Formulations for amylin agonist peptides
a technology of amylin agonist and amylin agonist, which is applied in the direction of peptide/protein ingredients, inorganic non-active ingredients, metabolic disorders, etc., can solve the problems of insulin secretory response disorder, ineffective or lost efficacy, and ineffective insulin work, so as to reduce the loss of biological activity, and prolong the effect of stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Liquid Pramlintide Formulation
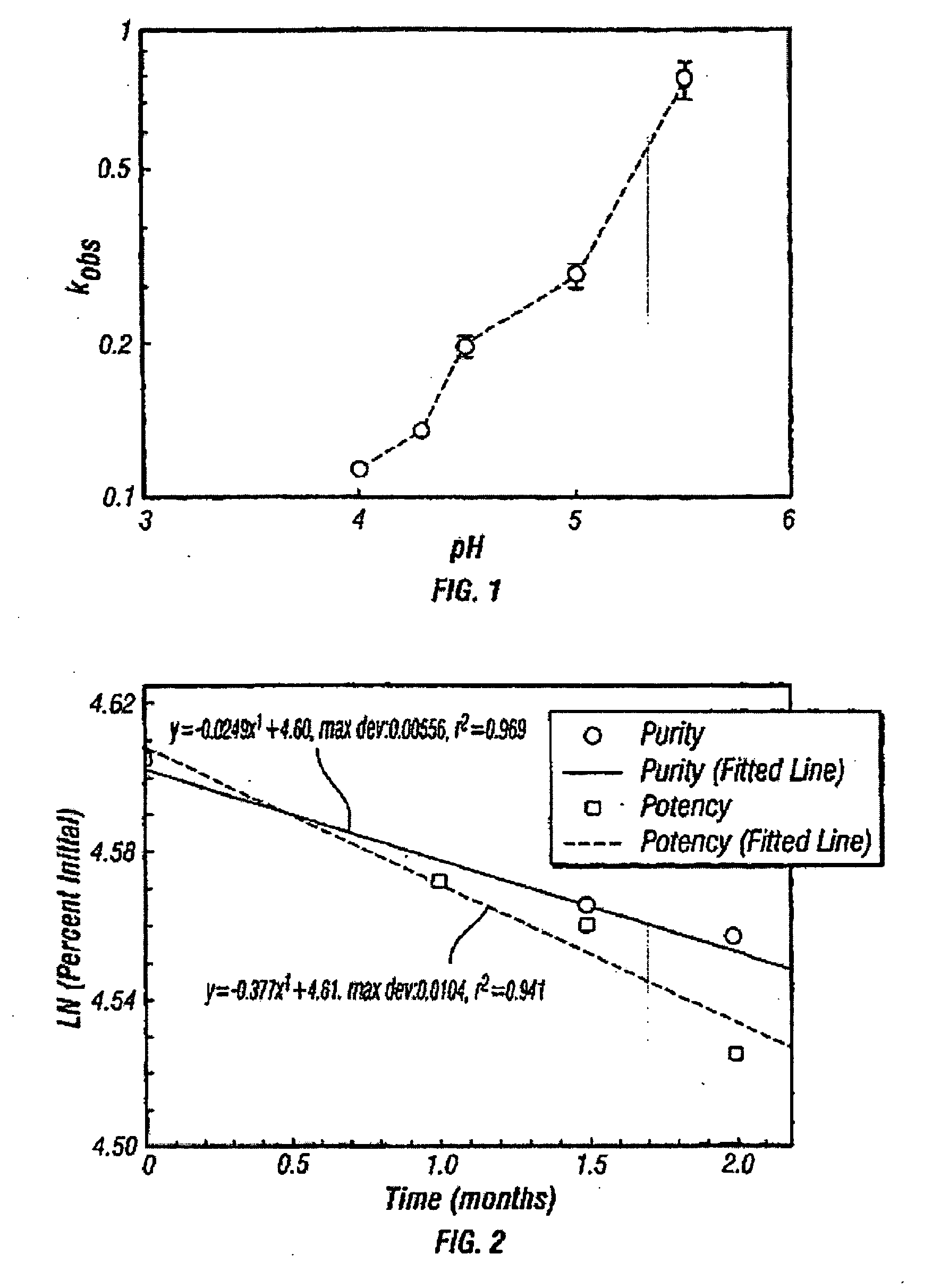
[0108]This example describes two preferred liquid formulations for pramlintide. Major degradation pathways for the peptide are deamidation and peptide bond hydrolysis. Therefore, the stability of the peptide was investigated in the pH region of 4.0-5.5 at 45° C. The pH-rate profile for the peptide in 60 mM acetate buffer, 4.1% mannitol, 0.3% m-cresol is shown in FIG. 1. It can be observed from this figure that Pro25,28,29 h-amylin over the pH range studied is most stable at pH 4.0. The following formulation was developed:
TABLE AINGREDIENTWeight (%) / RangePramlintide0.01-0.2Acetate (30 mM, pH 4.0 ± 0.1):sodium acetate trihydrate0.061glacial acetic acid0.153mannitol4.3m-cresol0.225Water For Injection (qs)100 mL
[0109]The above formulation with 0.01% drug showed an acceptably low irritancy in a rabbit subcutaneous irritancy study. The placebo of this formulation when tested in humans also showed an acceptably high level of tolerability and low irritancy. The...
example 2
Liquid Pramlintide Formulation
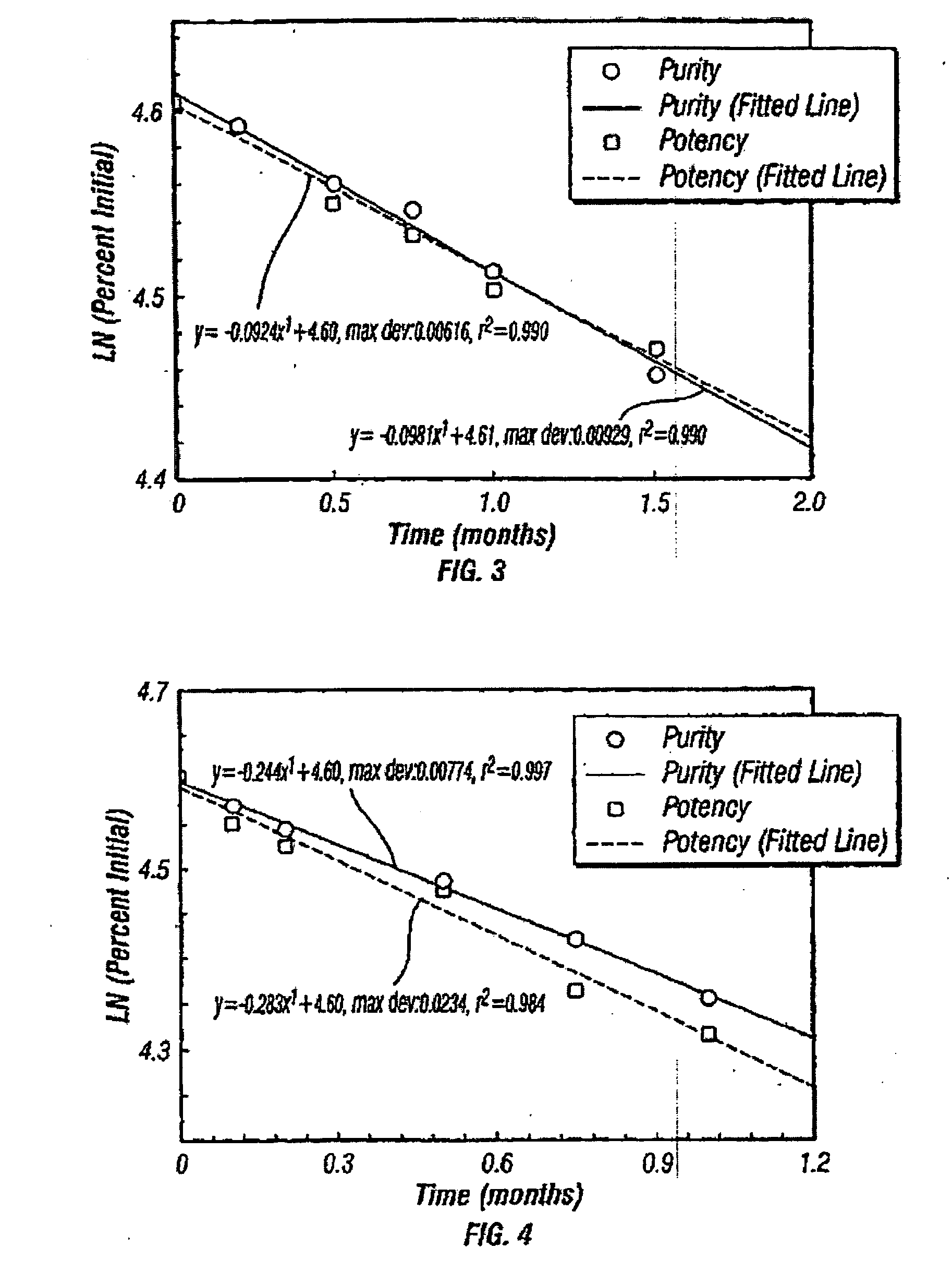
[0111]Table B describes second the peptide formulation with a shelf-life of greater than 4 years at 4° C. and greater than 60 days at 30° C. This formulation differs from the one in Table A in acetate buffer concentration. This formulation also showed no irritancy in a rabbit subcutaneous irritancy study. Additionally, the placebo did not show significant irritancy in humans. The shelf-lives of these formulations are at least as great as the formulation given in Table A, both formulations being novel parenteral peptide dosage forms with substantial shelf-lives.
TABLE BINGREDIENTWeight (%) / RangePramlintide0.01-0.2Acetate (20 mM, pH 4.0 ± 0.1):sodium acetate trihydrate0.049glacial acetic acid0.0985mannitol4.3m-cresol0.225Water for injection (qs)100 mL
example 3
Liquid Pramlintide Formulation and Insulin
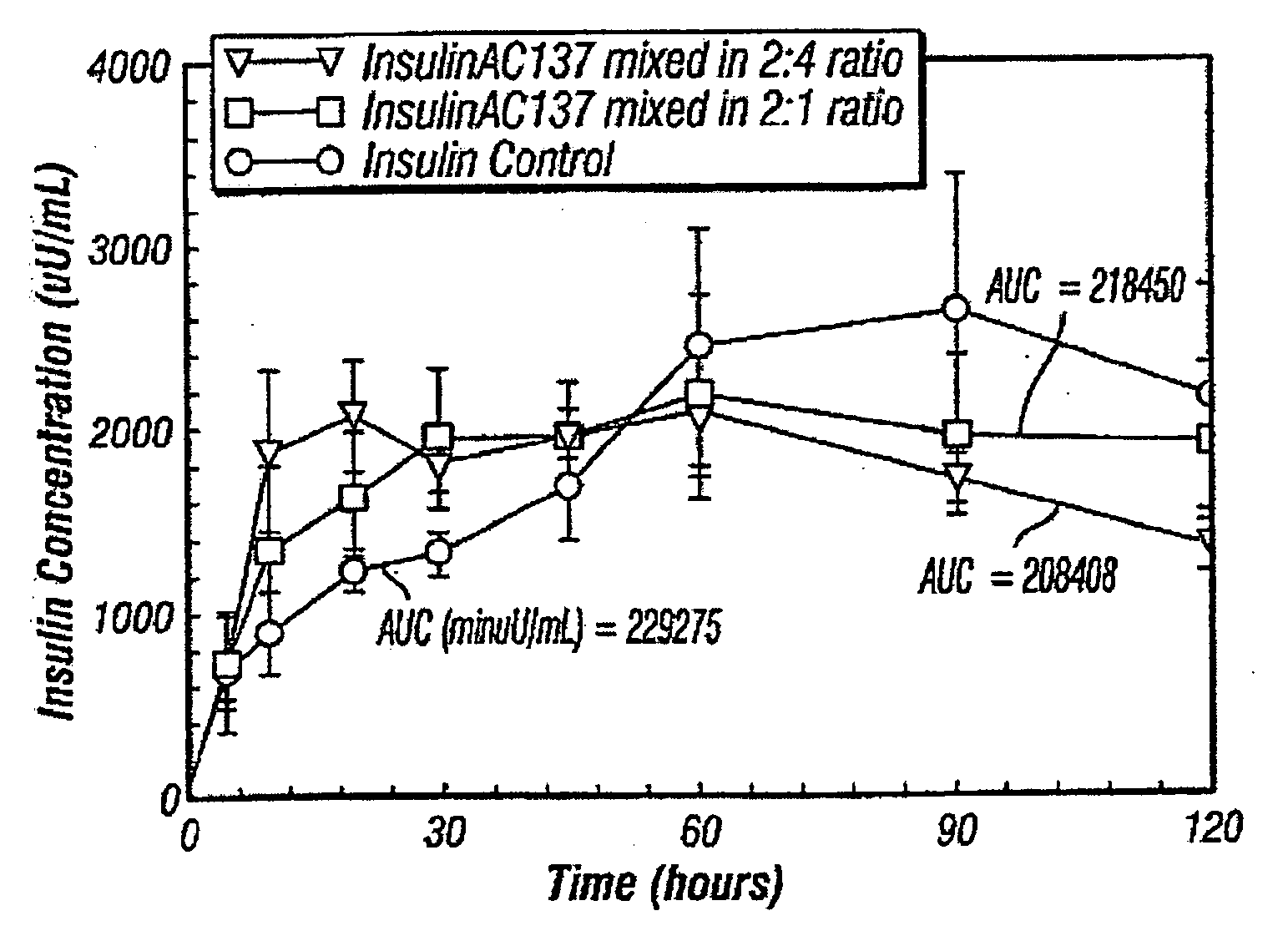
[0112]The formulations reported in Tables A and B are also compatible when mixed in a syringe with commercially available insulin products. Table C provides results of insulin compatibility study with Humulin® R. The results indicate the time to form a clear solution and criteria for compatibility, when the peptide formulation is mixed with Humulin R in a specific ratio in a syringe. As outlined by Brange et al, “Insulin Structure and Stability,” in Stability and Characterization of Protein and Peptide Drugs: Case Histories, 1993, Wang et al. (Ed), Plenum Press, NY, insulin has an isoelectric precipitation zone pH range of 4.5-6.5, within 1 pH unit from isoelectric point. Therefore, while not seeking to be bound by theory, if the pH of the insulin product drops from 7.2±0.2 to pH in the isoelectric precipitation range it may cause insulin to precipitate. Thus, clarity of the solution mixture was used as a criteria for preliminary compatibili...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com