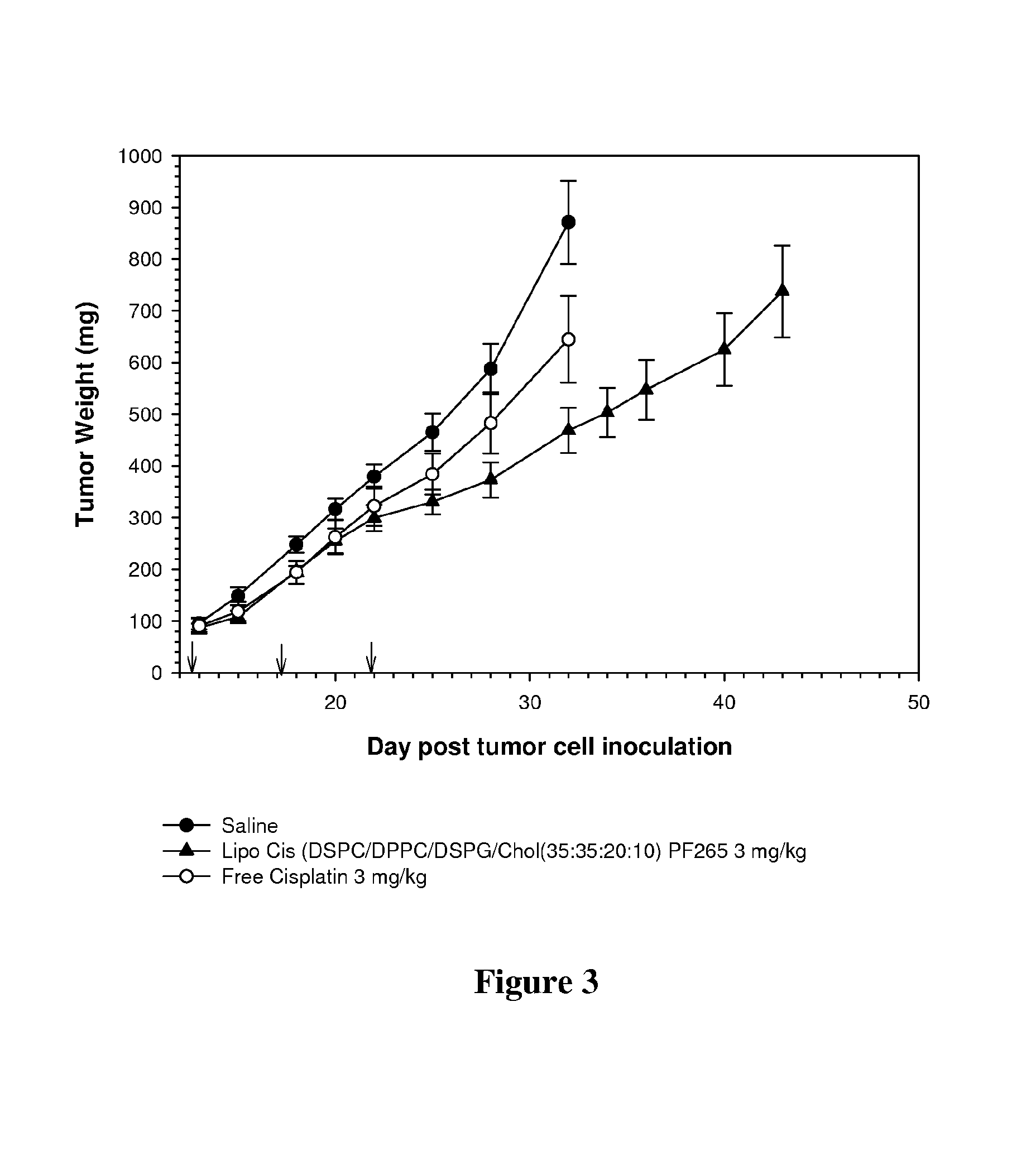
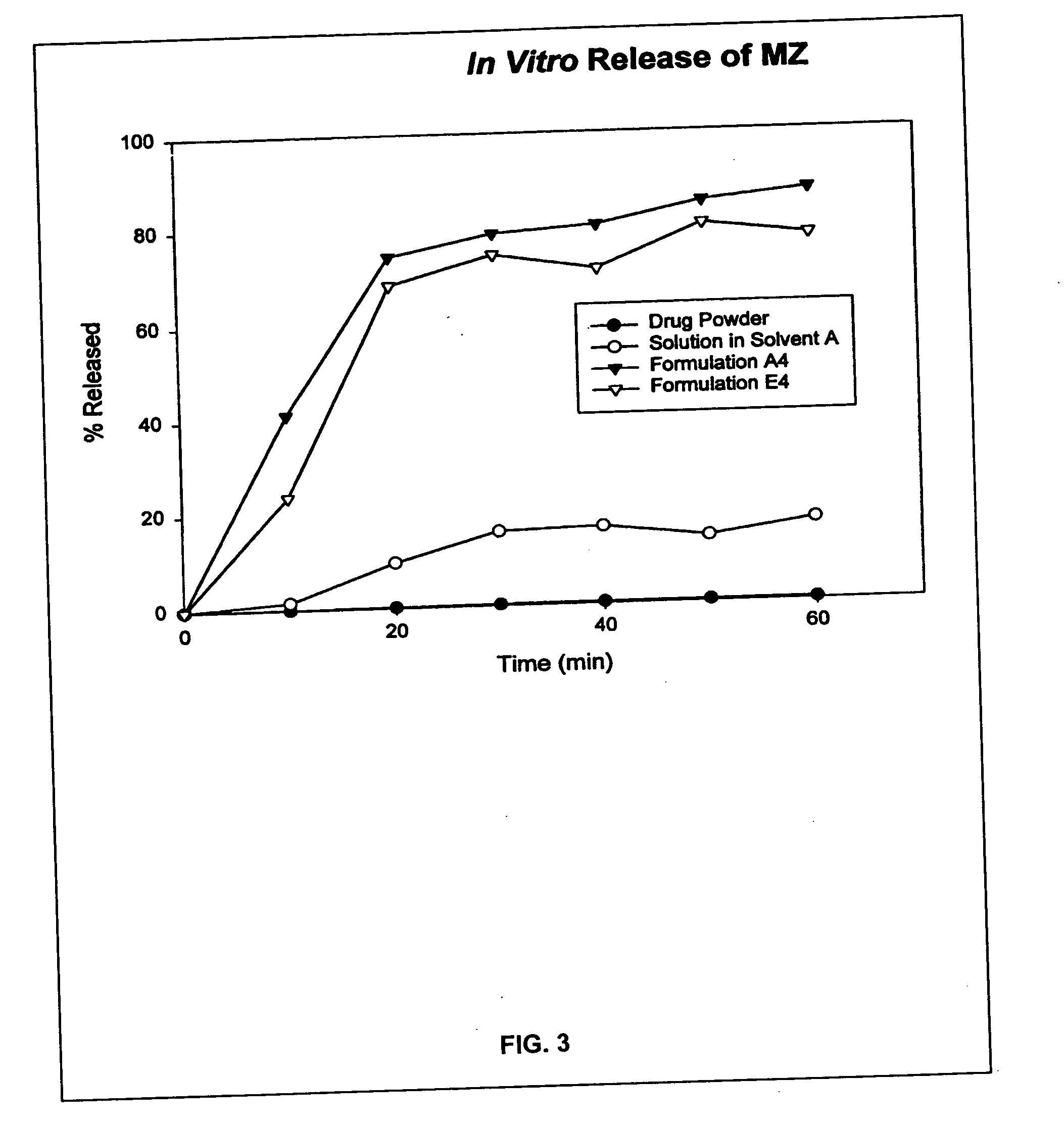
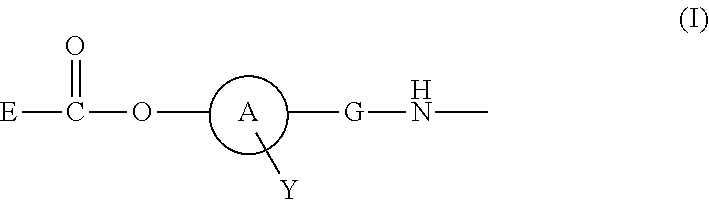Patents
Literature
88results about How to "Enhanced drug release" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Loading and release of water-insoluble drugs
Owner:BOSTON SCI SCIMED INC
Pharmaceutical formulations of potassium ATP channel openers and uses thereof
InactiveUS20060051418A1Enhanced drug releaseReduces food uptakeBiocideOrganic active ingredientsPotassium channel openerPotassium
Provided are immediate or prolonged administration of certain potassium ATP (KATP) channel openers to a subject to achieve novel pharmacodynamic, pharmacokinetic, therapeutic, physiological, metabolic and compositional outcomes in the treatment of diseases or conditions involving KATP channels. Also provided are pharmaceutical formulations, methods of administration and dosing of KATP channel openers that achieve these outcomes and reduce the incidence of adverse effects in treated individuals. Further provided are method of co-administering KATP channel openers with other drugs to treat diseases of humans and animals.
Owner:ESSENTIALIS INC
Chronotherapeutic dosage forms
InactiveUS20090169587A1Reduce releaseEnhanced drug releaseBiocideAntipyreticActive agentSurface-active agents
Owner:ENDO PHARMA INC
Chronotherapeutic dosage forms
InactiveUS20050118267A1Facilitated releaseFacilitates immediate release of drugOrganic active ingredientsAntipyreticActive agentPharmaceutical formulation
A chronotherapeutic pharmaceutical formulation comprising a core containing an active agent (e.g., a drug) and a surfactant and a delayed release compression coating comprising a natural or synthetic gum applied onto the surface of the core.
Owner:PENWEST PHARMA CO
Drug-Eluting Medical Device
InactiveUS20100233228A1Enhanced drug releaseImprove featuresOrganic active ingredientsBiocidePolyesterImmediate release
A drug-eluting medical device includes a catheter balloon completely or partially coated with paclitaxel in anhydrous crystalline form, having an immediate release and bioavailability of a therapeutically effective amount of paclitaxel at the intervention site. The balloon can be made of a polyether-polyamide block copolymer, or a polyester amide, or polyamide-12.
Owner:INVATEC TECH CENT
Salts of potassium ATP channel openers and uses thereof
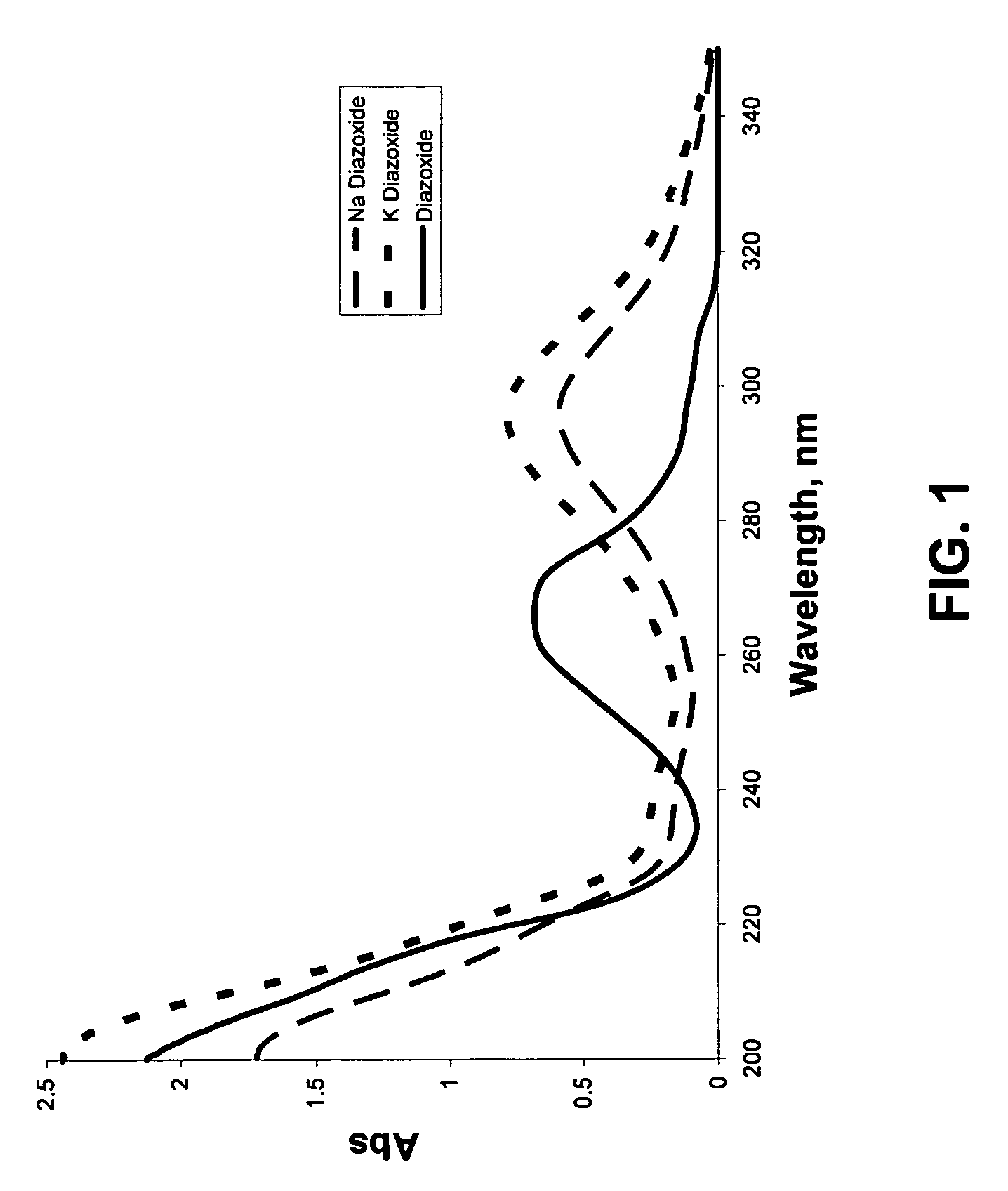
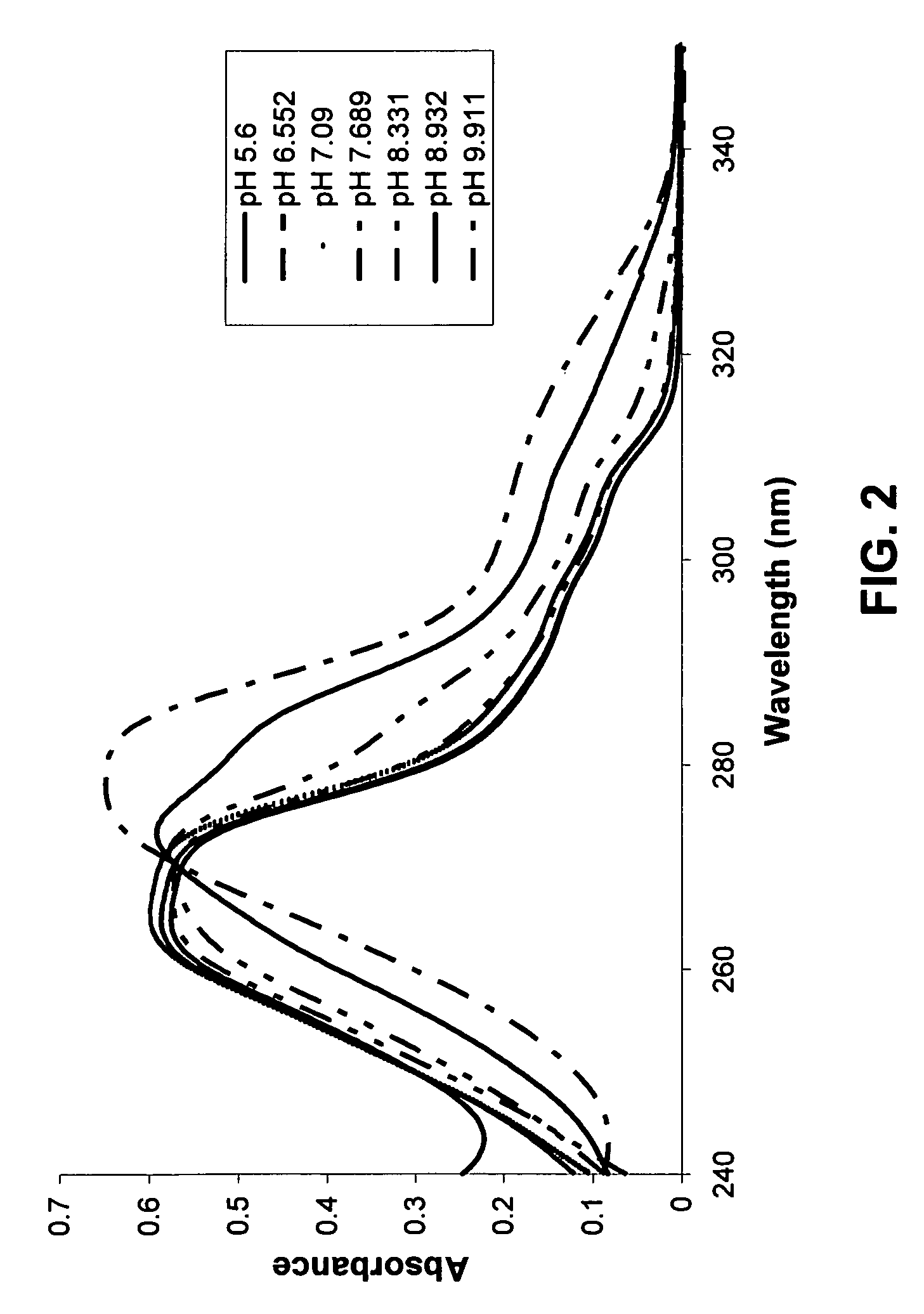
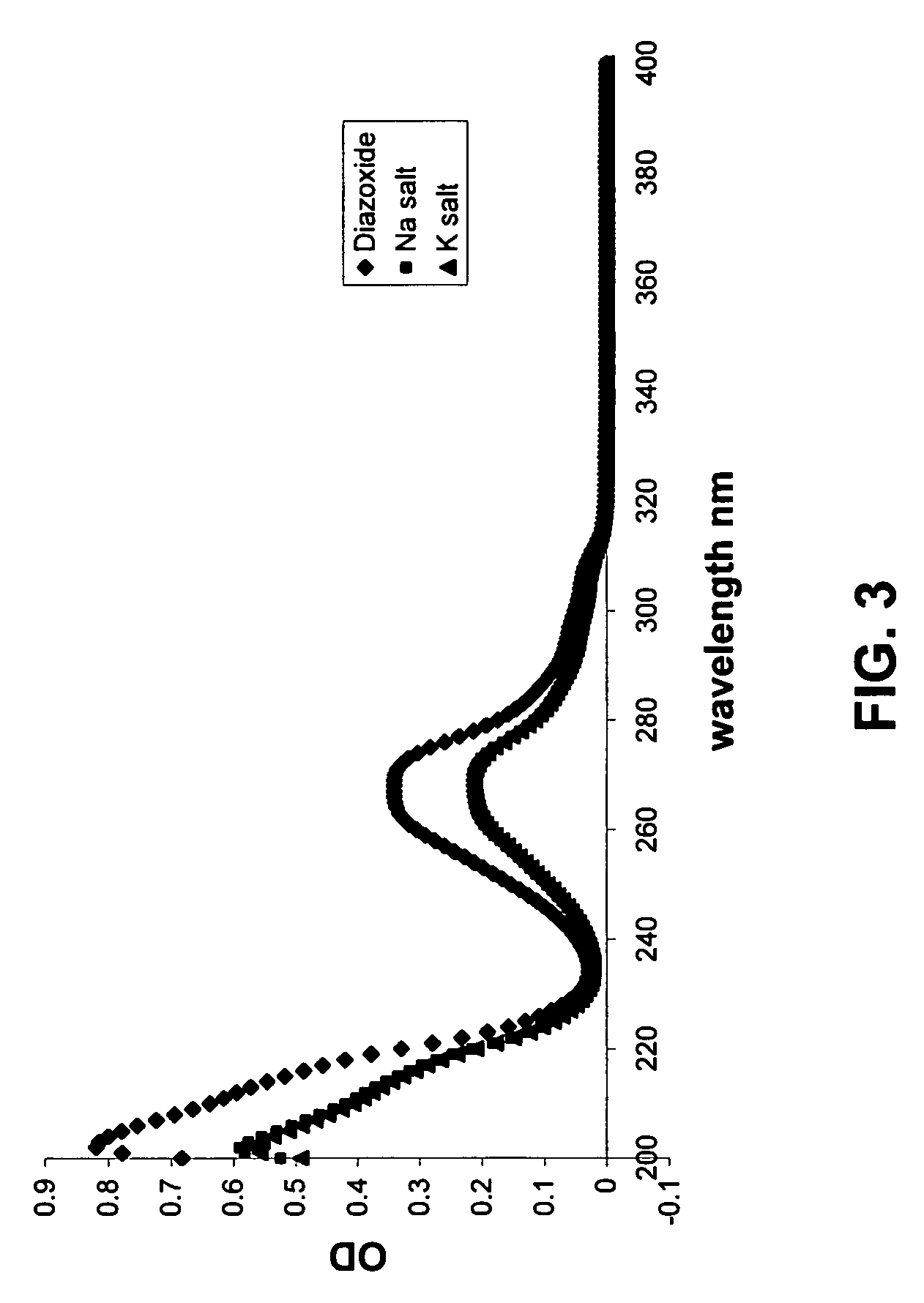
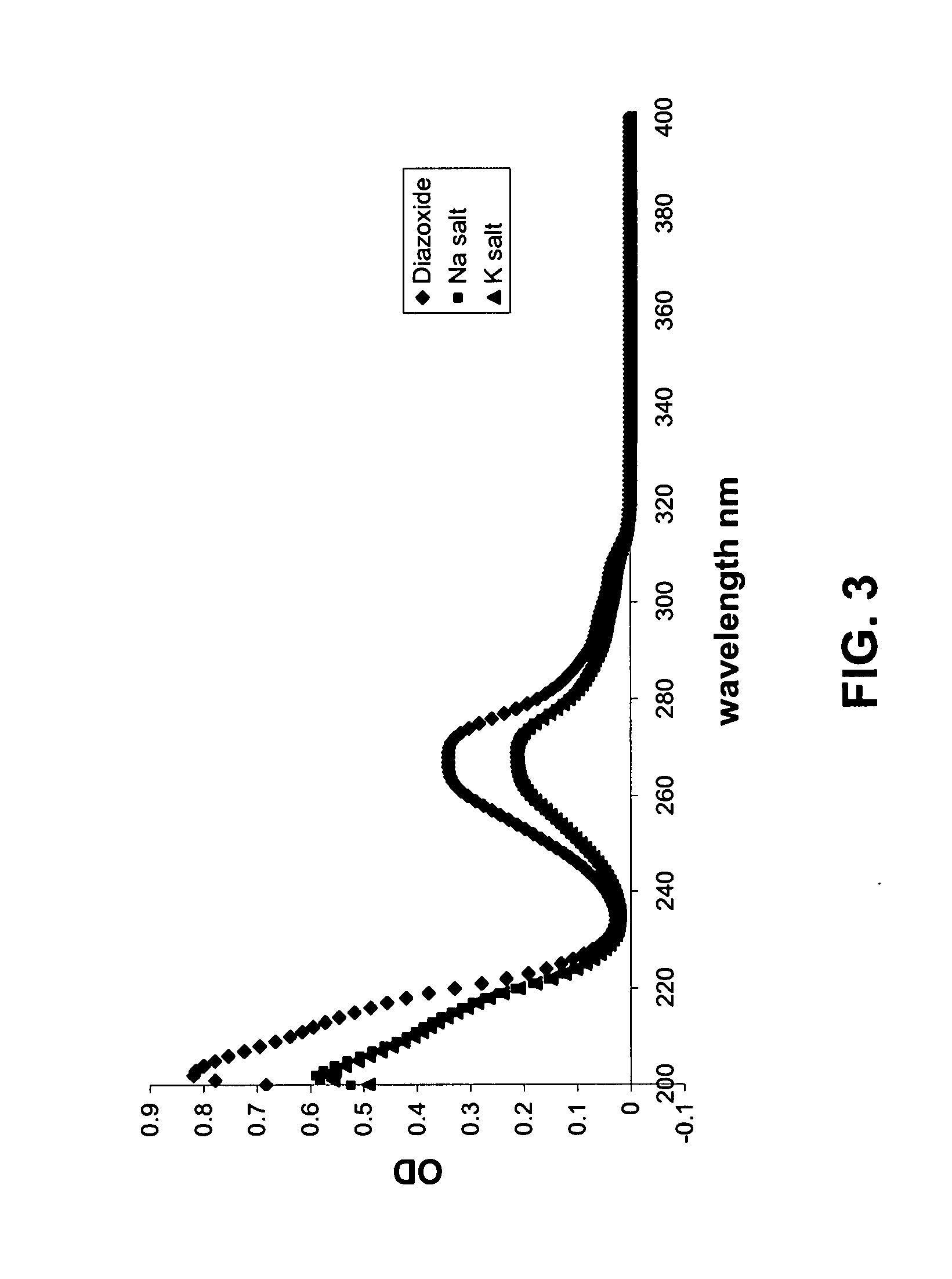
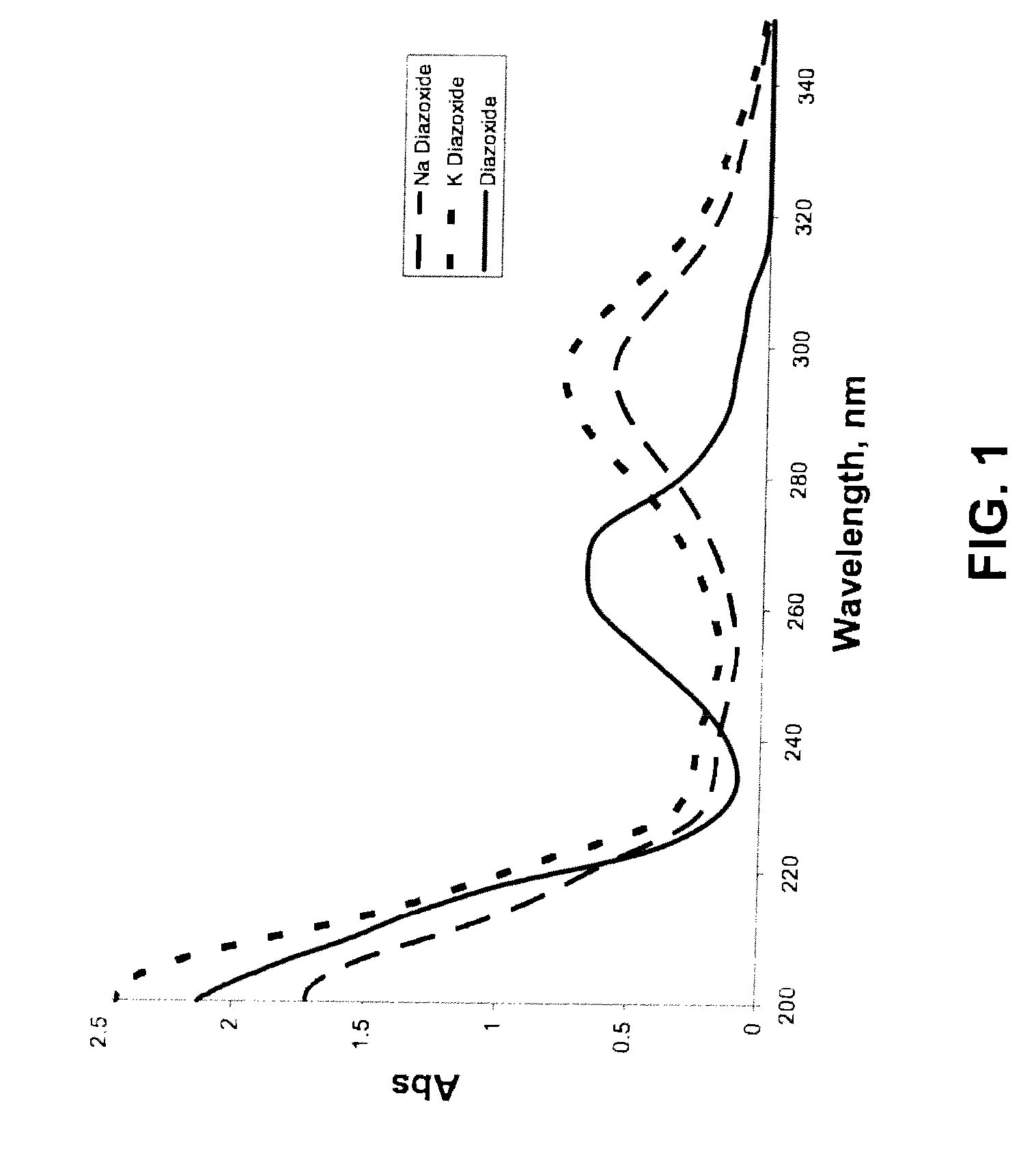
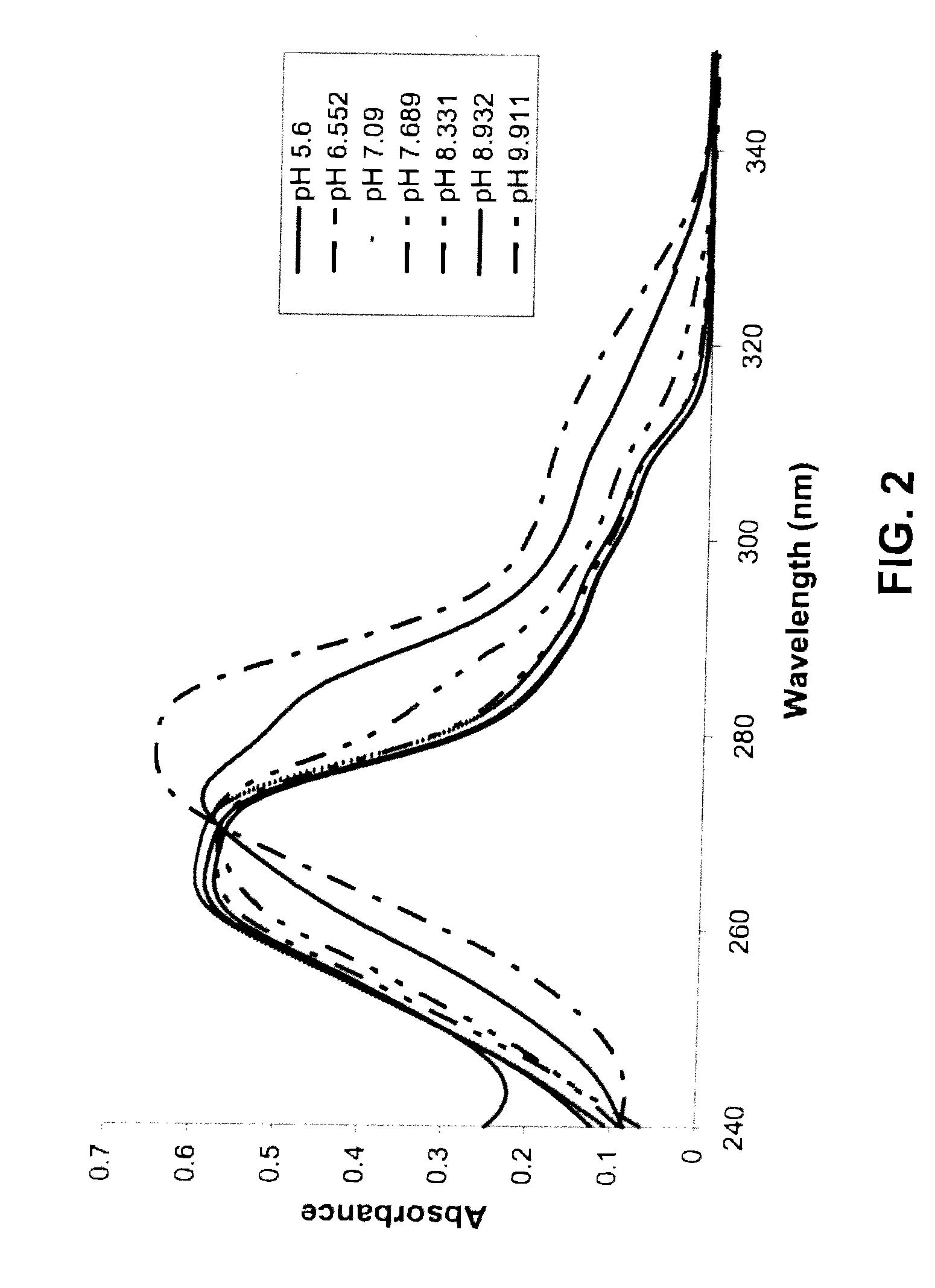
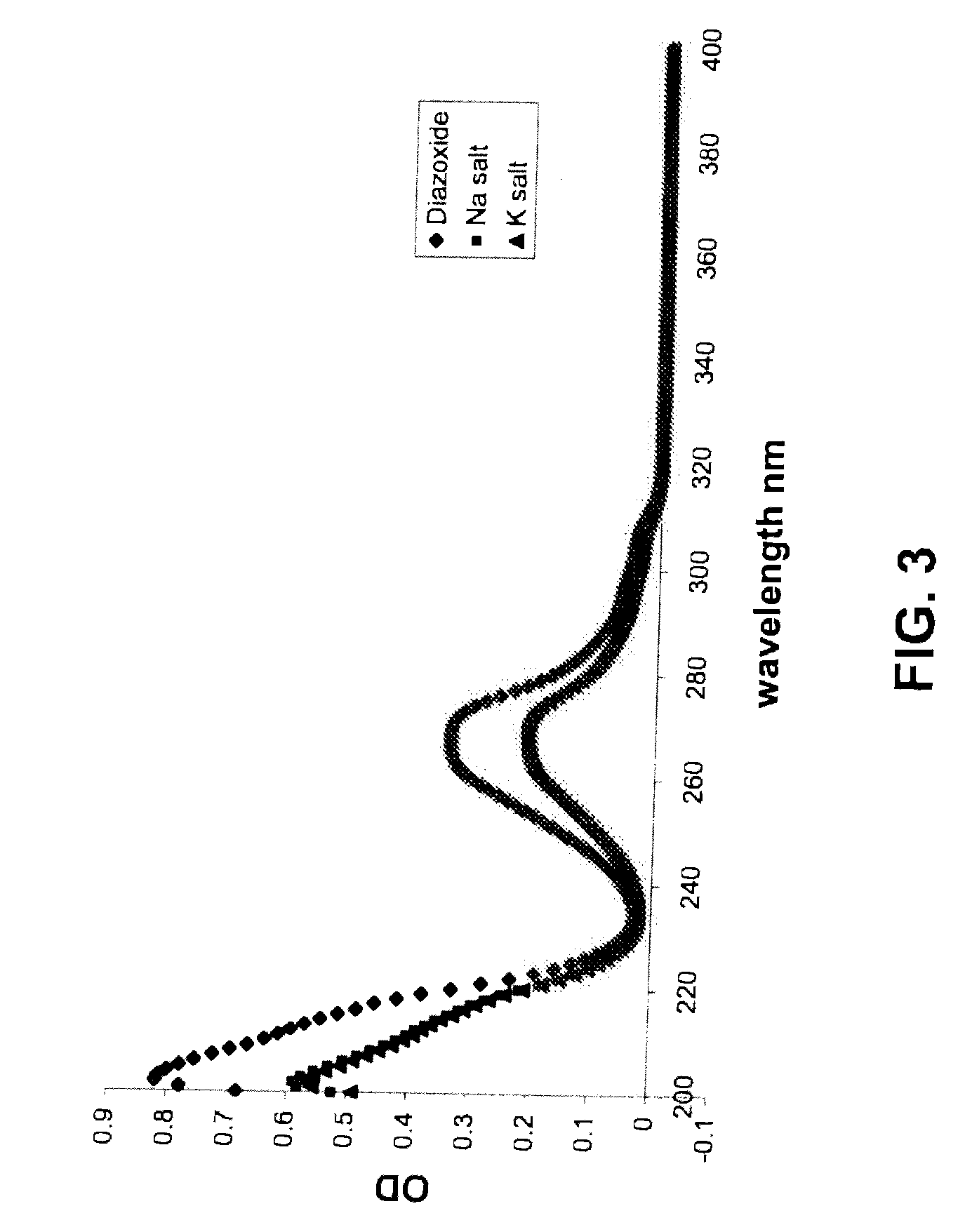
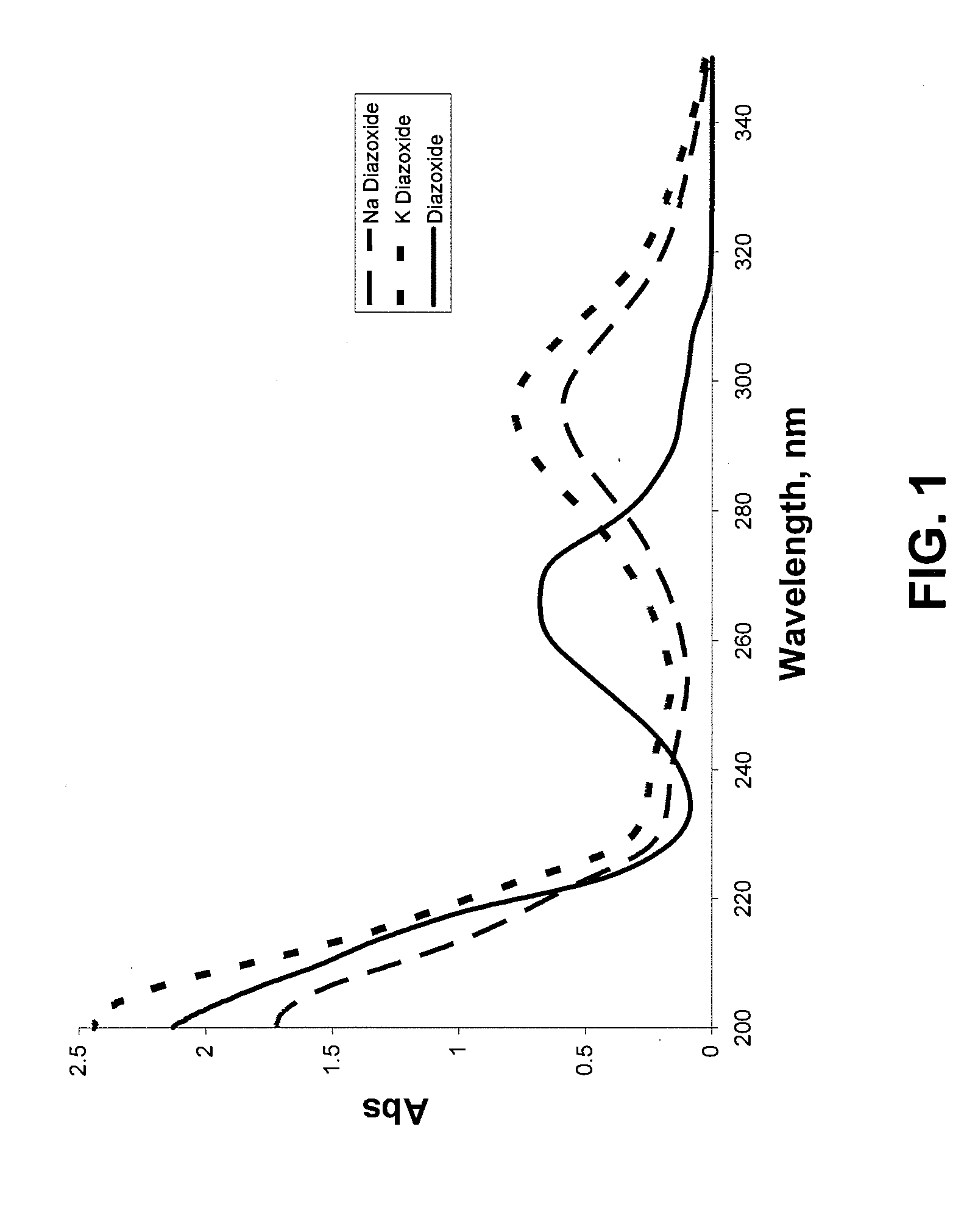
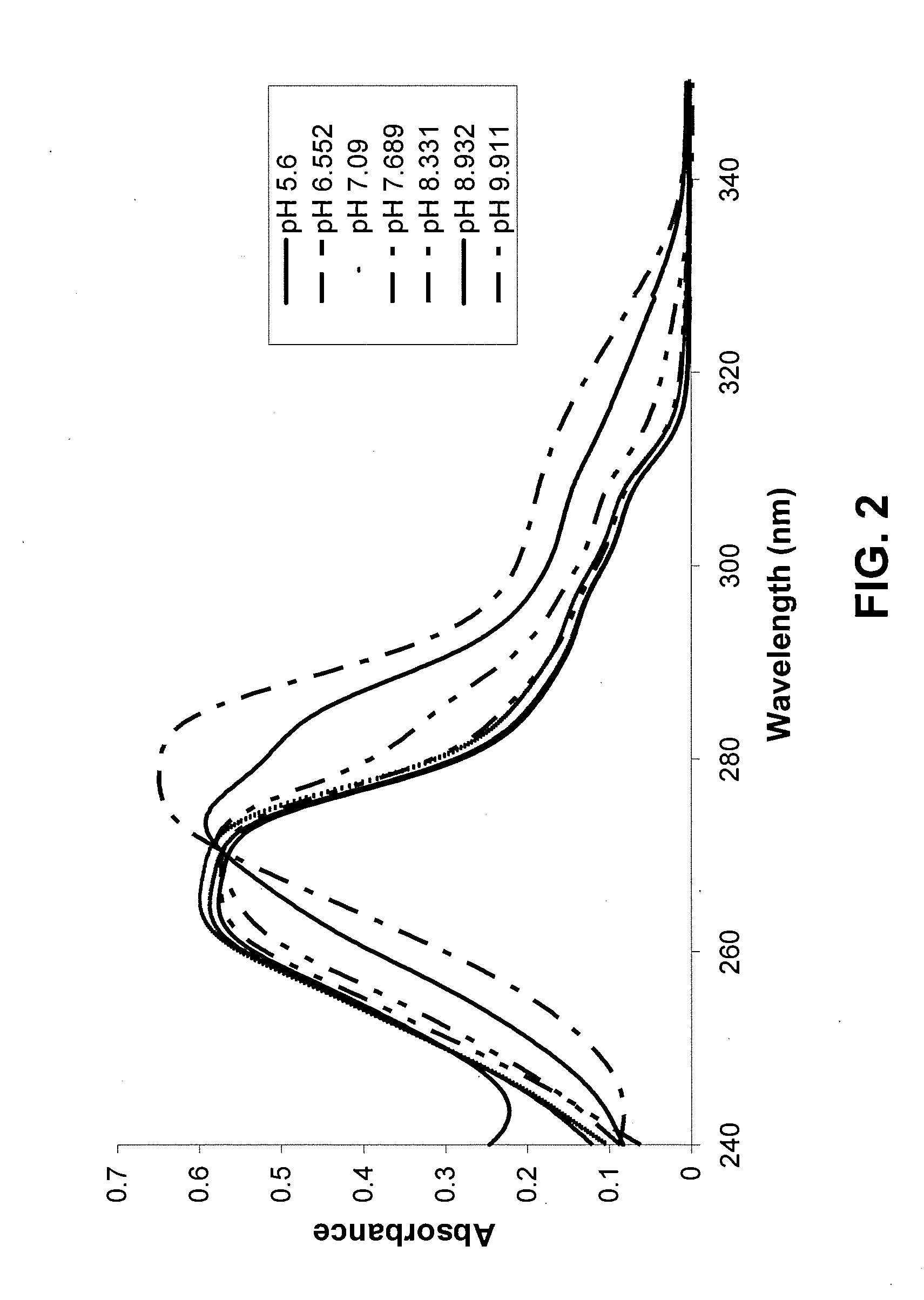
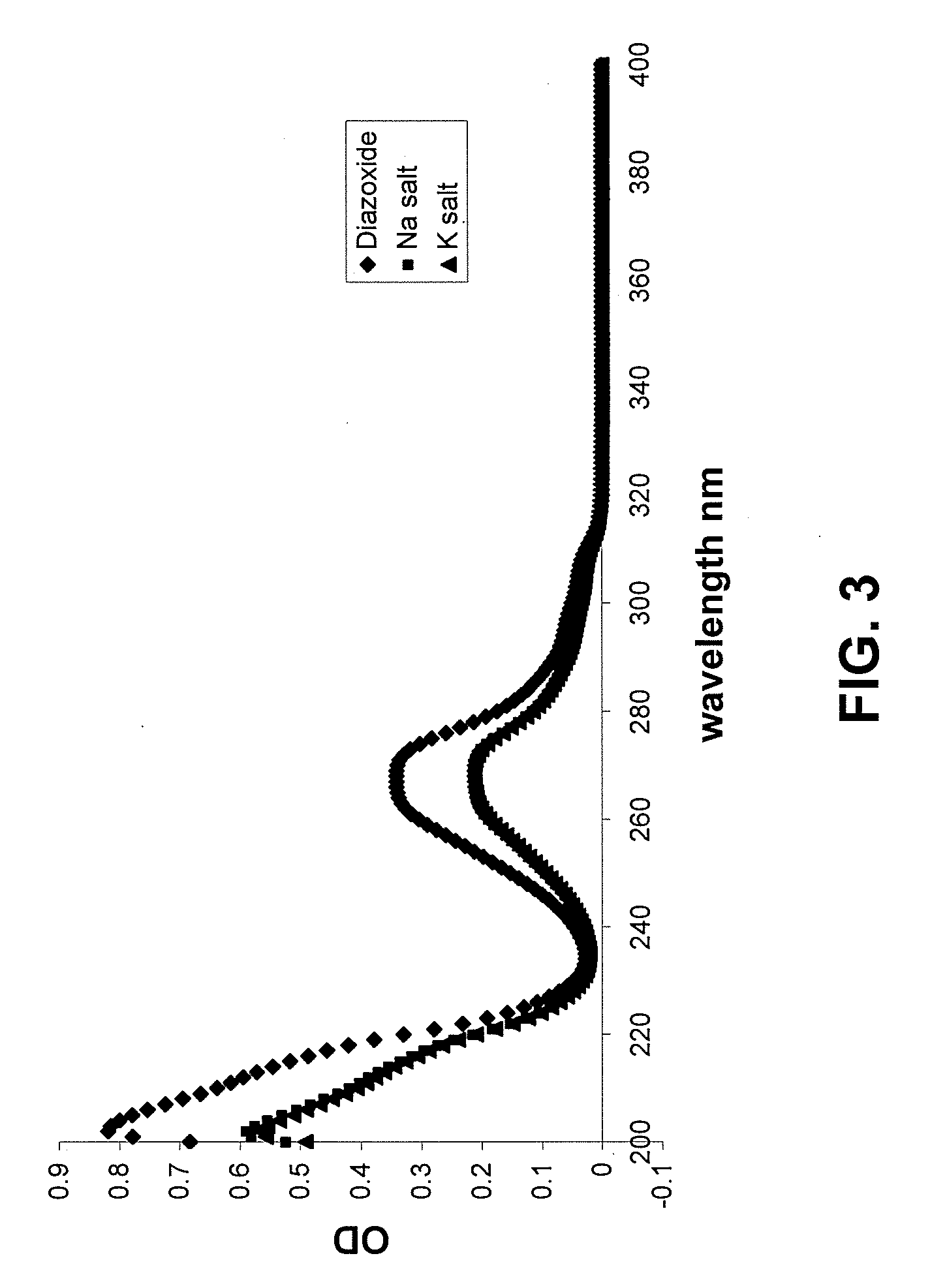
Provided are immediate or prolonged administration of certain salts of KATP channel openers such as diazoxide to a subject to achieve novel pharmacodynamic, pharmacokinetic, therapeutic, physiological, metabolic and compositional outcomes in the treatment of diseases or conditions involving KATP channels. Also provided are pharmaceutical formulations, methods of administration and dosing of the salts that achieve these outcomes and reduce the incidence of adverse effects in treated individuals. Further provided are method of co-administering the salts with other drugs to treat diseases of humans and animals.
Owner:ESSENTIALIS INC
Extended release tablet formulations of flibanserin and method for manufacturing the same
ActiveUS20080038346A1Reduce solubilitySufficiently slow releasePowder deliveryOrganic active ingredientsOral medicationExtended release tablets
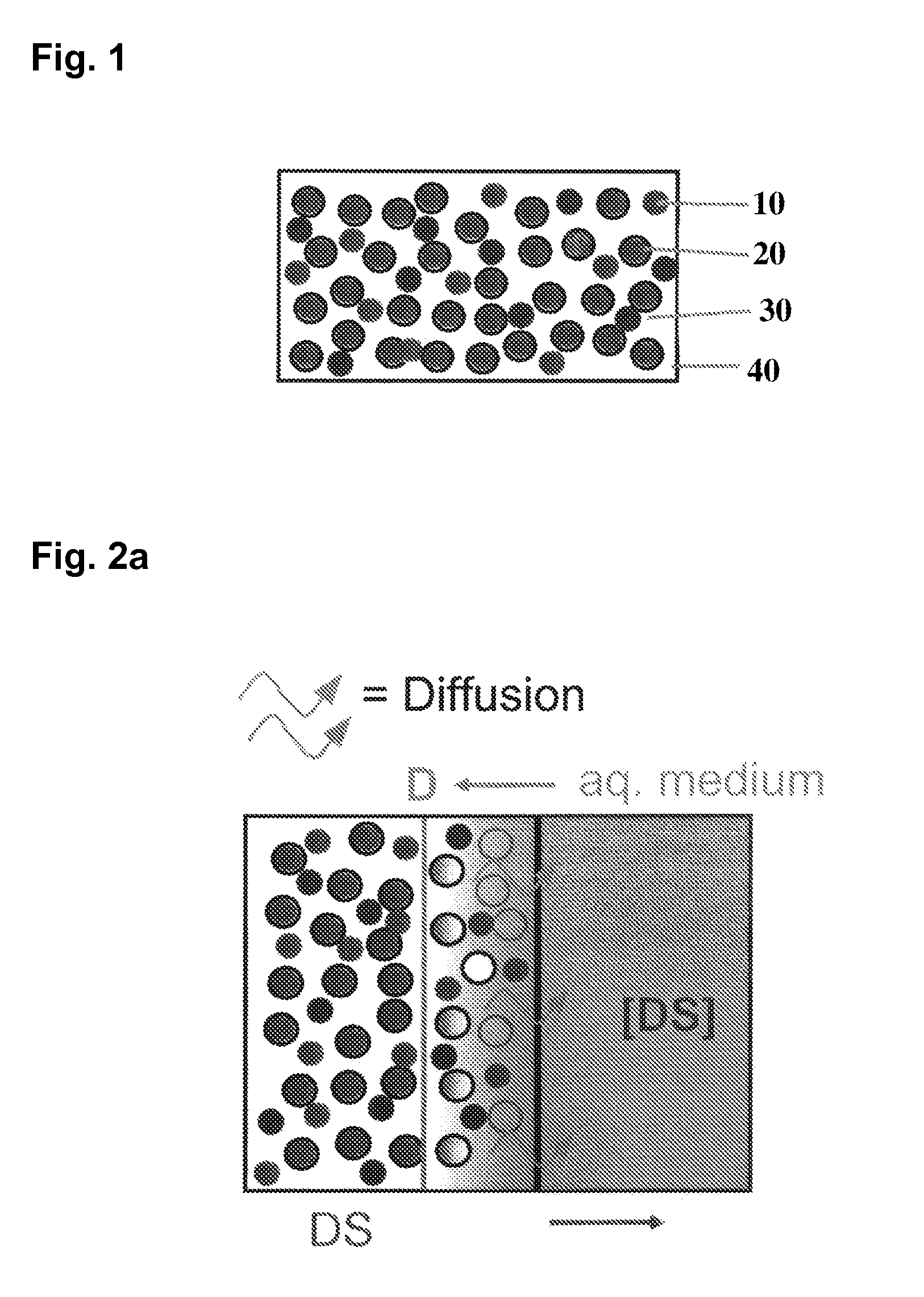
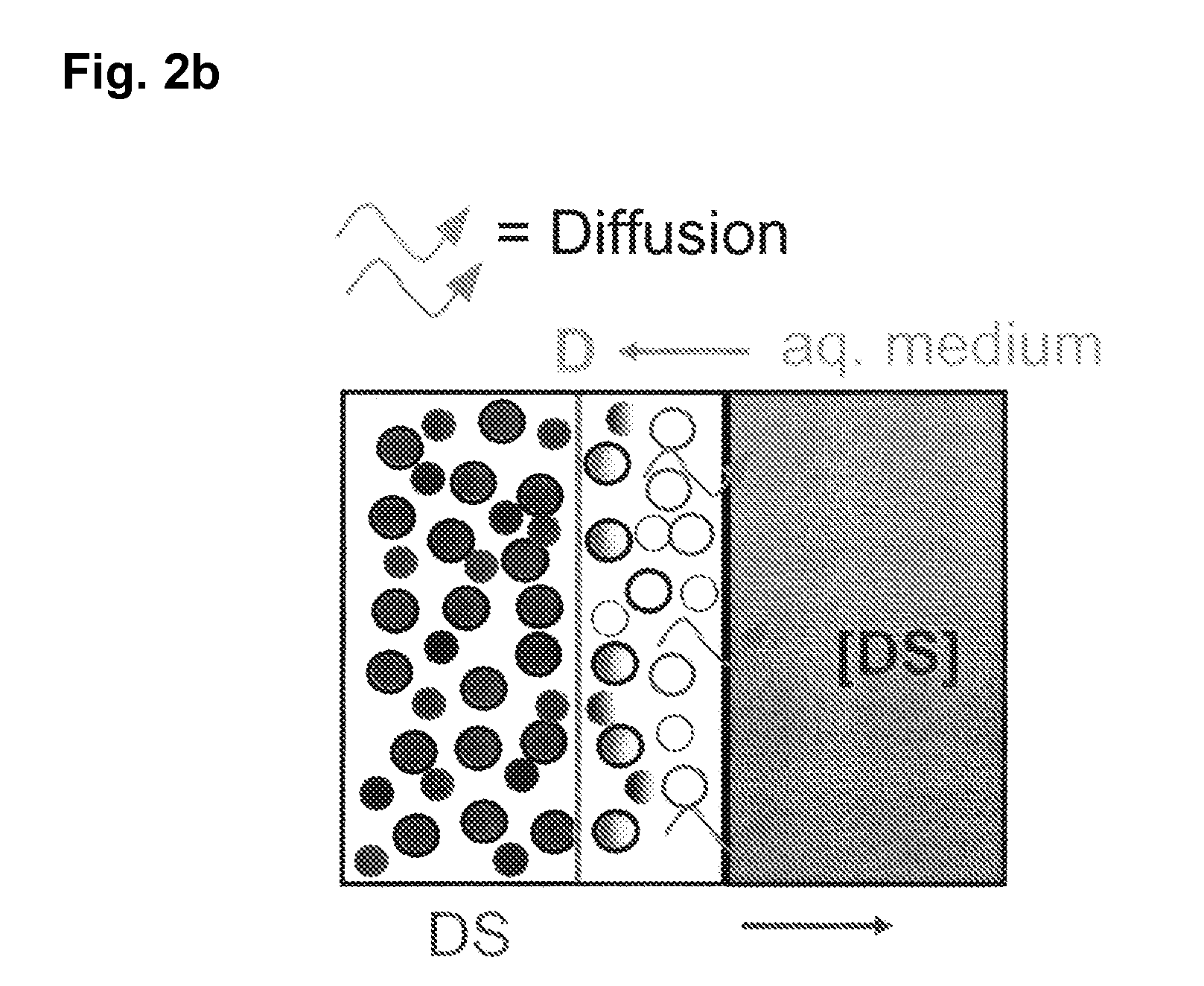
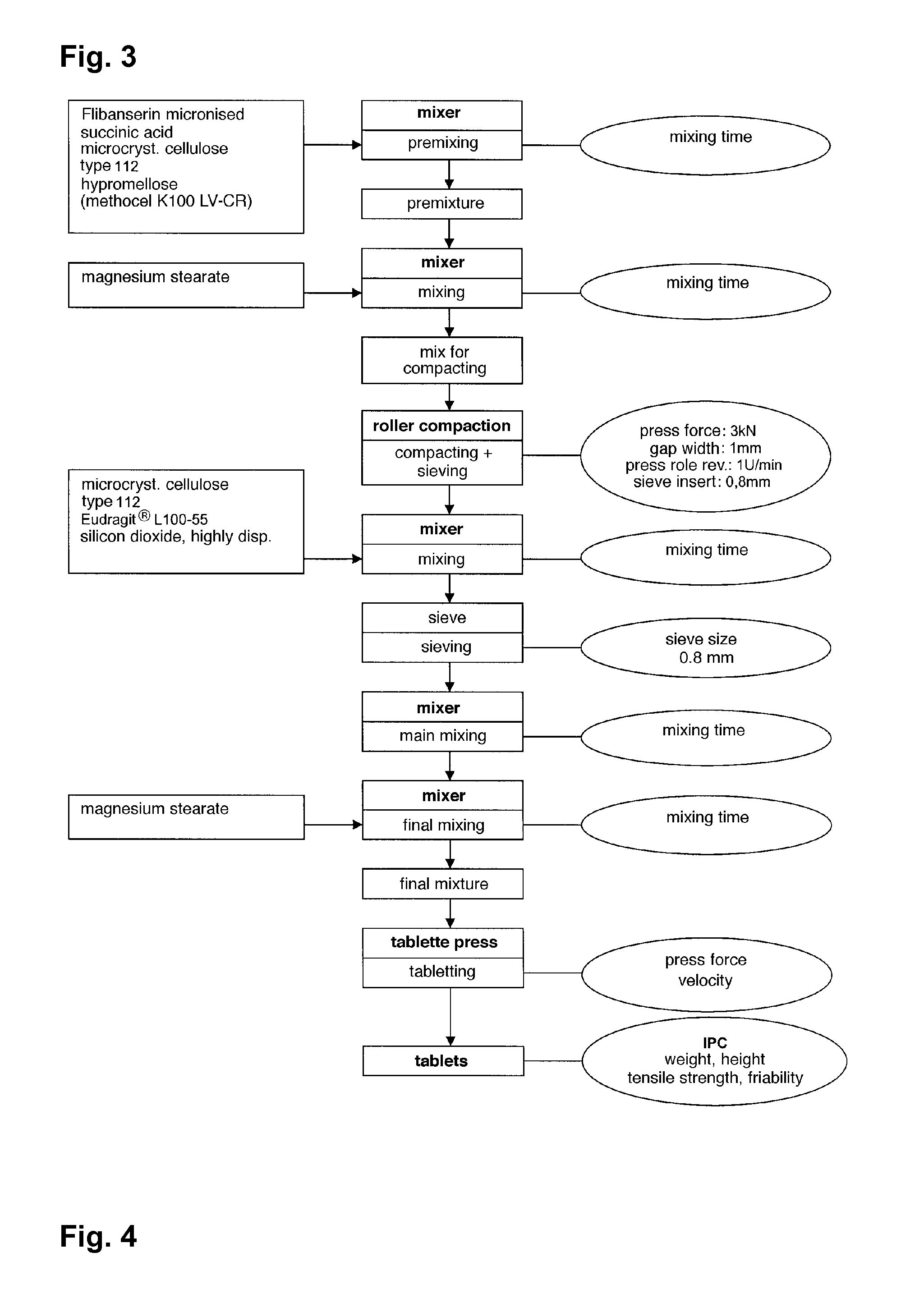
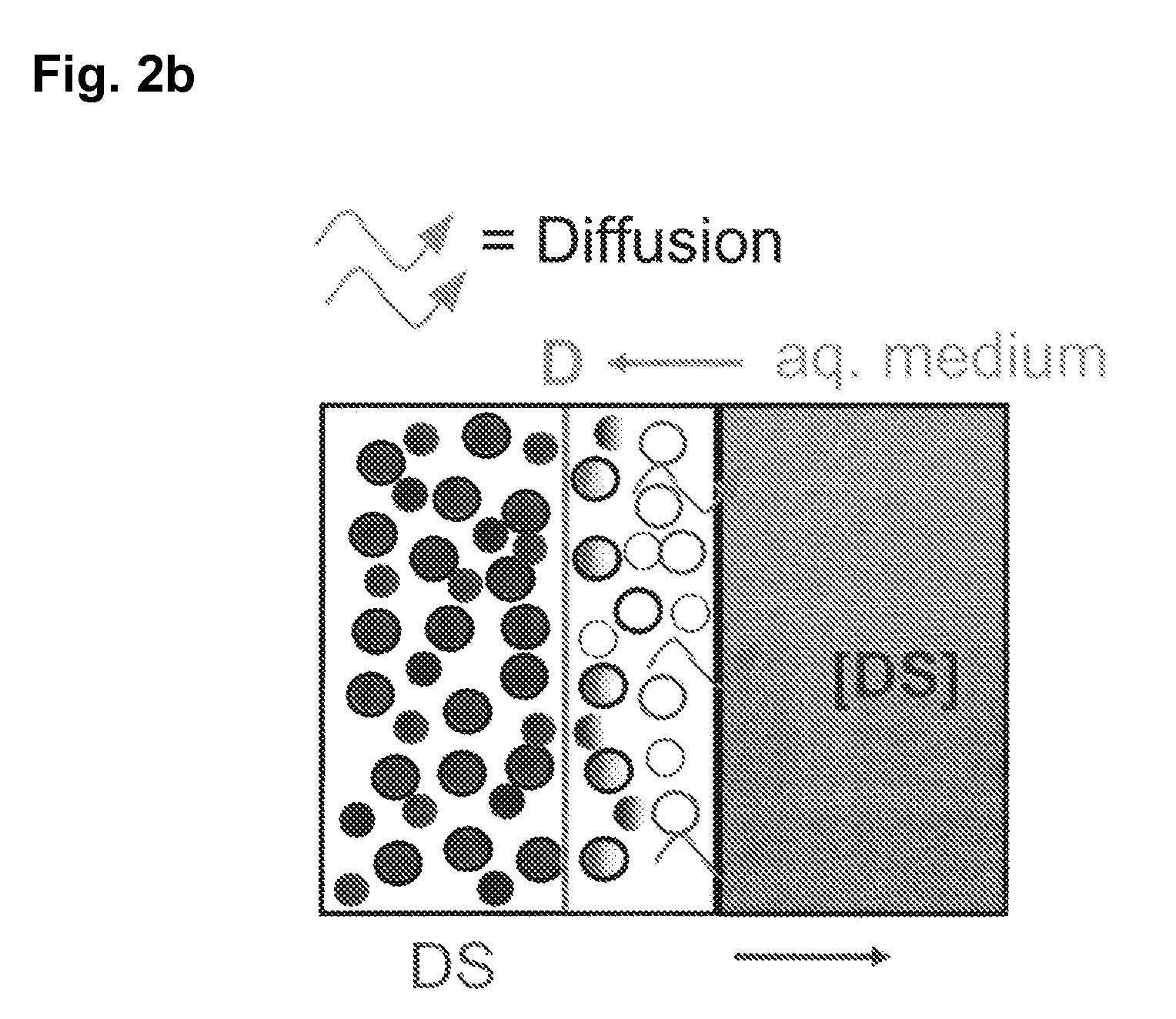
The invention is directed to a Pharmaceutical extended release system, particularly for oral administration, of a pH-dependent water-soluble active substance, comprising or essentially consisting of a) flibanserin or a pharmaceutically acceptable derivative thereof as active substance; b) one or more pharmaceutically acceptable pH-dependent polymers; c) one or more pharmaceutically acceptable pH-independent polymers; d) one or more pharmaceutically acceptable acids; and e) optionally one or more additives. The present invention provides a release profile of flibanserin which is independent on the pH in the gastrointestinal tract when administered orally resulting in a significantly improved bioavailability.
Owner:BOEHRINGER INGELHEIM INT GMBH
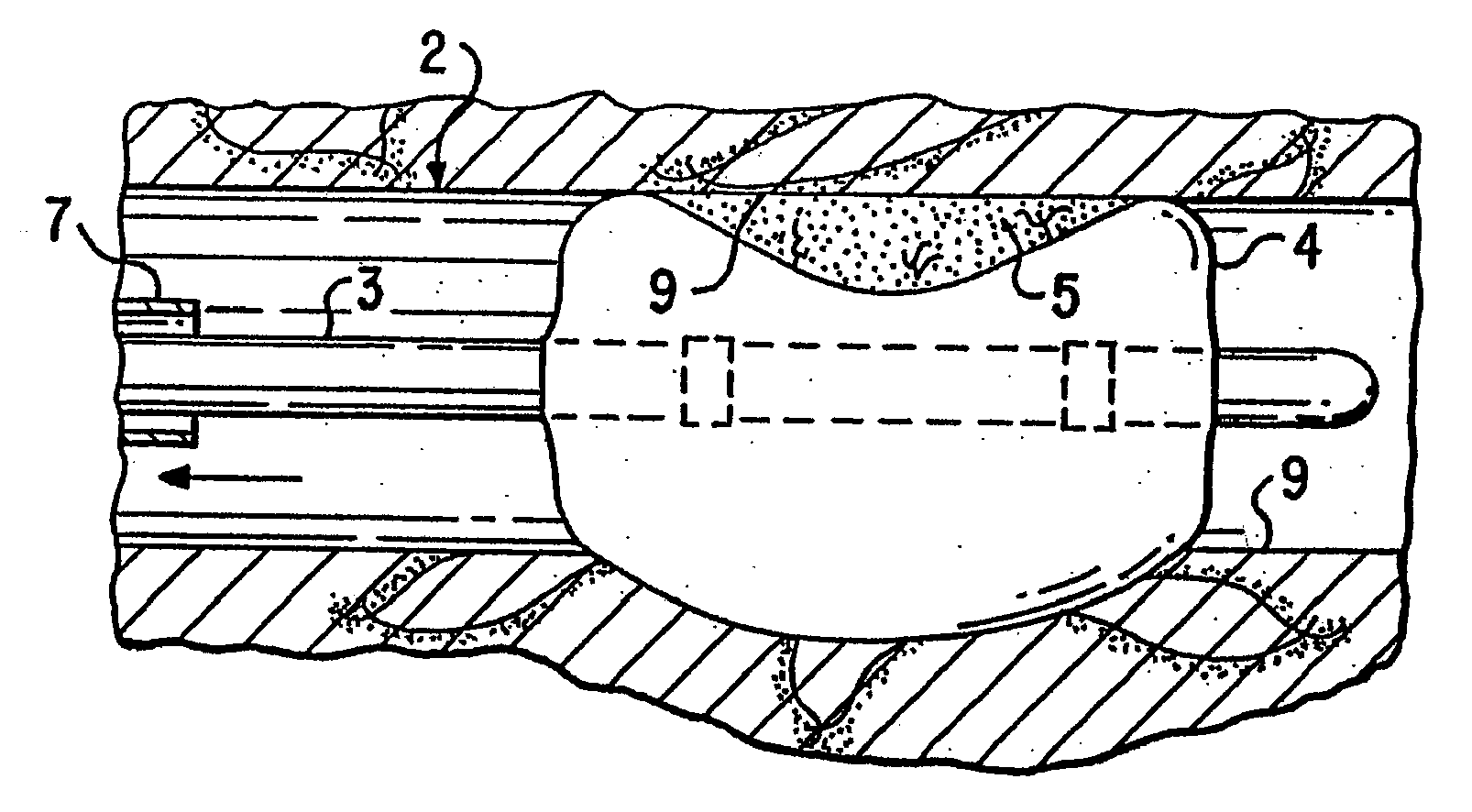
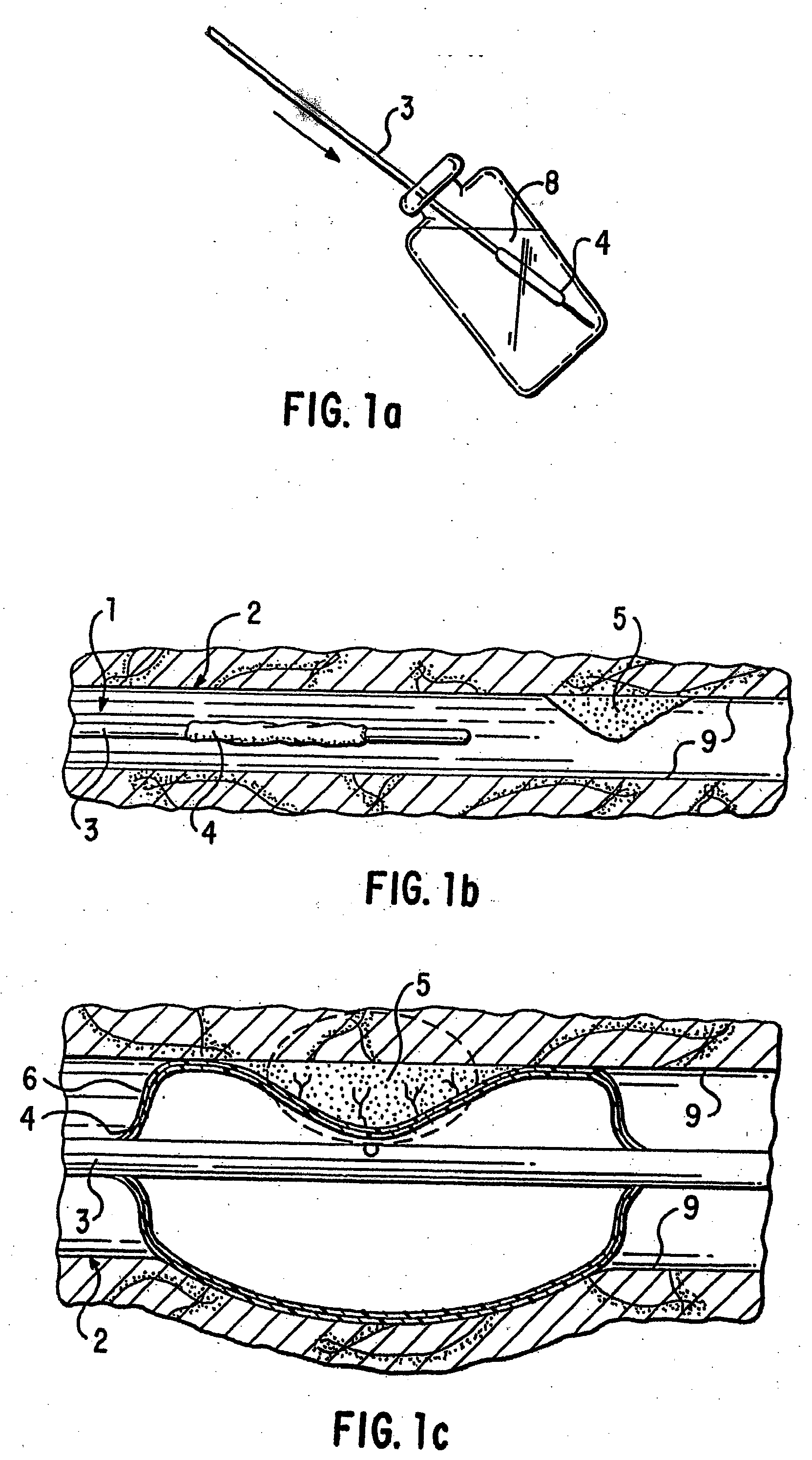
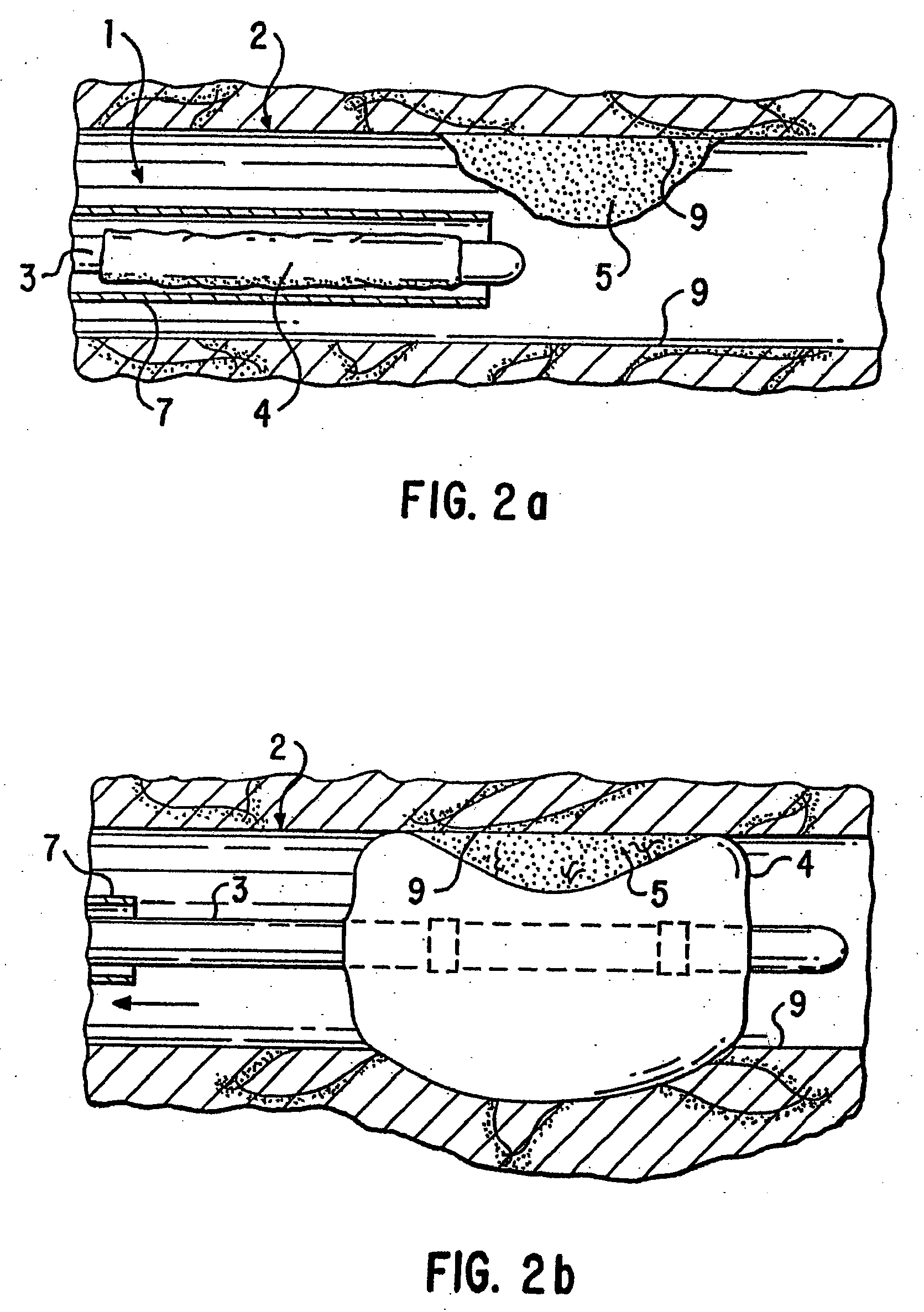
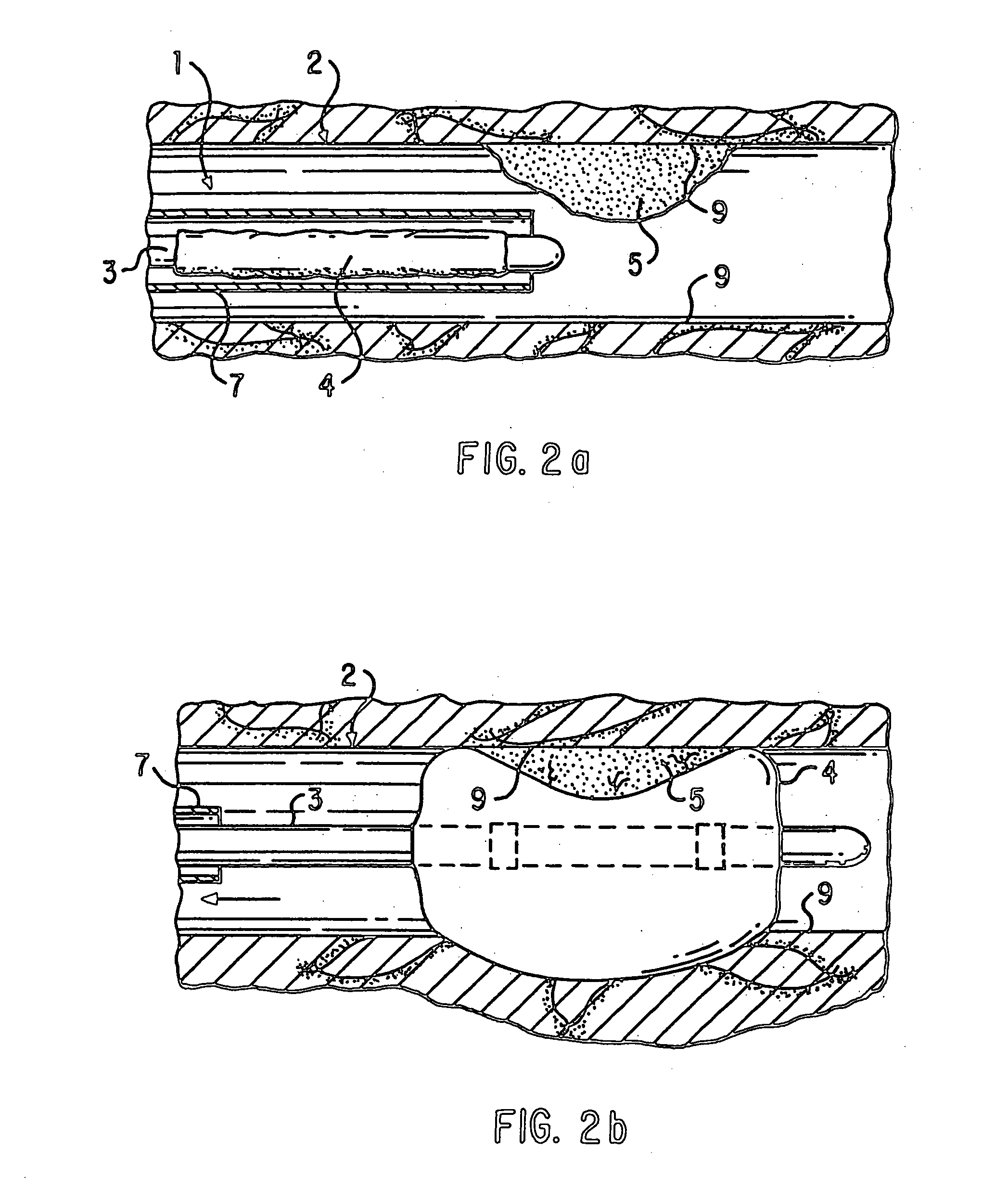
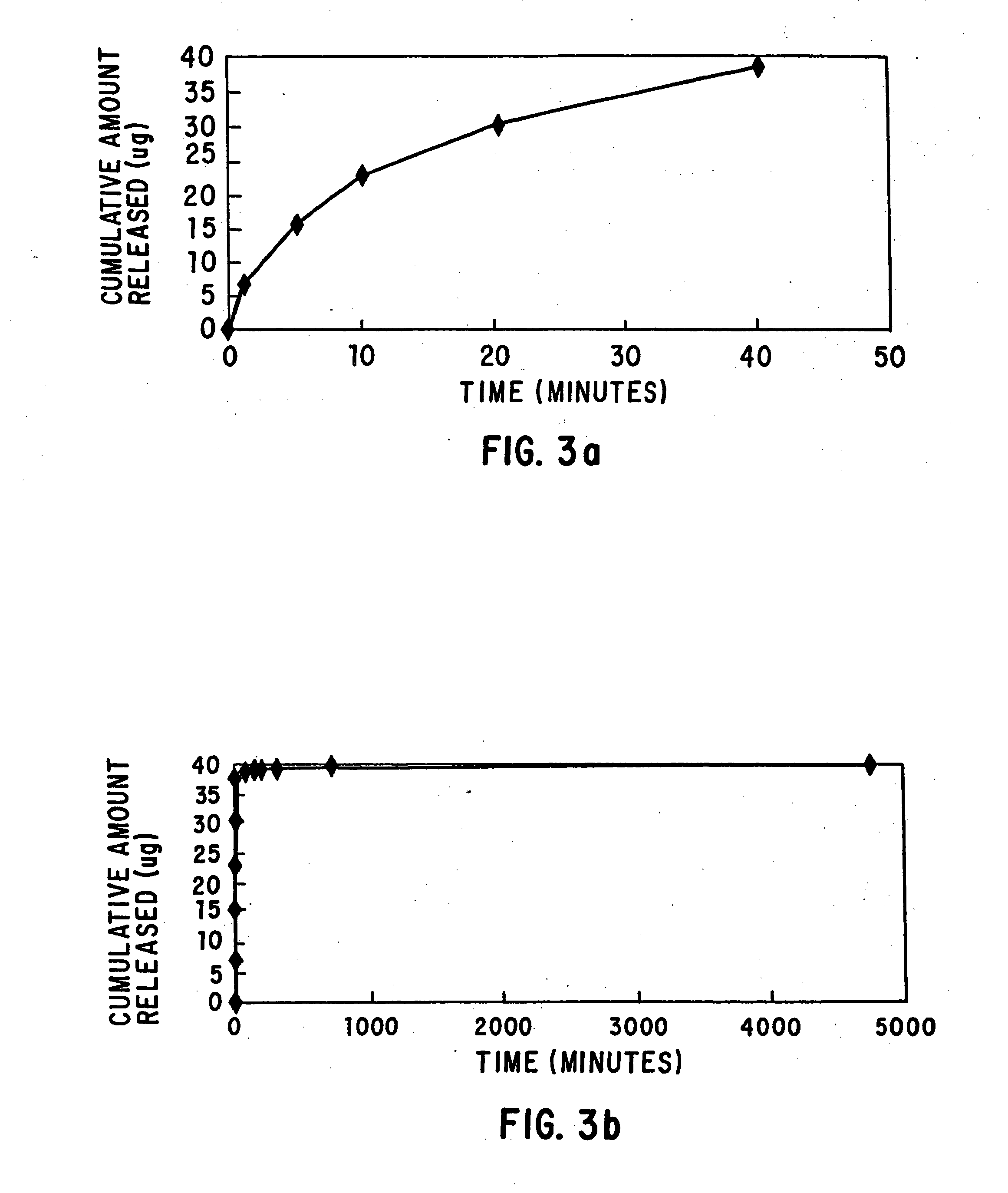
Loading and release of water-insoluble drugs
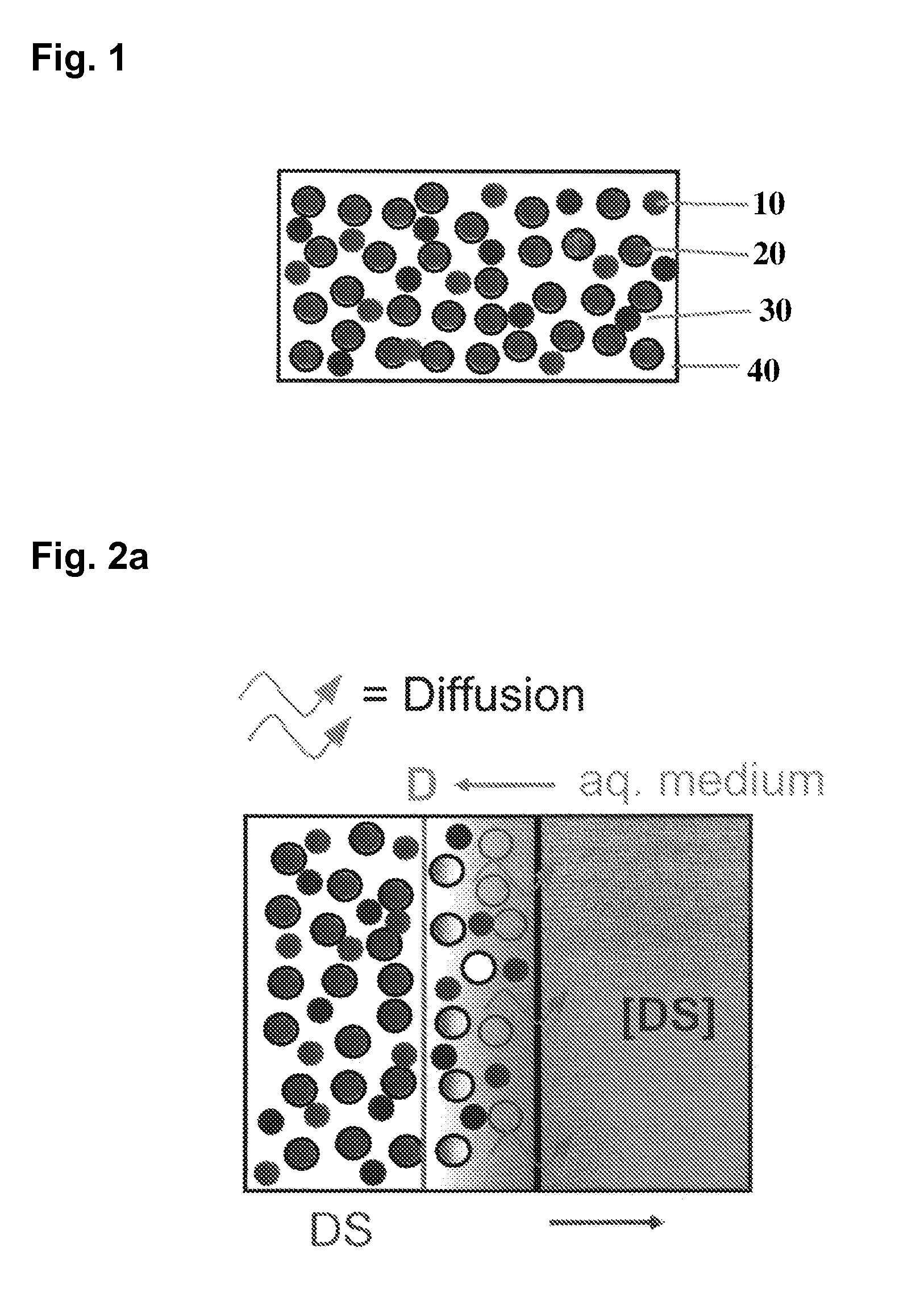
A medical device, polymer composition, and method for delivering substantially water-insoluble drugs to tissue at desired locations within the body. At least a portion of the exterior surface of the medical device is provided with a polymer coating. Incorporated in the polymer coating is a solution of at least one substantially water-insoluble drug in a volatile organic solvent. The medical device is positioned to a desired target location within the body, whereupon the drug diffuses out of the polymer coating.
Owner:SCI MED LIFE SYST
Parenteral and oral formulations of benzimidazoles
ActiveUS20090048322A1Good treatment effectImprove drug solubilityOrganic active ingredientsBiocideBenzimidazole derivativeMebendazole
Provided herein are drug delivery systems, such as self-nanoemulsifying drug delivery systems, self-emulsifying drug delivery systems and parenteral microemulsion formulations, suitable for parenteral or oral delivery to a subject. The drug delivery systems may comprise a benzimidazole derivative, e.g., mebendazole, an oil, a surfactant, a cosurfactant and a dipolar aprotic solvent in a microemulsion formulation. Also provided are methods for improving the bioavailability of a benzimidazole derivative during treatment of a pathophysiological condition by using a formulation combining a particular emulsion droplet diameter and ratio of the surfactant:cosurfactant therein, for increasing concentration and retention of a benzimidazole derivative in the lung via a parenterally administerable microemulsion with droplet size of about 35 nm to less than 100 nm and for defining hemolytically safe microemulsions of a benzimidazole derivative during a therapeutic treatment via a parenterally administerable microemulsion with a surfactant:cosurfactant content by weight of about 6% to 48%.
Owner:UNIV HOUSTON SYST
Patch
InactiveUS6211425B1Improve long-term stabilitySmooth releaseOrganic active ingredientsThin material handlingTransdermal patchDisease
A transdermal patch containing a drug comprising formoterol and / or a salt, a solvent for the drug, a pressure-sensitive adhesive comprising an ethylene / vinyl acetate copolymer resin and at least one of a filler and a plasticizer. The patch is substantially water-free. The patch serves to promote percutaneous absorption of the formoterol and / or a salt thereof and allow the efficacy of the drug to stably persist for a long period of time. The patch is thus effective for diseases such as asthma, which can be cured or prevented by exciting beta-receptors.
Owner:SAITAMA DAIICHI SEIYAKU
Liposome combination and the use thereof
InactiveUS20070231375A1Strong cytotoxicityEnhanced drug releaseBiocideEnergy modified materialsCancer cellSinglet oxygen
The present invention relates to a liposome combination, which wrapped hydrophilic drugs in water layer and wrapped hydrophobic drugs in lipid bilayer; hydrophobic drugs are photosensitizers. Using light with appropriate wavelength to activate the photosensitizer in the hydrophobic layer can result in the production of singlet oxygen and the free radical, and cause the oxidizing and breaking of the carbon chain of the phospholipid, and influences the stability of the liposome and the releases of the drug. The singlet oxygen and the free radical will attack the cancer cells at the same time as a result of combining the photodynamic- and chemo-effects.
Owner:TAIPEI MEDICAL UNIV
Salts of potassium atp channel openers and uses thereof
ActiveUS20070191351A1Enhanced drug releaseNervous disorderAmino preparation from aminesDiseasePotassium
Provided are immediate or prolonged administration of certain salts of KATP channel openers such as diazoxide to a subject to achieve novel pharmacodynamic, pharmacokinetic, therapeutic, physiological, metabolic and compositional outcomes in the treatment of diseases or conditions involving KATP channels. Also provided are pharmaceutical formulations, methods of administration and dosing of the salts that achieve these outcomes and reduce the incidence of adverse effects in treated individuals. Further provided are method of co-administering the salts with other drugs to treat diseases of humans and animals.
Owner:ESSENTIALIS INC
Salts of potassium atp channel openers and uses thereof
InactiveUS20090062264A1Inhibit and prevent progressionReducing insulin dosingMetabolism disorderDigestive systemDiseasePotassium channel opener
Provided are immediate or prolonged administration of certain salts of KATP channel openers such as diazoxide to a subject to achieve novel pharmacodynamic, pharmacokinetic, therapeutic, physiological, metabolic and compositional outcomes in the treatment of diseases or conditions involving KATP channels. Also provided are pharmaceutical formulations, methods of administration and dosing of the salts that achieve these outcomes and reduce the incidence of adverse effects in treated individuals. Further provided are method of co-administering the salts with other drugs to treat diseases of humans and animals.
Owner:ESSENTIALIS INC
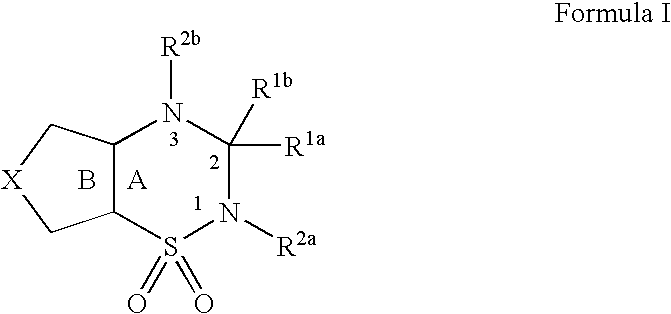
Polymer Conjugate Of Folic Acid Or Folic Acid Derivative
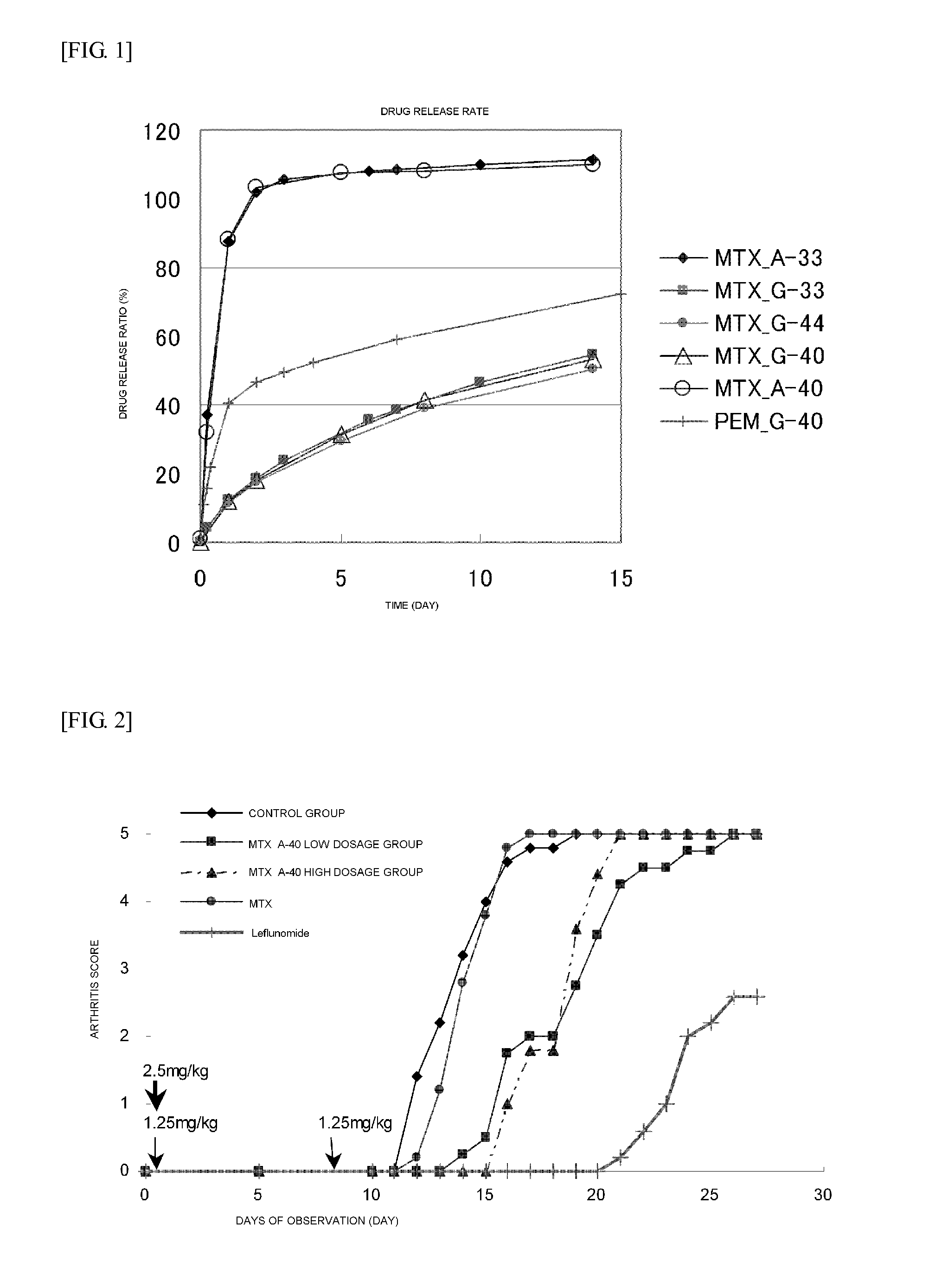
ActiveUS20110294980A1Practical chemical stabilityExcellent in vivo drug release efficiencyOrganic active ingredientsPeptide/protein ingredientsDrug release rateSide chain
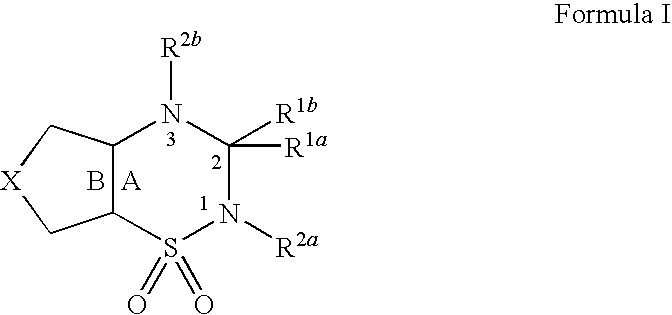
Disclosed is a polymer conjugate of folic acid or a folic acid derivative, wherein an amide bond is not used. The compound has chemical stability and adequate drug release rate in the living organism. Specifically disclosed is a polymer conjugate of folic acid or a folic acid derivative, wherein a substituent represented by formula (I) is bonded to a carboxy group of a block copolymer which is composed of a polyethylene glycol and a polymer having a carboxy group in a side chain, or a pharmacologically acceptable salt thereof.[In the formula, A represents a monocyclic or fused aromatic group; G represents an optionally substituted (C1-C6) alkylene group; Y represents a hydrogen atom or a substituent; and E represents a residue of folic acid or a folic acid derivative.]
Owner:NIPPON KAYAKU CO LTD
Noninvasively low-frequency ultrasonic apparatus for the brain therapy
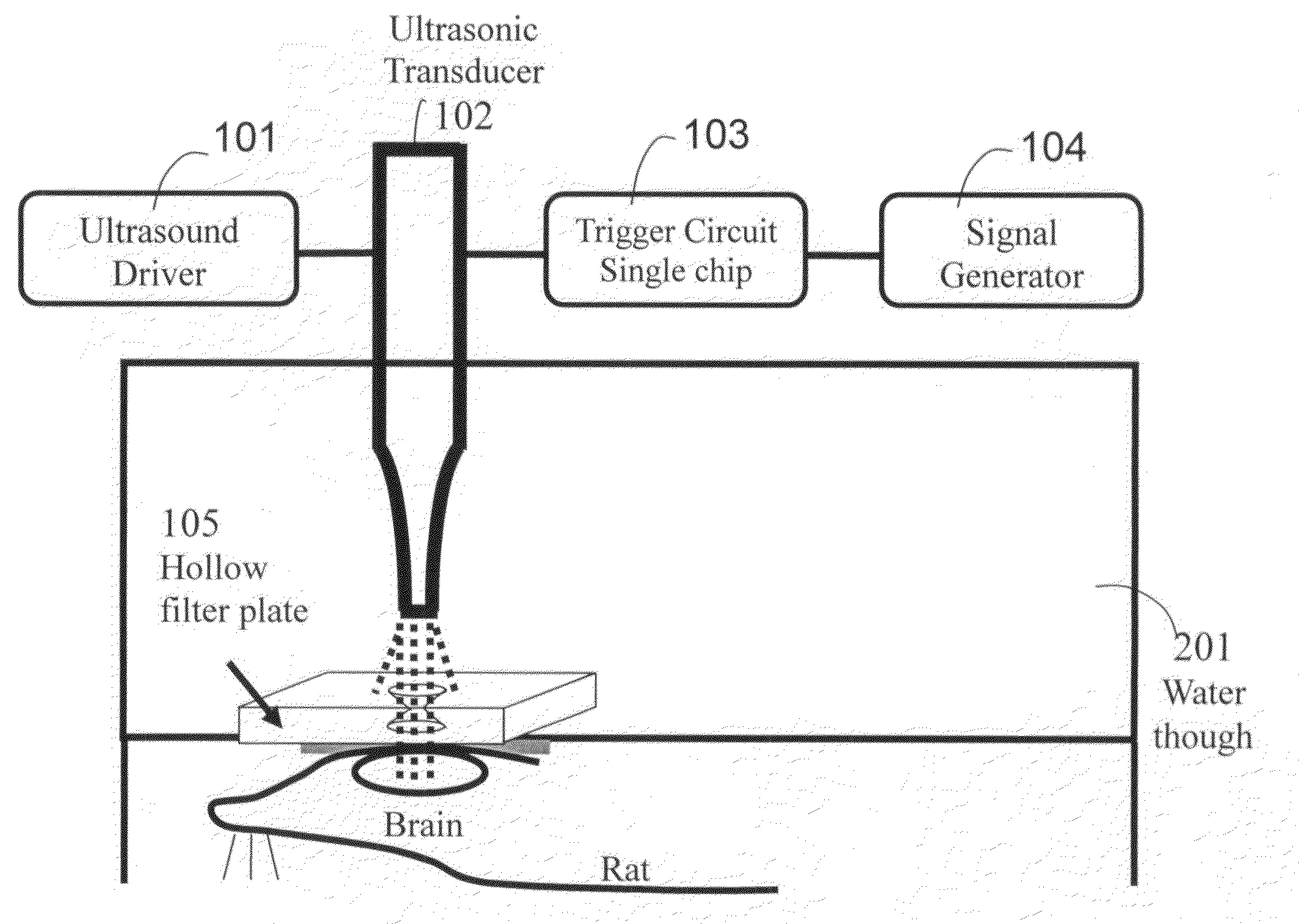
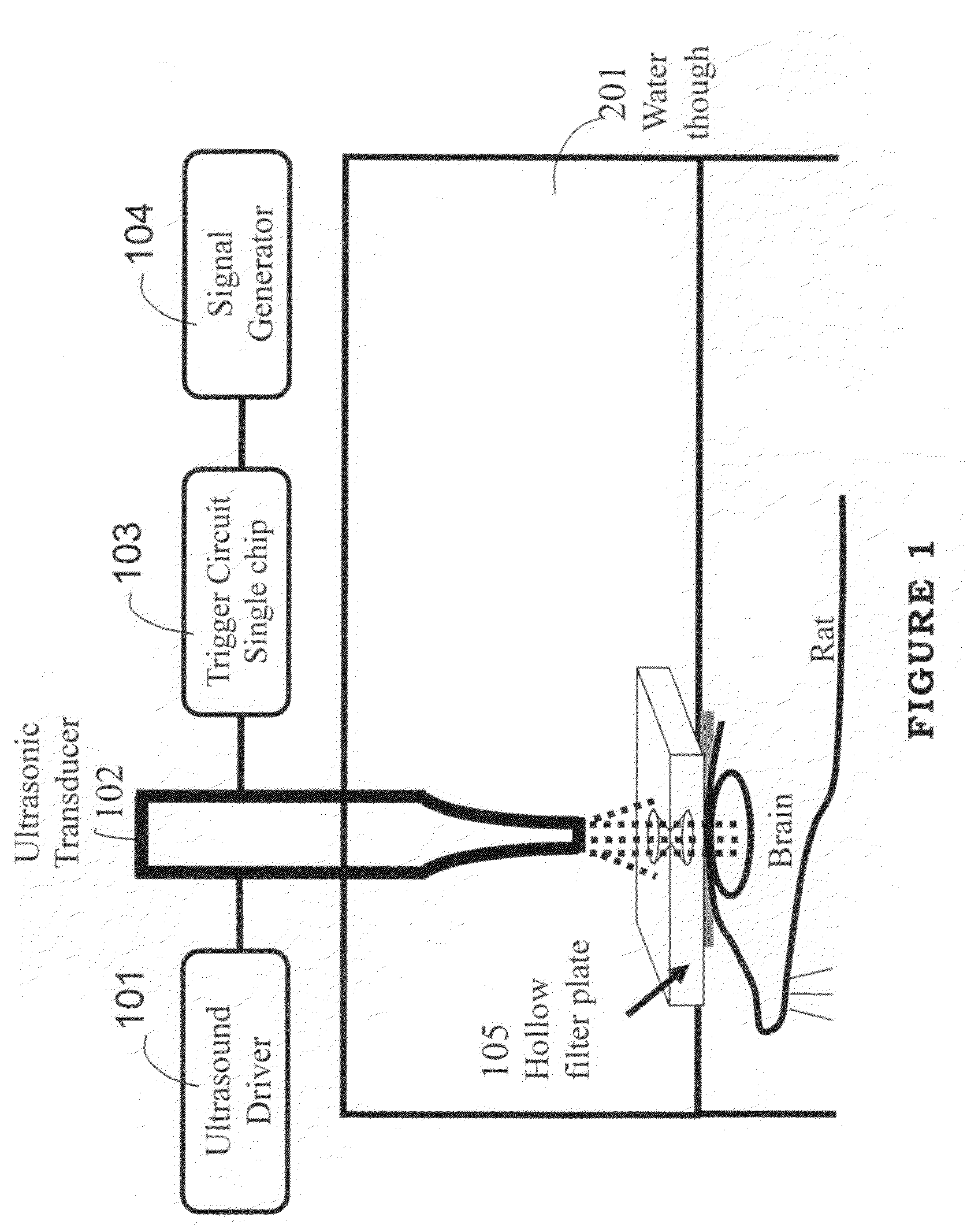
InactiveUS20100010394A1Increase the areaReduce absorptionUltrasound therapyChiropractic devicesSonificationMedicine
Ultrasonic energy has been proven that at suitable frequency range (670-kHz to 2-MHz), ultrasound can be focused to a specific target, and the concentrated energy has sufficient high acoustic pressure so that capable of inducing localized blood-brain barrier (BBB) disruption, which has an important implication on noninvasively delivering drug into brain.
Owner:CHANG GUNG UNIVERSITY
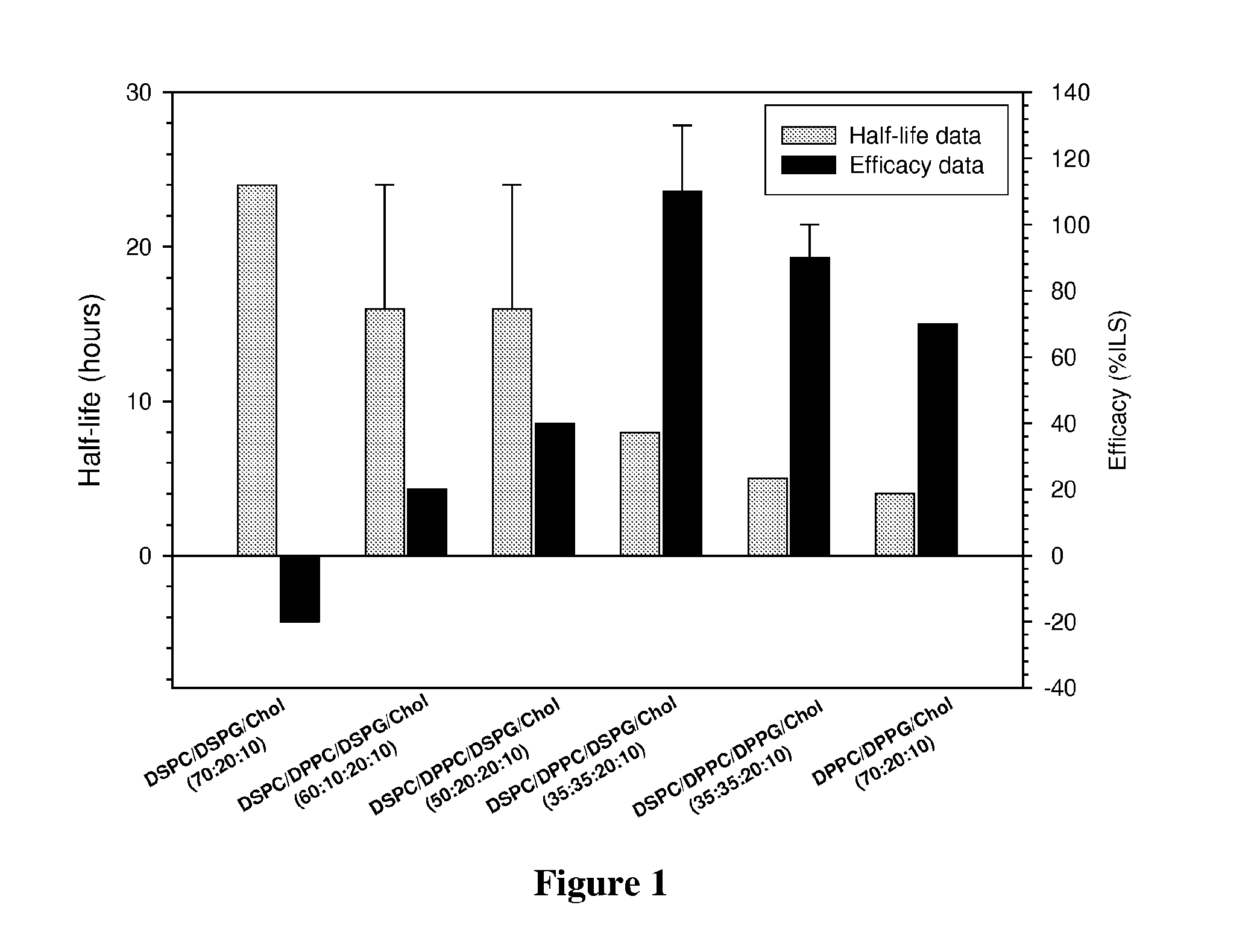
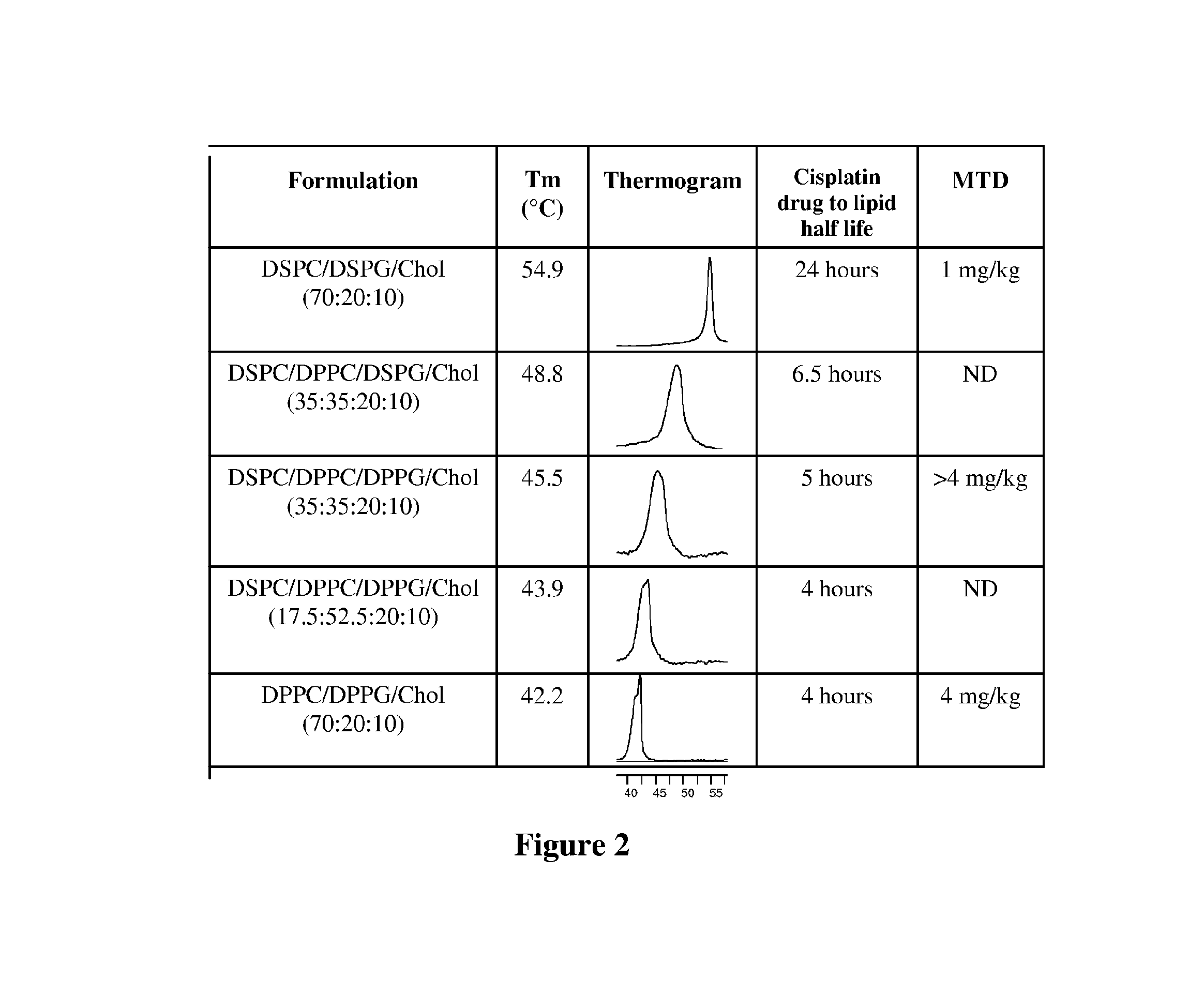
Platinum drug formulations
InactiveUS20160058704A1Improve therapeutic indexSufficient drug loadingBiocideHeavy metal active ingredientsPlatinumPlatinum Agents
Owner:CELATOR PHARMA INC
Parenteral and oral formulations of benzimidazoles
ActiveUS20050038096A1Good treatment effectImprove drug solubilityBiocideDispersion deliveryBenzimidazole derivativePolyol
Pharmaceutical compositions of a benzimidazole or a benzimidazole derivative are disclosed. For example, in certain embodiments the pharmaceutical compositions include a benzimidazole, a polyol, and a dipolar aprotic solvent. In other embodiments, pharmaceutical compositions include a benzimidazole, an oil, a dipolar aproptic solvent, and a surfactant. In certain embodiments, the benzimidazole is mebendezole. The pharmaceutical compositions are formulated for delivery to a subject by any means, and include formulations for oral and parenteral delivery.
Owner:UNIV HOUSTON SYST
Salts of potassium atp channel openers and uses thereof
InactiveUS20120238554A1Inhibit and prevent progressionReducing insulin dosingOrganic chemistryMetabolism disorderDiseasePotassium
Provided are immediate or prolonged administration of certain salts of KATP channel openers such as diazoxide to a subject to achieve novel pharmacodynamic, pharmacokinetic, therapeutic, physiological, metabolic and compositional outcomes in the treatment of diseases or conditions involving KATP channels. Also provided are pharmaceutical formulations, methods of administration and dosing of the salts that achieve these outcomes and reduce the incidence of adverse effects in treated individuals. Further provided are method of co-administering the salts with other drugs to treat diseases of humans and animals.
Owner:ESSENTIALIS INC
Extended release tablet formulations of flibanserin and method for manufacturing the same
ActiveUS8545886B2Reduce solubilitySufficiently slow releasePowder deliveryNervous disorderExtended release tabletsOral medication
The invention is directed to a Pharmaceutical extended release system, particularly for oral administration, of a pH-dependent water-soluble active substance, comprising or essentially consisting ofa) flibanserin or a pharmaceutically acceptable derivative thereof as active substance;b) one or more pharmaceutically acceptable pH-dependent polymers;c) one or more pharmaceutically acceptable pH-independent polymers;d) one or more pharmaceutically acceptable acids; ande) optionally one or more additives.The present invention provides a release profile of flibanserin which is independent on the pH in the gastrointestinal tract when administered orally resulting in a significantly improved bioavailability.
Owner:BOEHRINGER INGELHEIM INT GMBH
Parenteral and oral formulations of benzimidazoles
ActiveUS7419996B2Good treatment effectImprove drug solubilityBiocideDispersion deliveryBenzimidazole derivativeMebendazole
Pharmaceutical compositions of a benzimidazole or a benzimidazole derivative are disclosed. For example, in certain embodiments the pharmaceutical compositions include a benzimidazole, PEG 400, and a dipolar aprotic solvent. In other embodiments, pharmaceutical compositions include a benzimidazole, an oil, a dipolar aproptic solvent, and a surfactant. In certain embodiments, the benzimidazole is mebendezole. The pharmaceutical compositions are formulated for delivery to a subject by any means, and include formulations for oral and parenteral delivery.
Owner:UNIV HOUSTON SYST
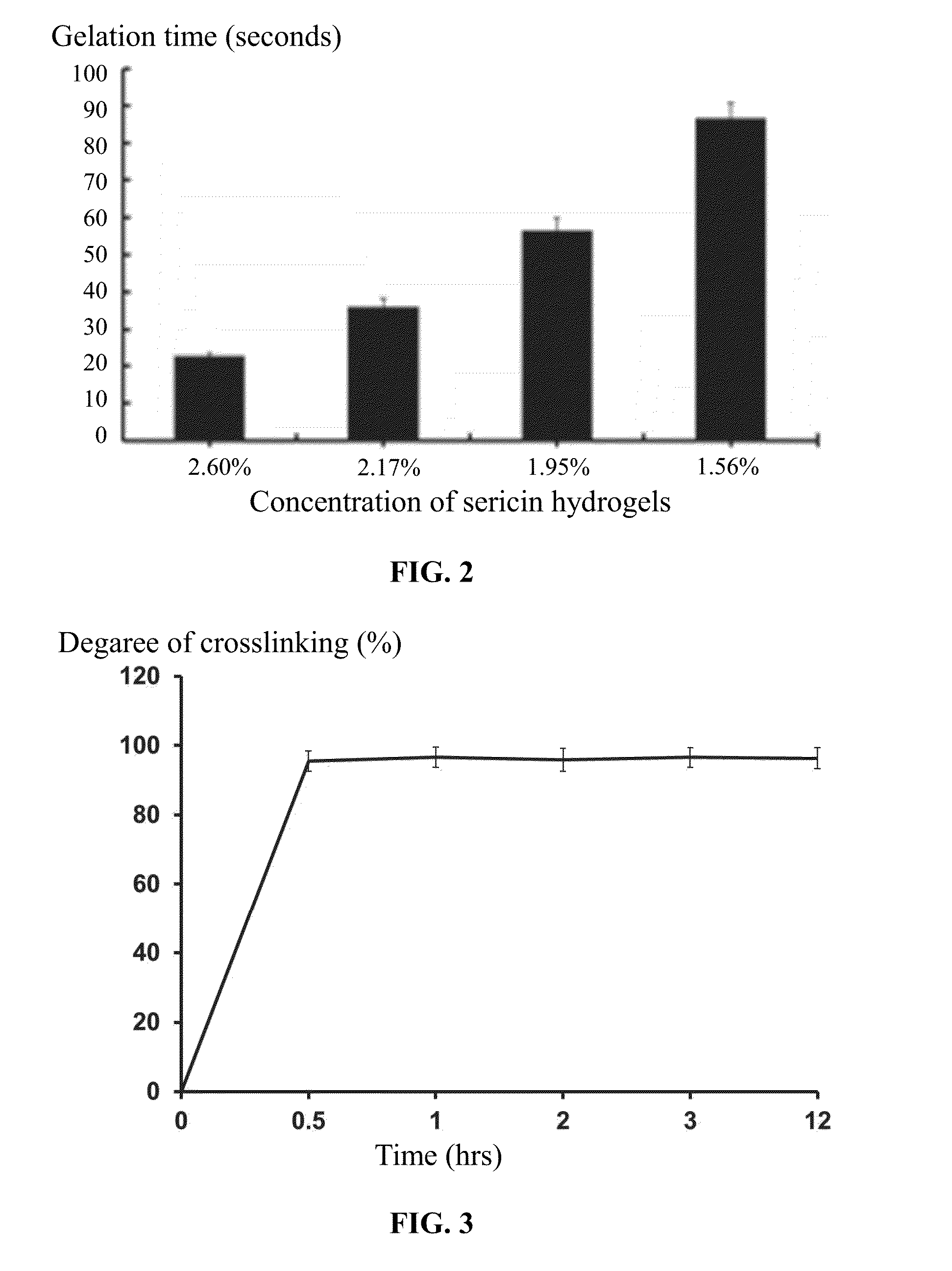
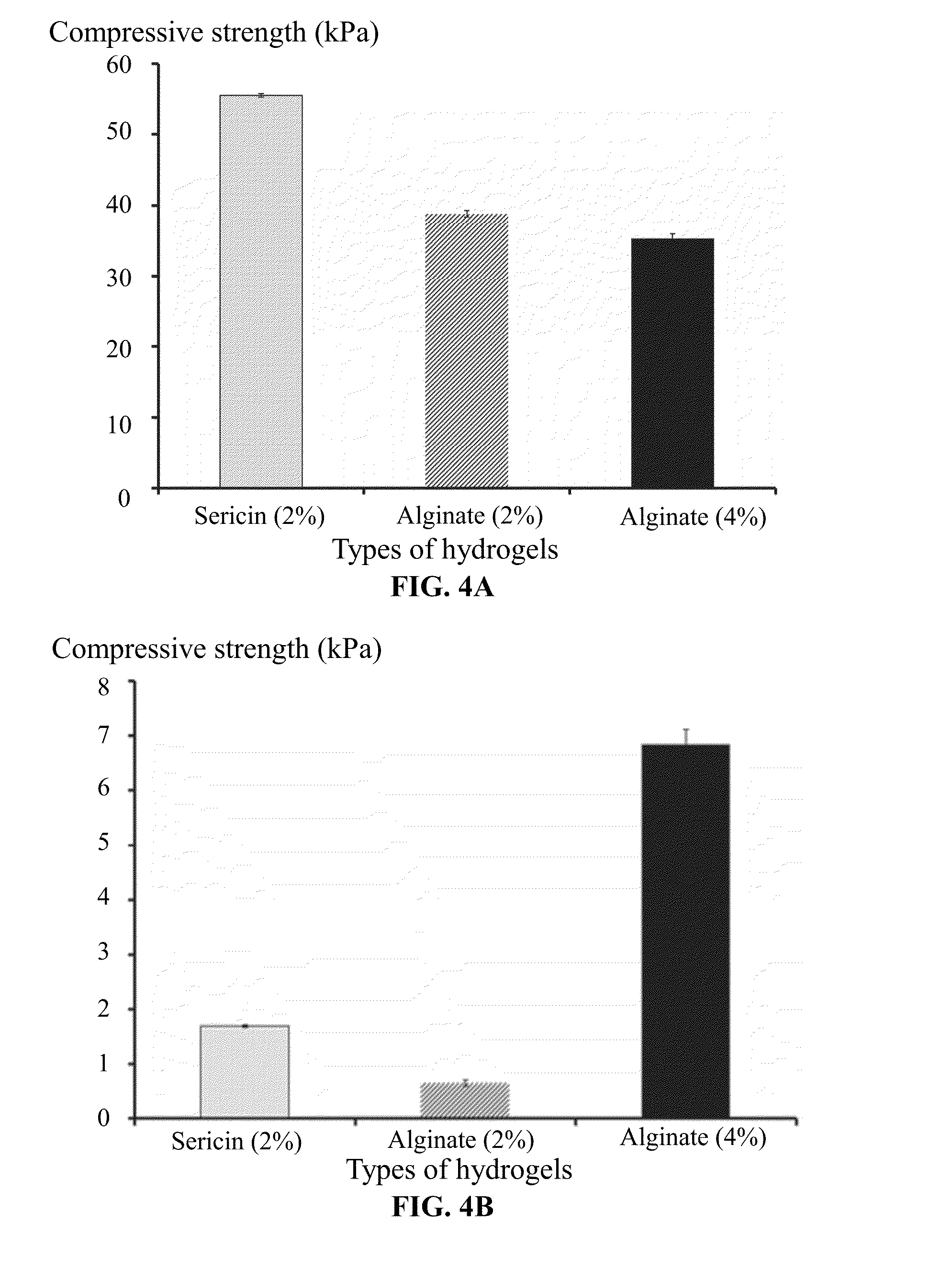
Methods of preparing and using sericin hydrogel
InactiveUS20160136241A1Good biocompatibilityPromote cell adhesionUltrasonic/sonic/infrasonic diagnosticsNervous disorderAqueous solutionSericin
A method for preparing a sericin hydrogel, the method including: 1) weighing a cocoon of a fibroin-deficient mutant silkworm, Bombyx mori, extracting the cocoon by an aqueous solution of LiBr or LiCl, dialyzing an extracted solution to yield a sericin solution having a concentration of a non-degraded sericin of between 0.1 and 4 wt. %; and 2) concentrating the sericin solution to a concentration of between 1.5 and 10 wt. %, adding a crosslinking agent to the concentrated sericin solution at a ratio of between 2 and 500 μL of the crosslinking agent per each milliliter of the sericin solution, fully blending the crosslinking agent with the concentrated sericin solution, and keeping a resulting mixture at the temperature of between 4 and 45° C. for between 5 s and 36 hrs to yield a hydrogel.
Owner:XIEHE HOSPITAL ATTACHED TO TONGJI MEDICAL COLLEGE HUAZHONG SCI & TECH UNIV
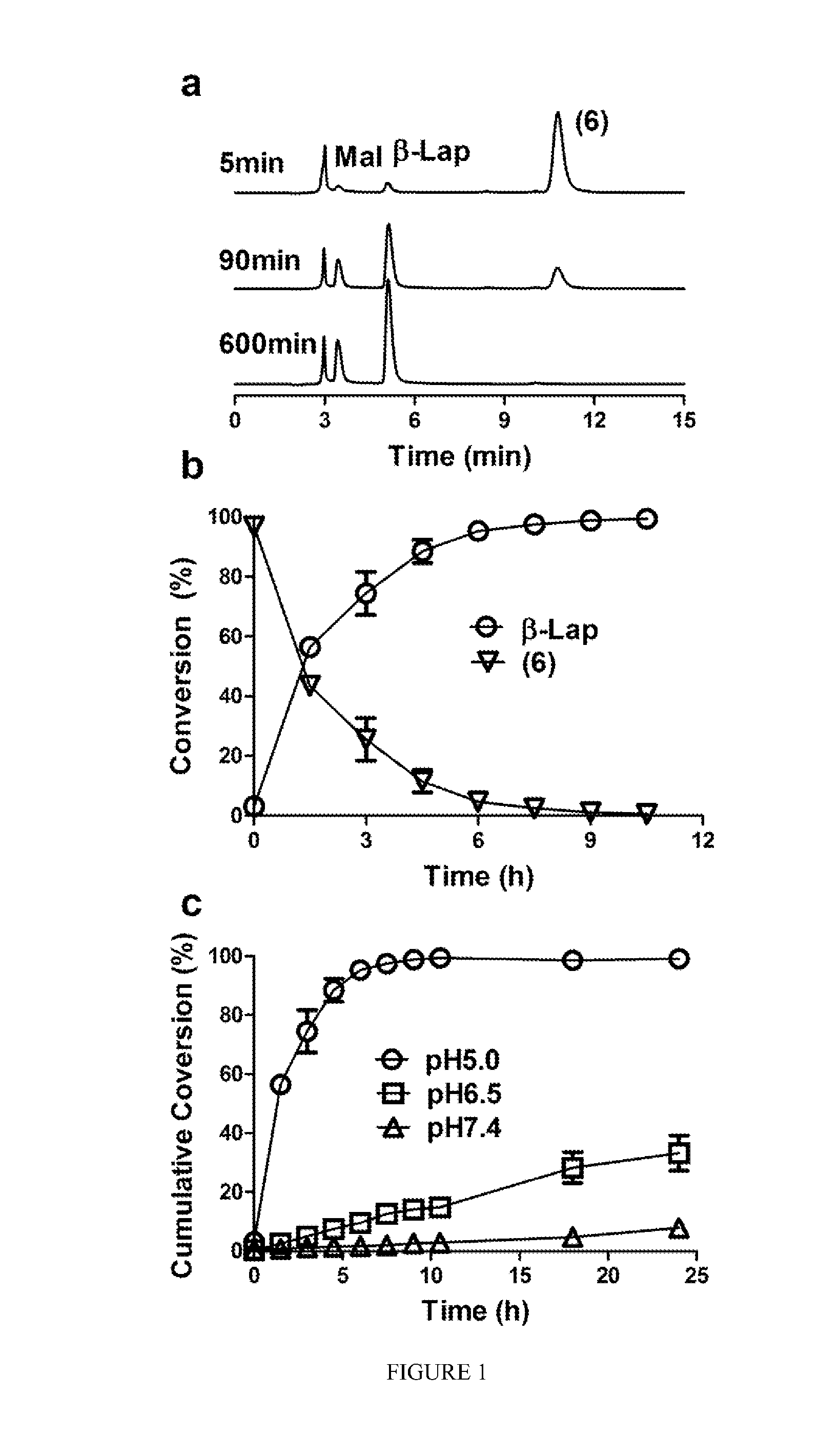
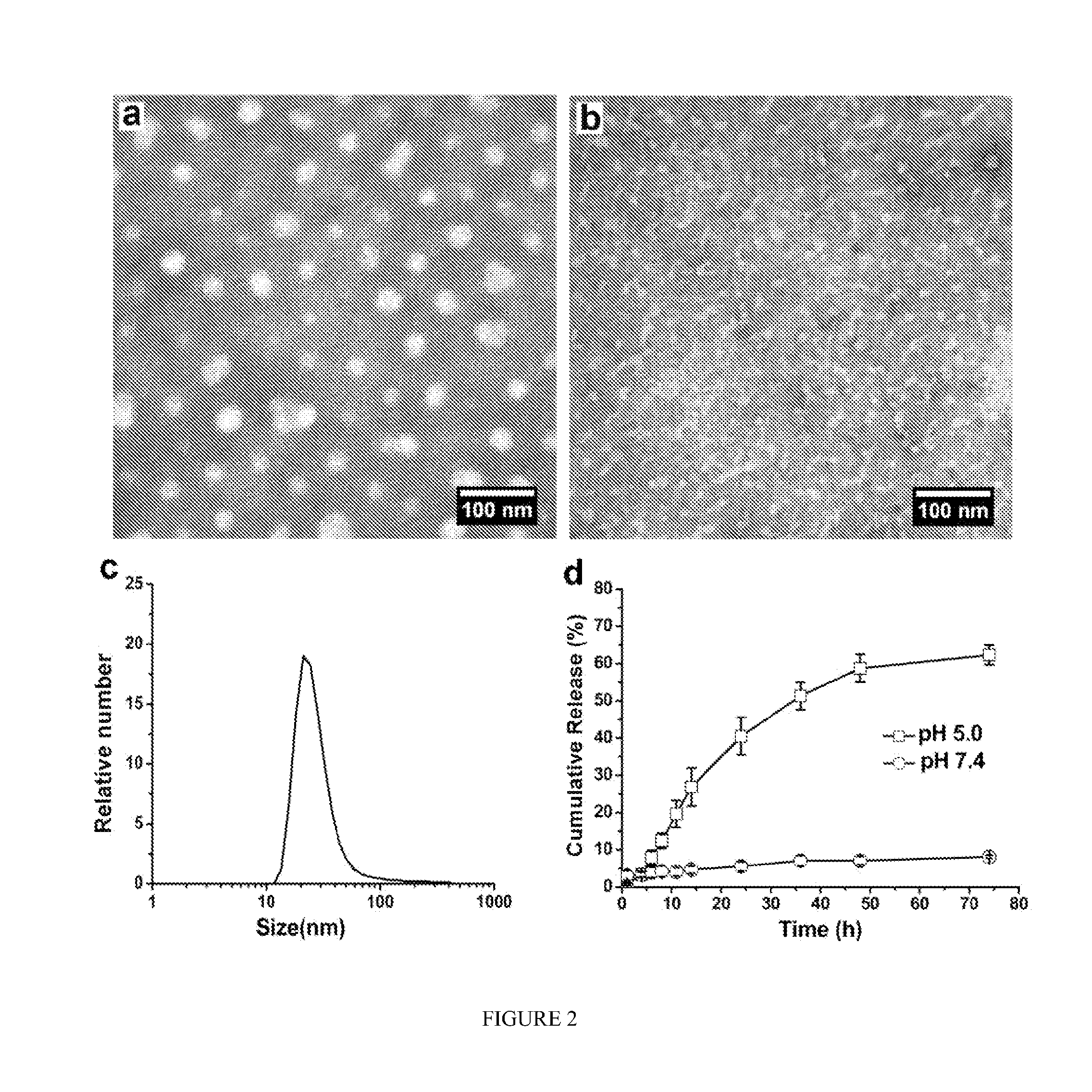
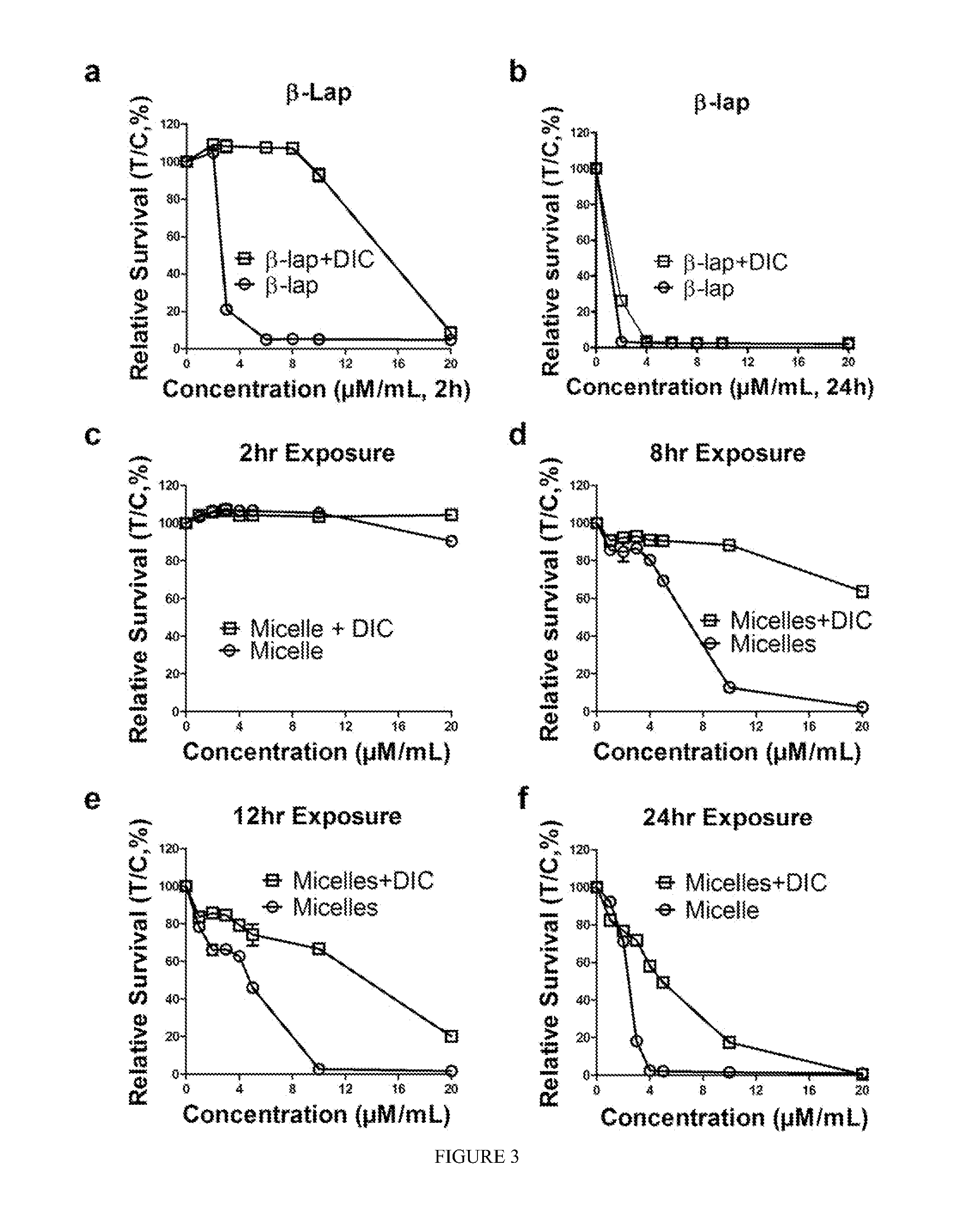
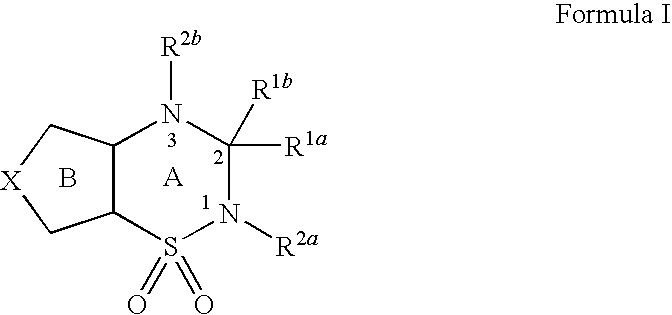
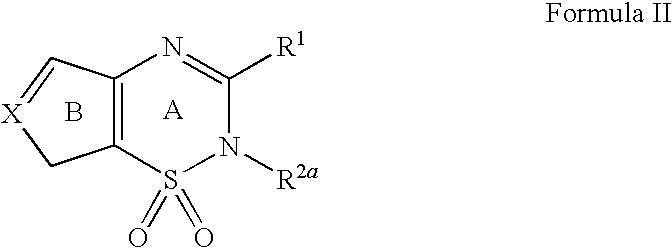
pH-SENSITIVE COMPOSITIONS FOR DELIVERY OF BETA LAPACHONE AND METHODS OF USE
ActiveUS20130331426A1Improve drug specificity and bioavailabilityUseful lifeBiocideOrganic chemistryArylPolymer
Disclosed herein are compounds comprising a polymer conjugated with a pH-sensitive prodrug of beta-lapachone, wherein the compound is capable of forming a micelle, and wherein the pH-sensitive prodrug comprises a pH-sensitive linker selected from the group consisting of: an aryl imine and an aliphatic imine. Also provided are micelles comprised of such polymer-prodrug conjugates. Further provided are methods for treating cancer with the micelles.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
Pharmaceutical formulations of potassium atp channel openers and uses thereof
InactiveUS20090148525A1Enhanced drug releaseReduces food uptakePowder deliveryMetabolism disorderDiseasePotassium
Provided are immediate or prolonged administration of certain potassium ATP (KATP) channel openers to a subject to achieve novel pharmacodynamic, pharmacokinetic, therapeutic, physiological, metabolic and compositional outcomes in the treatment of diseases or conditions involving KATP channels. Also provided are pharmaceutical formulations, methods of administration and dosing of KATP channel openers that achieve these outcomes and reduce the incidence of adverse effects in treated individuals. Further provided are method of co-administering KATP channel openers with other drugs to treat diseases of humans and animals.
Owner:ESSENTIALIS INC
Liposome having inner water phase containing sulfobutyl ether cyclodextrin salt
ActiveUS8871253B2Efficient packagingIncrease release rateBiocideAnimal repellantsCompound (substance)Γ cyclodextrin
A liposome comprising bilayer and inner water phase is disclosed. Said inner water phase contains sulfobutyl ether cyclodextrin and active compound. Said sulfobutyl ether cyclodextrin is sulfobutyl ether α-cyclodextrin, sulfobutyl ether β-cyclodextrin, or sulfobutyl ether γ-cyclodextrin.
Owner:CSPC ZHONGQI PHARM TECH (SHIJIAZHUANG) CO LTD
Gastric floating slow-release administrating system and its three-dimensional printing forming preparation method
InactiveCN1709222AEnhanced drug releaseCompensate for the reduction in drug release areaPharmaceutical delivery mechanismHydroxypropylmethyl celluloseSpecific weight
The present invention discloses an endogastric flotation slow-released administration system, including medicine-loaded shell body and medicine-loaded kernel body, in which said kernel body is placed in the interior of said shell body, in the interior of said kernel body the air is held, and the specific weight of said endogastric flotation show-released medicine is less than 1g / cm. Said invention also provides the method for preparing endogastric flotation slow-released administration system by utilizing three-dimensional printing forming technique.
Owner:HUAZHONG UNIV OF SCI & TECH +1
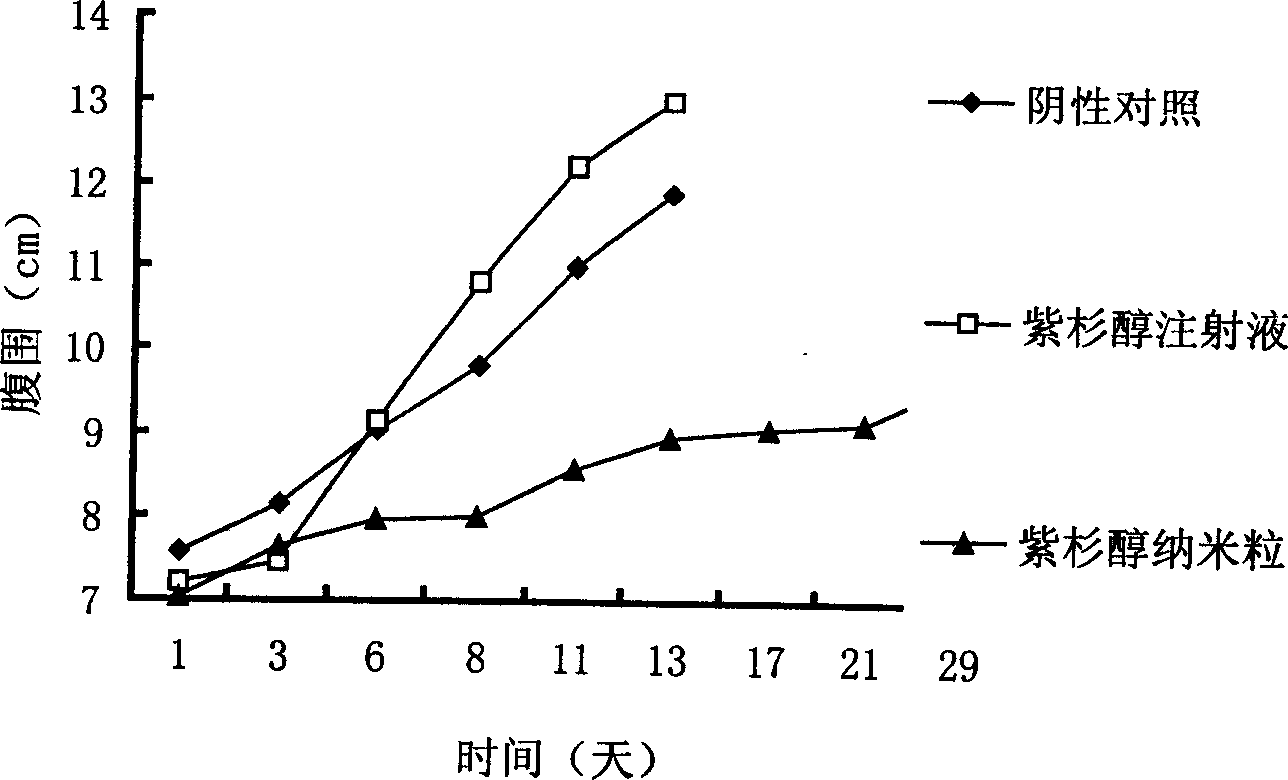
Taxadol slow release nano-particle, its preparation method and application
InactiveCN1823740AEnhanced drug releaseFacilitated releaseOrganic active ingredientsPharmaceutical non-active ingredientsPolyvinyl alcoholNanoparticle
A slow-releasing taxusol nanoparticle used for anticancer medicine is prepared through dissolving biodegradable high-molecular polyester, poloxamer 188 (or 407) or polyethylene glycol and taxusol in acetone, adding it to the aqueous solution of polyvinyl alcohol or poloxamer 188, removing acetone, centrifugal separation, washing with distilled water and freeze drying.
Owner:INST OF BIOMEDICAL ENG CHINESE ACAD OF MEDICAL SCI +1
Coated Pelvic Implant Device and Method
InactiveUS20120022321A1Promote healingAdvantageous antimicrobialAnti-incontinence devicesBandagesPolypropylene meshImplanted device
Implant systems and methods are provided to include a treatment material with a pelvic implant device. The pelvic implant device, such as an incontinence sling, can include the treatment coating combination of polycarbonate (PC) and an infection prevention material, such as InhibiZone® (IZ) technology. The treatment material can be coated onto, or impregnation or integrated with, polypropylene mesh in order to prevent infection and promote healing.
Owner:AMS RES CORP
Pharmaceutical formulations of potassium atp channel openers and uses thereof
InactiveUS20090149451A1Enhanced drug releaseReduces food uptakeMetabolism disorderDigestive systemDiseasePotassium
Provided are immediate or prolonged administration of certain potassium ATP (KATP) channel openers to a subject to achieve novel pharmacodynamic, pharmacokinetic, therapeutic, physiological, metabolic and compositional outcomes in the treatment of diseases or conditions involving KATP channels. Also provided are pharmaceutical formulations, methods of administration and dosing of KATP channel openers that achieve these outcomes and reduce the incidence of adverse effects in treated individuals. Further provided are method of co-administering KATP channel openers with other drugs to treat diseases of humans and animals.
Owner:ESSENTIALIS INC
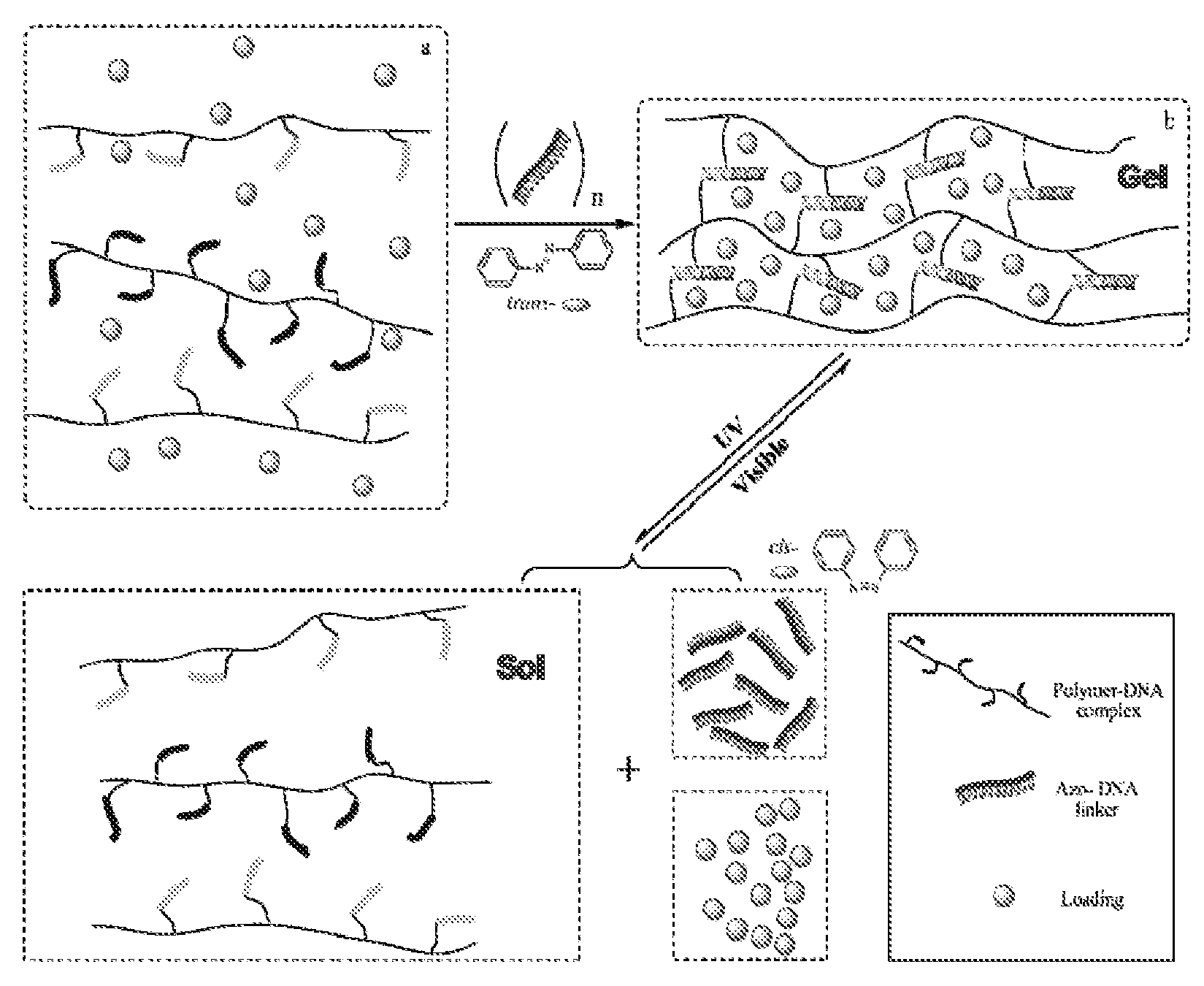
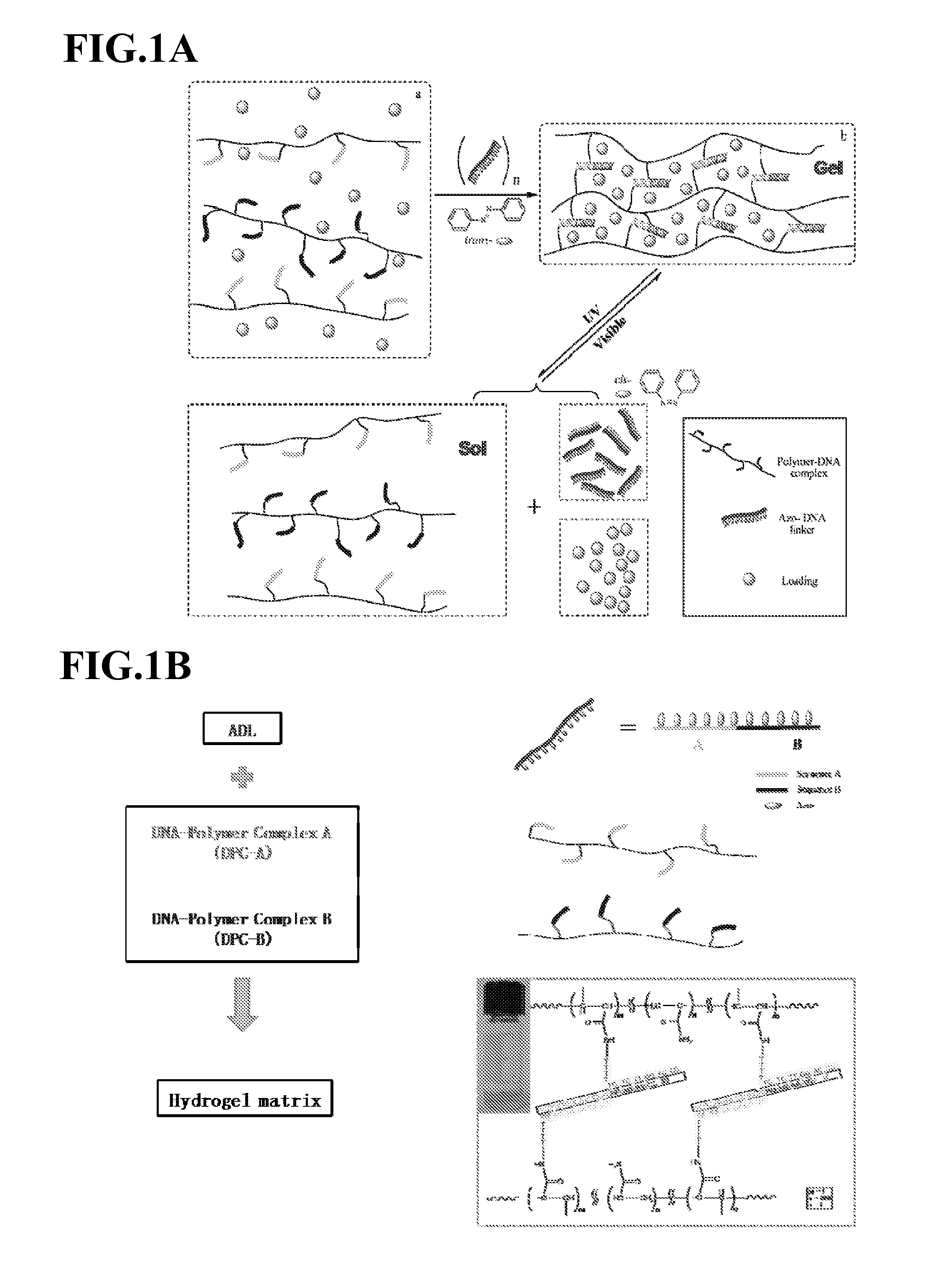
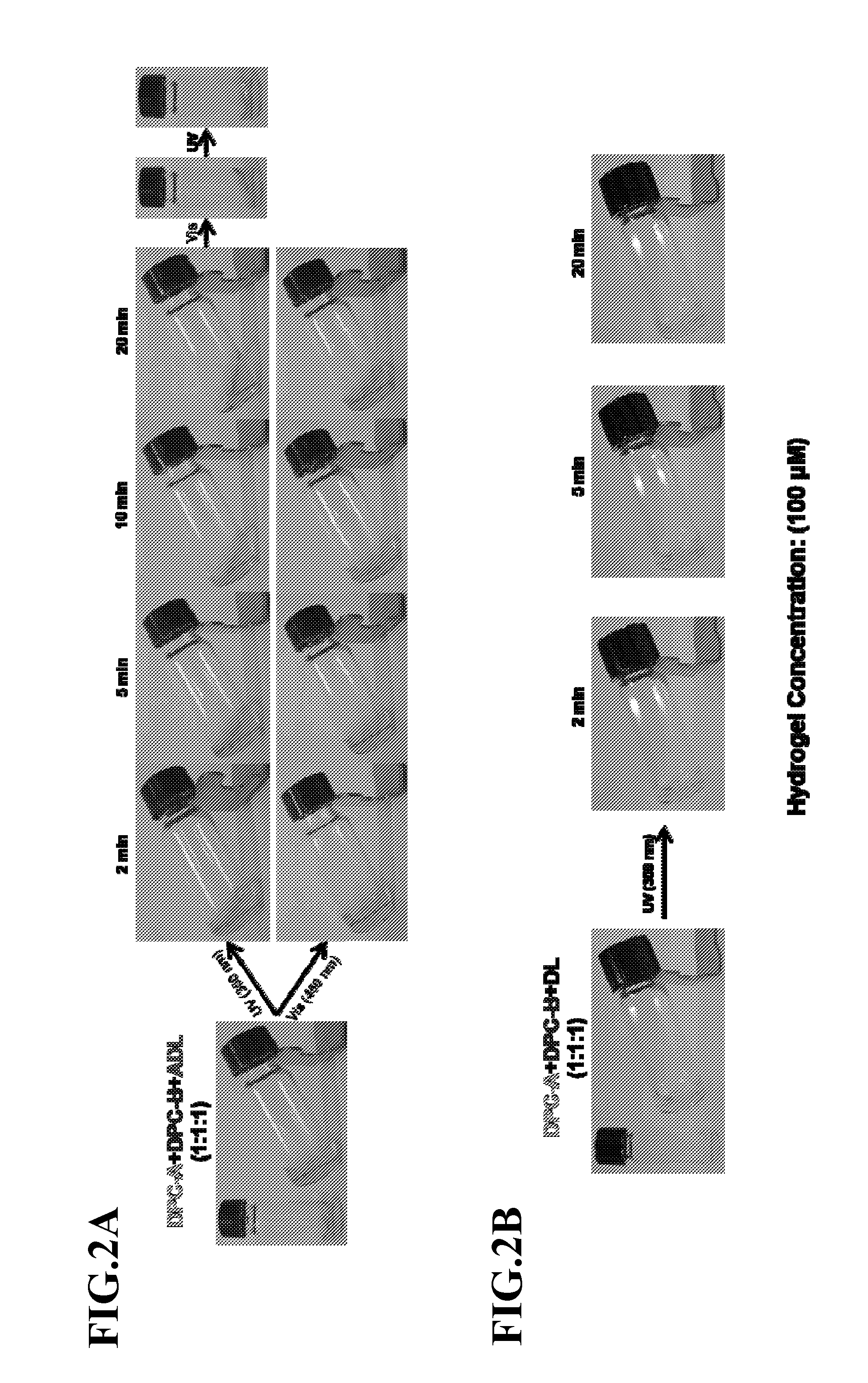
Photoregulated Reversible Hydrogels for Delivery and Releasing of Drugs and Other Therapeutical Reagents
InactiveUS20120228520A1Improve spatial resolutionFaster and deep penetrationOintment deliveryPharmaceutical non-active ingredientsCross-linkIsomerization
A novel hydrogel delivery systems useful for encapsulating and releasing pharmaceuticals or chemicals is disclosed where water soluble polymers containing crosslinker repeating units that associate or dissociate with complementary crosslinking repeating units or separate linkers to reversibly crosslink the hydrogel. In an exemplary embodiment, a DNA crosslinked hydrogel displays photoreversibility. An exemplary hydrogel delivery system comprises DNA polymer conjugates, wherein complementary DNA sequences are crosslinked with polymer chains and hybridization of the DNA sequences is controlled by photoresponsive moieties. Such hydrogels can be used to release drug molecules and / or other therapeutic reagents. The exemplary hydrogel employs photosensitive azobenzene moieties that are incorporated into the DNA crosslinker units. The azobenzene moieties respond to different wavelengths of light so that the state of azobenzene isomerization is induced by the proportion of visible and UV light irradiated. The isomer state of the azobenzene dictates whether the complementary DNA sequences hybridize to cross link the DNA polymer conjugates. Thus, irradiation of light (visible or UV) can transform the hydrogel network between a sol and any of multiple gel states to regulate the degree of crosslinking between complementary DNA sequences and, therefore, provide a profile of release of a hydrogel encapsulated pharmaceutical or other chemical.
Owner:UNIV OF FLORIDA RES FOUNDATION INC
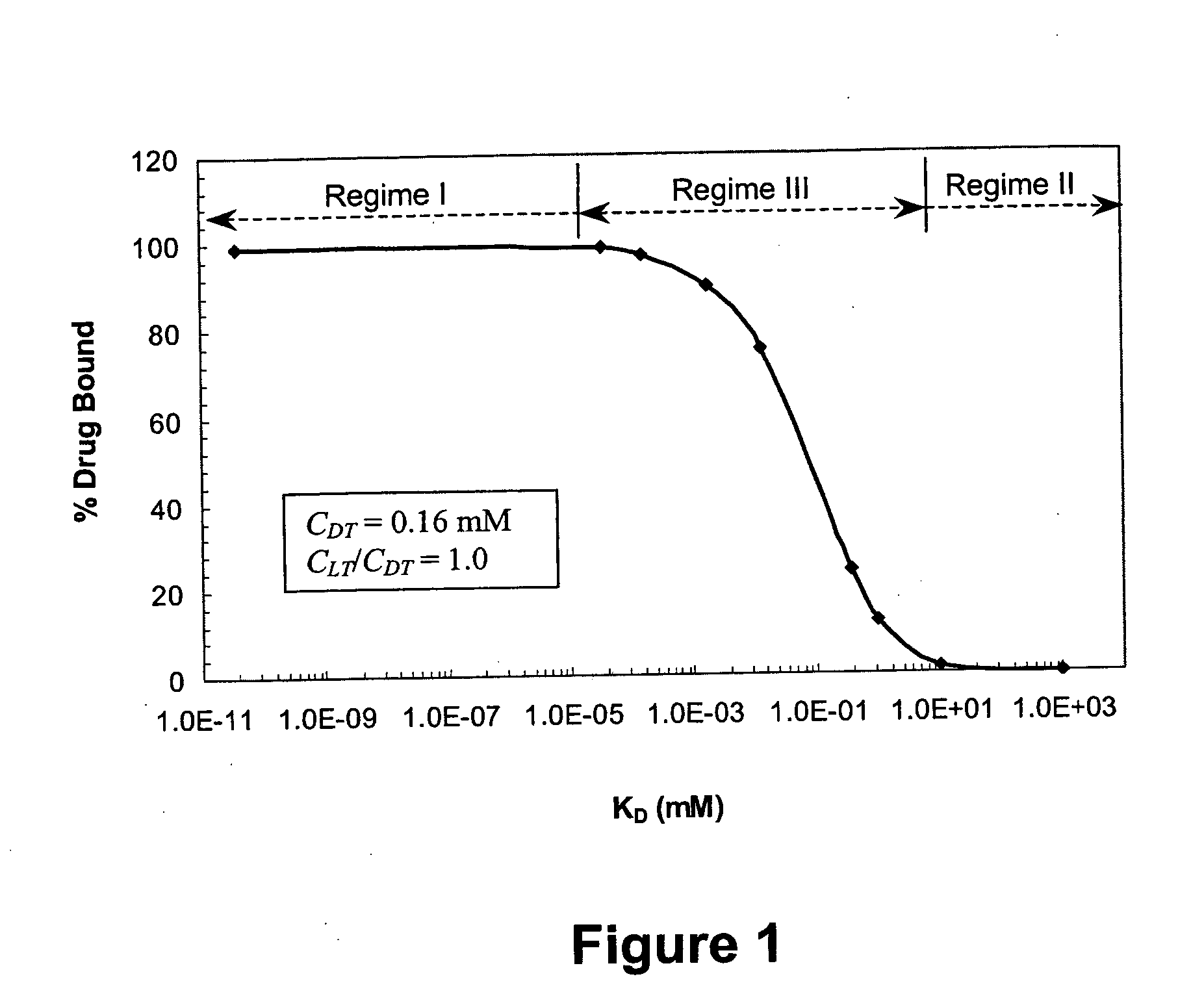
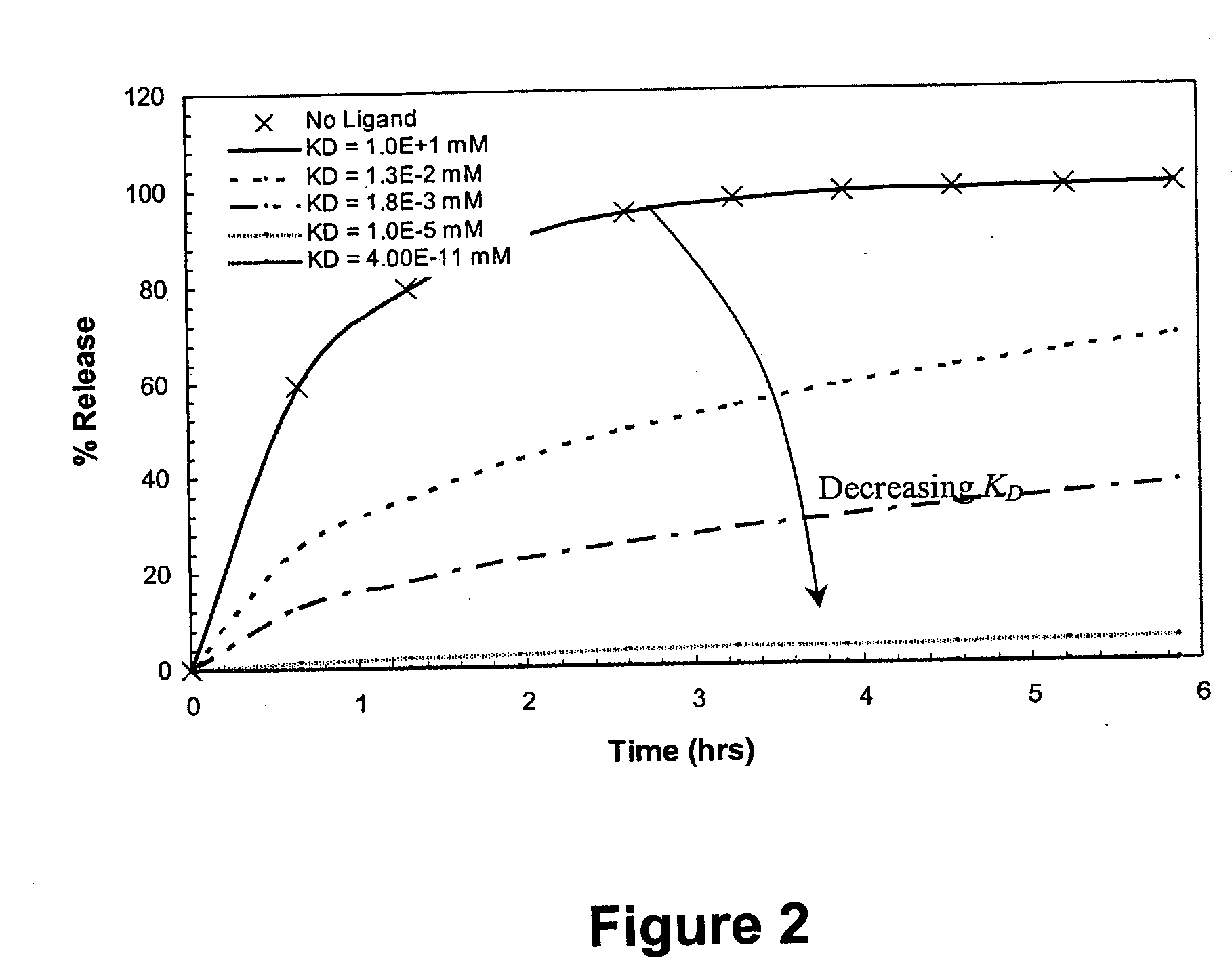
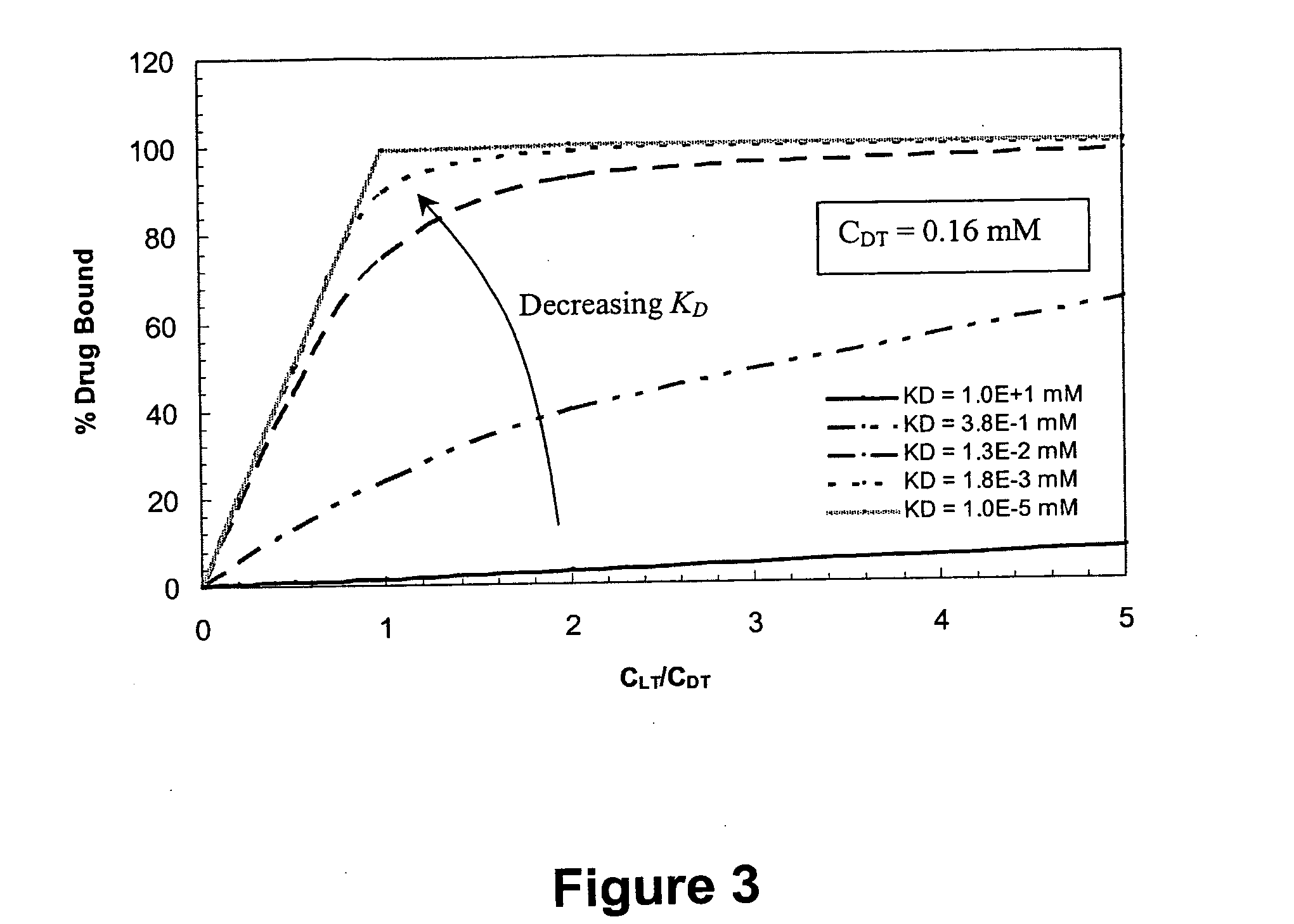
Ligand-mediated controlled drug delivery
InactiveUS20060153919A1Enhanced drug releaseImprove publishing efficiencyPowder deliveryOintment deliveryClinical settingsActive agent
Disclosed are systems and methods that can be utilized to define and control the delivery rate of a biological agent from a carrier matrix such as a biocompatible hydrogel. The carrier matrices of the present invention can include ligands incorporated within the matrix at a predetermined concentration level (CLT). In addition, the ligands within the matrix can display a particular, predetermined affinity for the biologically active agents to be delivered by the system. In particular, the affinity between the ligand and the biologically active agent can have a known predetermined dissociation constant (KD). When utilizing the system, the agent can be incorporated within the matrix due to association of the agent with the ligand. In addition, the agent can be protected from side reactions due to the association of the agent with the ligand. Through particular selection of the parameters CLT and KD, the rate of release of the biologically active agent from the matrix can be controlled. The disclosed methods and systems can be advantageously used in both in vivo clinical settings and ex vivo settings, such as tissue engineering applications.
Owner:CLEMSON UNIV RES FOUND
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com