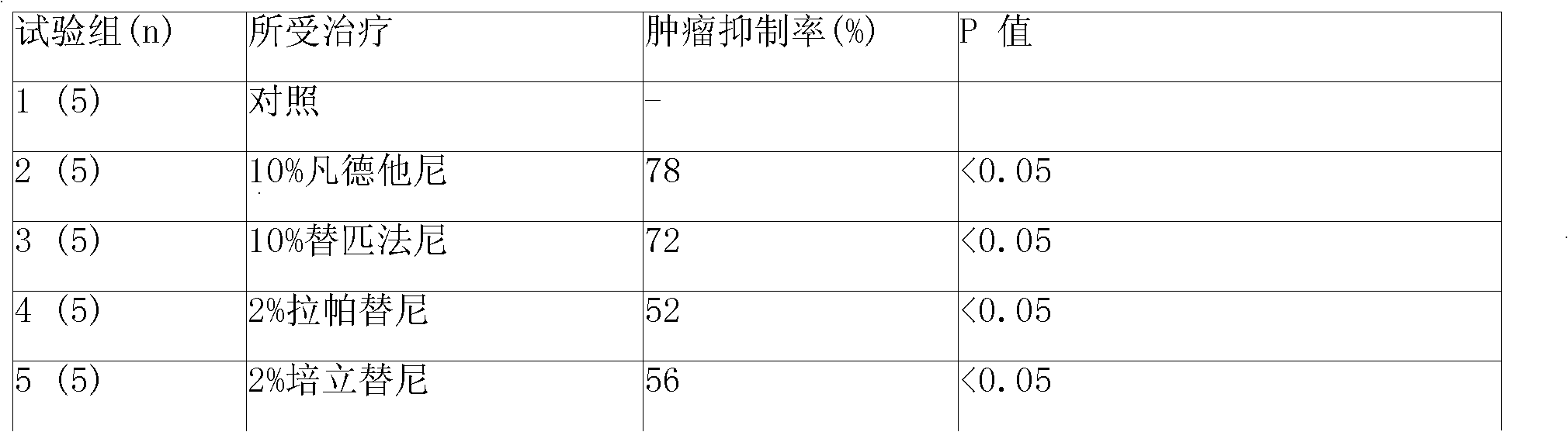
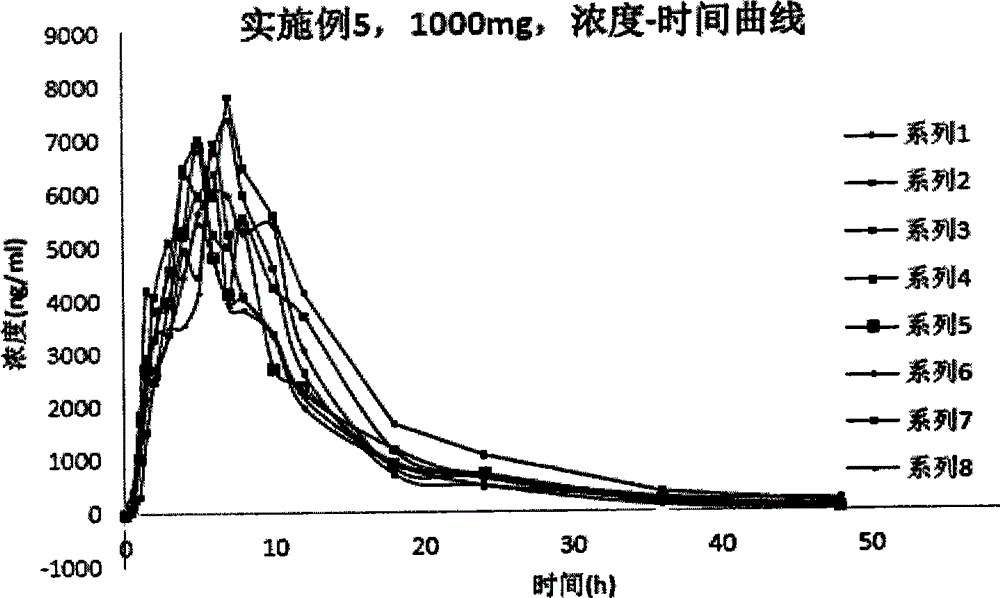
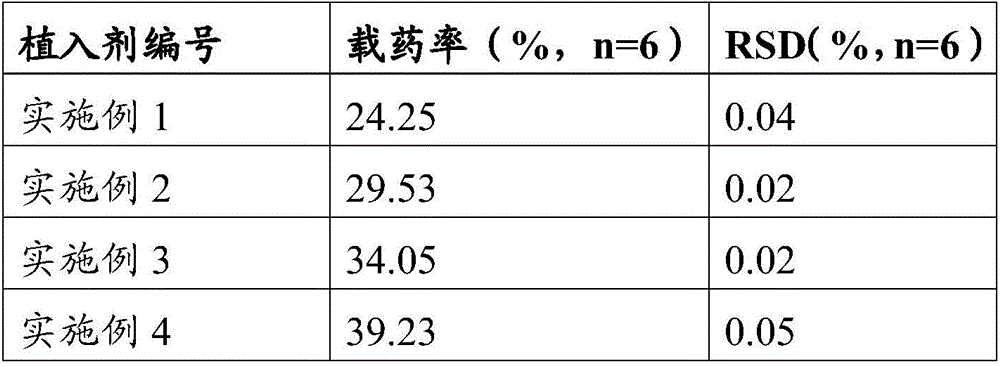
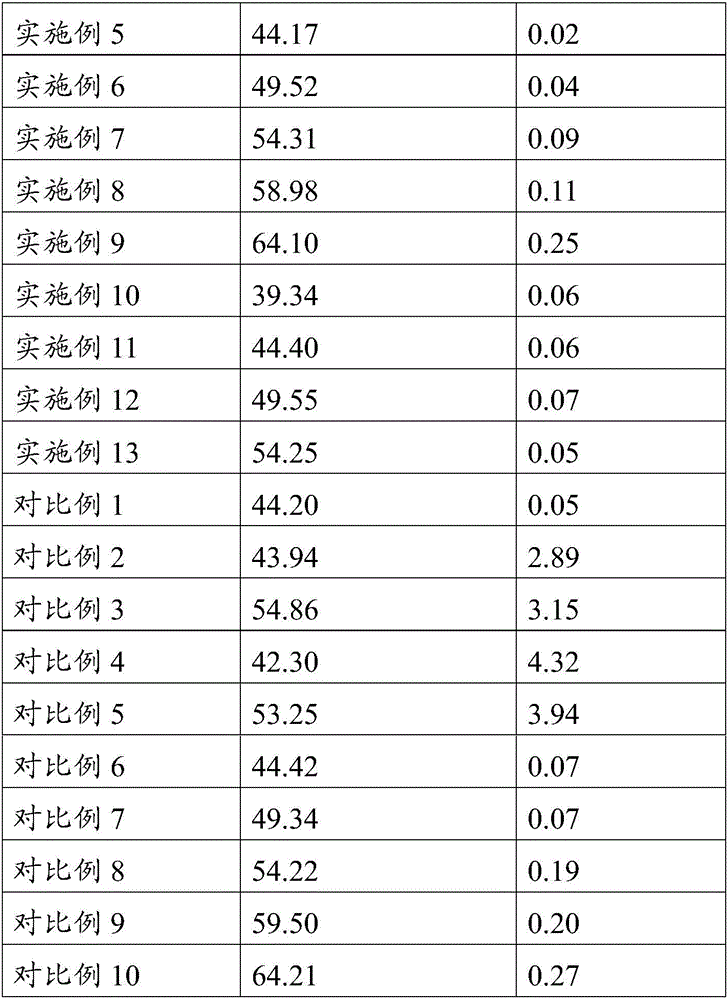
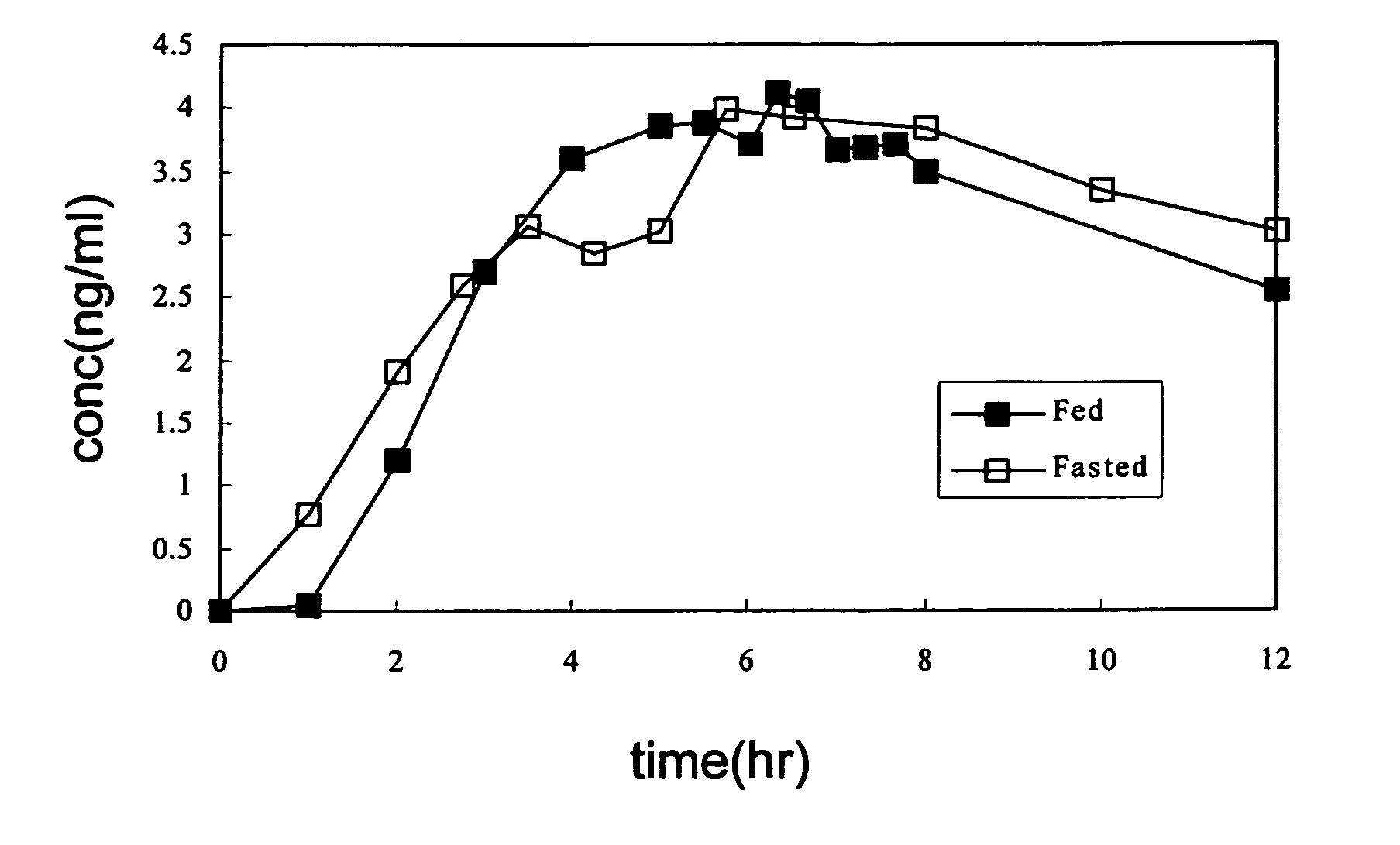
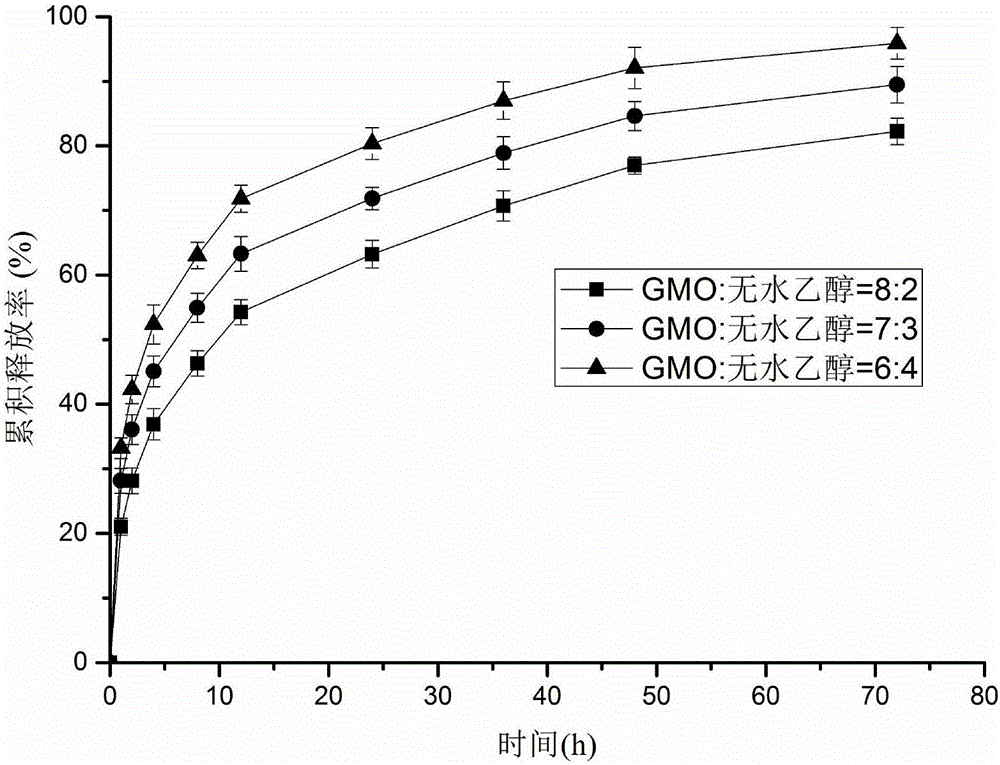
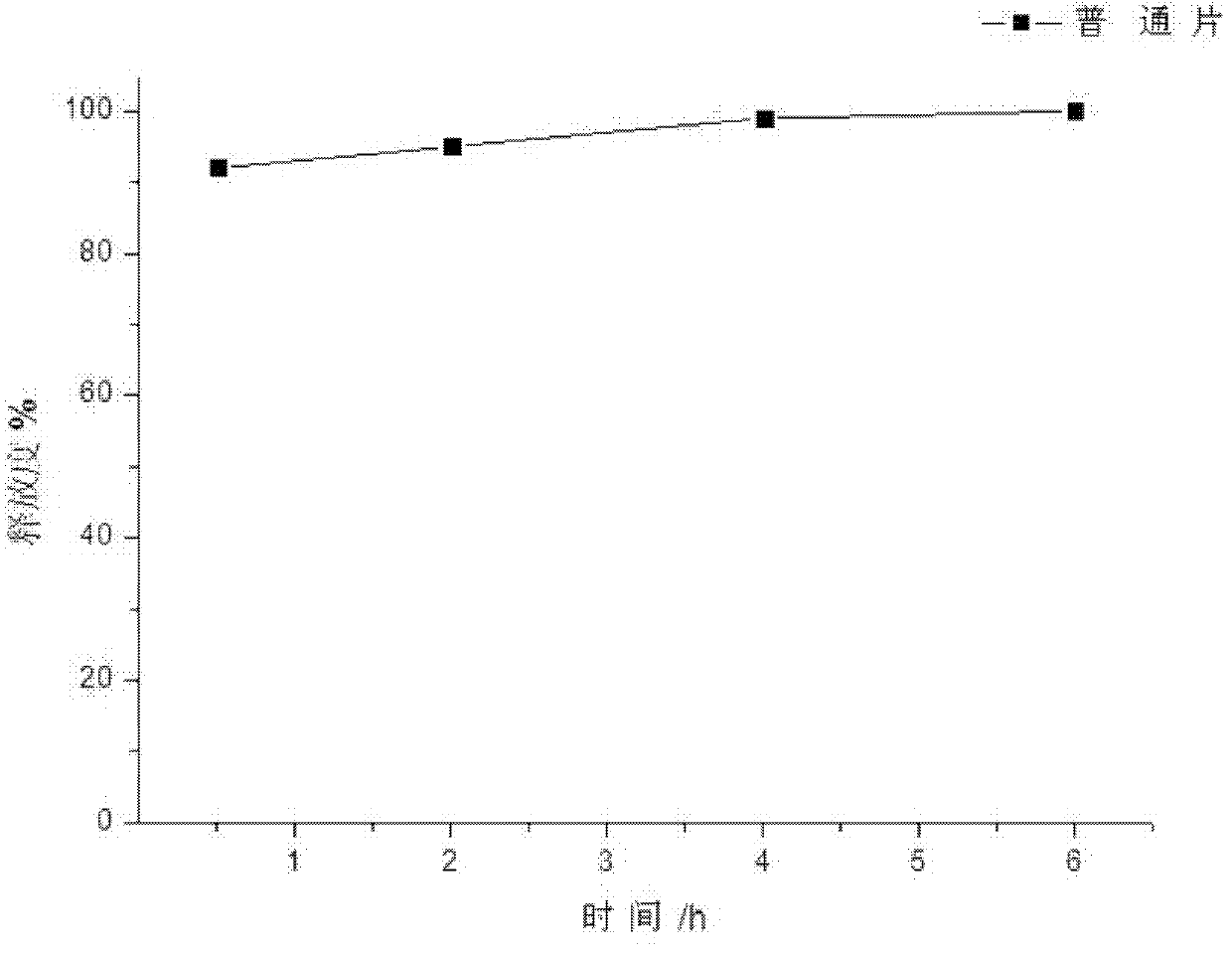
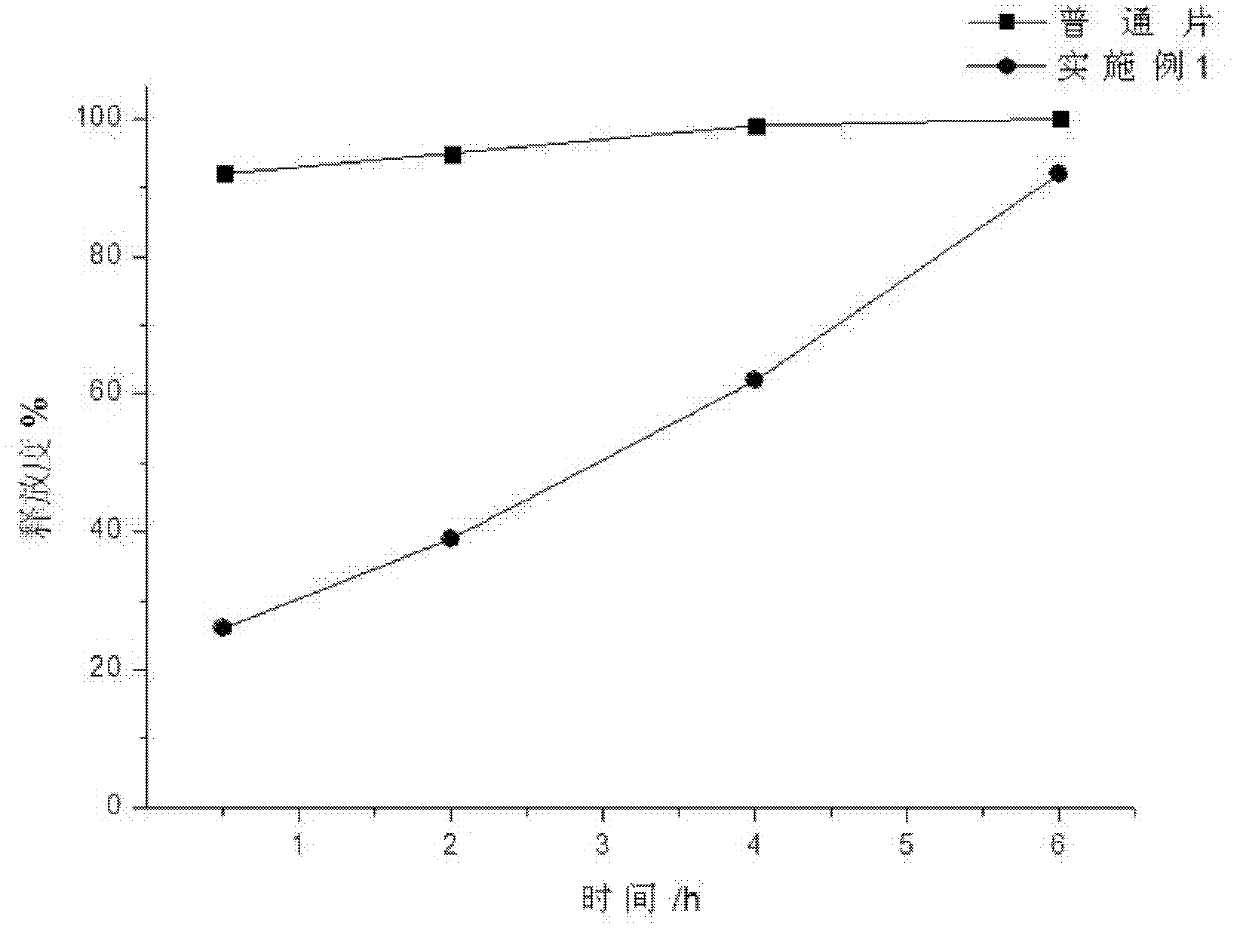
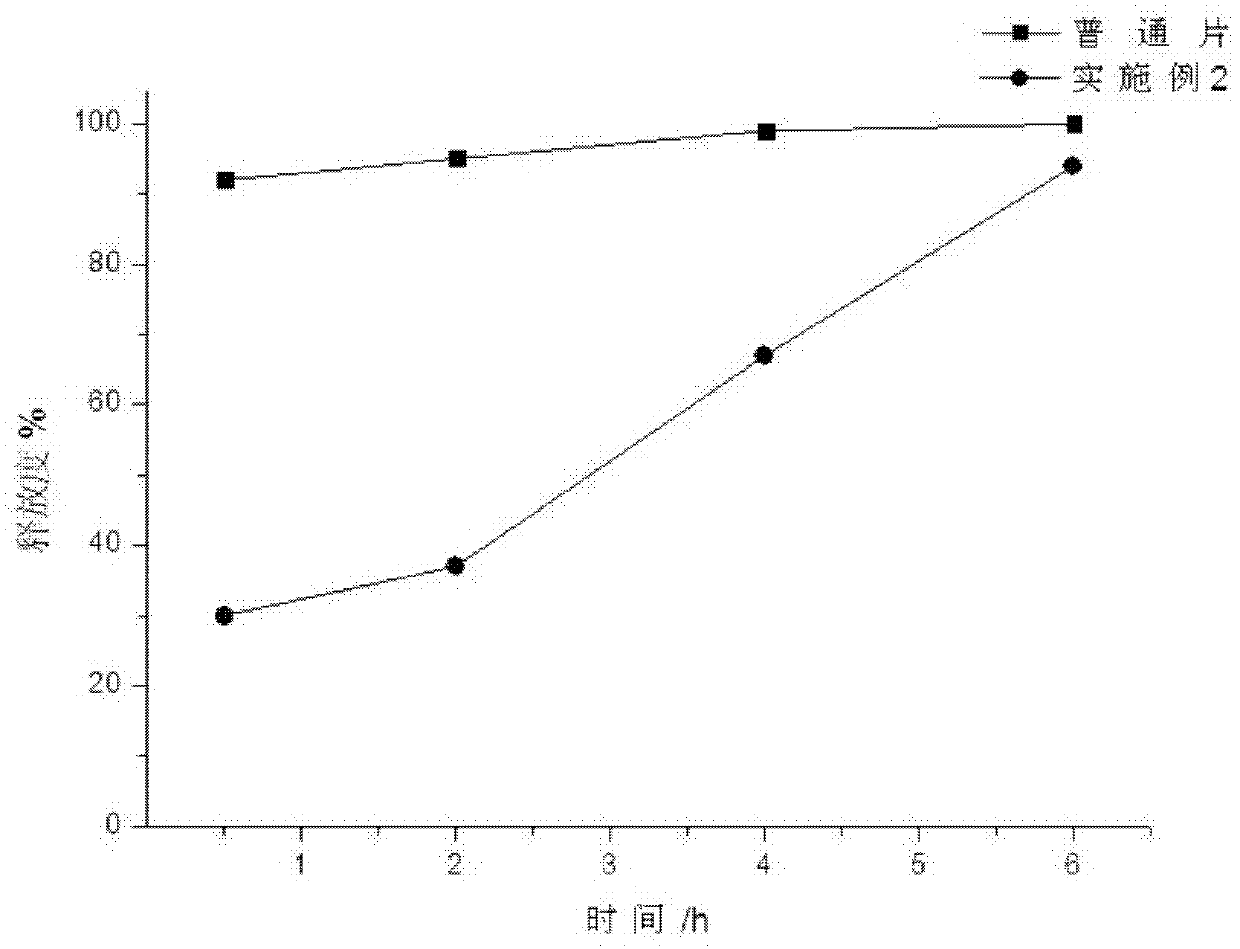
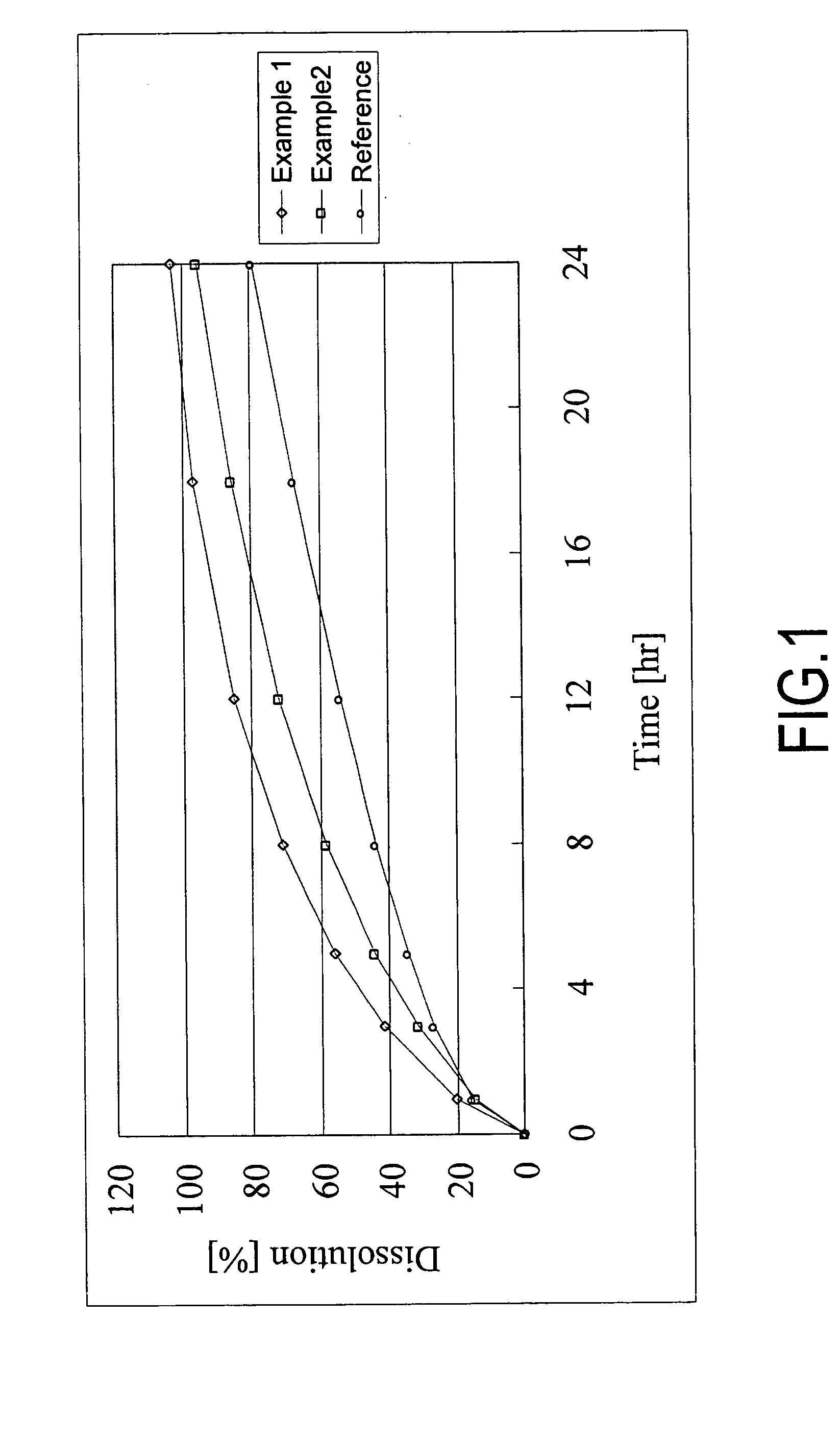
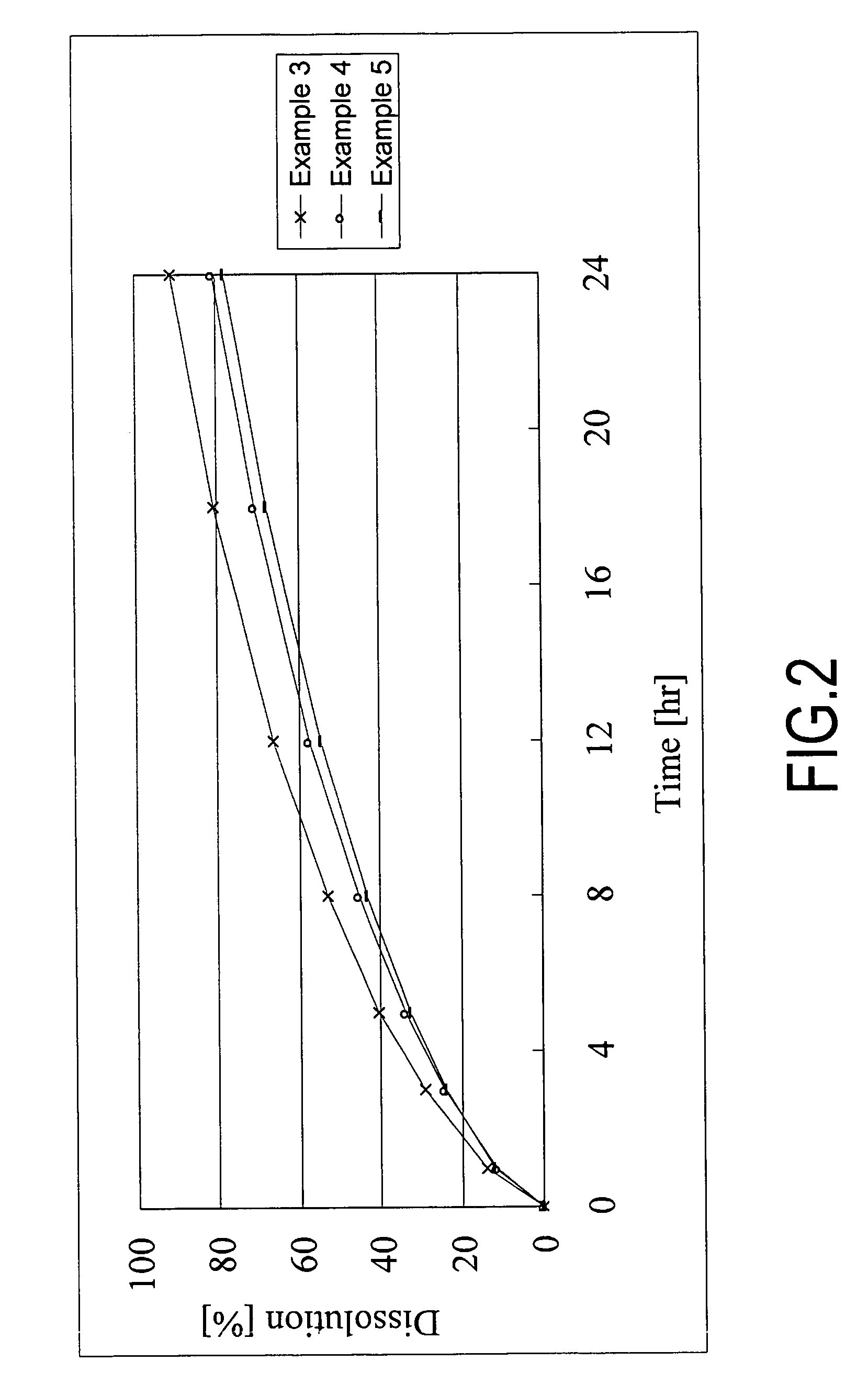
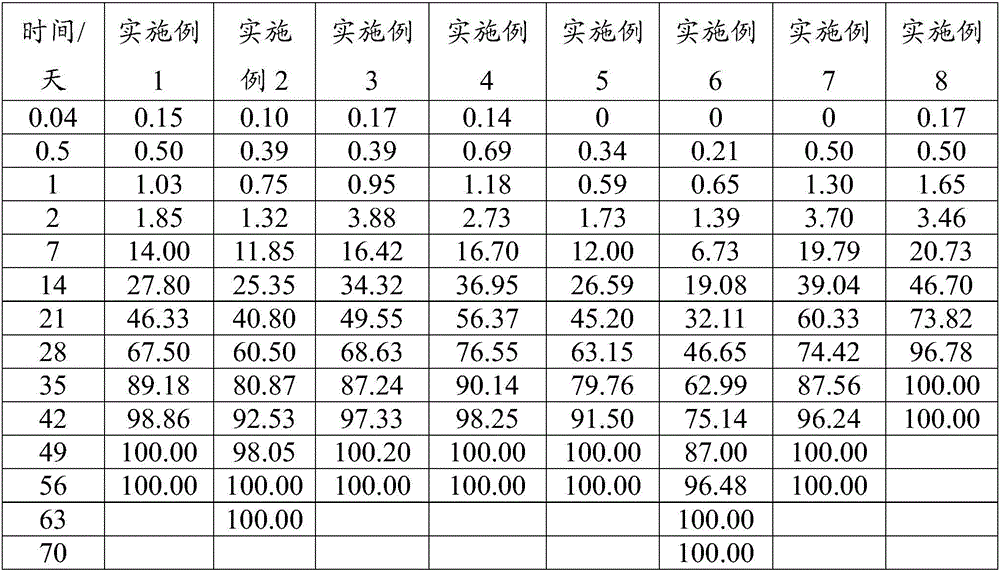
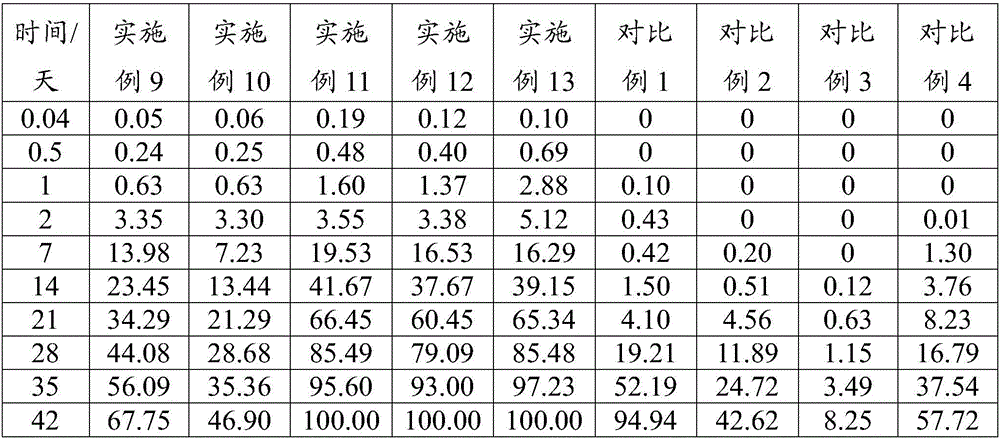
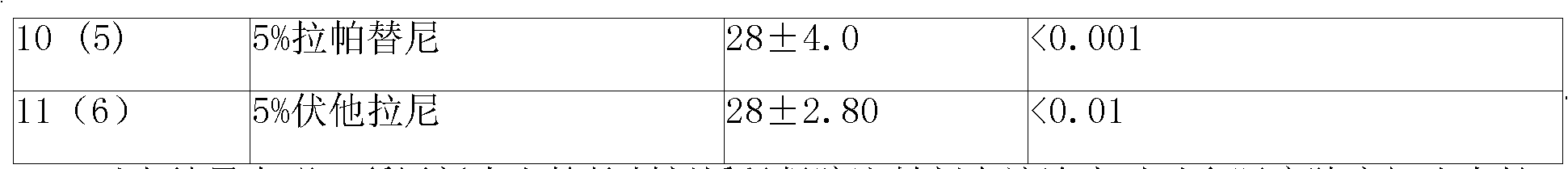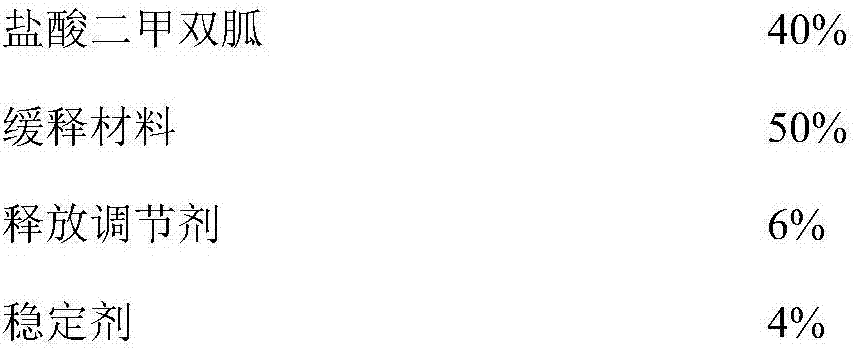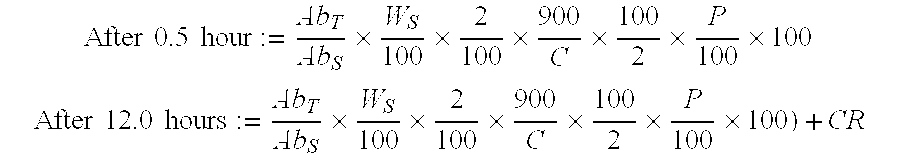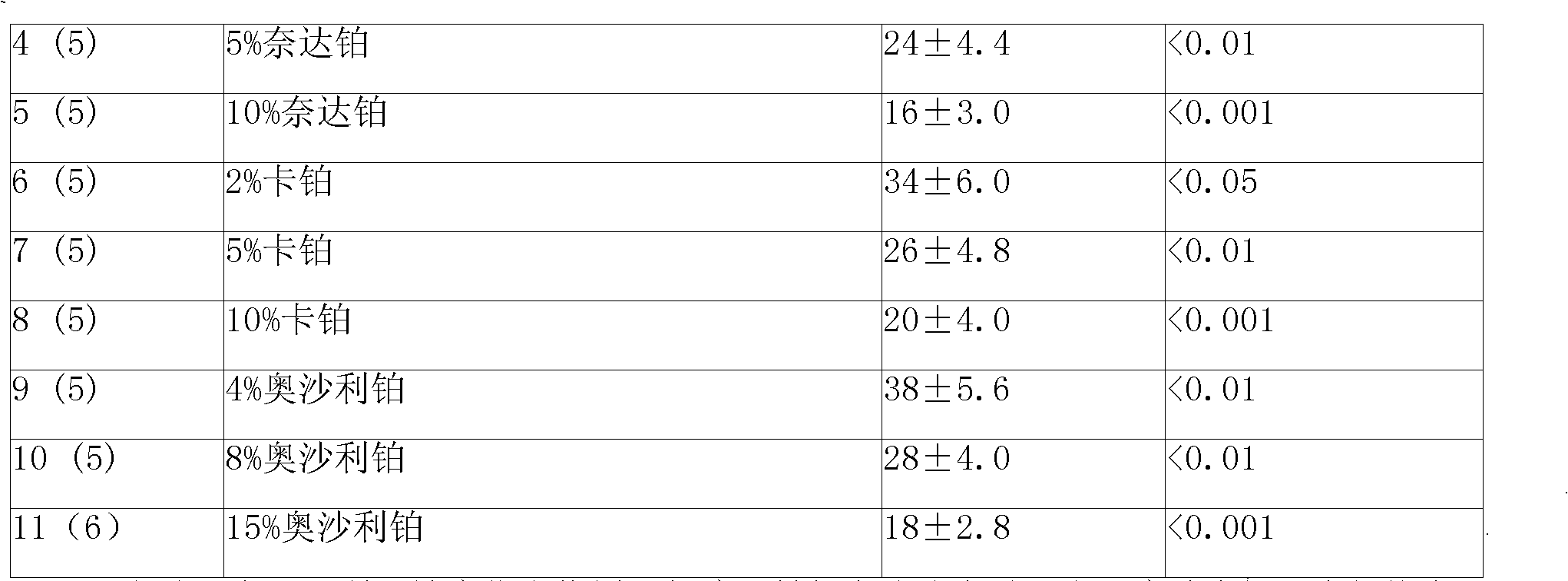Patents
Literature
67 results about "Release modulator" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A release modulator, or neurotransmitter release modulator, is a type of drug that modulates the release of one or more neurotransmitters. Examples of release modulators include monoamine releasing agents such as the substituted amphetamines (which induce the release of norepinephrine, dopamine, and/or serotonin) and release inhibitors such as botulinum toxin A (which inhibits acetylcholine release by inactivating SNAP-25, thereby preventing exocytosis from occurring).
Formulations for amylin agonist peptides
InactiveUS7312196B2Loss of biological activityLong-term stabilityPowder deliveryPeptide/protein ingredientsPrefilled SyringePharmaceutical formulation
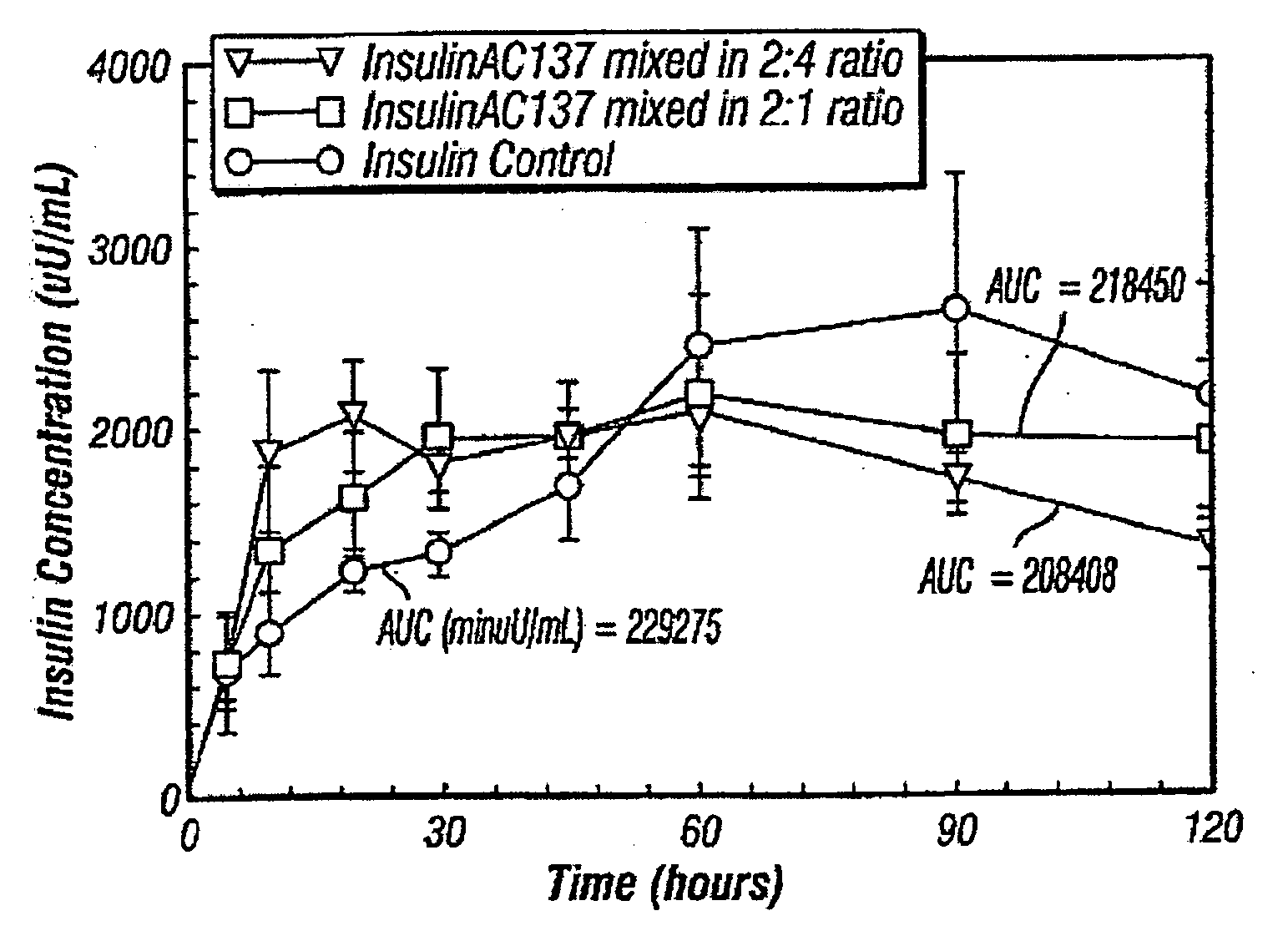
The present invention is concerned with a pharmaceutical formulation comprising an amylin agonist and optionally a buffer, a tonicifier or stabilizer, and a preservative in a container, for example, a vial, prefilled cartridge, prefilled syringe or disposable pen. This formulation may be in liquid, gel, solid or powdered form for delivery, for example, via nasal, pulmonary, oral, sublingual, buccal, transdermal, or parenteral routes. Formulation with biocompatible polymers and release modifiers, such as sugars, can facilitate controlled release after injection, minimizing the number of administrations to a patient. These formulations maintain stability upon storage under refrigerated or room temperature conditions. Such formulations can be further combined with insulin for administration to a patient.
Owner:ASTRAZENECA PHARMA LP
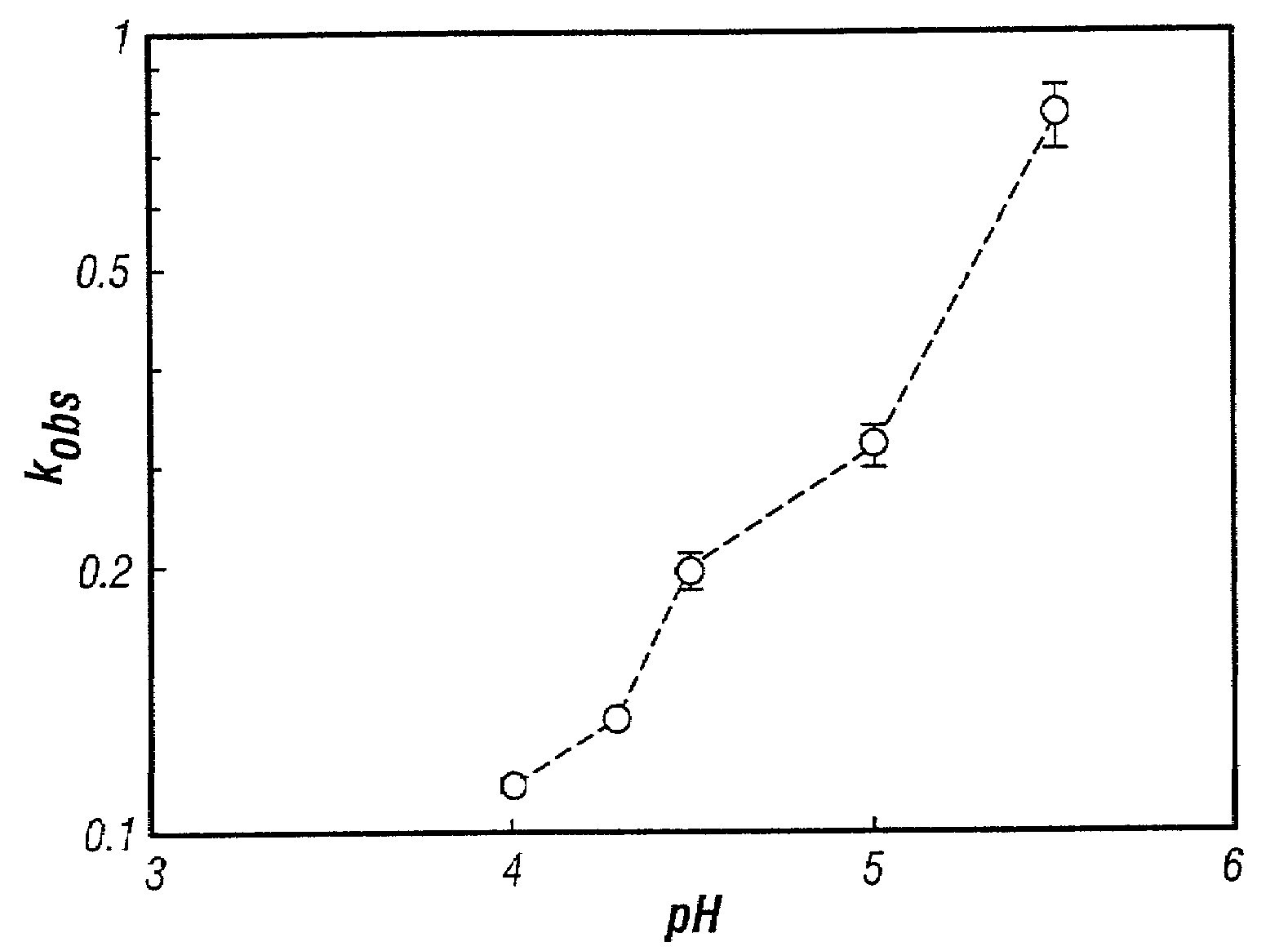
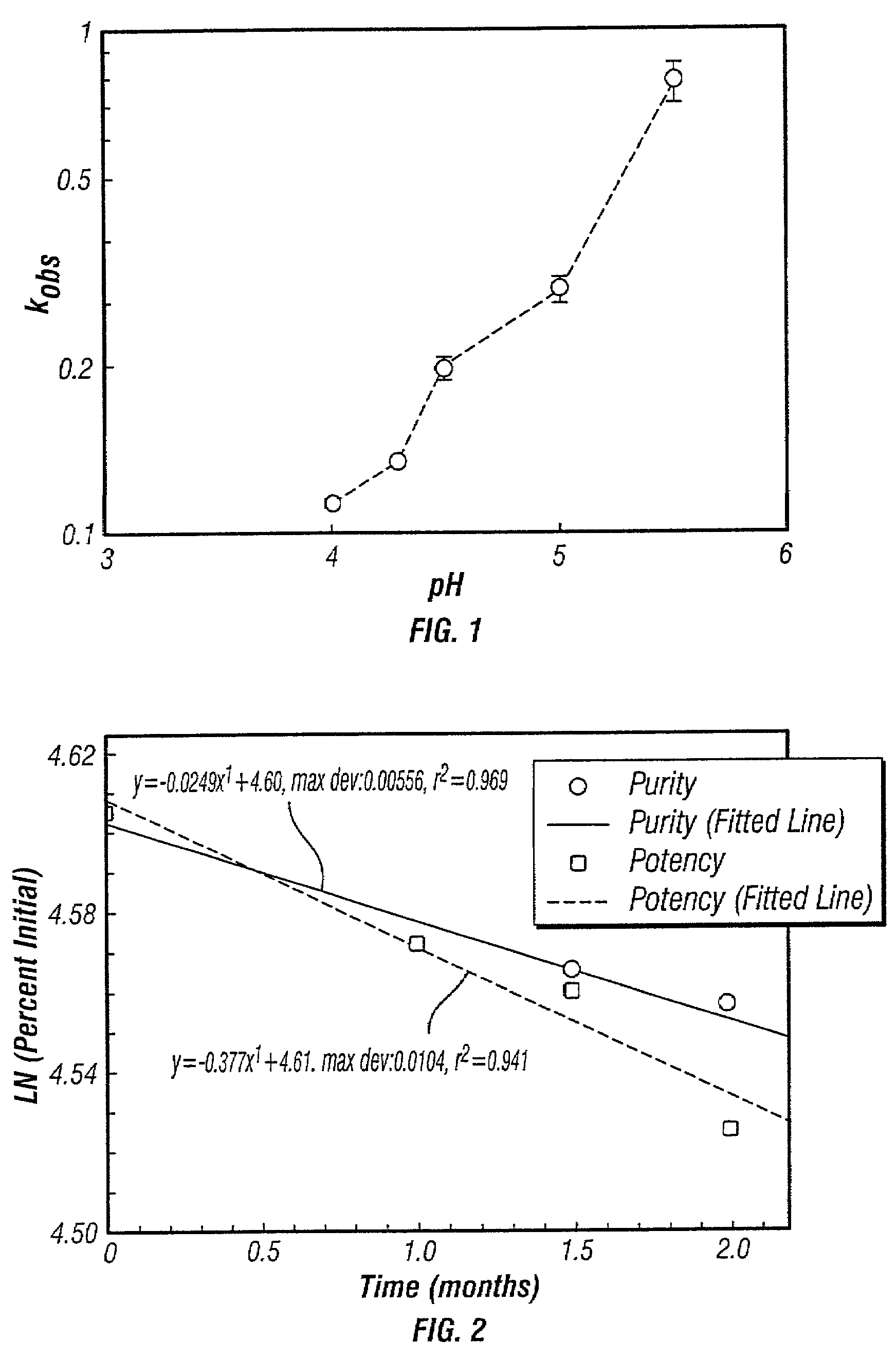
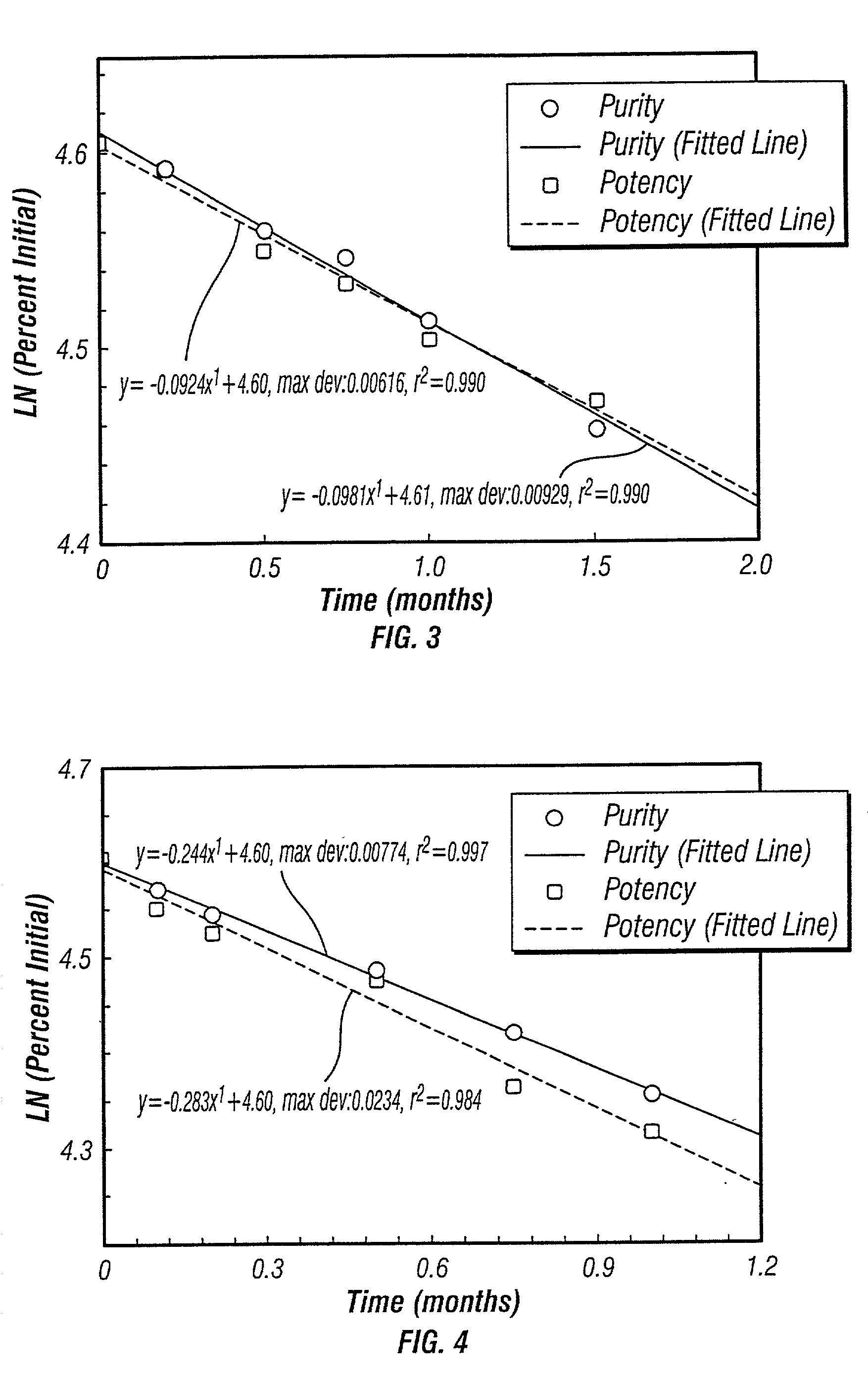
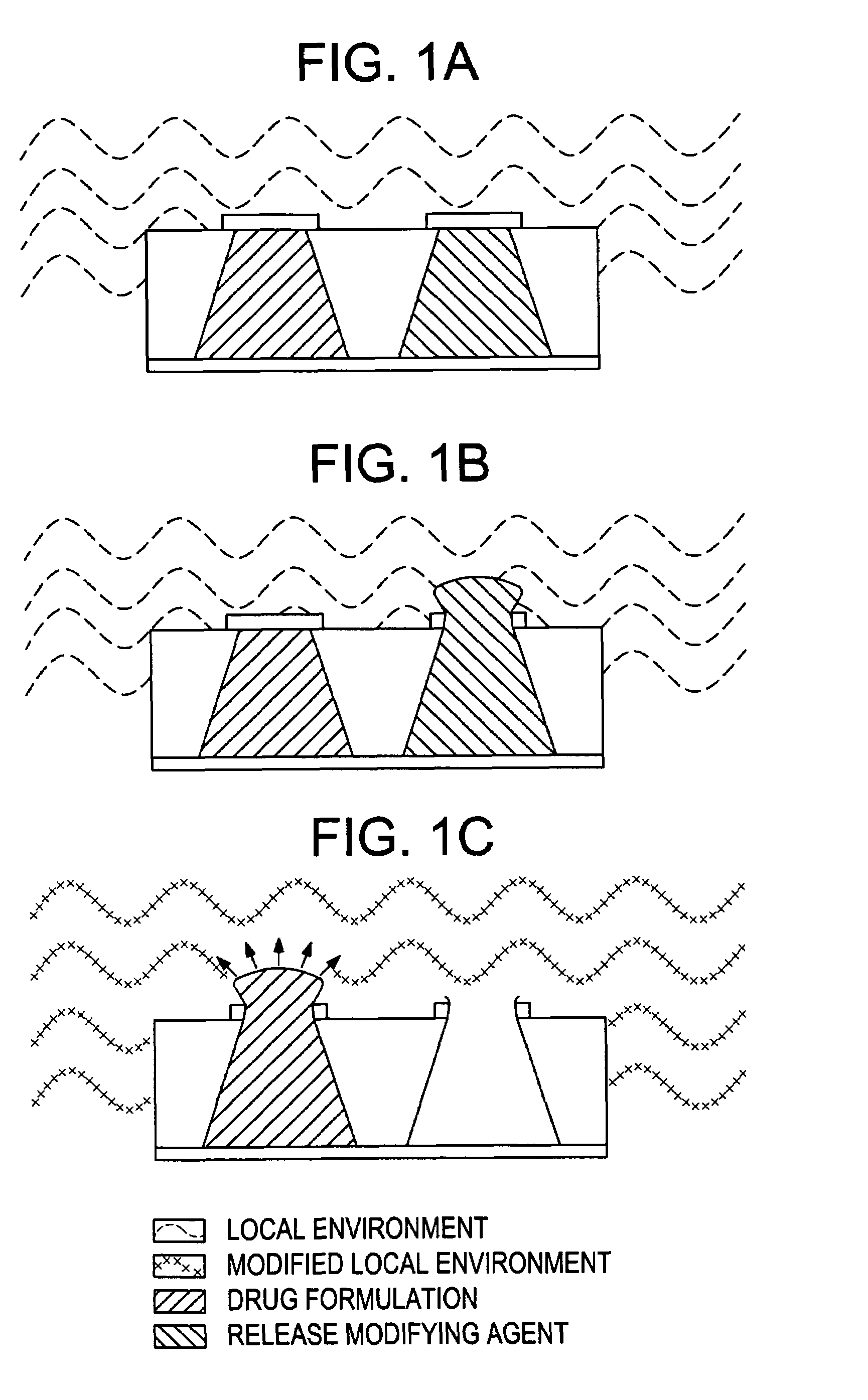
Control of drug release by transient modification of local microenvironments
InactiveUS7488316B2Facilitated releasePrevent oxidationStentsPeptide/protein ingredientsRelease modulatorMedicine
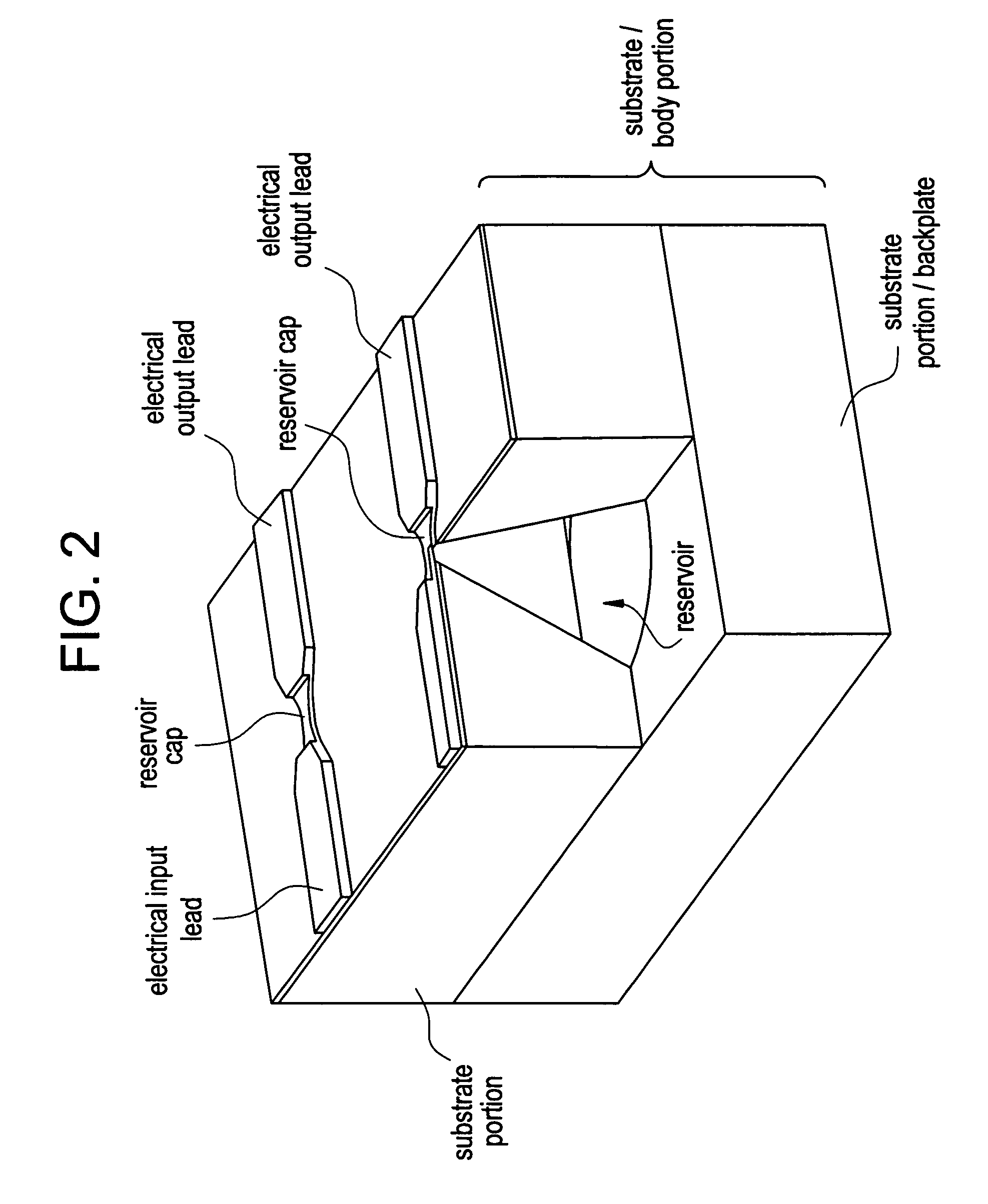
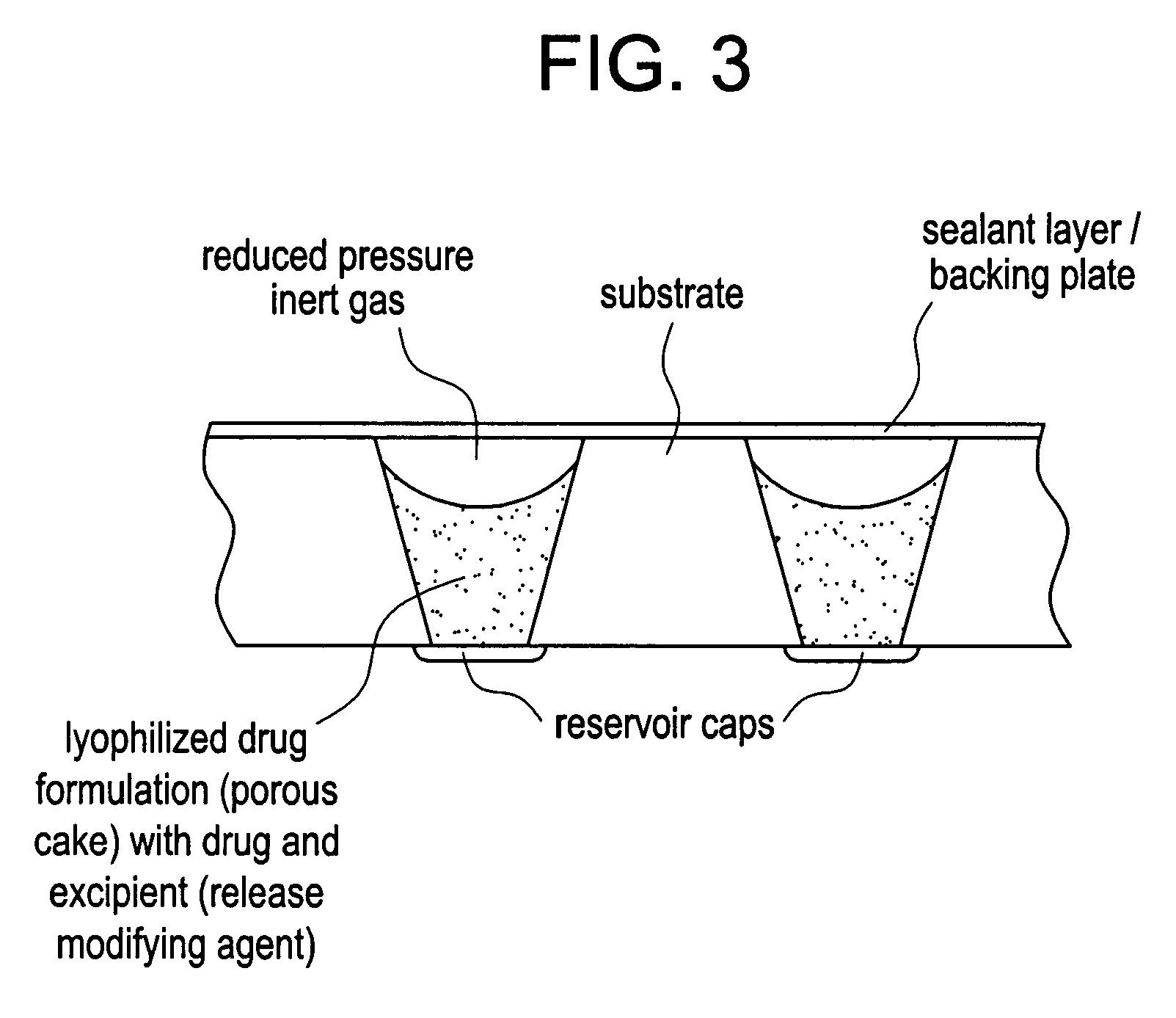
Methods, formulations, and devices are provided for enhancing drug delivery from a medical device. The method is provided for increasing the rate or quantity of a drug formulation released from an implantable drug delivery device, which method comprises the step of providing a release-modifying agent within or proximate to the implantable drug delivery device, in a manner effective to inhibit gelation, aggregation, or precipitation of the drug formulation being released from the device. The drug formulation and the release-modifying agent may be stored together in at least one reservoir in the implantable drug deliver device. Alternatively, the release-modifying agent may be stored in one or more reservoirs separate from the drug formulation.
Owner:MICROCHIPS BIOTECH INC
Formulations for amylin agonist peptides
InactiveUS20090018053A1Retain stabilityShort term mixing compatibilityPowder deliveryPeptide/protein ingredientsPrefilled SyringePharmaceutical formulation
The present invention is concerned with a pharmaceutical formulation comprising an amylin agonist and optionally a buffer, a tonicifier or stabilizer, and a preservative in a container, for example, a vial, prefilled cartridge, prefilled syringe or disposable pen. This formulation may be in liquid, gel, solid or powdered form for delivery, for example, via nasal, pulmonary, oral, sublingual, buccal, transdermal, or parenteral routes. Formulation with biocompatible polymers and release modifiers, such as sugars, can facilitate controlled release after injection, minimizing the number of administrations to a patient. These formulations maintain stability upon storage under refrigerated or room temperature conditions. Such formulations can be further combined with insulin for administration to a patient.
Owner:AMYLIN PHARMA INC
Anticancer sustained-release gel injection
InactiveCN101336890APharmaceutical delivery mechanismPharmaceutical non-active ingredientsLestaurtinibSolvent
The invention relates to an anticancer slow-release gel injection which contains slow-release microspheres containing an angiogenesis inhibitor, an amphiphilic block polymer, a solvent and a slow-release agent, wherein the composition of the amphiphilic block polymer and the non-organic solvent exhibit the property of temperature-sensitive gelation. After the in vivo injection, the injection turns into a stagnant and biodegradable water-insoluble gel and the gel slowly releases the drug contained therein for a plurality of weeks to a plurality of months. After the intratumoral or peritumoral injection, the anticancer slow-release gel injection can significantly reduce the general drug reactions and is used for treating tumors of different stages. The angiogenesis inhibitor is selected from SU5416, SU6668, bosutinib, sprycel, erlotinib, vandetanib, gefitnib, canertinib, lapatinib, lestaurtinib, masitinib, vatalanib, mubritinib, tandutinib, nilotinib, marimastat, nilotinib, pelitinib, telatinib, sunitinib, sorafenib, zarnestra, sirolimus, imatinfb, lenalidomide and thalidomide.
Owner:济南基福医药科技有限公司
Composite sulfur enveloped slow release fertilizer
InactiveCN101003454ALong release periodImprove permeabilityFertiliser formsFertilizer mixturesEpoxyRelease modulator
This invention provides a sustained-release compound fertilizer with sulfur envelope. The compound fertilizer is composed of fertilizer core and an envelope. The envelope comprises a sulfur film layer as inner or middle layer, and a sulfur-containing thermosetting polymer film layer with average sulfur weight percentage of 20-90%. The thermosetting polymer is one or more of polyurethane, epoxy resin and unsaturated polyester. The sulfur film layer may contain release regulator. The envelope has a total average thickness of 20-90 mums, and the sulfur film layer has an average thickness of 5-70 mums. The compound fertilizer has such advantages as long release period, and higher reliability.
Owner:NANJING UNIV OF SCI & TECH
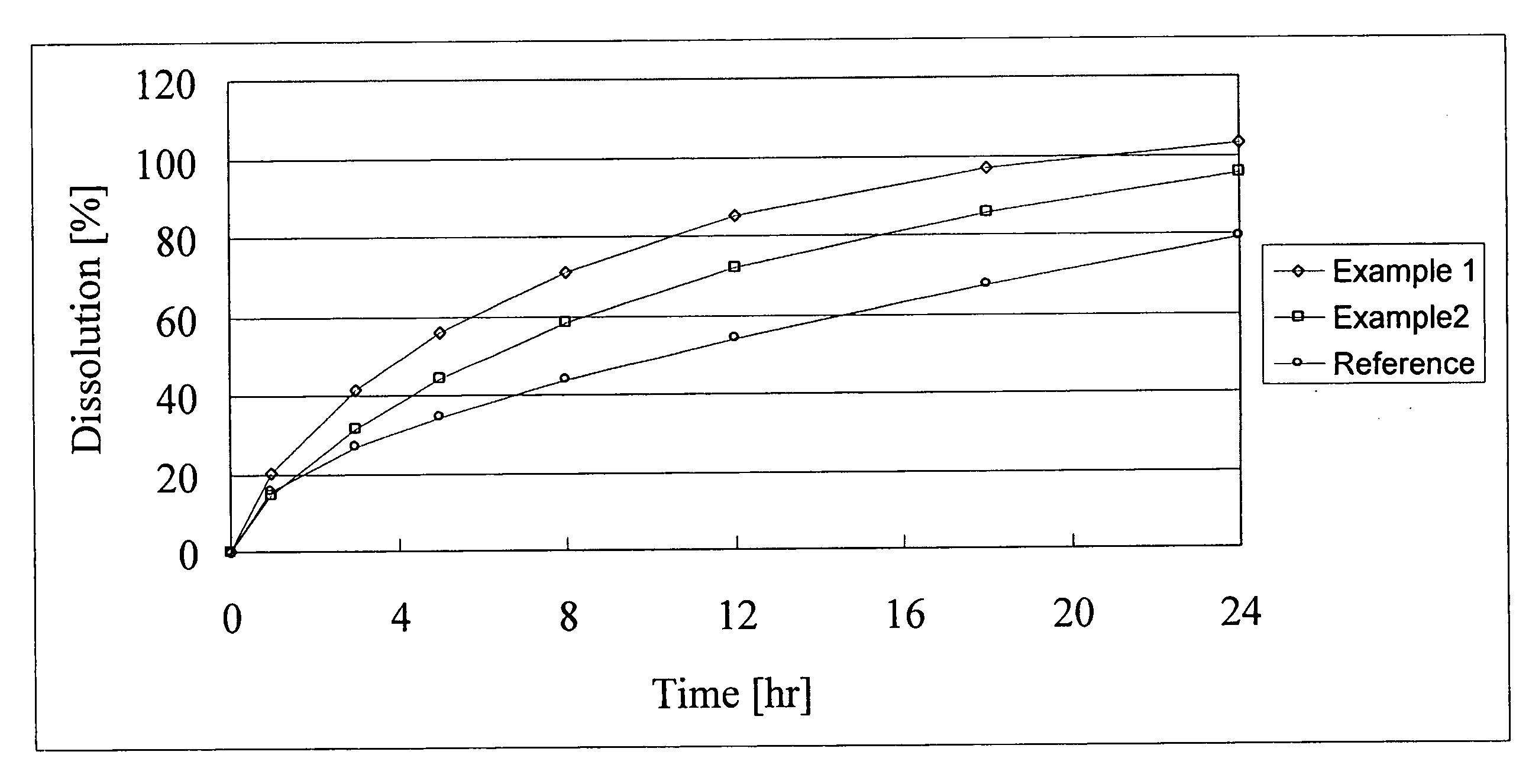
Drug sustained release composition sparingly soluble or slightly soluble in water, and preparation method thereof
InactiveCN106822039ANo significant release delay periodNo obvious burst phenomenonMacromolecular non-active ingredientsMicrocapsulesNon solventRelease modulator
The present invention discloses a drug sustained release composition sparingly soluble or slightly soluble in water, comprising non-solvent based raw material: a releasing conditioning agent. In the composition, the releasing conditioning agent is added into preparation raw materials and can be used for effectively adjusting the releasing speed of the drug sustained release composition sparingly soluble or slightly soluble in the water, so that a phenomenon of obvious releasing delaying period and burst releasing of the drug sustained release composition sparingly soluble or slightly soluble in the water is avoided, the good sustained release performance is obtained, the therapeutic plasma concentration can be kept for several weeks or even more, the stabilization is relatively good, and the releasing behavior of the drug can be maintained after long-time storage. At the same time, the present invention also discloses a preparation method of the drug sustained release composition sparingly soluble or slightly soluble in the water.
Owner:AC PHARMA CO LTD
Sustained-Release Microspheres and Methods of Making and Using Same
InactiveUS20080131513A1Improve stabilityEnhancing local deliveryAntibacterial agentsBiocideOrganic solventRelease modulator
Provided, among other things, are compositions and methods for making sustained-release microspheres, as well as a microsphere delivery system for the sustained release of an active agent. The microsphere delivery system comprises a homogeneous mixture of biodegradable polymer, active agent, and a so-called release-modifying agent (including a pH-stabilizing agent), and provides protected and sustained release of active agents from the microsphere delivery system. According to the invention, the microspheres preferably are produced by an oil-in-water emulsion method that involves the production of a homogeneous oil phase prepared by mixing active agent and a release-modifying agent, such as arginine, with biodegradable polymer, each dissolved in organic solvent. The homogeneous oil phase desirably is then dispersed in an aqueous phase containing an emulsifying agent, followed by solvent removal, to produce the microspheres in which the active agent and release-modifying agent are distributed homogeneously throughout the biodegradable polymer matrix.
Owner:FRESENIUS KABI USA LLC +1
Anticancer sustained-release gel injection containing taxone medicine
InactiveCN101283976APharmaceutical delivery mechanismPharmaceutical non-active ingredientsTreatment effectLactide
A sustained-release gel injection comprises sustained-release microspheres containing taxanes drug, amphiphilic block copolymer, solvent and release regulator, wherein the mixture of the amphiphilic block copolymer and the solvent has a temperature sensitive gelatinization characteristic, and can automatically become non-flowing degradable water insoluble gel, which can locally and slowly release drug at tumor foci for several weeks to several months, after injection into body. The preparation can be injected into or around a tumor for treating solid tumors at different stages. The preparation has the effects of controlling residual tumor cells relapsed after operation and tumors that can not be excised via operation, controlling complications at late stage of tumors, and enhancing treatment effect of chemotherapy and radiotherapy (particularly radioactive particles). The taxanes are selected from docetaxel, taxol, epitaxol, hydroxyl taxol and deacetyltaxol; the amphiphilic block copolymer is PLGA-PEG-PLGA copolymer, wherein the PEG has molecular weight of 1200-1600, accounting for 20 wt% of the amphiphilic block copolymer; and in the glycolide-lactide copolymer, the mol ratio of glycolide and lactide is 6:1.
Owner:济南基福医药科技有限公司
Gastric floating capsule of 'Zuo Jin Wan' and preparation method thereof
The invention provides a capsule for treating gastric diseases such as gastritis and peptic ulcer, as well as the preparing process, wherein the capsule is prepared from Zuojin extract and stomach floating auxiliary material by a weight ratio of 1:0.5-5, Zuojin extract is prepared from coptis root and evodia rutaecarpa by a weight ratio of 6:1 through extracting, the stomach floating auxiliary material comprises hydrophilic gel material, foaming agent and release modifier. The preparing process comprises the steps of coating evodia rutaecarpa extract volatile oil with beta-cyclodextrin, merging evodia rutaecarpa dross and coptis root, extracting with ethanol, mixing the extract with auxiliary materials homogeneously, packing into capsules, and heating for hot-setting.
Owner:NANJING UNIVERSITY OF TRADITIONAL CHINESE MEDICINE
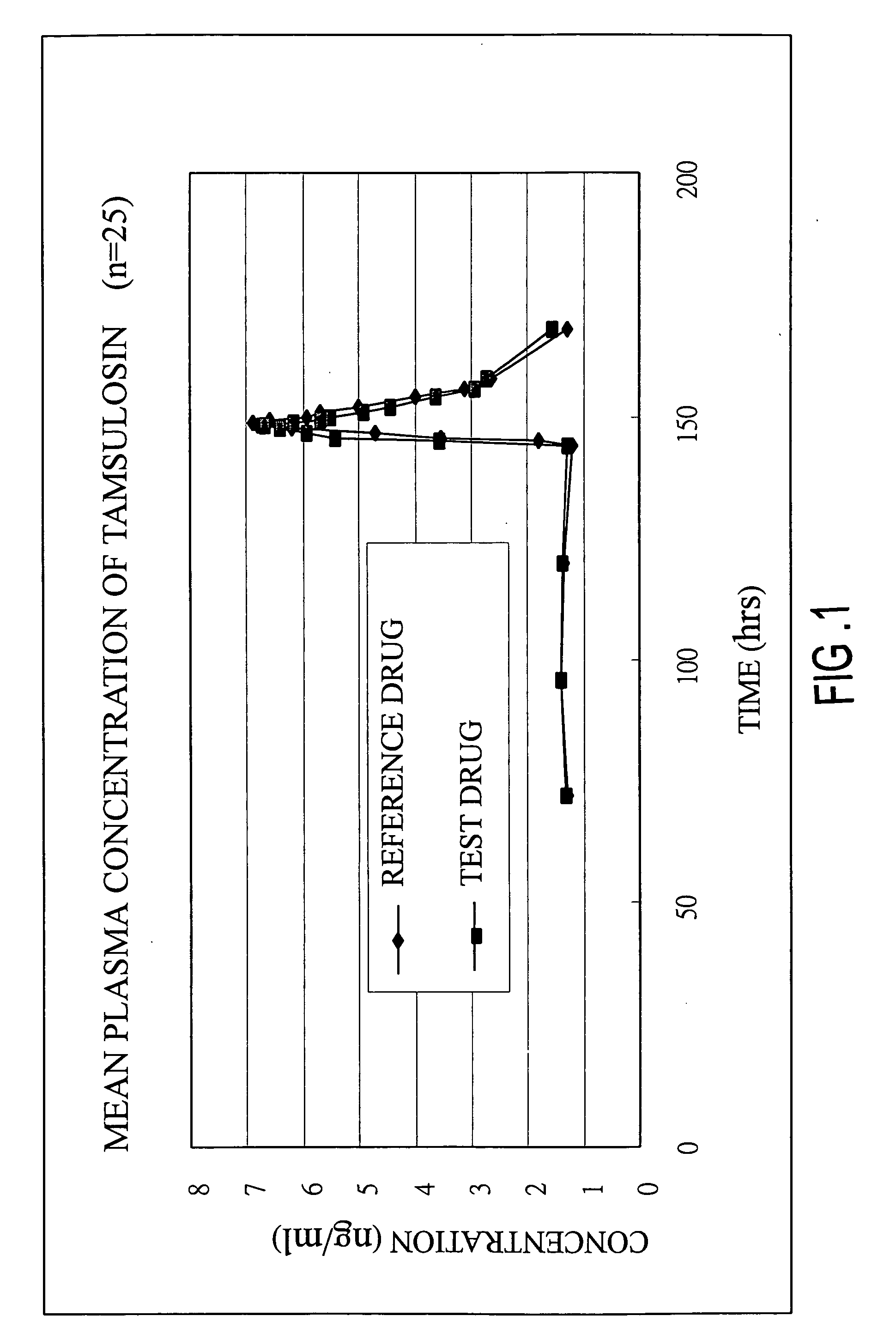
Sustained release tamsulosin formulations
A sustained release tamsulosin formulation contains tamsulosin, a hydrophobic polymer, a microsphere forming agent and a diluent. The hydrophobic polymers include pH-dependent and pH-independent polymers are used as the release-modulating agent to control the dissolution profile of tamsulosin formulation so that the formulation releases tamsulosin slowly and continuously as the formulation passed through the stomach and gastrointestinal tract.
Owner:STANDARD CHEM PHARM
Hot melt pressure-sensitive adhesive and preparation method thereof
InactiveCN102580101AGuaranteed thermal stabilityGood drug releaseOil/fats/waxes non-active ingredientsSheet deliveryElastomerRelease modulator
The invention relates to a hot melt pressure-sensitive adhesive suitable for percutaneous administration of hydrophilic medicines, and a preparation method of the hot melt pressure-sensitive adhesive, belonging to the technical field of the hot melt pressure-sensitive adhesive. The hot melt pressure-sensitive adhesive is prepared by introducing polar groups into the molecules of styrene-isoprene-styrene thermoplastic elastomer and adding a hydrophilic medicine release regulator, so that the characteristic of lipotropy of the traditional styrene-isoprene-styrene hot melt pressure-sensitive adhesive matrix can be changed. According to the invention, the release action of the hydrophilic medicine in the styrene-isoprene-styrene hot melt pressure-sensitive adhesive matrix can be obviously improved.
Owner:DALIAN UNIV OF TECH
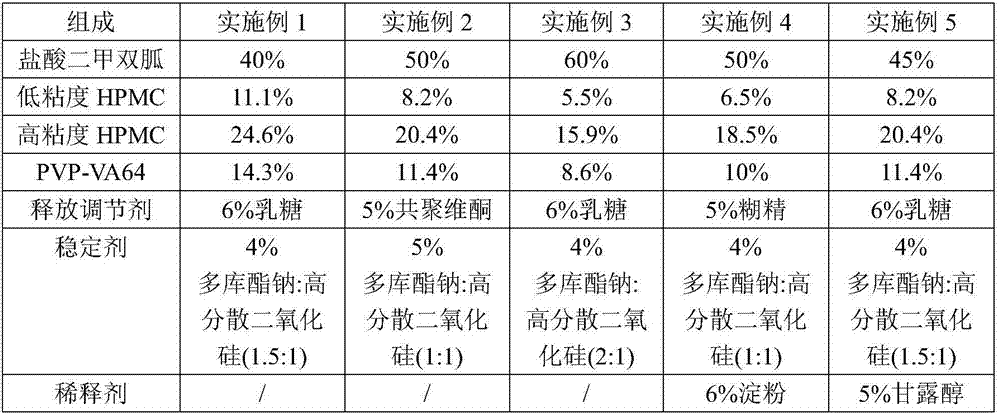
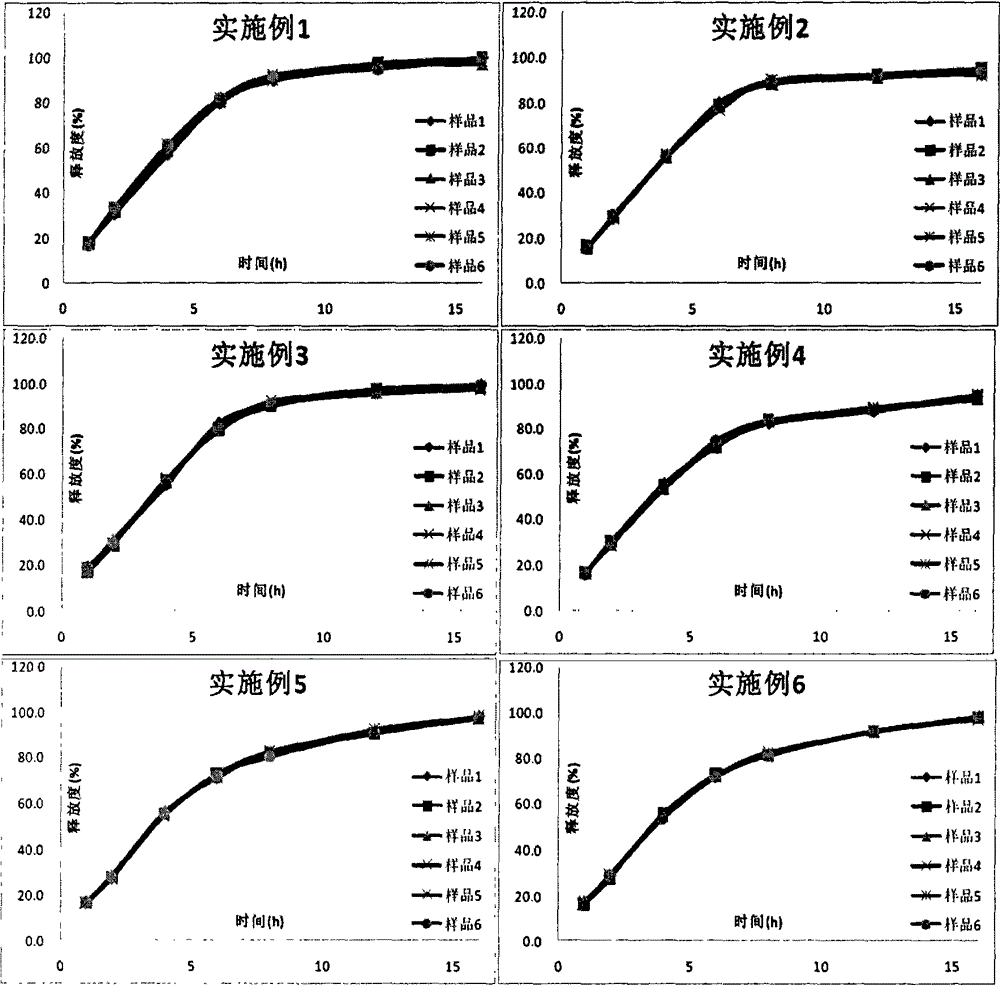
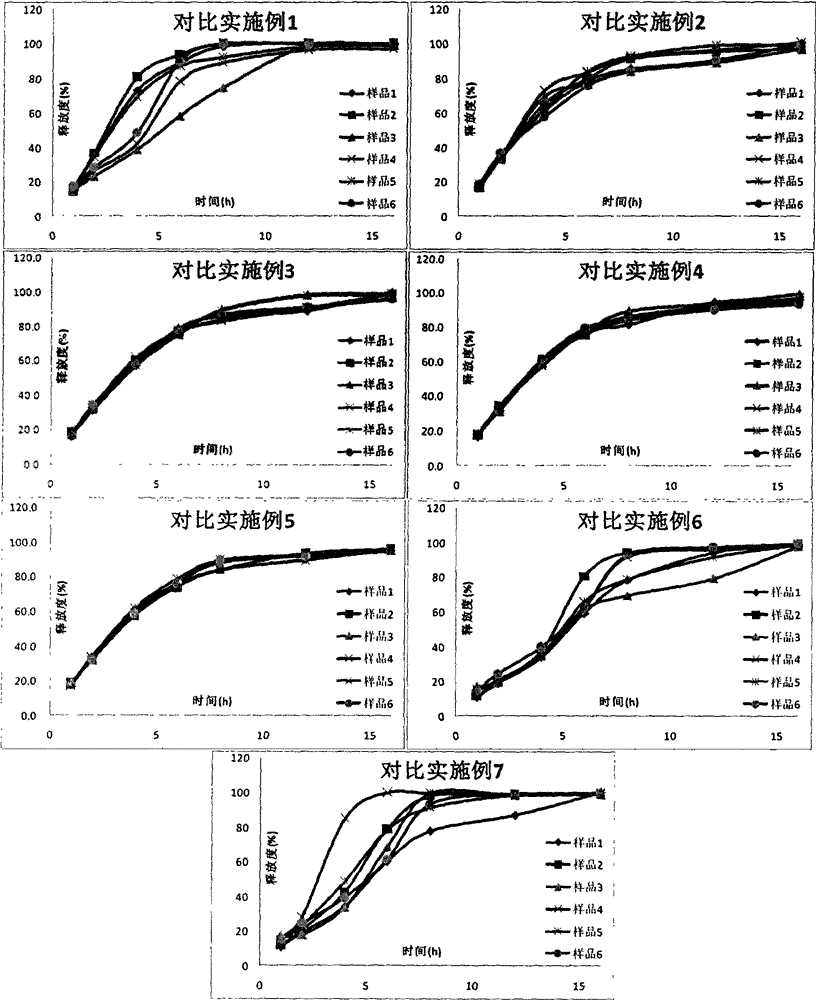
Metformin hydrochloride sustained-release tablet and preparation method thereof
ActiveCN107184559AImprove water retentionReduce water retentionOrganic active ingredientsMetabolism disorderMetformin HydrochlorideDrug release
The invention discloses a metformin hydrochloride sustained-release tablet and a preparation method thereof. The metformin hydrochloride sustained-release tablet is prepared by the following steps of using a hydroxypropyl methylcellulose and PVP-VA64 mixture as the sustained-release material, adding a certain amount of stabilizer and release adjuster, and preparing by a hot melting extrusion technique. The metformin hydrochloride sustained-release tablet has the advantages that the sustained-release material and the metformin hydrochloride are tightly bonded, the medicine is more smoothly and slowly released, the effect is obviously better than the in-vitro release conditions of the sustained-release tablet prepared by the conventional wet type granulating tabletting method and the market product (Glucophage SR), and the stability is better; under the condition of no coating, the adverse odor of the metformin hydrochloride can be covered, and the compliance of a patient is improved; the preparation technology is simple, the energy consumption is less, the solvent residue is avoided, the other impurities are not introduced in the whole process, and the continuous production can be easily realized.
Owner:广东赛康药业有限公司 +1
Metformin hydrochloride osmotic pump controlled release tablet and preparation method thereof
ActiveCN105878204AImproved controlled releaseGood compressibilityOrganic active ingredientsMetabolism disorderRelease modulatorAdhesive
The invention relates to the field of pharmaceutical preparations and particularly provides a controlled release tablet of metformin hydrochloride and a preparation method of the controlled release tablet. The controlled release tablet provided by the invention contains a tablet core, an insoluble semi-permeable membrane and a drug release micro-pore, wherein the tablet core contains metformin hydrochloride, an adhesive, a release regulator, an absorption accelerant and a lubricating agent. The metformin hydrochloride in the controlled release tablet provided by the invention can be stably released at a constant speed, so that absorption of drugs is more facilitated, and a better in vivo pharmacokinetic curve is realized. Moreover, the preparation method of the controlled release tablet is simple and easy in process, and the technical defect of poor compressibility of the tablet is solved.
Owner:HEFEI LIFEON PHARMA
Implant and preparation method thereof
ActiveCN106580868AShorten mixing timeAvoid or reduce the phenomenon of degradationPharmaceutical delivery mechanismPharmaceutical non-active ingredientsOrganic solventRelease modulator
The invention relates to an implant and a preparation method thereof. The implant is prepared from components of raw materials in parts by weight as follows: 25-65 parts of a water-insoluble / micro-soluble medicine, 35-75 parts of a water-insoluble polymer and 0-10 parts of a release modifier. The preparation method of the implant comprises steps as follows: (1) the water-insoluble / micro-soluble medicine and the release modifier are dissolved in an organic solvent, and a water-insoluble / micro-soluble medicine solution is obtained; (2) the water-insoluble polymer and the water-insoluble / micro-soluble medicine solution obtained in the step (1) are mixed, the organic solvent is removed and a solid mixture is obtained; (3) the solid mixture obtained in the step (2) is put into a hot melt extruder to be extruded and cut, alternatively, the solid mixture in the step (2) is pressed and formed, and the implant is obtained. With the adoption of the preparation method of the implant, the phenomena of medicine crystallization or aggregation and degradation of the polymer can be effectively reduced or avoided, and long-term release of a medicine is facilitated.
Owner:AC PHARMA CO LTD
Sustained release tamsulosin formulations
A sustained release tamsulosin formulation contains tamsulosin, a hydrophobic polymer, a microsphere forming agent and a diluent. The hydrophobic polymers include pH-dependent and pH-independent polymers are used as the release-modulating agent to control the dissolution profile of tamsulosin formulation so that the formulation releases tamsulosin slowly and continuously as the formulation passed through the stomach and gastrointestinal tract. The present invention further relates to a method for preparing the sustained release tamsulosin formulation.
Owner:STANDARD CHEM & PHARMA
Zuojin helicobacter-pylori-resistant floating tablets and preparation method thereof
InactiveCN102058703AReasonable workmanshipNovel dosage formDigestive systemPill deliveryTherapeutic effectAlkaloid
The invention relates to Zuojin helicobacter-pylori-resistant floating tablets and a preparation method thereof. The floating tablets are characterized by comprising 0.2 to 0.75 part of Zuojin alkaloid extract, 0.1 to 0.4 part of hydrophilic macromolecular material, 0.1 to 0.4 part of floating auxiliary agent, 0.01 to 0.1 part of foaming agent, 0.04 to 0.1 part of release regulator and a proper amount of starch, wherein the final total amount is 1 part. The preparation method comprises the following steps of: mixing the Zuojin alkaloid extract, 70 to 90 percent of the hydrophilic macromolecular material, the floating auxiliary agent, the foaming agent, the release regulator and the proper amount of starch in part uniformly, sieving the mixture with a sieve of 60 to 100 meshes, preparing a soft material by using water or 2 to 10 percent polyvinyl pyrrolidone ethanol solution or ethanol solution as an adhesive, sieving with a sieve of 10 to 24 meshes to prepare granules, drying the granules in the air at the temperature of 30 to70 DEG C, and mixing the rest hydrophilic macromolecular material, magnesium stearate in an amount accounting for 1 percent of the total amount and the granules, wherein the total amount is 1 part; and tableting the mixture by using a single-punch tablet press, wherein the weight of each tablet is 500mg, and the hardness is controlled between 30N and 60N. The tablets have the characteristics of constant and complete medicament release, reduced administration amount and administration times, and enhanced treatment effect; and the Zuojin helicobacter-pylori-resistant floating tablets for treating Hp infected stomach diseases are prepared with excellent prescription, compact pharmacological basis, reasonable processes and novel formulation, and are favorable for overcoming the defects of western medicaments, digging the treasure of the traditional Chinese medicine and exerting the treatment advantages of the traditional Chinese medicine.
Owner:SHUNDE POLYTECHNIC
Anticancer sustained-release gel injection containing stines medicine
The invention relates to an anticancer sustained release gel injection with stine drugs, comprising sustained release microspheres with stine drugs, a amphiphilic block copolymer, solvent and releasing moderator; wherein, the mixture of the amphiphilic block copolymer and the solvent possesses sensitive gelation property; after in vivo injection, the injection can be transformed into nonflowing, biodegradable gel insoluble in water; the insoluble gel can release the contained drugs in local tumor for weeks, even months. Intra-tumor injection or local injection can be used to treat different tumors and unresectable tumors, control late complications and the postoperative residual tumor cell recurrent, reinforce the effect of radiotherapy and chemotherapy and the effect of radiotherapy particles; nimustine and carmustine and other stines; the amphiphilic block copolymer is a PLGA-PEG-PLGA copolymer with the molecular weight of PEG 1200-1600 accounting for 20% of the amphiphilic block copolymer weight; in the poly lactide coglycolide copolymer, the molar ratio of glycolide and lactide is 6:1.
Owner:济南基福医药科技有限公司
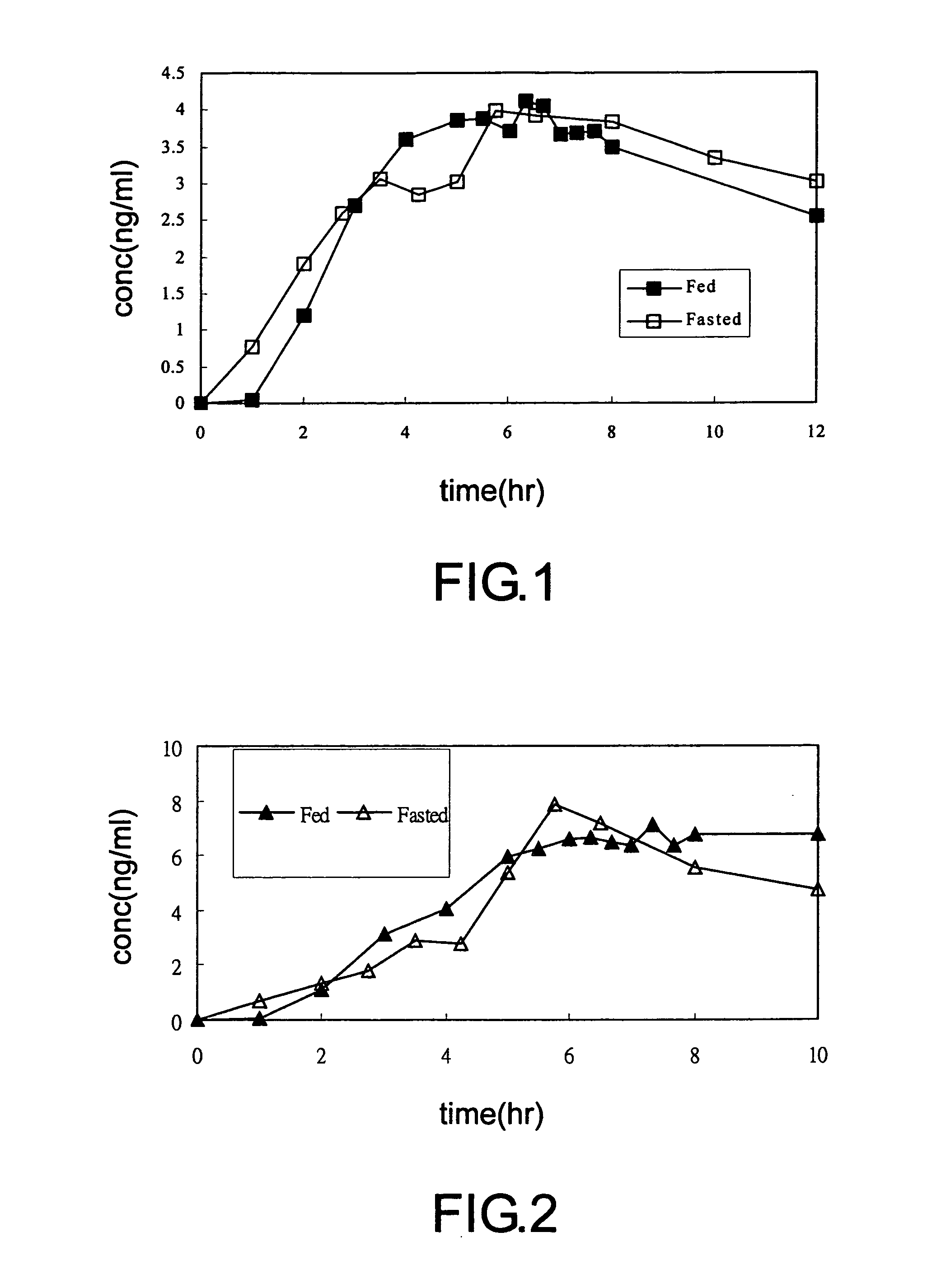
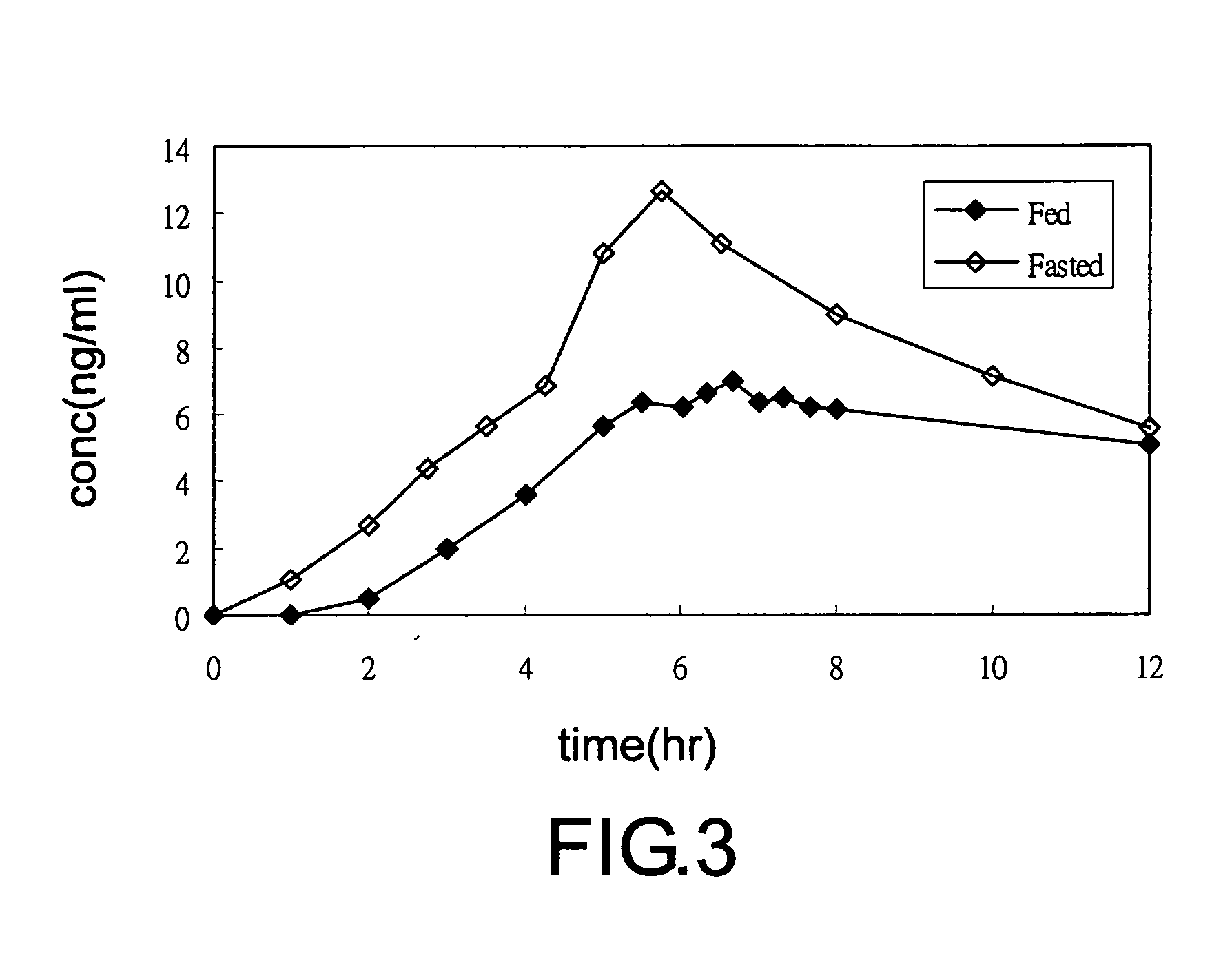
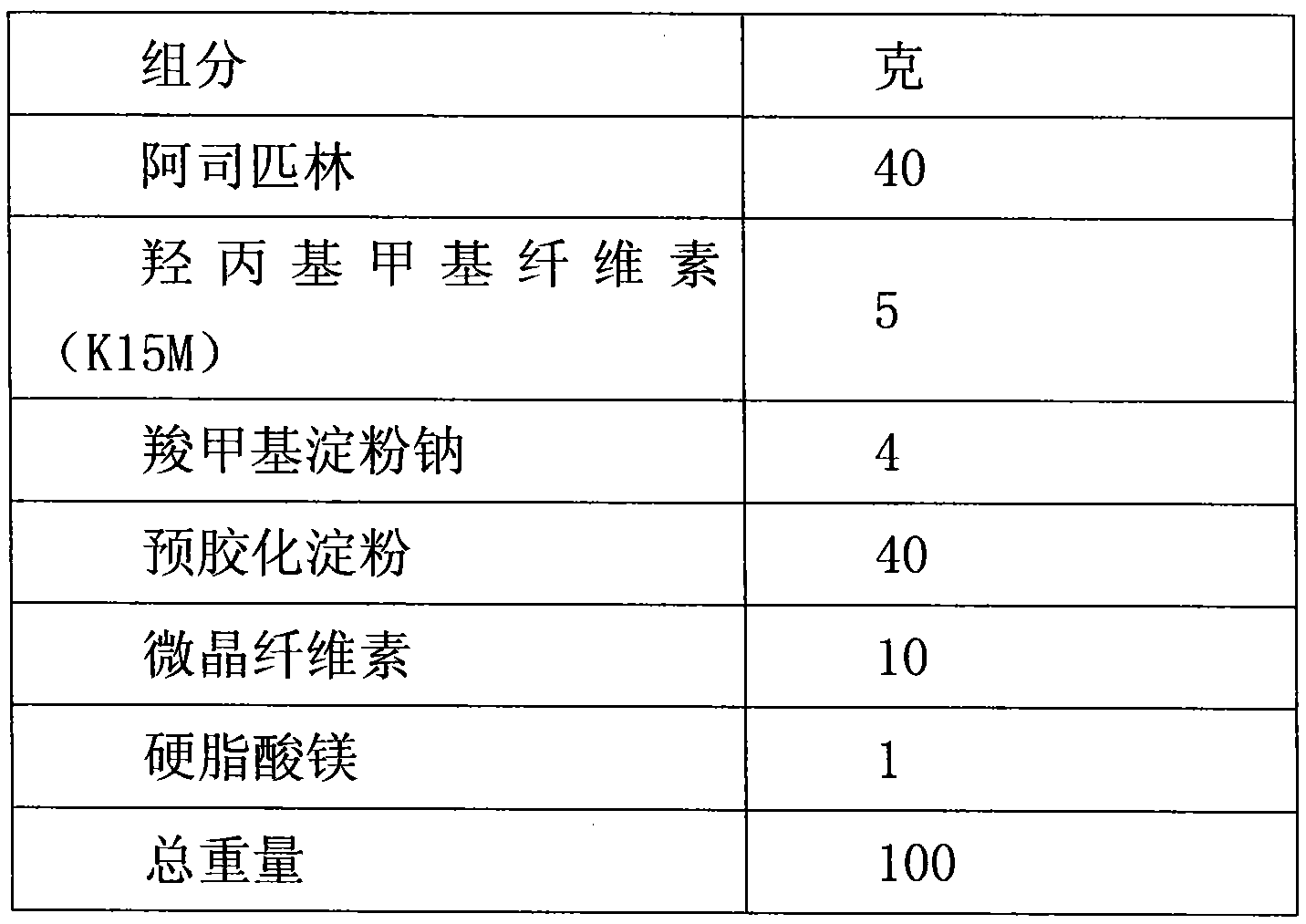
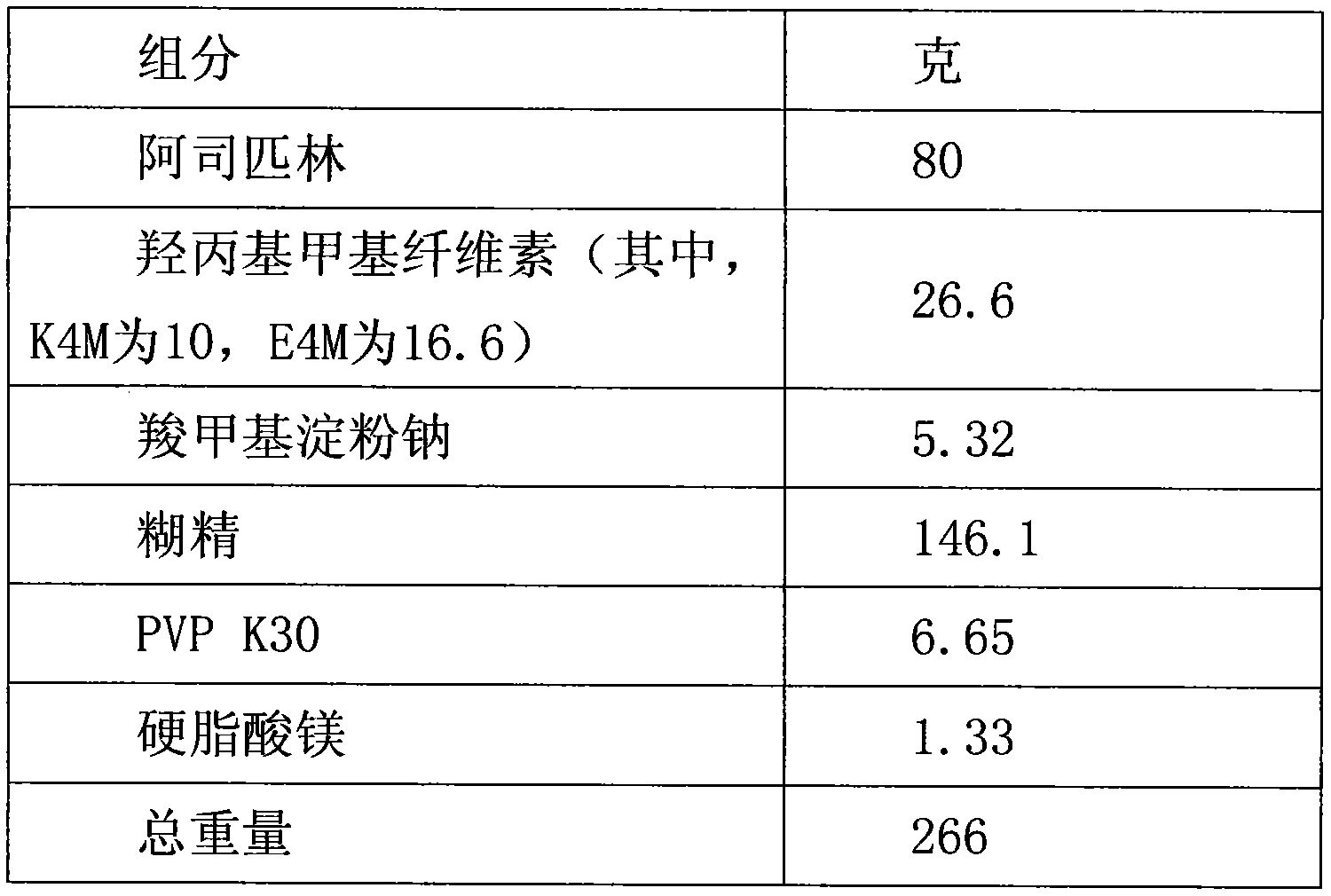
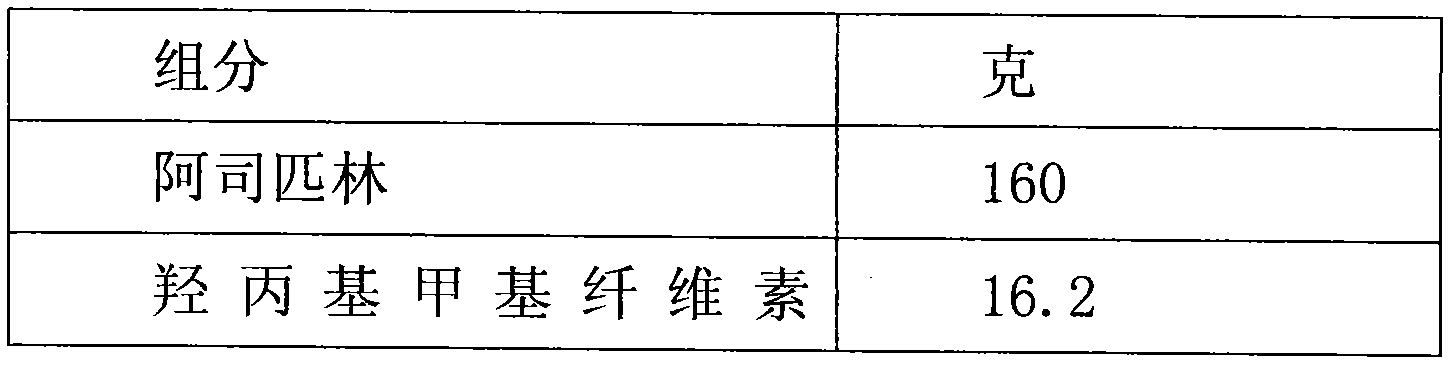
Aspirin enteric sustained-release capsule and preparation method thereof
InactiveCN104069085AAvoid irritationOrganic active ingredientsAntipyreticIrritationSustained Release Capsule
The invention provides an aspirin enteric sustained-release capsule and a preparation method thereof, and relates to the technical field of medicament production. The formula of the capsule comprises the following components: 30-60 percent of aspirin, 30-60 percent of packing, 1-10 percent of release regulating agent and 2-20 percent of a frame material, wherein the packing is one or more of mannitol, sorbitol, xylitol, lactose, saccharose, glucose, starch, dextrin, pregelatinized starch and microcrystalline cellulose; the release regulating agent is one or more of sodium carboxymethyl starch, polyvinylpolypyrrolidone, hydroxypropylcellulose and microcrystalline cellulose; the frame material is one or more of hydroxypropyl methyl cellulose, ethyecellulose, carbomer, sodium alginate and stearic acid. About 100mg of the aspirin enteric sustained-release capsule is taken once a day conventionally, irritation of the capsule to gastric mucosa is avoided due to existence of the enteric capsule after a patient takes the aspirin enteric sustained-release capsule, the capsule is dissolved after entering the intestinal tract, and the content micro-tablet of the capsule slowly release aspirin, so that the aspirin enteric sustained-release capsule is very convenient for patients who have cardiovascular diseases and need to take medicines for a long time.
Owner:HEFEI JINYUE PHARMA
Modified release pharmaceutical compositions
InactiveUS8865213B2Improve complianceReduce dosing frequencyBiocidePowder deliveryRelease modulatorBULK ACTIVE INGREDIENT
Disclosed herein are multiparticulate modified release pharmaceutical compositions comprising: (a) a first portion comprising an active ingredient, at least one surfactant and at least one release modifying agent and (b) a second portion comprising an active ingredient and optionally a release modifying agent. Particularly, the active ingredient in the first portion is a calcium channel blocker and the modified release composition is in the form of a multiparticulate tablet.
Owner:USV LTD +1
Cubic liquid crystal in-situ gel injection of local anesthetic, and preparation method of injection
ActiveCN106491519AAvoid breedingImprove stabilityAerosol deliveryOintment deliveryMass ratioAmide local anesthetics
The invention relates to cubic liquid crystal in-situ gel injection of local anesthetic, and a preparation method of the injection. The injection is prepared from the local anesthetic, a liquid crystal material and an organic solvent and / or a release regulator; the local anesthetic is amide local anesthetic and the mass concentration of the local anesthetic is 0.1 to 8 w / w%; the organic solvent is an organic solvent which is mutually soluble with water, the liquid crystal material is mono-oleic acid glycerolipid, and the mass ratio of the liquid crystal material to the organic solvent is (1-9):1; and the release regulator is selected from at least one of medium chain triglyceride, oleic acid, tocopherol and tocopheryl acetate, and the mass concentration of the release regulator is 0 to 30 w / w%. The cubic liquid crystal in-situ gel injection of the local anesthetic has very good long-acting sustained release effect, low sudden release raten high compliance and small adverse effect, can reduce administration times and can avoid the peak valley phenomenon.
Owner:GUANGZHOU NEWORLD PHARMA CO LTD
Pharmaceutical compostions comprising kisspeptin or derivatives thereof
InactiveUS20160074320A1Peptide/protein ingredientsPharmaceutical delivery mechanismImmediate releaseSilicone Elastomers
The present invention relates to pharmaceutical compositions comprising a peptide that stimulates the release of gonadotropins and sexual steroids. More specifically, the present invention provides pharmaceutical compositions comprising kisspeptin, preferably in the kp-10 form, or derivatives thereof, for use in ovulation cycle inducing and / or infertility treatment programs. The formulations according to the present invention belong to two main groups: injectable solutions and implantable formulations. The injectable solutions according to the present invention can be divided into immediate release solutions and prolonged action solutions. The implantable formulations according to the present invention can be prepared using an RTV silicone elastomer, a rapid vulcanization silicone elastomer or a rapid vulcanization silicone elastomer with a release modulator.
Owner:OURO FINO SAUDE ANIMAL +1
Capecitabine pharmaceutical composition and preparation method thereof
ActiveCN103301079AConstant concentrationLow densityOrganic active ingredientsDigestive systemSide effectAdditive ingredient
The invention relates to a capecitabine pharmaceutical composition for treating gasatric cancer, and a preparation method thereof. The pharmaceutical composition is prepared by taking capecitabine as bulk pharmaceutical chemicals and a foaming agent, a release modifier, a binding agent, a lubricant and the like as auxiliary ingredients through adopting pharmaceutical acceptable preparation technology including wet-process pelletizing or dry-process pelletizing and then tabletting. The capecitabine is prepared into gastric floating tablets which can target towards the stomach and can be kept in the stomach for longer time and slowly release pharmaceutics, the curative effect of the capecitabine on the gastric cancer can be enhanced, the peak valley change of blood concentration of pharmaceutics can be reduced, the toxic and side effects of the pharmaceutics can be reduced, and the effecting time of the pharmaceutics can be prolonged.
Owner:QILU PHARMA
Antibiotic compositions of modified release and process of production thereof
InactiveUS20090111788A1Minimize adverse effectsEasy and cost-effectiveAntibacterial agentsBiocideAntibiotic YBULK ACTIVE INGREDIENT
Novel modified release pharmaceutical compositions wherein the composition comprises at least one antibiotic(s) preferably amoxicillin or its pharmaceutically acceptable salts, esters, polymorphs, isomers, prodrugs, solvates, hydrates, or derivatives thereof either alone or in combination with other antibiotic(s) as active ingredient, with at least one release modifying agent(s) for controlling the release of the beta lactam antibiotic optionally with one or more other pharmaceutically acceptable excipient(s) is provided, wherein the dosage form provides a release of not more than about 60% of the antibiotic in about 30 minutes and not less than about 70% of the antibiotic after 8 hours when subjected to in vitro dissolution study or when tested in vivo. Further, the compositions of the present invention which when tested in a group of healthy humans provide a mean peak plasma concentration (Cmax) after at least about 0.5 hour of administration of the dosage form. The present invention also provides process of preparing such dosage form and methods of using such dosage form.
Owner:PANACEA BIOTEC
Triamcinolone acetonide microsphere preparation and preparation method thereof
ActiveCN109700770AImprove efficiencyHigh yieldOrganic active ingredientsSkeletal disorderRelease modulatorMicrosphere
The invention provides a preparation method of a triamcinolone acetonide microsphere preparation. The method comprises the steps of: (1) dissolving PLGA in a volatile and well-soluble organic solventso as to form a homogeneous-phase solution; (2) adding triamcinolone acetonide to the homogeneous-phase solution described in the step (1) to be dissolved to obtain a homogeneous-phase solution with afinal viscosity of 5-500cp; and (3) feeding the homogeneous-phase solution described in the step (2) to a cup-shaped container in the center of a turntable device, wherein the liquid in the cup-shaped container passes over a cup rim, the solution hits a lateral disc-shaped turntable under the action of centrifugal force and gravity so as to form microdroplets, the formed microdroplets continue tohit a more lateral disc-shaped turntable, and after two or more hitting, the microdroplets fly out of the turntable and are solidified to form microspheres. The microsphere preparation obtained by the method has outstanding sustained release ability, and requires no additional release regulator, and the sustained release period can be as long as 1-3 months.
Owner:ZHEJIANG SUNDOC PHARMA SCI & TECH CO LTD
Altrenogest slow-release microsphere and preparation method thereof
ActiveCN108403667AReduce releaseReduce the number of dosesOrganic active ingredientsPharmaceutical non-active ingredientsFlavorSide effect
The invention relates to an altrenogest slow-release microsphere and a preparation method thereof. The preparation method comprises the following steps of dissolving altrenogest and a macromolecular material into an organic solvent, so as to form an oil phase; dissolving a releasing regulator and a flavor masking agent into a surfactant solution to form a water phase, and slowly adding the oil phase into a certain ratio of water phase, so as to form an O / W emulsion; adding the formed O / W emulsion into the residual water phase, and enabling the organic solvent to completely volatize; centrifuging, washing, and drying, so as to obtain the medicine microsphere. The altrenogest slow-release microsphere has the advantages that the slow-release effect is realized; after one-time administration,the altrenogest can be continuously and stably released for six days, so as to realize good slow release effect, and reduce the administration times of clinical medicine use; the occurrence of toxic or side effect and poor reaction is reduced, the palatability of the medicine is improved, the packaging rate is high and reaches 75% or above, the appearance is round, the granularity is large, and the dispersivity is good.
Owner:QILU ANIMAL HEALTH PROD +1
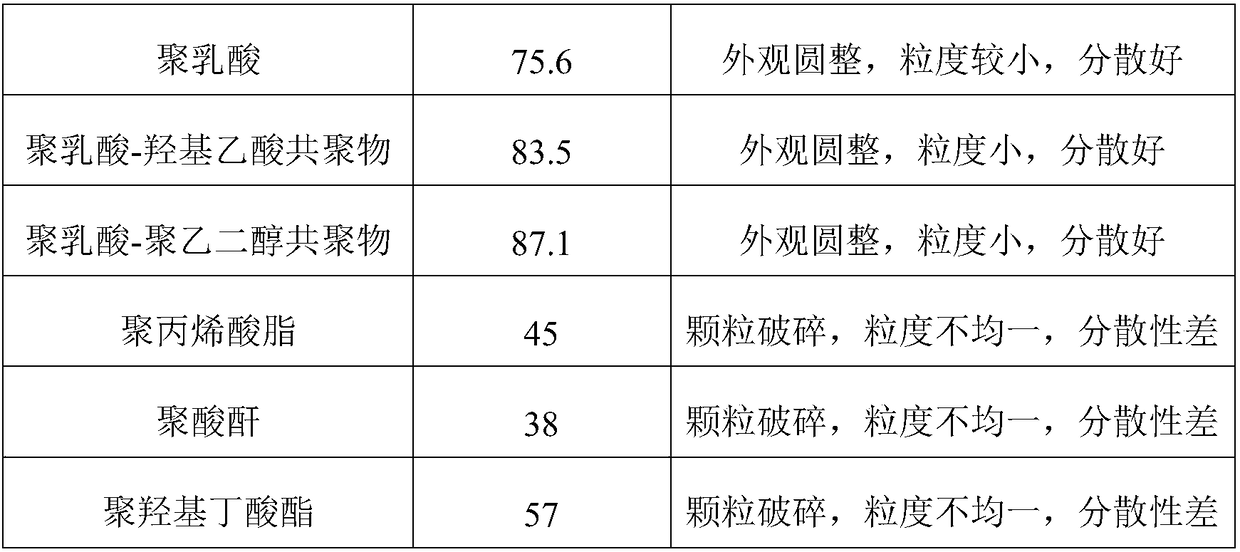
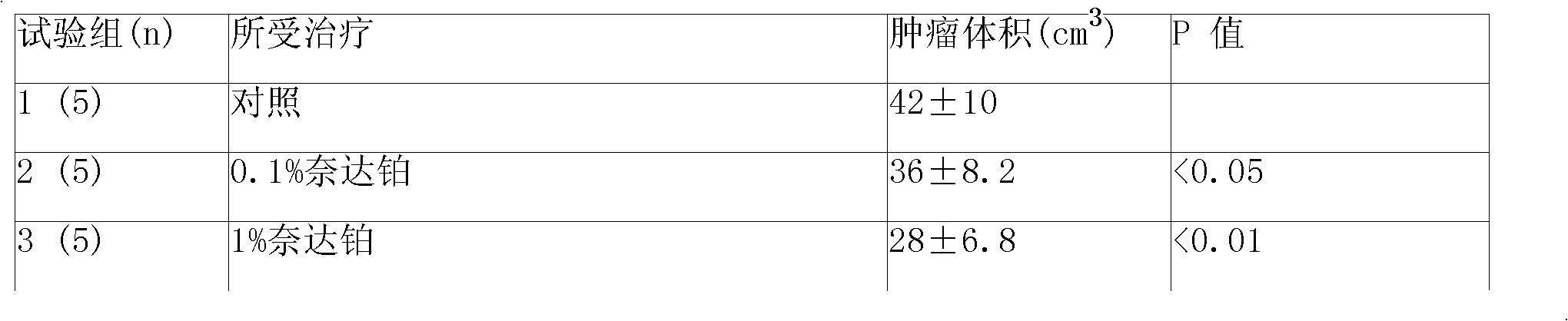
Anticancer sustained-release gel injection containing platinum compound
InactiveCN101283978APharmaceutical delivery mechanismPharmaceutical non-active ingredientsPicoplatinSolvent
An anticancer sustained-release gel injection sustained-release microspheres containing platinum-based compound, amphiphilic block copolymer, solvent and release regulator, the mixture of amphiphilic block copolymer and solvent has a temperature sensitive gelatinization characteristic, and can automatically become non-flowing degradable water insoluble gel, which can locally and slowly release drug at tumor foci for several weeks to several months, after injection into body. The adjuvant in the sustained-release microspheres is poly(lactic acid)-glycollic acid copolymer; and the amphiphilic block copolymer is PLGA-PEG-PLGA copolymer. The preparation can be injected into artery, inside a tumor or around a tumor with remarkably reduced systemic toxicity of drug, and can be used for solid tumors at different stages. The platinum-based compound is selected from nedaplatin, carboplatin, cisplatin, enloplatin, ormaplatin, sulfato-1,2-diaminocyclohezane-platinum (SHP), picoplatin, cyclopentylamine platium, zeniplatin, spiroplatin, lobaplatin, iproplatin, oxaliplatin, cycloplatin, and dexormaplatin. The preparation can be used with radioactive particles and can enhance chemotherapy effect.
Owner:济南基福医药科技有限公司
Sustained release alfuzosin hydrochl formulation and method for their production
A sustained release alfuzosin hydrochloride formulation contains alfuzosin hydrochloride as about 1% to about 5% by weight of the formulation, hydrophilic polymers as about 35% to about 75% by weight of the formulation, hydrophobic polymers as about 10% to about 30% by weight of the formulation, disintegrating agents as 10% to 30% by weight of the formulation and a binder as about 2% to about 12% by weight of the formulation. The hydrophilic and hydrophobic polymers are used as the release-modulating agent to control the dissolution profile of the alfuzosin hydrochloride formulation so that the formulation releases alfuzosin hydrochloride slowly and continuously as the formulation passed through the gastrointestinal tract. The present invention also relates to a method for preparing the above formulation.
Owner:STANDARD CHEM & PHARMA
Carmustine sustained-release implantation agent for curing entity tumour
InactiveCN101176707APharmaceutical delivery mechanismPharmaceutical non-active ingredientsTreatment effectWhole body
The invention relates to a slow-release implant agent of carmustine for tumor treatment, which is characterized in comprising effective amounts of carmustine, slow-release excipients and releasing regulatory agents in the slow-release implant agent. The excipients comprise macromolecules which are biologically compatible and degradable, mainly p (LAEG-EOP) and p (DAPG-EOP). The releasing regulatory agents are selected from one or more items from mannitol, sorbitol, xylitol, oligosaccharide, chitin, potassium salt, sodium salt, hyaluronic acid, collagen, chondroitin, gelatin and albumin. The slow-release implant agent slowly releases carmustine on the local tumor in the process of degradation and absorption, so the argent can evidently reduce the systemic toxicity and simultaneously apply to the effective drug concentration control on the local tumor. Therefore, the argent can be applied separately or combined with the non-surgical treatments such as chemotherapy drug and radiotherapy, which can be also widely used for tumor treatment of different phases, not only selectively improving the drug concentration on local tumor but also reinforcing the therapeutic effect of non-surgical treatments such as chemotherapy drug and radiotherapy.
Owner:SHANDONG LANJIN PHARMA
Risperidone slow-release composition and preparation method thereof
The invention discloses a risperidone slow-release composition and a preparation method thereof. The risperidone slow-release composition disclosed by the invention comprises non-solvent preparation raw materials, namely risperidone, a water-insoluble polymer and a release regulating agent, wherein the release regulating agent comprises organic lipophilic substances. The preparation method of the risperidone slow-release composition comprises the following steps: (1) dissolving the non-solvent preparation raw materials into an organic solvent so as to form inner oil phase; (2) dissolving a surfactant into an aqueous medium so as to form outer water phase; (3) adding the inner oil phase obtained in the step (1) into the outer water phase, preparing an emulsion, performing solvent evaluation or solvent extraction to harden micro particles in the solution, collecting the micro particles, washing, and drying, thereby obtaining risperidone slow-release microspheres. The risperidone slow-release composition disclosed by the invention is stable enough, can be continuously released for weeks or longer, and can be regulated according to specific medicine properties or treatment demands.
Owner:AC PHARMA CO LTD
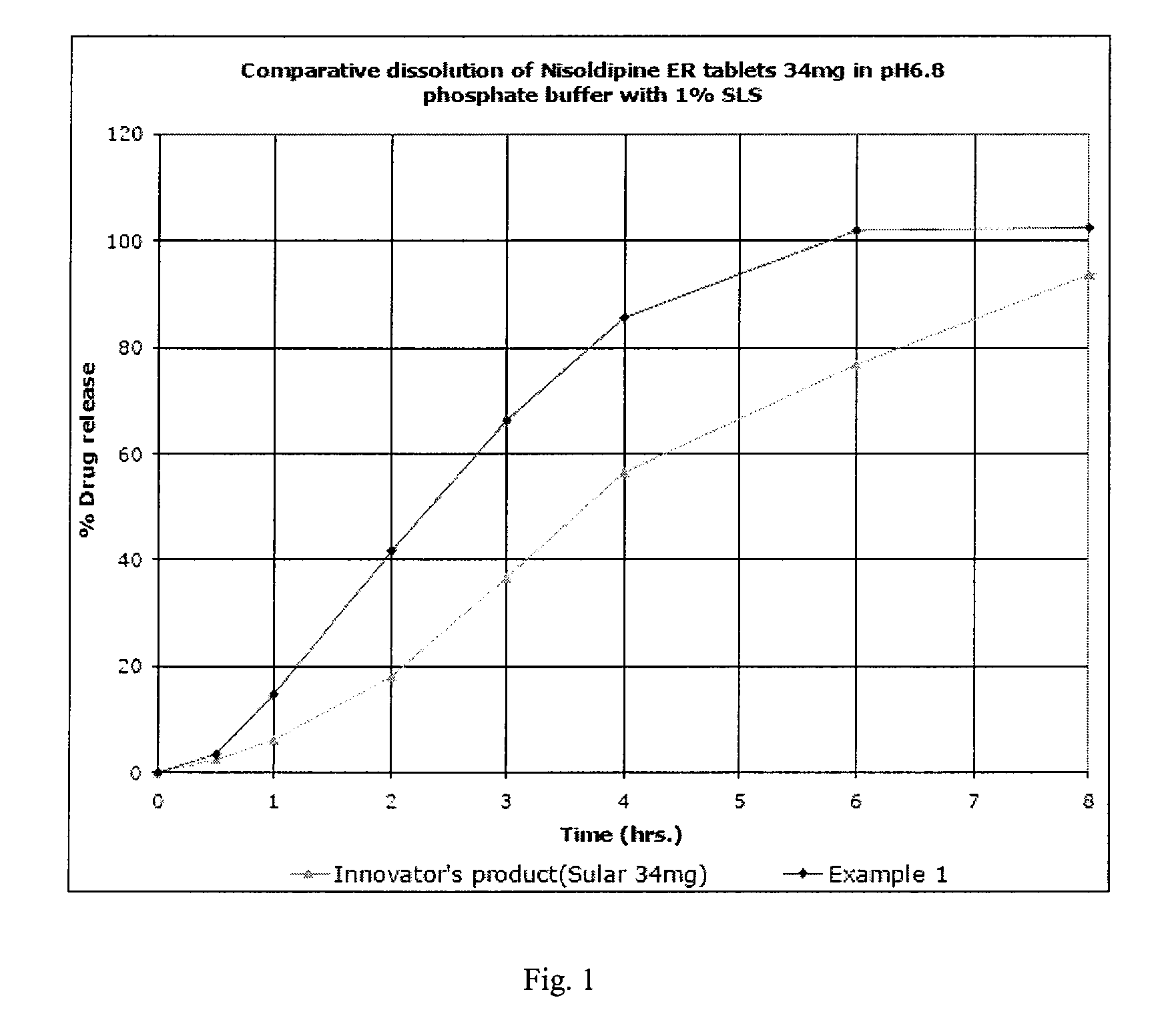
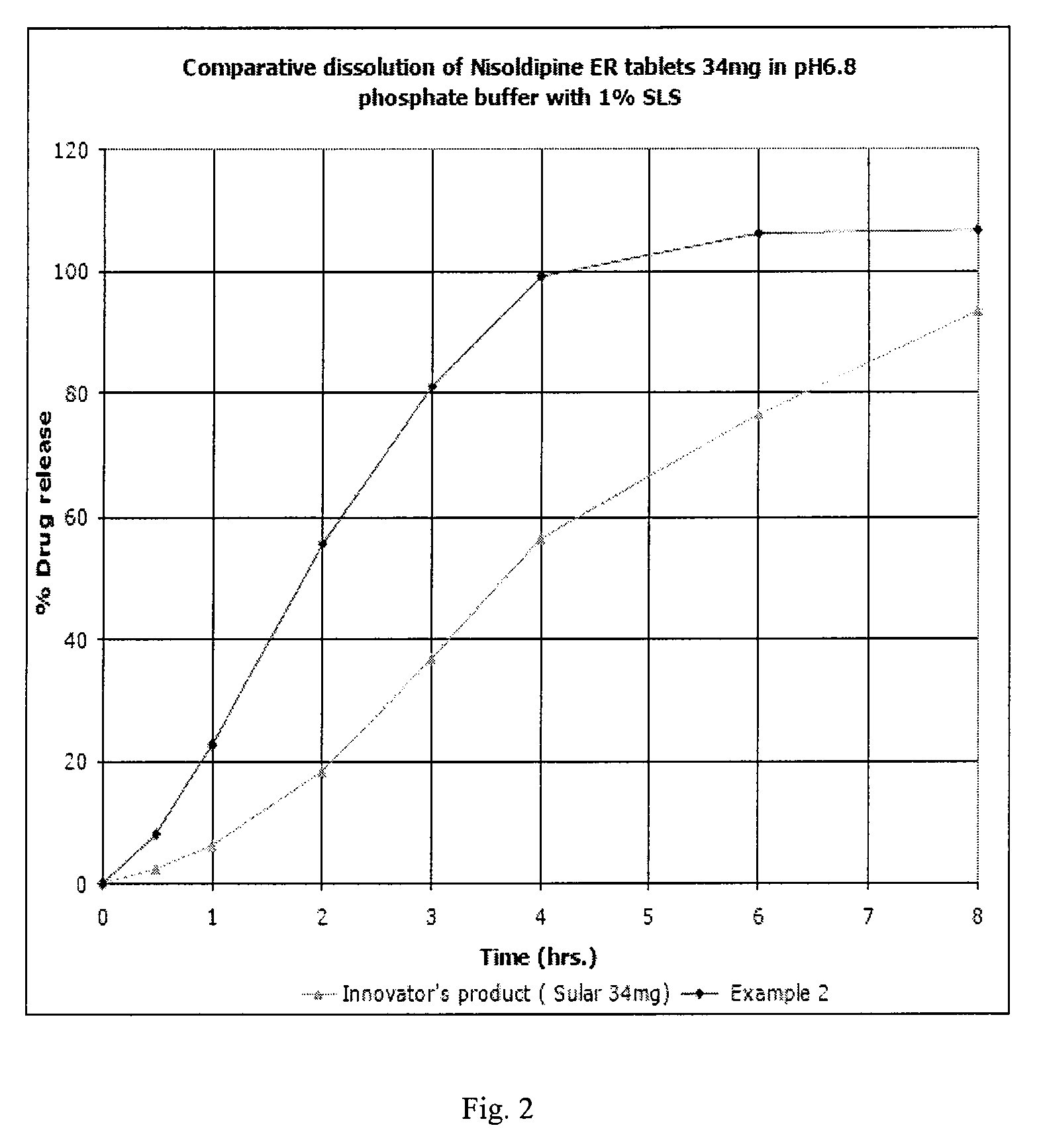
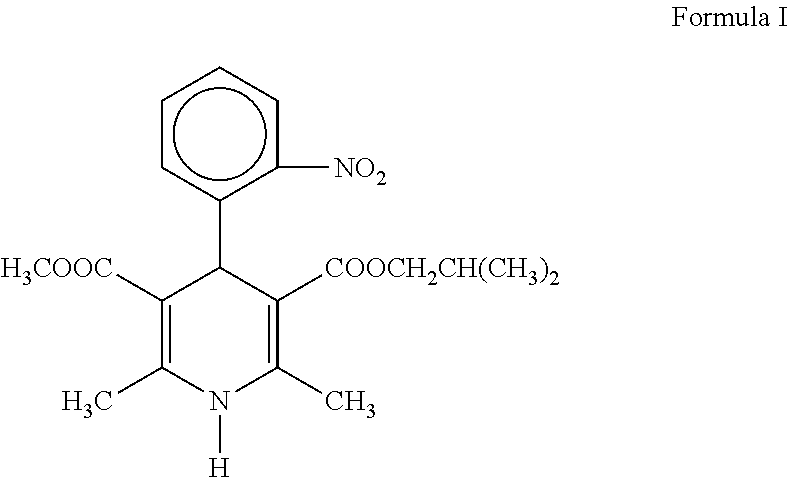
Topical composition comprising a dihydropyridine calcium antagonist
InactiveUS20100010052A1Improve bioavailabilityEnhanced drug releaseBiocideMuscular disorderDihydropyridineRelease modulator
A topical composition comprising a dihydropyridine calcium antagonist, a stiffening agent and a release modifier. The stiffening agent comprises a fatty alcohol, a fatty acid sorbitane ester, or a fatty acid glycerol ester, having a hydrocarbon chain containing 12 to 22 carbon atoms and having a melting point of about 45 to 750° C. The release modifier comprises a fatty alcohol, a fatty alcohol glycol ether, a fatty acid alkyl ester, a fatty acid glycerol ester, or a fatty acid sorbitane ester, having a hydrocarbon chain containing 12 to 18 carbon atoms and having a melting point of about −10 to 400° C. Use of such a composition for the treatment and / or prophylaxis of a dermal or mucosal disorder, preferably an anorectal disorder associated with high anal pressure or anal sphincter spasm.
Owner:MOBERG DERMA AB
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com