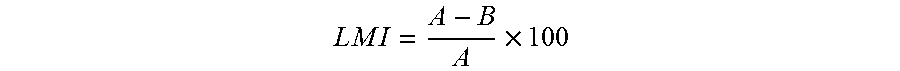Patents
Literature
49 results about "Mebendazole" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Mebendazole is used to treat intestinal worm infections such as pinworm, roundworm, and hookworm..
Parenteral and oral formulations of benzimidazoles
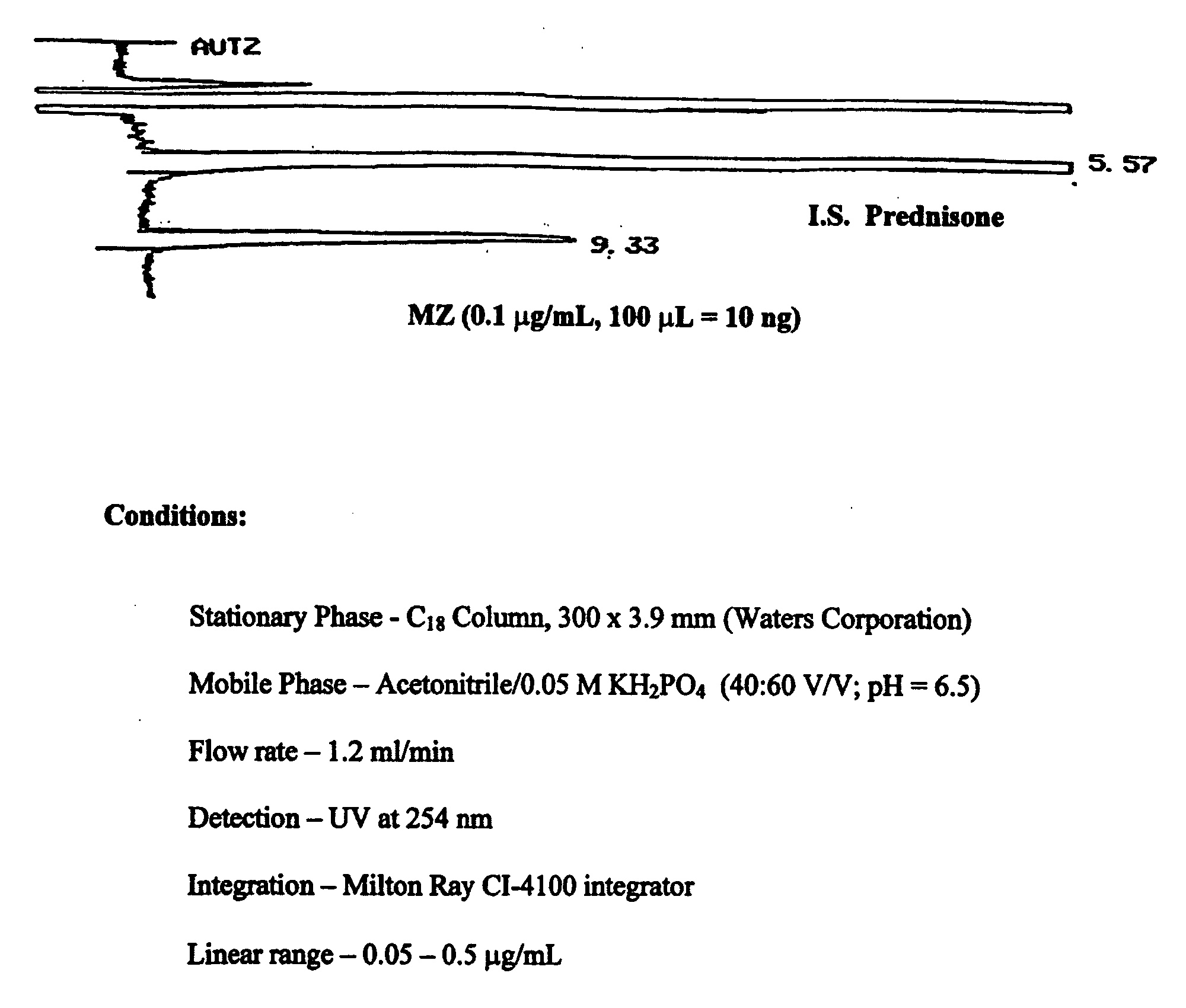
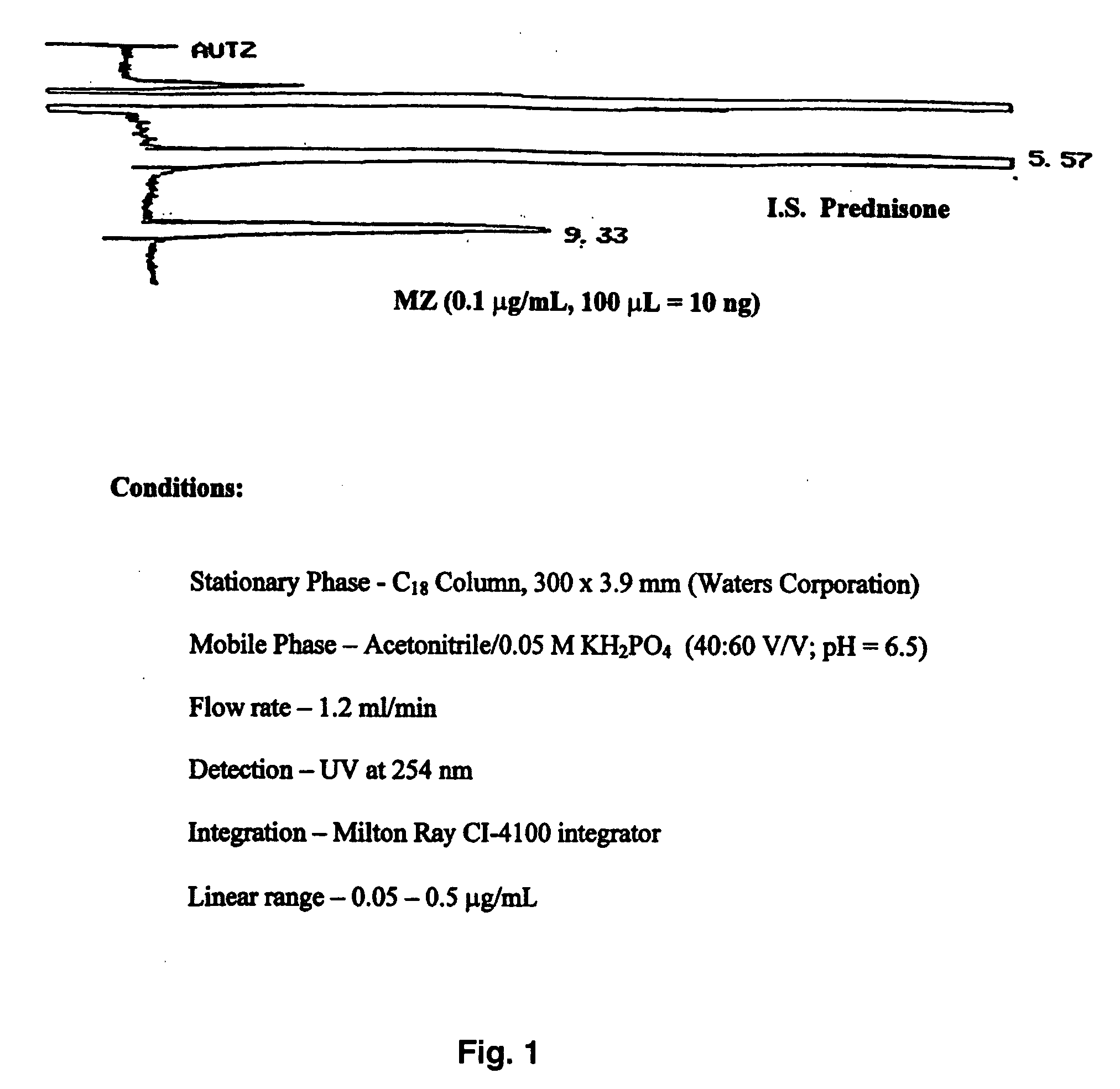
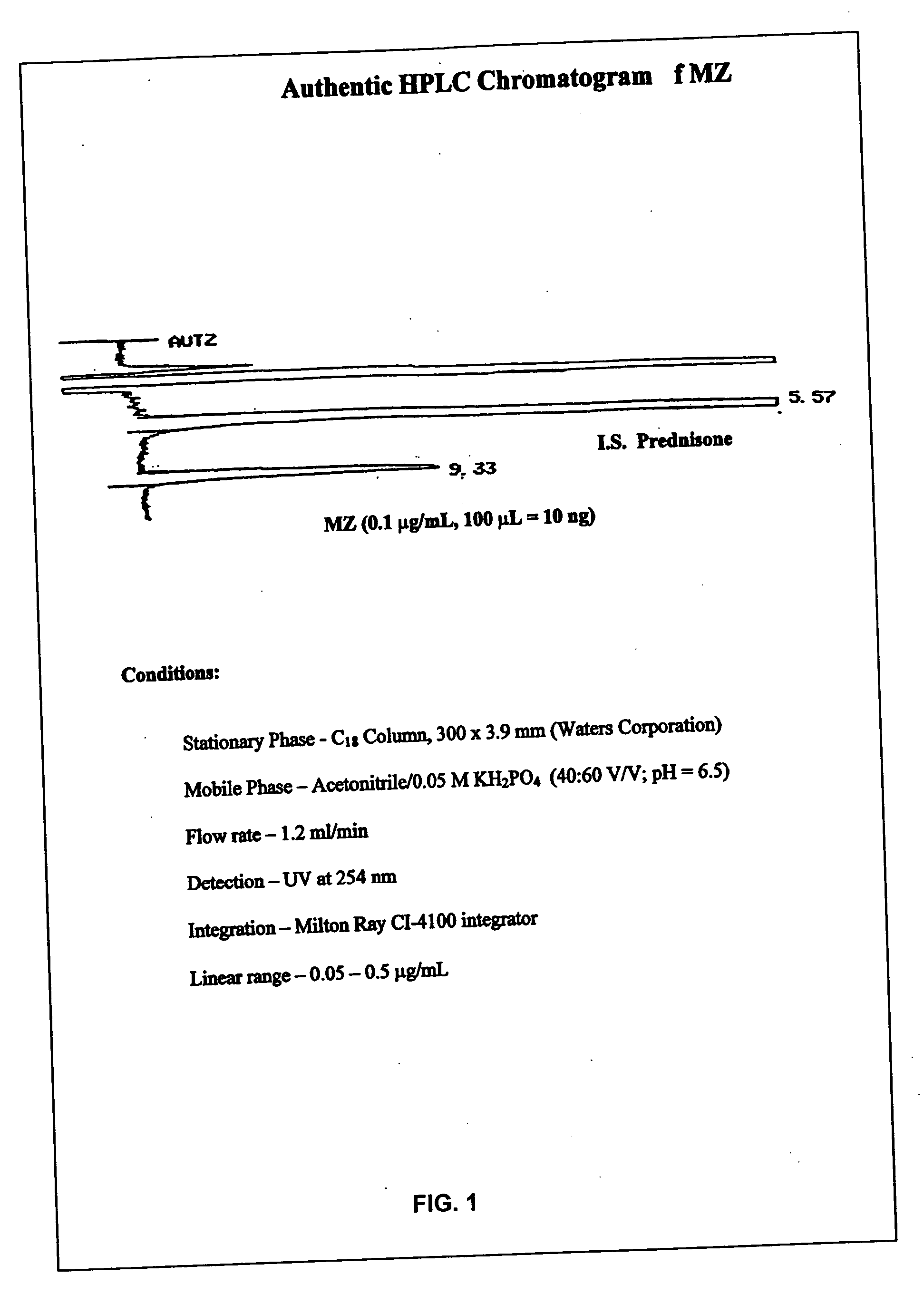
ActiveUS20090048322A1Good treatment effectImprove drug solubilityOrganic active ingredientsBiocideBenzimidazole derivativeMebendazole
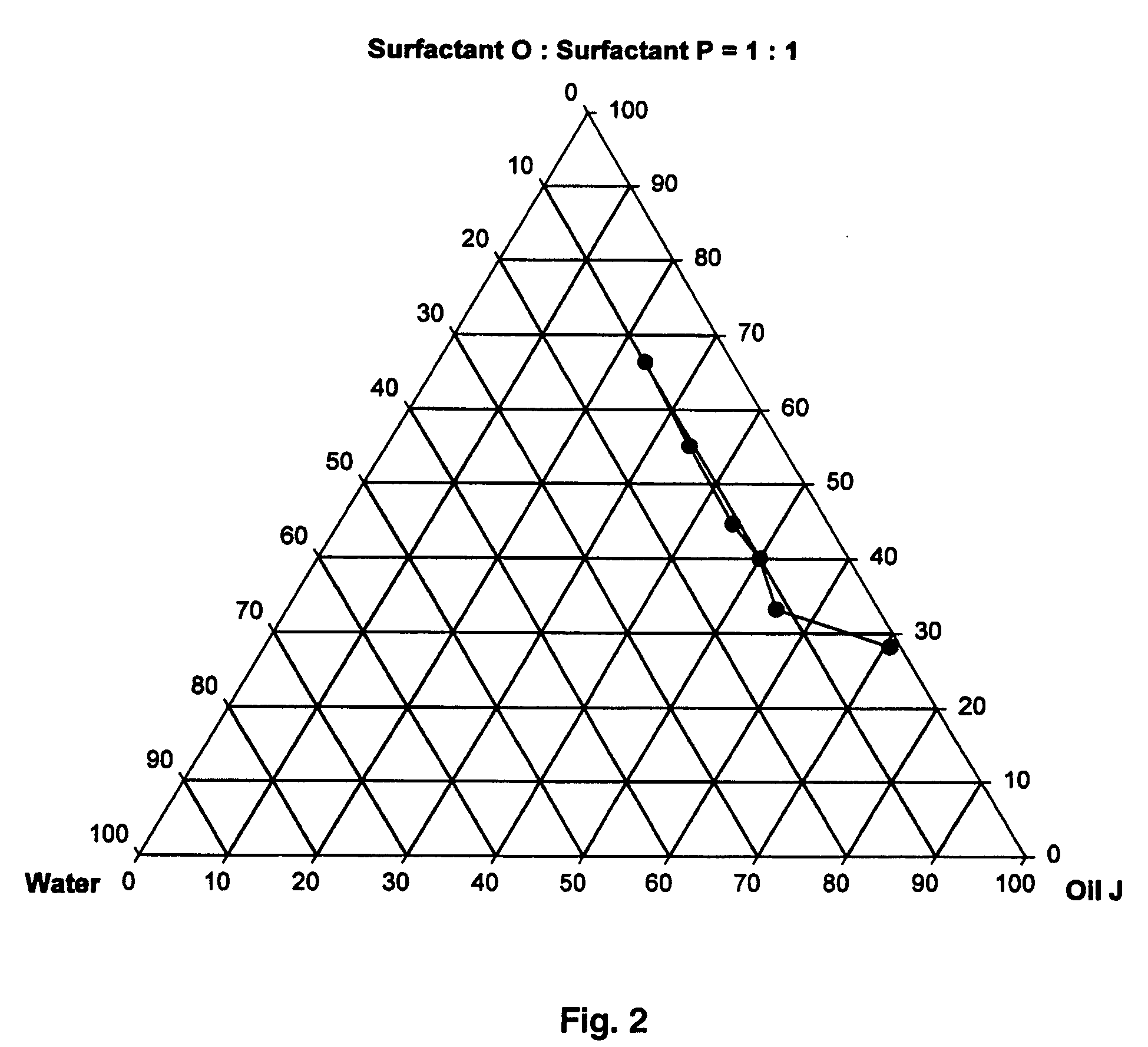
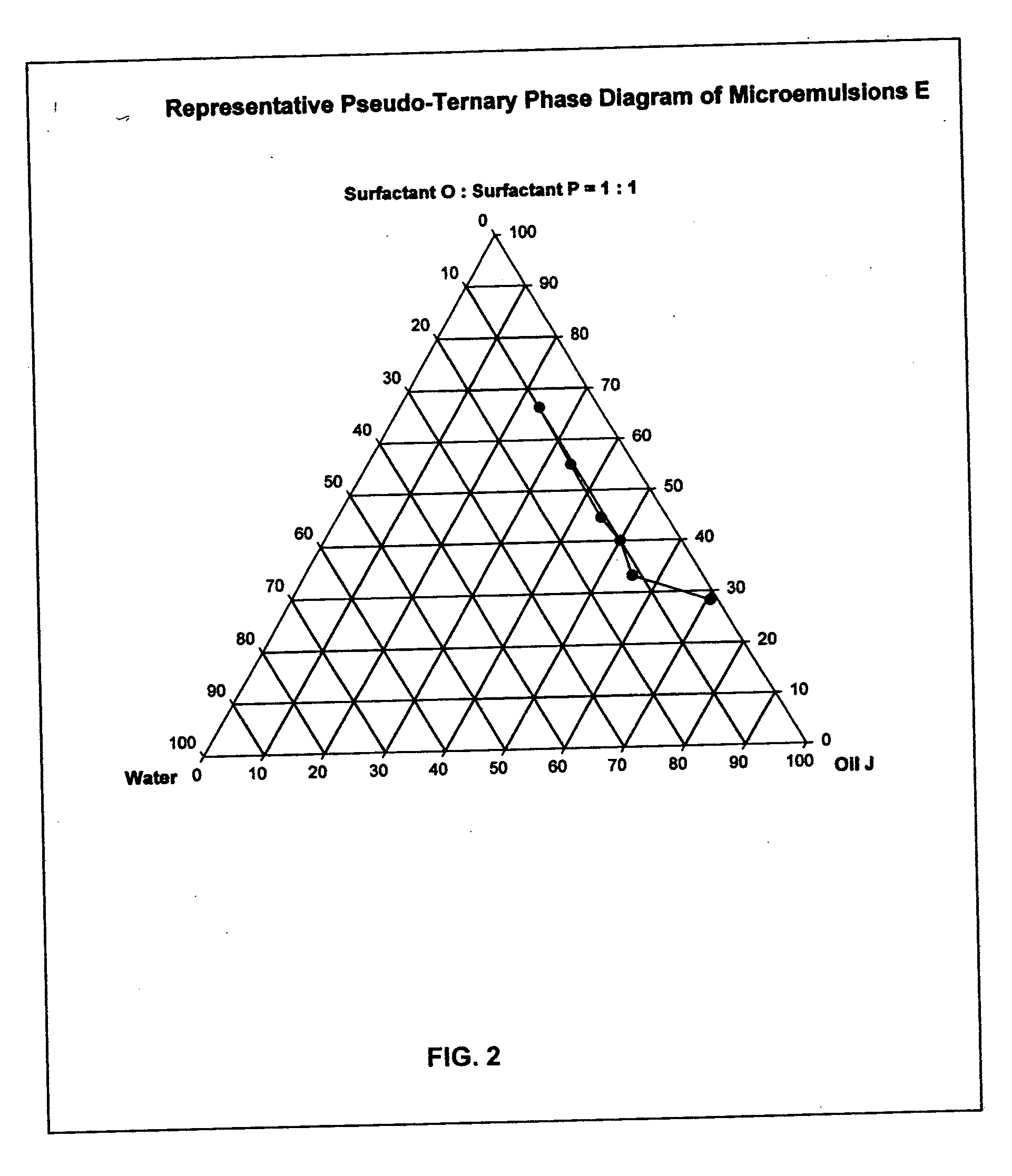
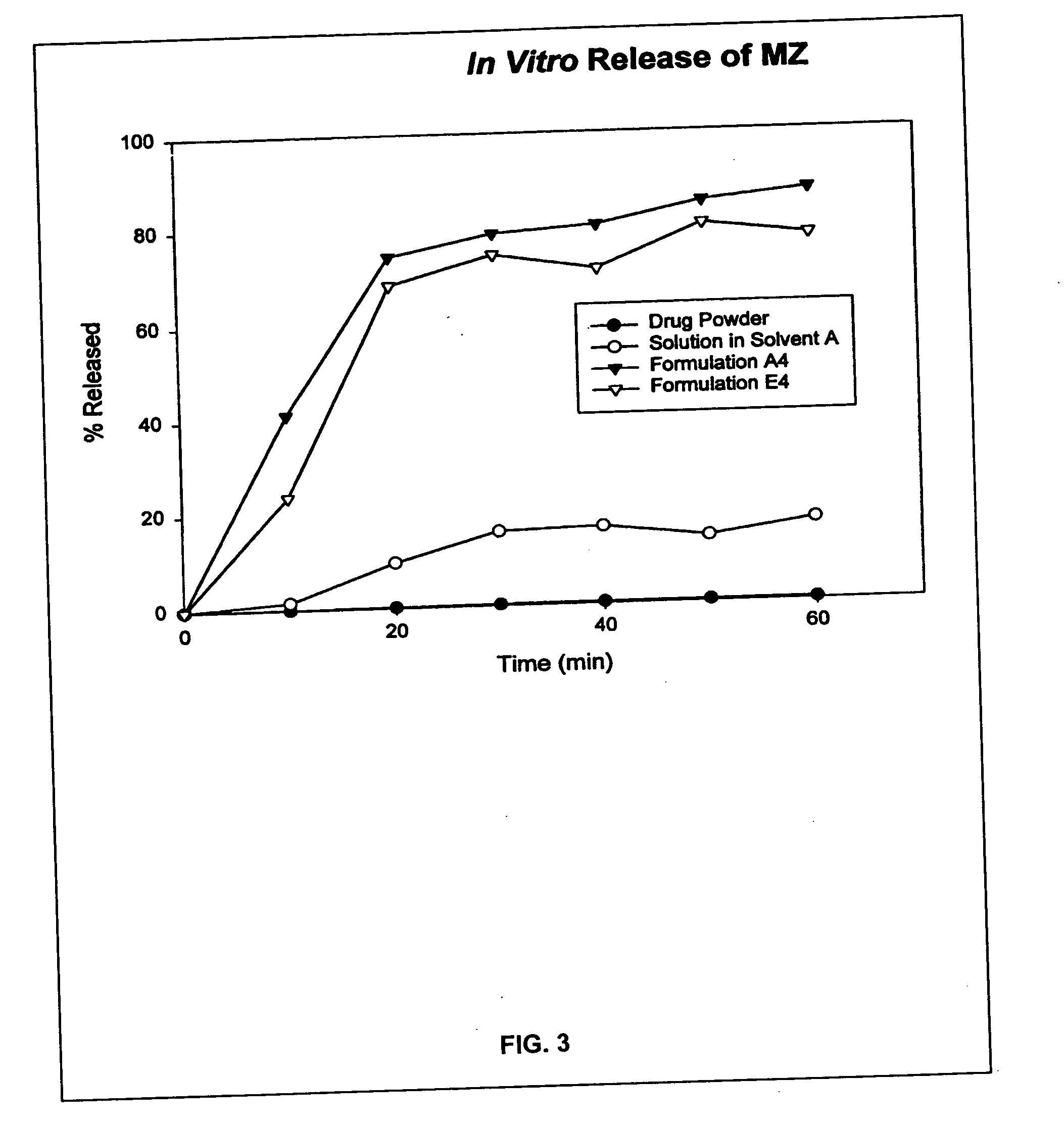
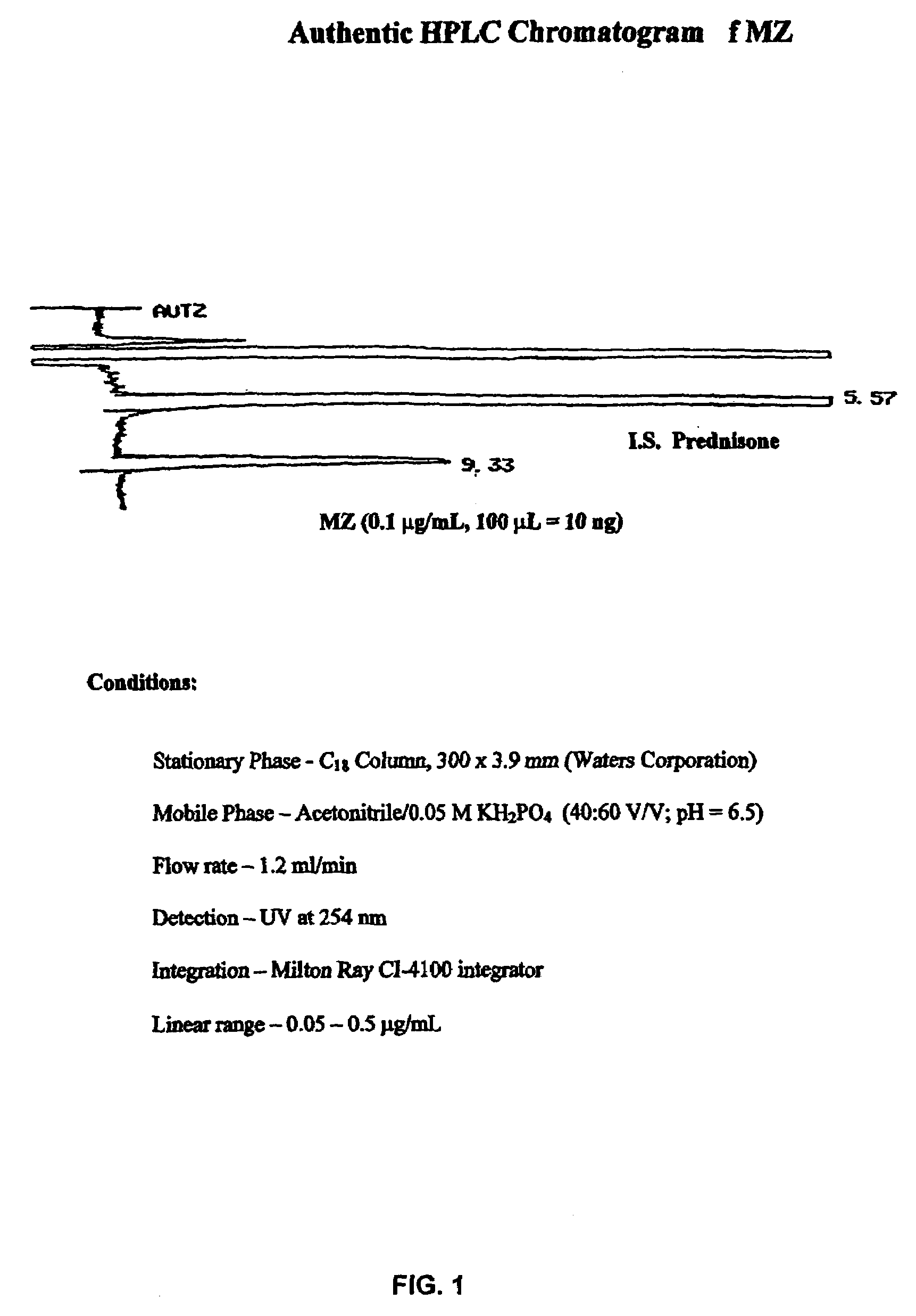
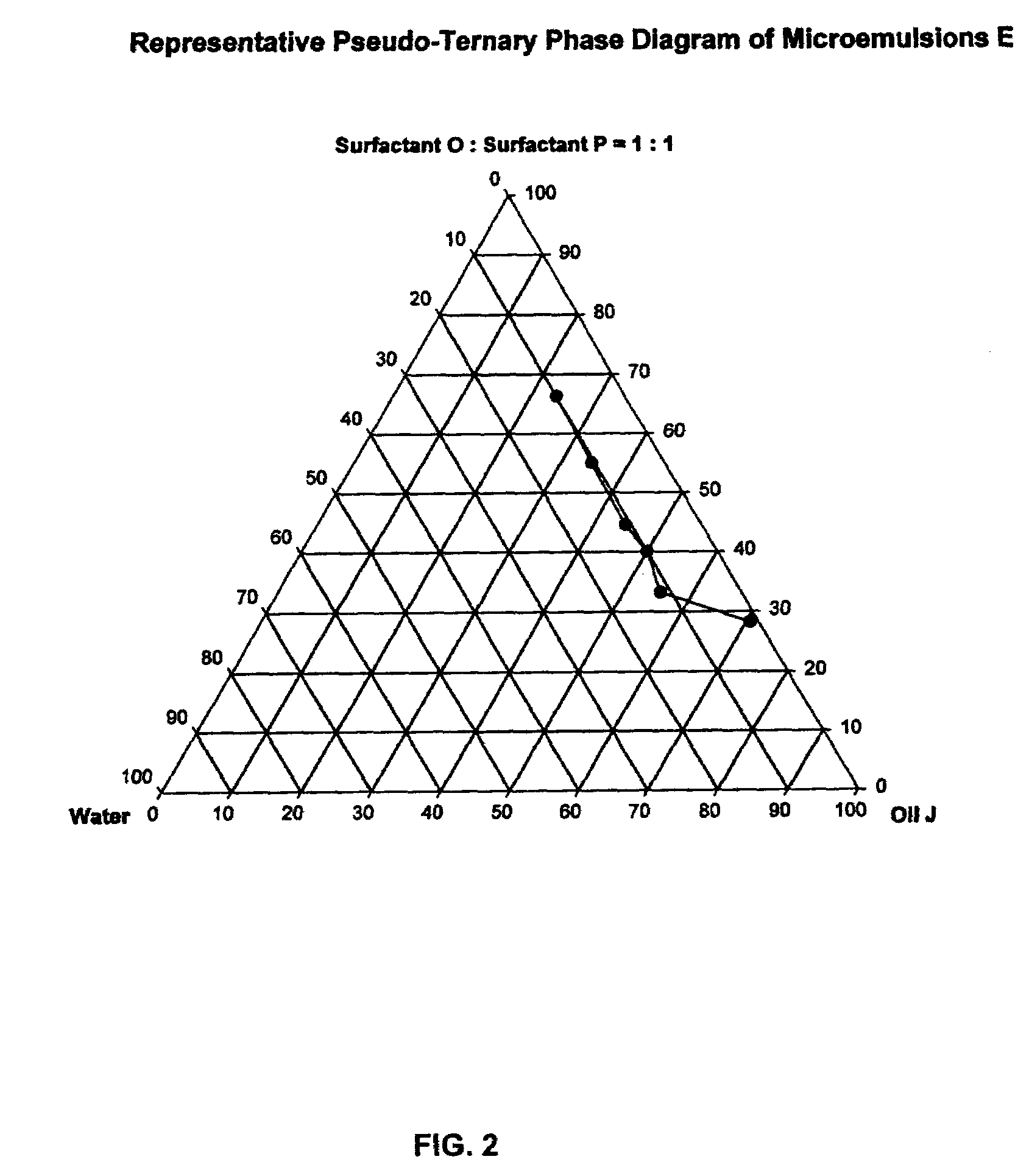
Provided herein are drug delivery systems, such as self-nanoemulsifying drug delivery systems, self-emulsifying drug delivery systems and parenteral microemulsion formulations, suitable for parenteral or oral delivery to a subject. The drug delivery systems may comprise a benzimidazole derivative, e.g., mebendazole, an oil, a surfactant, a cosurfactant and a dipolar aprotic solvent in a microemulsion formulation. Also provided are methods for improving the bioavailability of a benzimidazole derivative during treatment of a pathophysiological condition by using a formulation combining a particular emulsion droplet diameter and ratio of the surfactant:cosurfactant therein, for increasing concentration and retention of a benzimidazole derivative in the lung via a parenterally administerable microemulsion with droplet size of about 35 nm to less than 100 nm and for defining hemolytically safe microemulsions of a benzimidazole derivative during a therapeutic treatment via a parenterally administerable microemulsion with a surfactant:cosurfactant content by weight of about 6% to 48%.
Owner:UNIV HOUSTON SYST
Parenteral and oral formulations of benzimidazoles
ActiveUS20050038096A1Good treatment effectImprove drug solubilityBiocideDispersion deliveryBenzimidazole derivativePolyol
Pharmaceutical compositions of a benzimidazole or a benzimidazole derivative are disclosed. For example, in certain embodiments the pharmaceutical compositions include a benzimidazole, a polyol, and a dipolar aprotic solvent. In other embodiments, pharmaceutical compositions include a benzimidazole, an oil, a dipolar aproptic solvent, and a surfactant. In certain embodiments, the benzimidazole is mebendezole. The pharmaceutical compositions are formulated for delivery to a subject by any means, and include formulations for oral and parenteral delivery.
Owner:UNIV HOUSTON SYST
Parenteral and oral formulations of benzimidazoles
ActiveUS7419996B2Good treatment effectImprove drug solubilityBiocideDispersion deliveryBenzimidazole derivativeMebendazole
Pharmaceutical compositions of a benzimidazole or a benzimidazole derivative are disclosed. For example, in certain embodiments the pharmaceutical compositions include a benzimidazole, PEG 400, and a dipolar aprotic solvent. In other embodiments, pharmaceutical compositions include a benzimidazole, an oil, a dipolar aproptic solvent, and a surfactant. In certain embodiments, the benzimidazole is mebendezole. The pharmaceutical compositions are formulated for delivery to a subject by any means, and include formulations for oral and parenteral delivery.
Owner:UNIV HOUSTON SYST
Composition for preventing or treating degenerative brain diseases including compound downregulating expression of BACE1 proteins
ActiveUS20150018297A1Reduce expressionSuppress generationBiocideSugar derivativesEfavirenzRaloxifene Hydrochloride
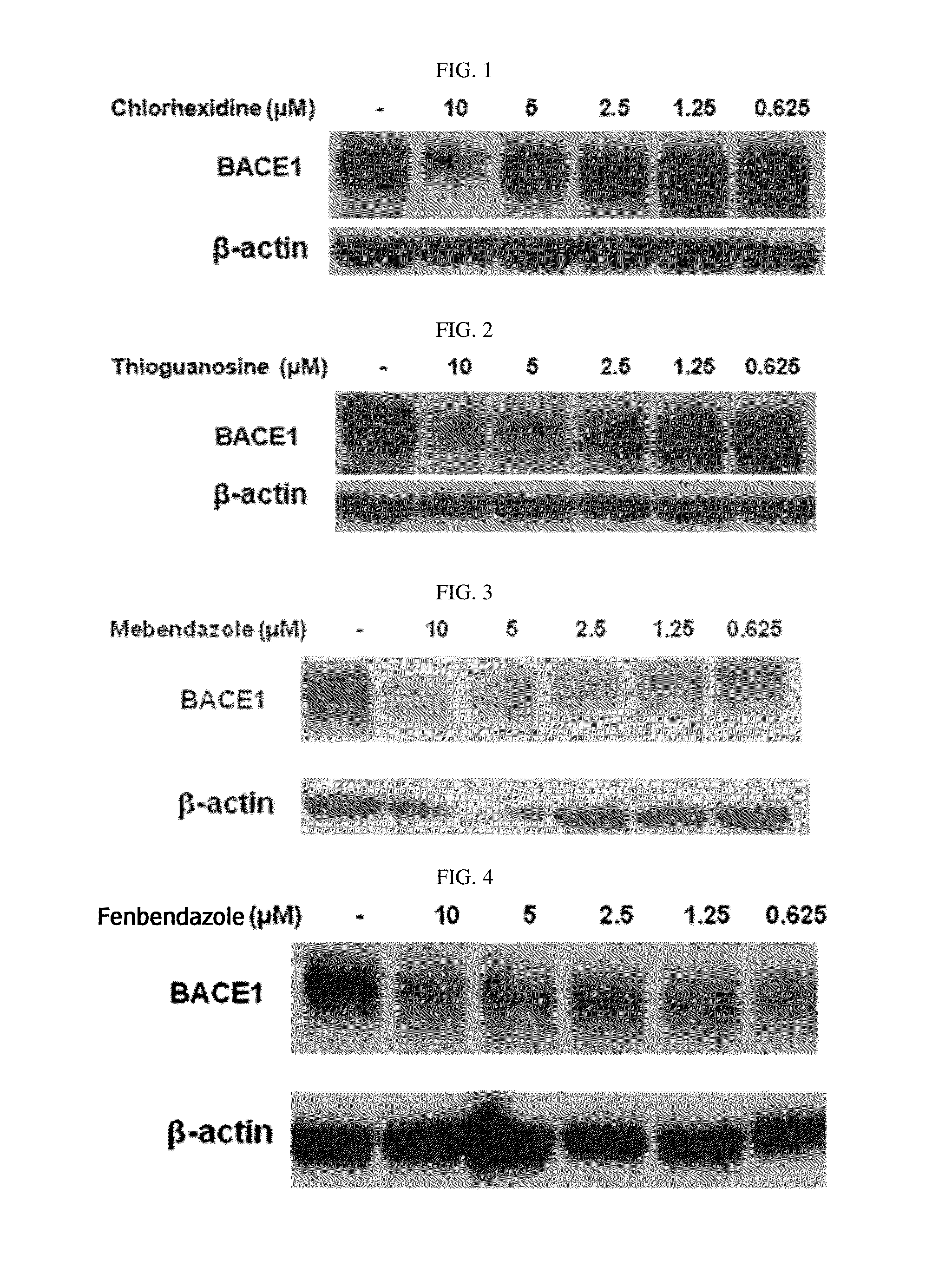
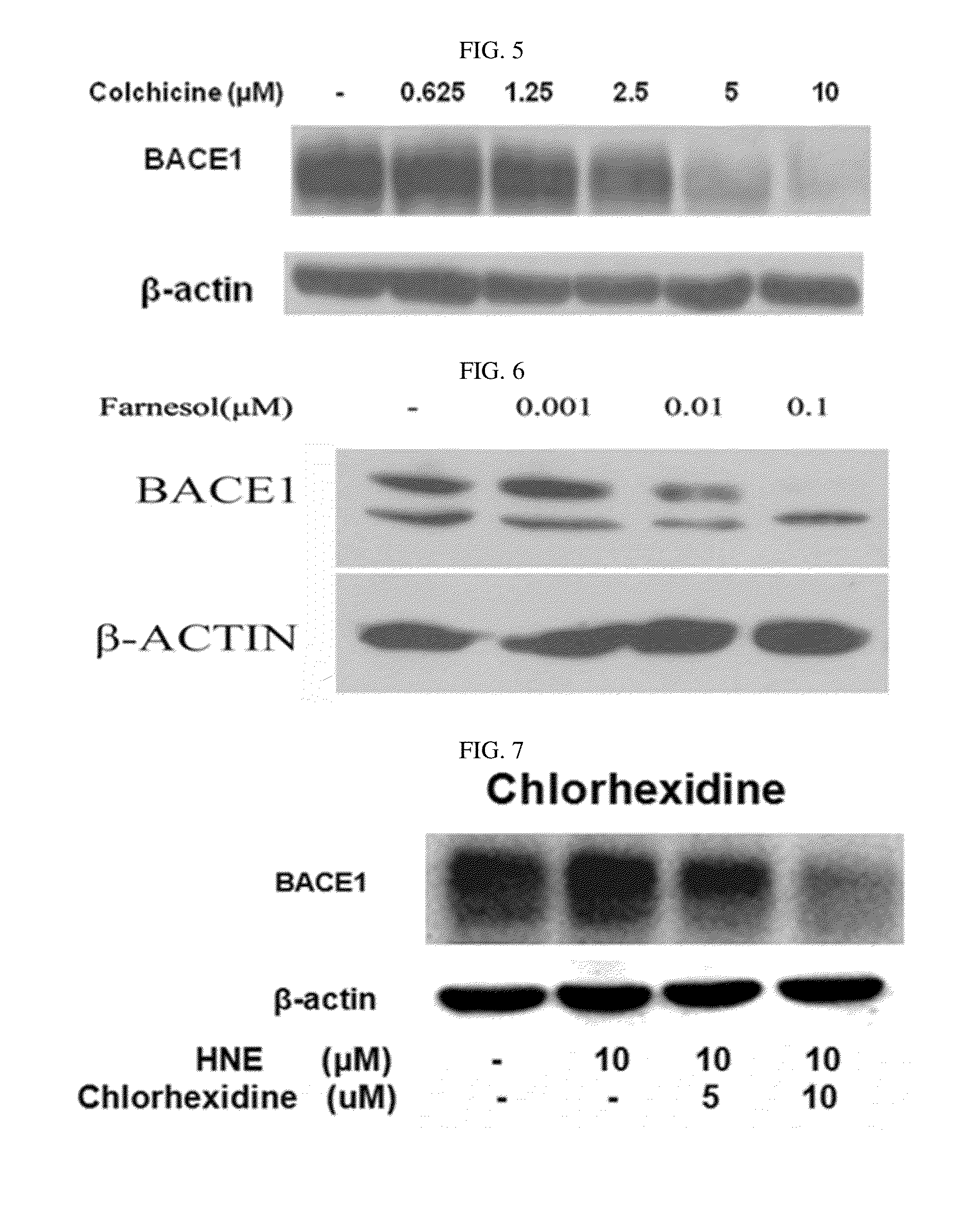
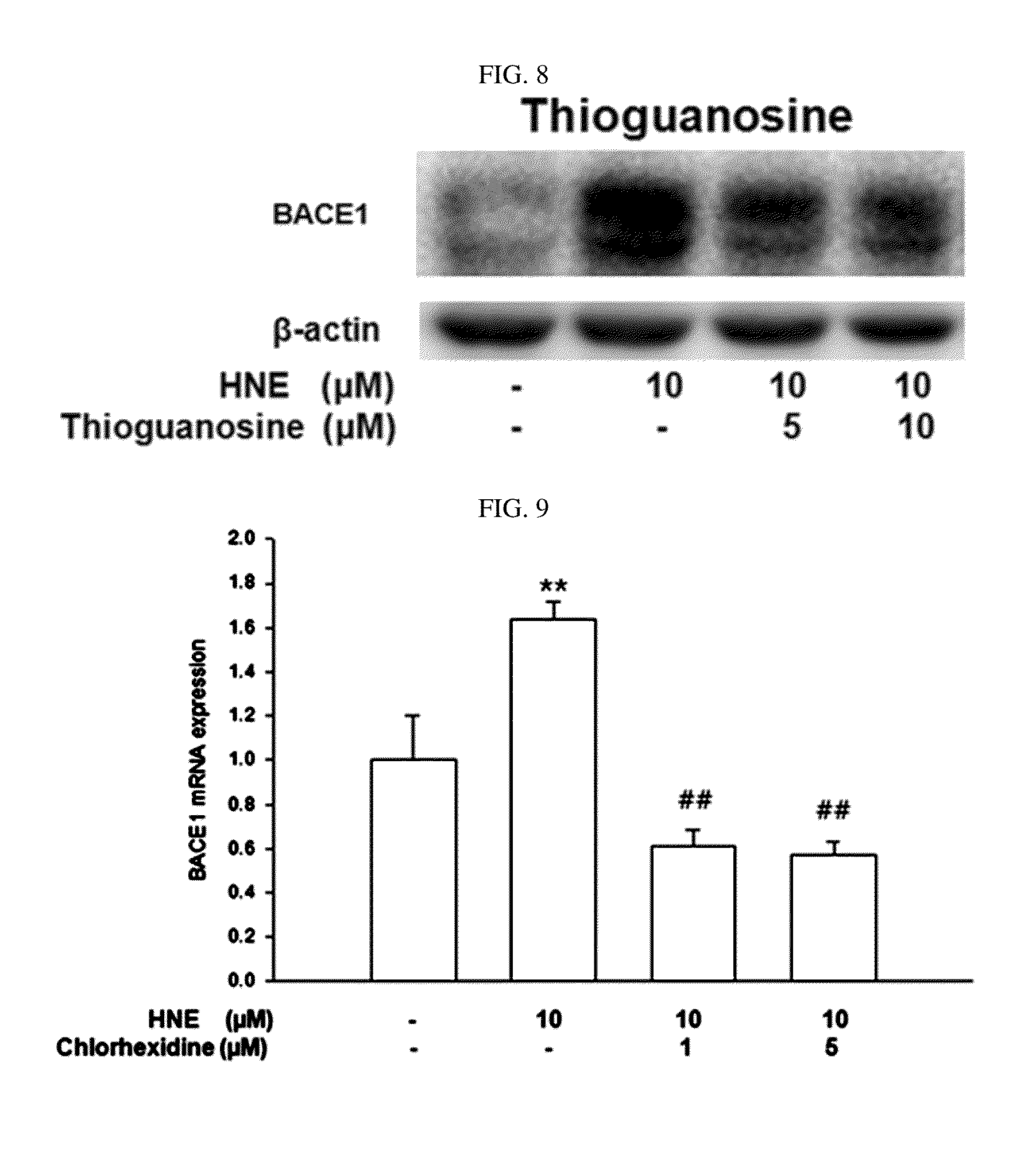
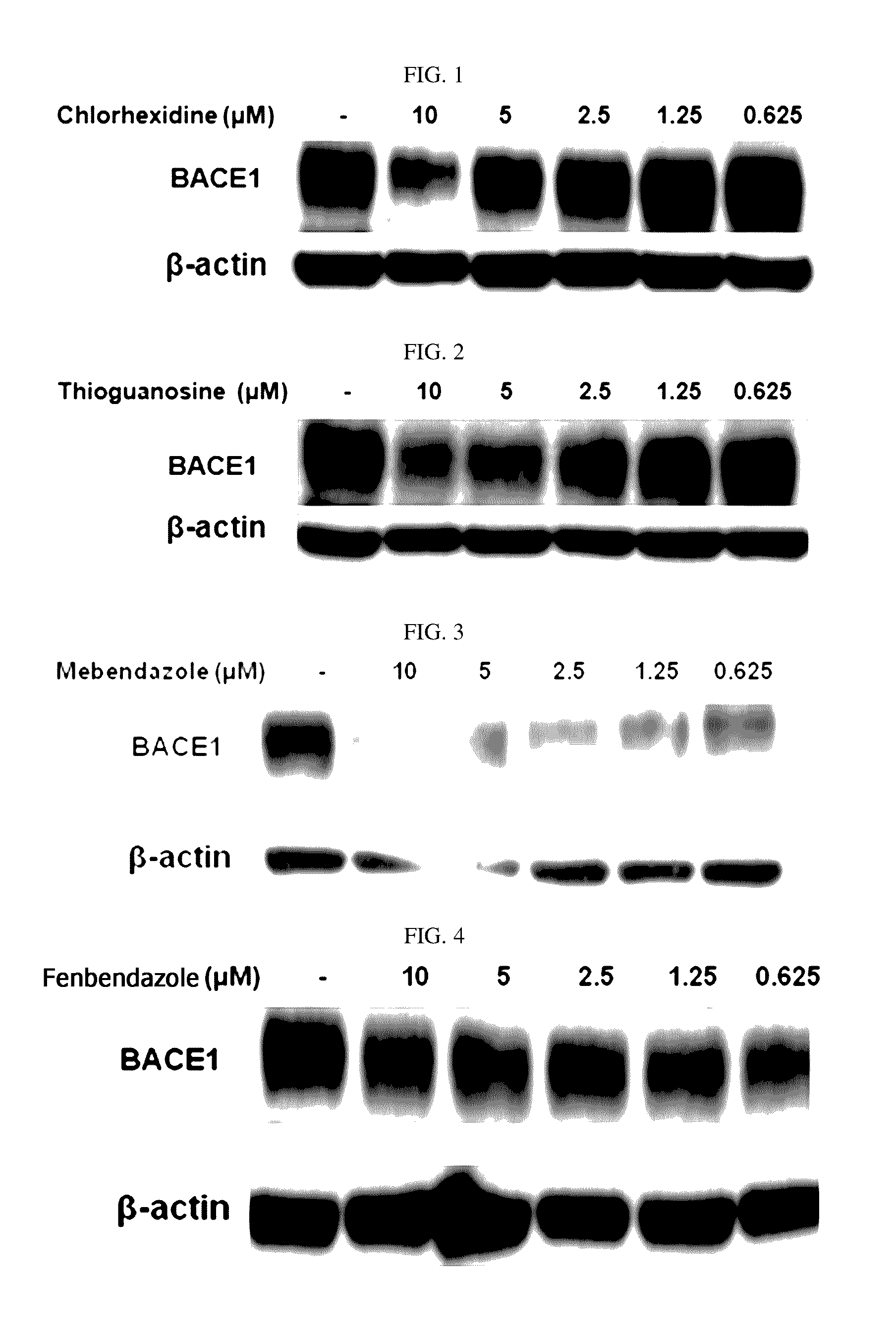
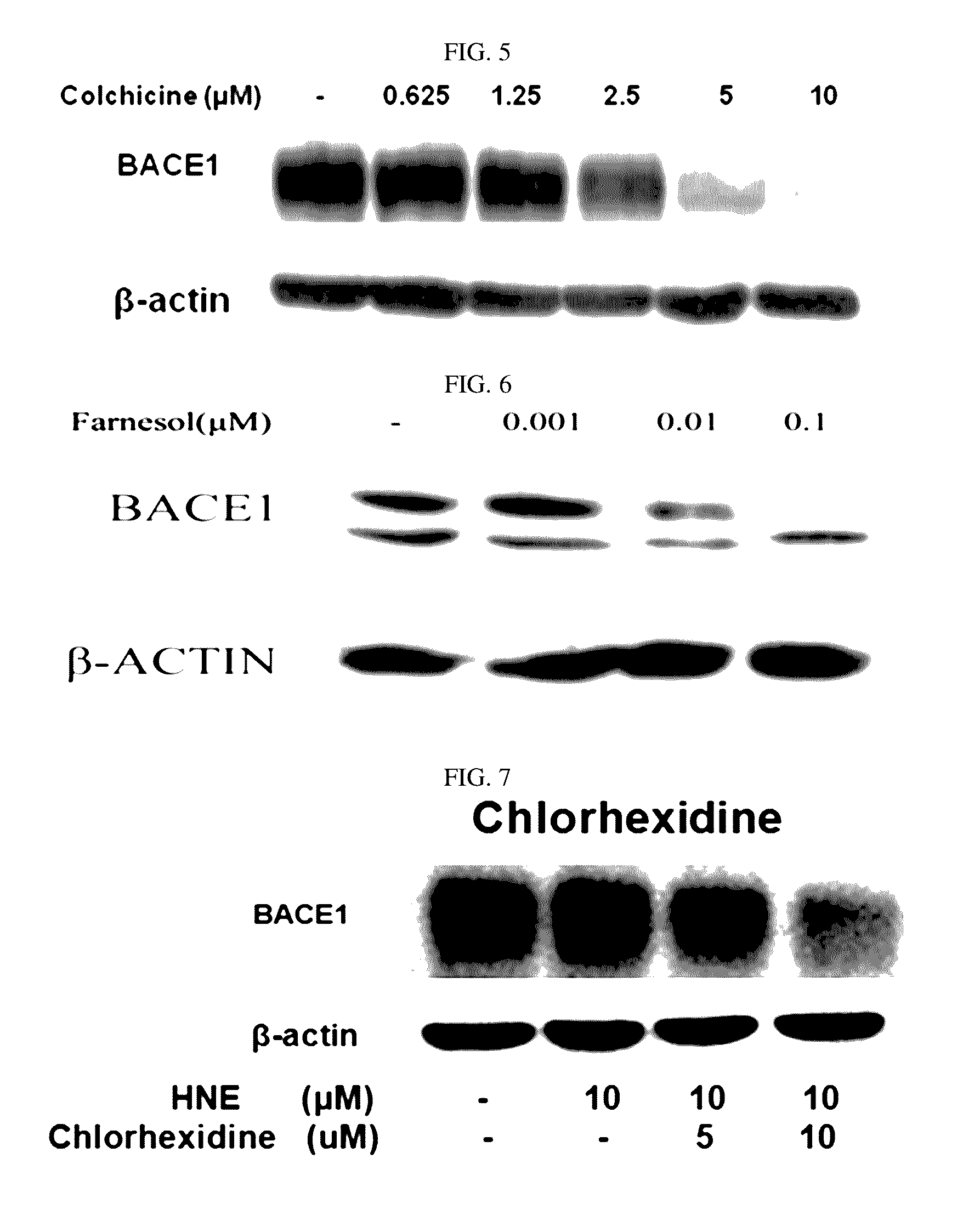
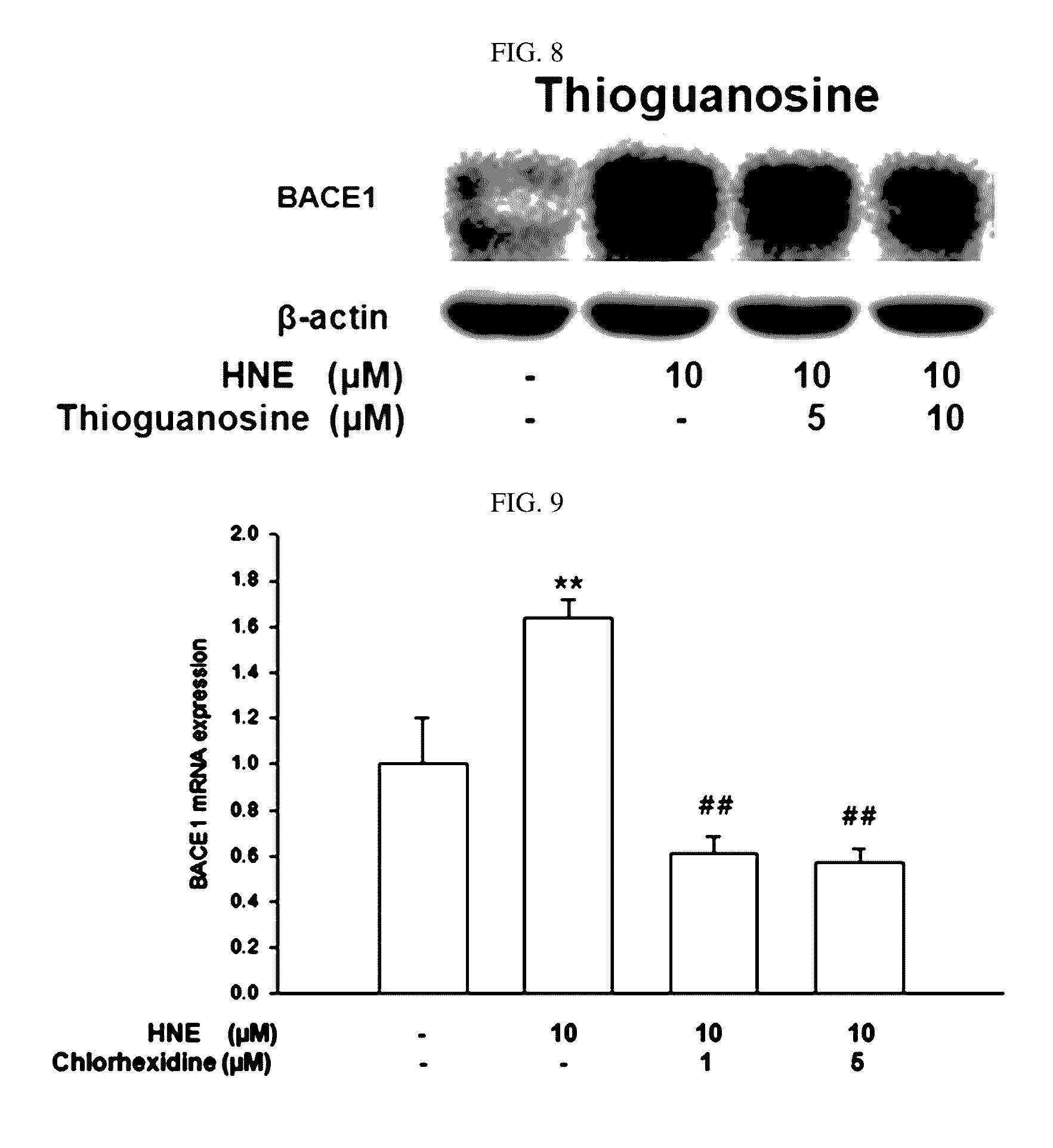
The present disclosure relates to a pharmaceutical composition, a health functional food composition, and a method for preventing or treating a brain disease or diabetes. The pharmaceutical composition, health functional food composition, and method includes at least one active ingredient that is chlorhexidine, thioguanosine, mebendazole, fenbendazole, colchicine, farnesol, trimethobenzamide hydrochloride, disulfuram, azathioprine, mebeverine hydrochloride, zaprinast, tosufloxacin hydrochloride, efavirenz, thiostrepton, probenecid, entacapone, harmine hydrochloride, flunisolide, thimerosal, hexestrol, sulfaquinoxaline sodium salt, monensin sodium salt, raloxifene hydrochloride, 2-chloropyrazine, or topotecan.
Owner:RES & BUSINESS FOUND SUNGKYUNKWAN UNIV
Fast extraction and LC-MS-MS detection method of benzimidazole and thiazole type residual medicine in aquatic product
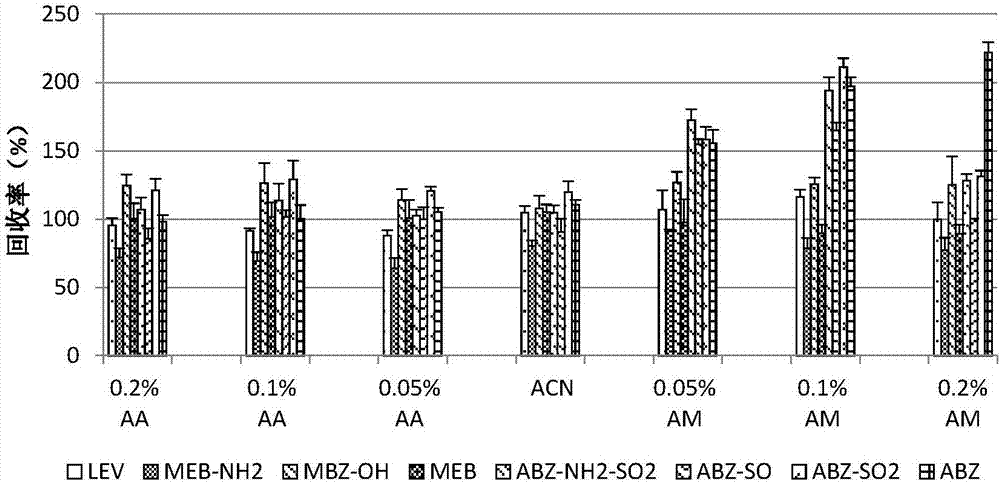
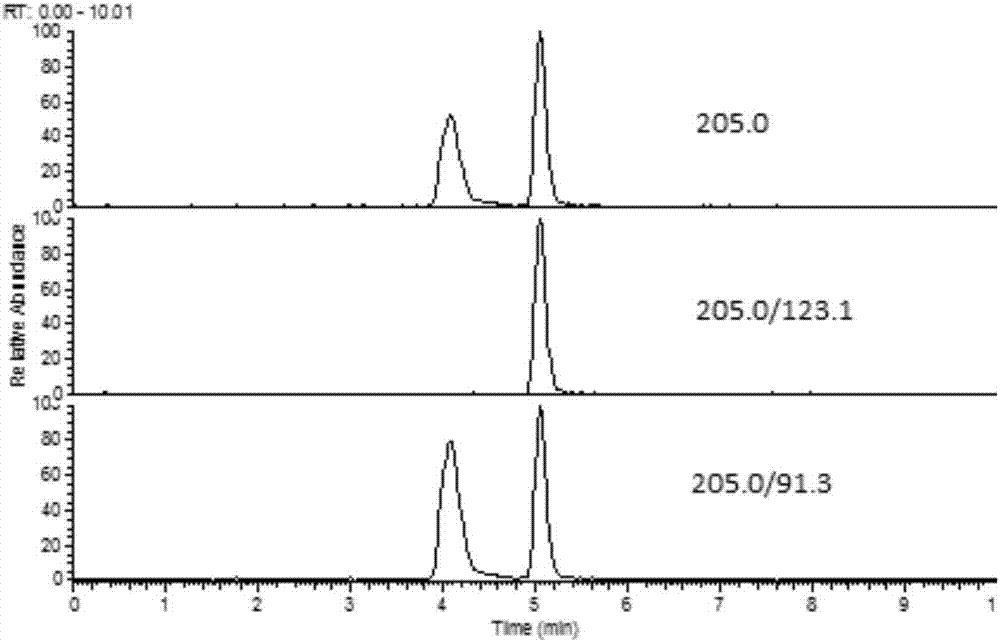
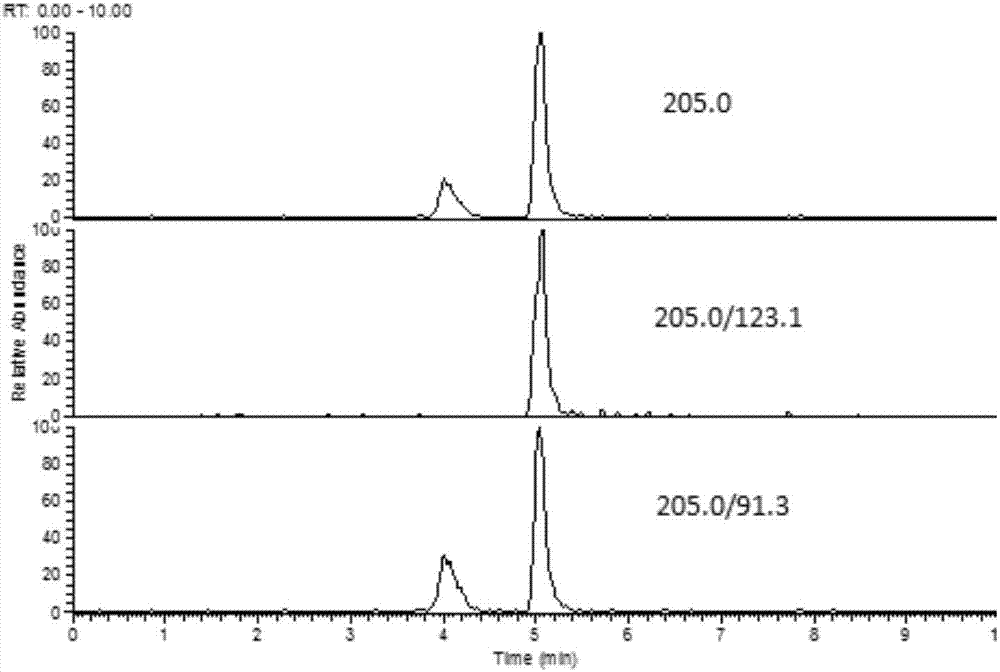
The invention discloses a fast extraction and LC-MS-MS detection method of benzimidazole and thiazole type residual medicine in an aquatic product. The method comprises the following steps that (1) muscle samples of the aquatic product are weighed; internal standard deuteratedalbendazole, deuterated mebendazole, magnesium sulfate, sodium chloride and acetonitrile are added for vortex extraction; (2) C18 powder and neutral alumina are added into extraction liquid; after swirling, nitrogen gas is used for blow-drying; filtering is performed after redissolution; (3) filter liquid is subjected to LC-MS-MS quantitative measurement; an internal standard method is used for calculating the residual quantity of levamisol, amino-mebendazole, hydroxyl-mebendazole, mebendazole, albendazole sulfoxide, albendazole sulphone, albendazole amino sulfone and albendazole. The method has the advantages that the sample treatment is simple, convenient and fast; meanwhile, the pH value of a liquid chromatography water phase and a cleaning agent is preferentially selected; the residual quantity of various benzimidazole and thiazole type medicines in the aquatic product can be simultaneously measured; the sensitivity is high; the repeatability is good; the accuracy is high; the lowest quantification of the medicine detection can reach 1mu g / kg.
Owner:YANGTZE RIVER FISHERIES RES INST CHINESE ACAD OF FISHERY SCI
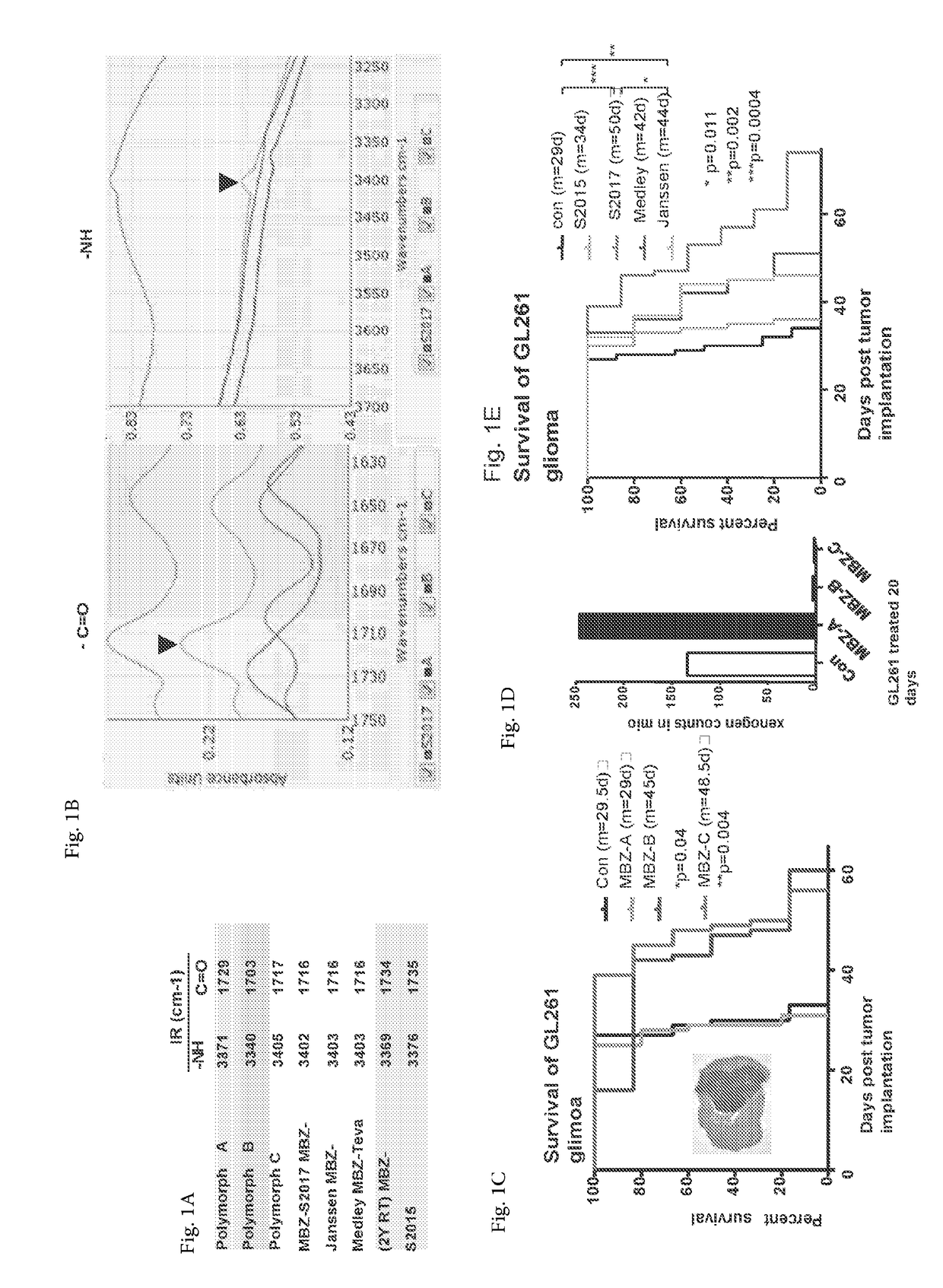
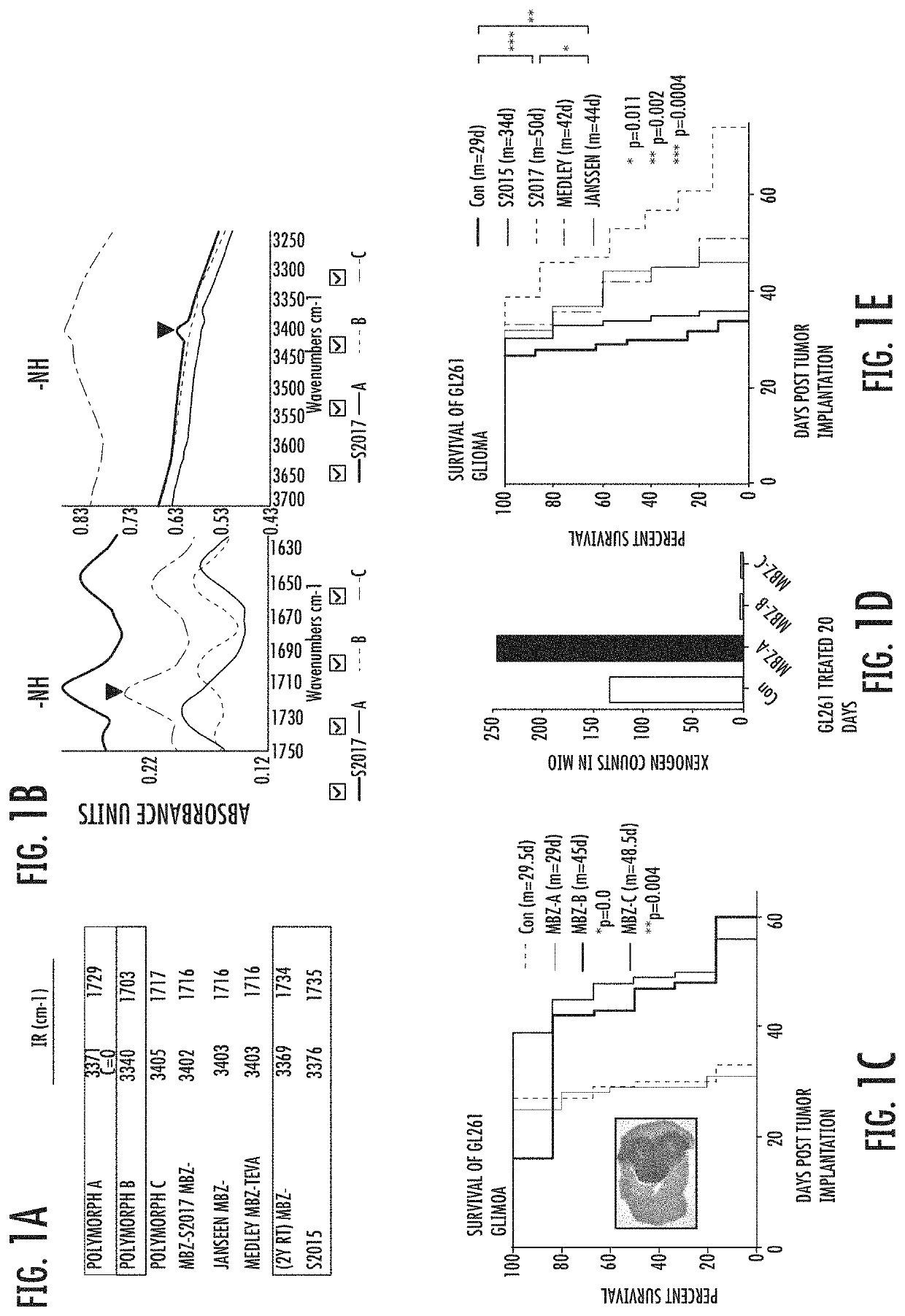
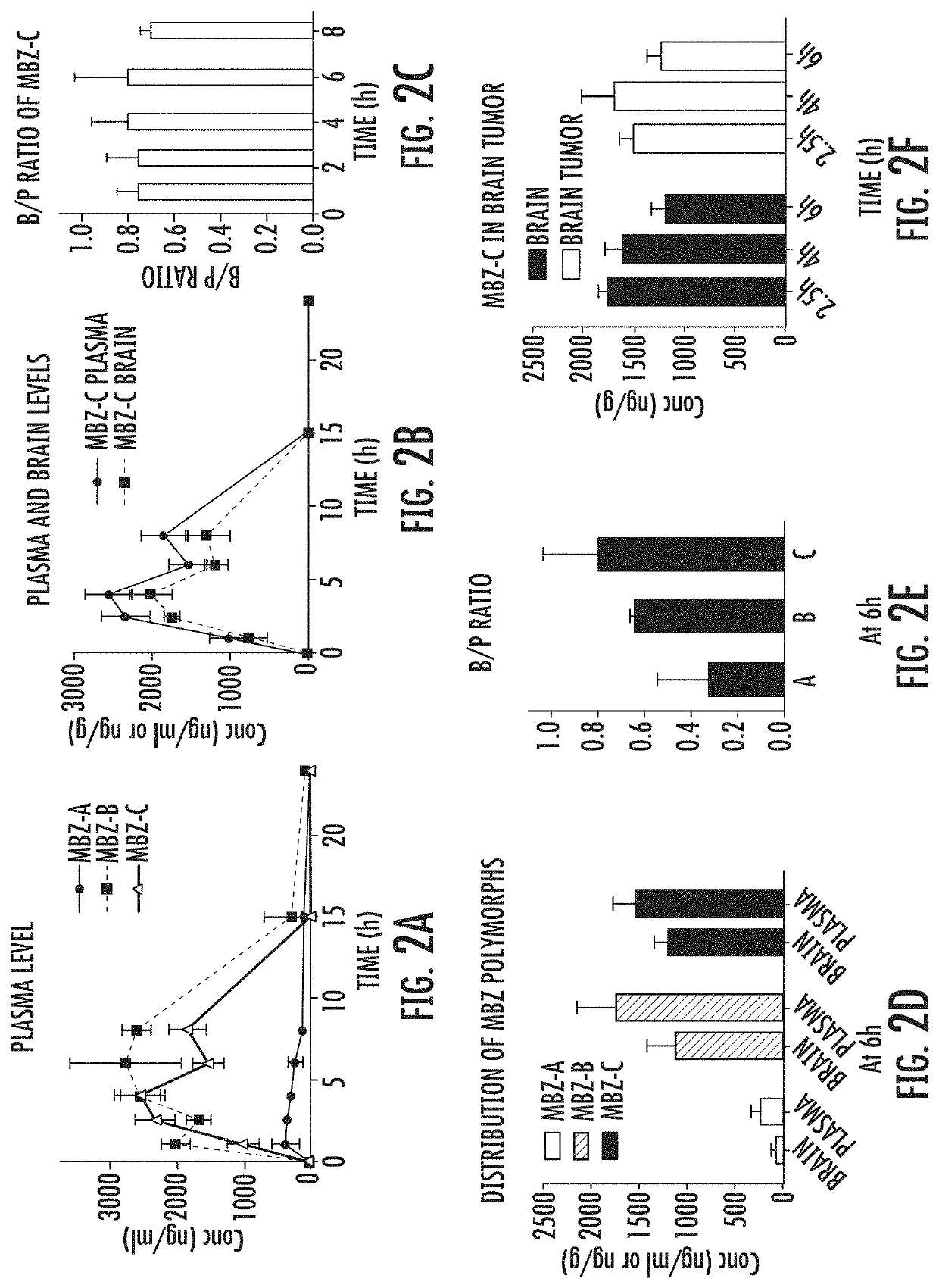
Mebendazole polymorph for treatement and prevention of tumors
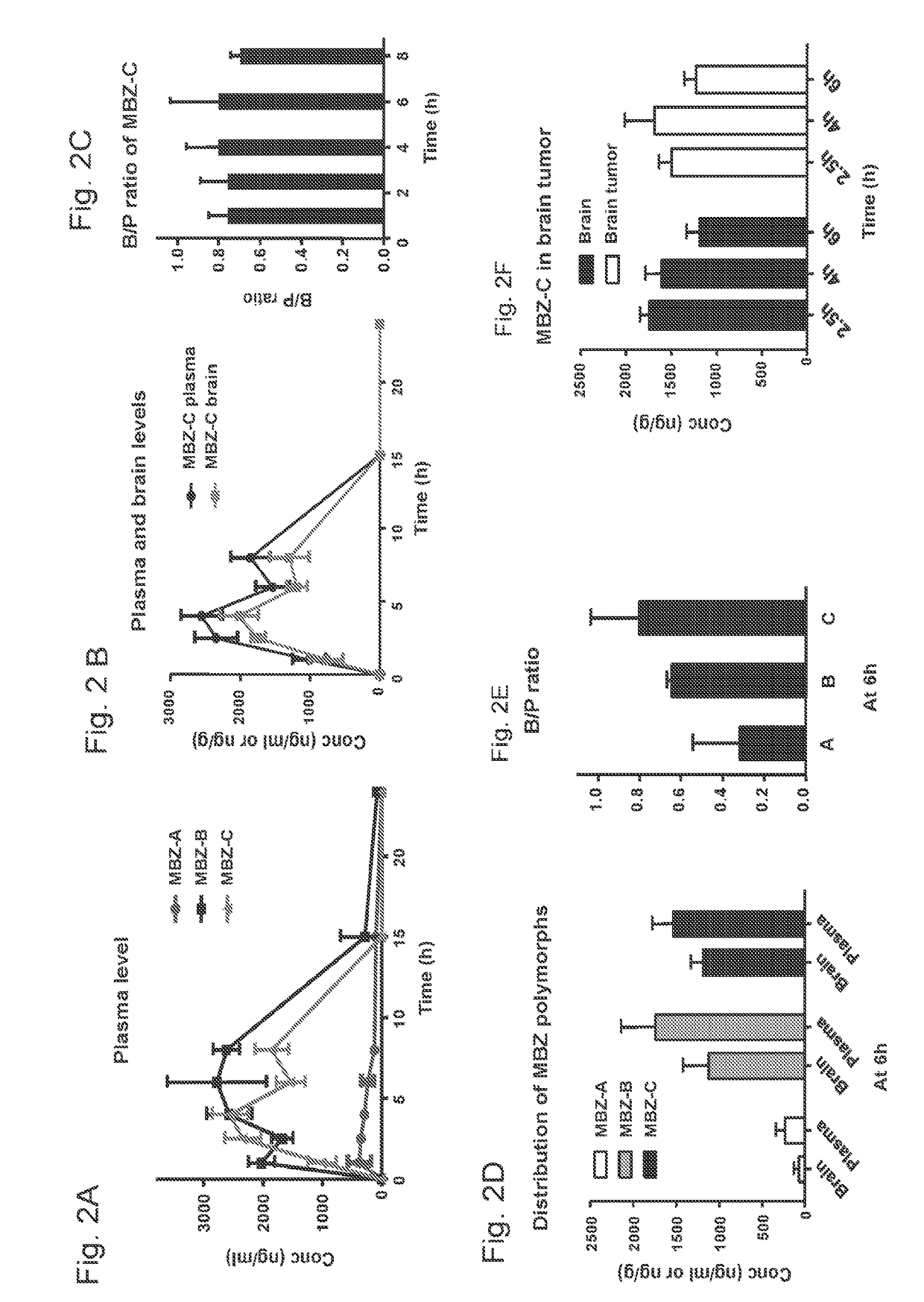
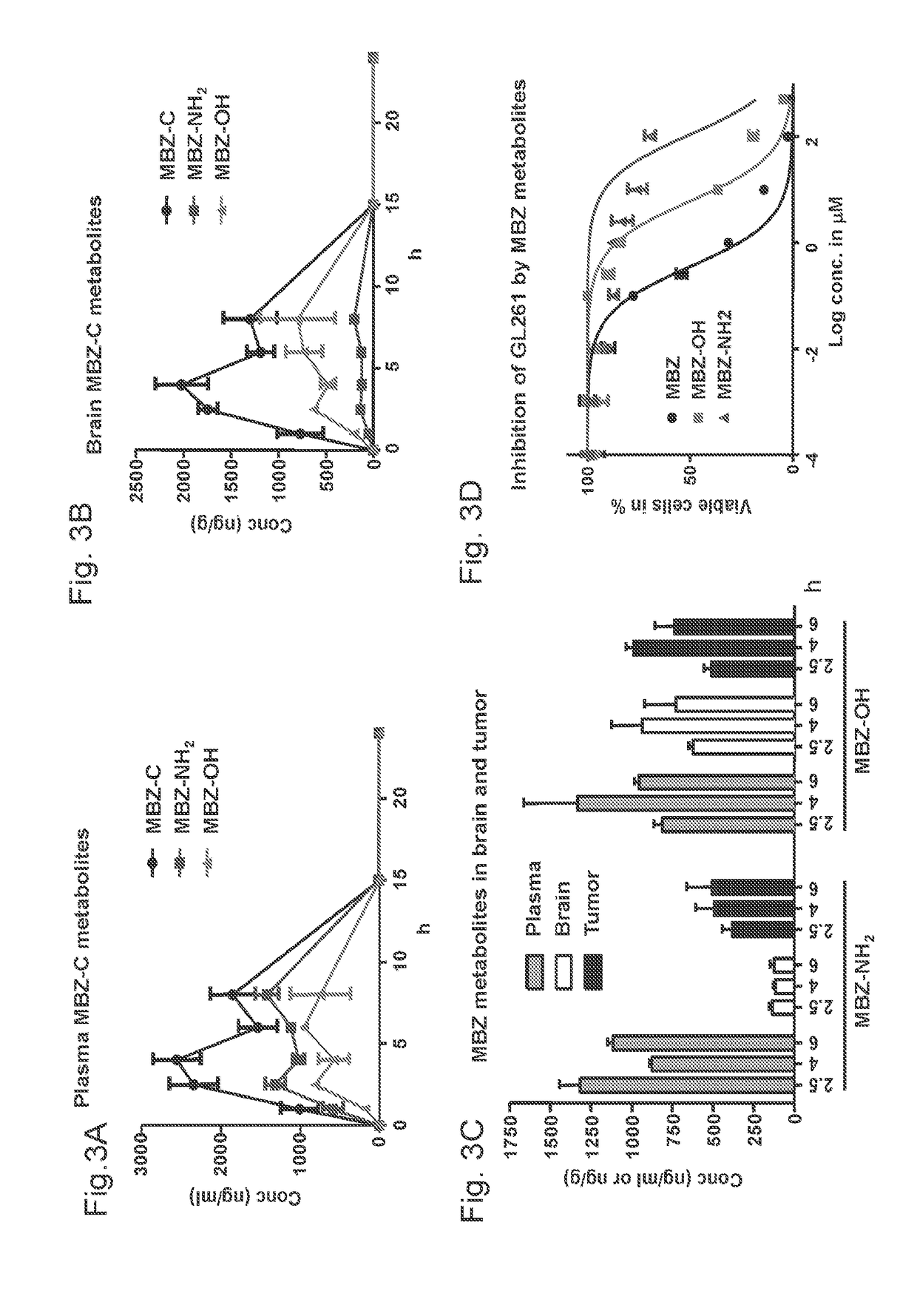
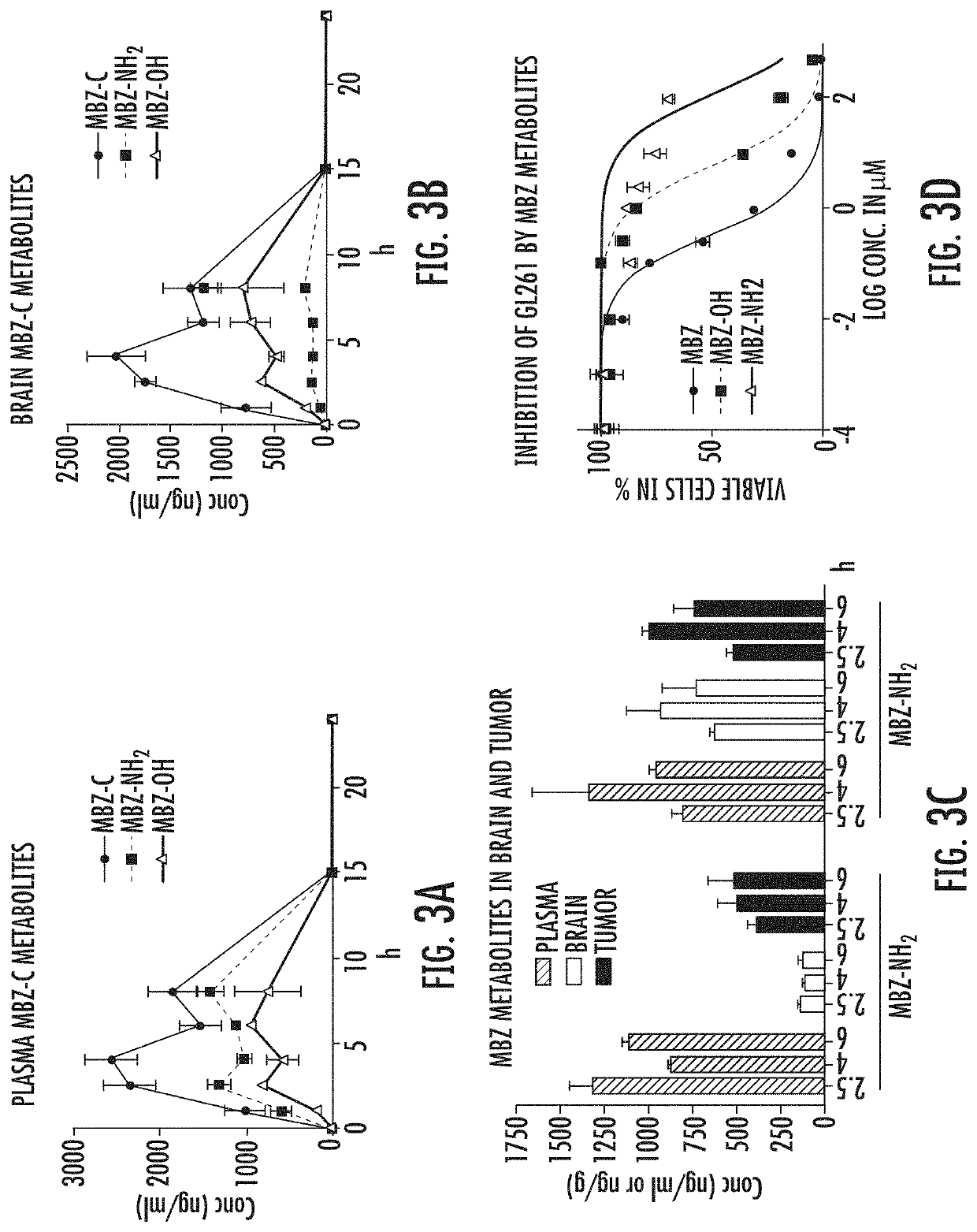
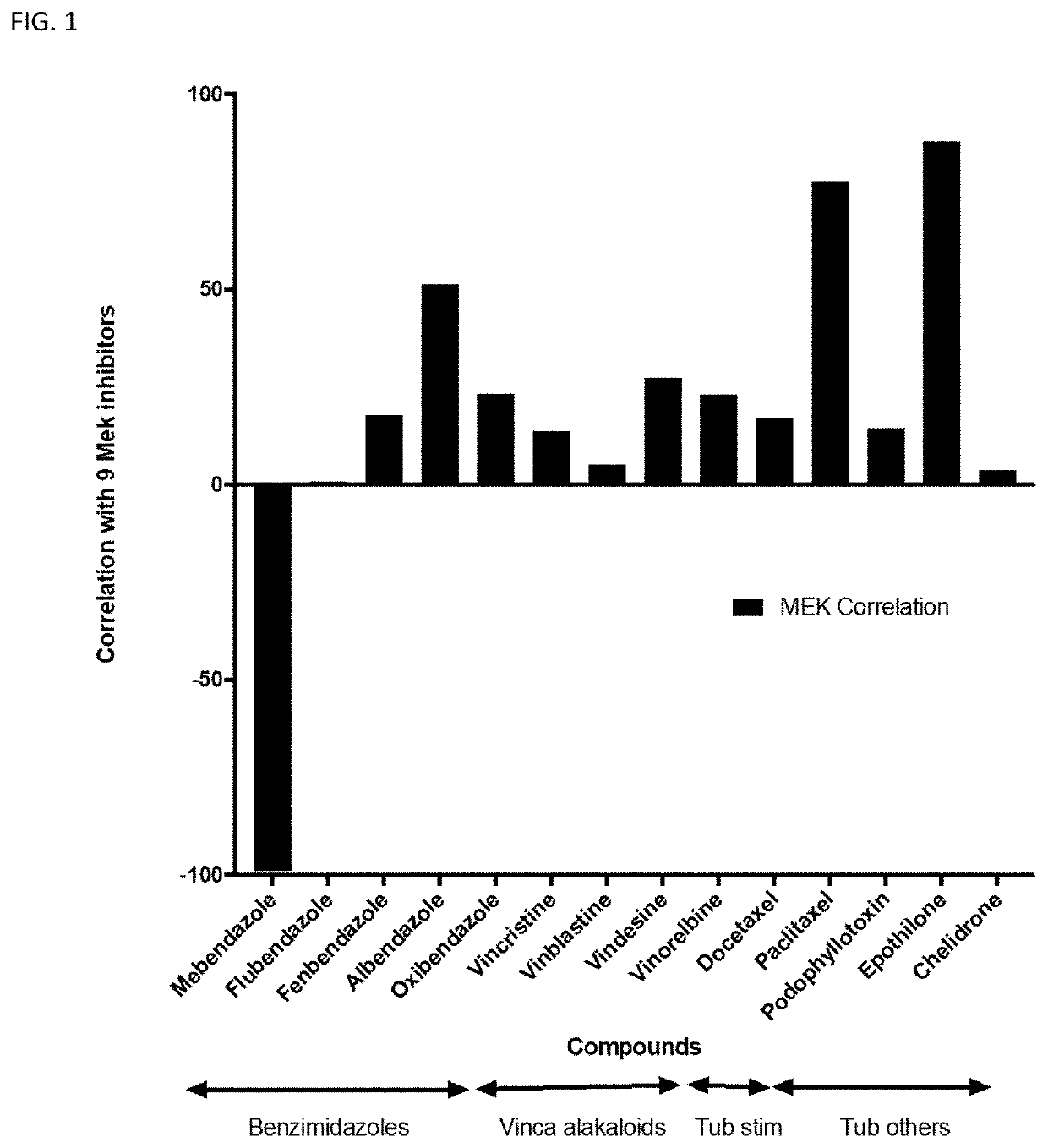
Mebendazole is an antiparasitic drug with over 40 years of safe use. Recently mebendazole was repurposed for glioblastoma therapy. Three polymorphs of mebendazole exist, but the relative polymorph content for existing drugs varies, and the therapeutic anti-cancer relevance of the different polymorphs was unknown. As an oral drug mebendazole polymorph C is a superior form, and it reaches the brain and brain tumors in effective concentrations. Efficacy is further improved by combining mebendazole with a P-glycoprotein inhibitor. Mebendazole may also be used for therapy of other cancers, as well as a chemo-preventative agent.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
Anti-parasite medicine for aquatic cultication
InactiveCN1899301AReduce dosageAddressing drug resistanceOrganic active ingredientsSolution deliveryAquatic animalAntiparasitic agent
Owner:TIANJIN SHENGJI GRP CO LTD
Compound ivermectin injection and preparation method thereof
InactiveCN104306388AGood insect resistanceBroad spectrum insect resistanceOrganic active ingredientsPharmaceutical delivery mechanismSulfite saltPyrrolidinones
The present invention discloses a composing prescription of a compound ivermectin injection and a preparation method of the compound ivermectin injection, and belongs to the veterinary medicine technology. The compound ivermectin injection comprises main drugs, a solvent and an antioxidant so as to prepare the stable high-concentration compound ivermectin injection. The injection has characteristics of advanced technology, good stability, broad spectrum, high efficiency, low irritation on the injected site, easy use, and clinical efficiency superior to the single-formula injection. According to the present invention, the raw materials comprise 0.5-5.0% of ivermectin, 2-20.0% of mebendazole, 0.5-5.0% of levamisole hydrochloride, 0.1-1% of the antioxidant, and the balance of the solvent, wherein the antioxidant is one or a mixture selected from thiourea, L-cysteamine hydrochloride, vitamin C, ethylenediamine, sodium hydrogen sulfite, anhydrous sodium sulfite, sodium formaldehydesulfoxylate dihydrate, sodium metabisulfite and the like, and the solvent is one or a mixture selected from propylene glycol, ethanol, glycerol, glycerol formal, dimethylformamide, dimethylacetamide, 2-pyrrolidone and the like.
Owner:JIANGXI NUCLEAR IND TIANDIHE PHARMA
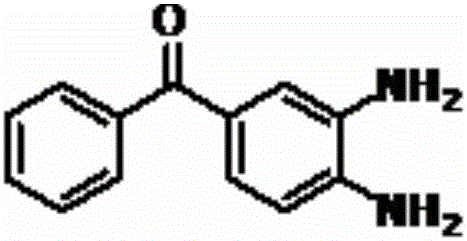
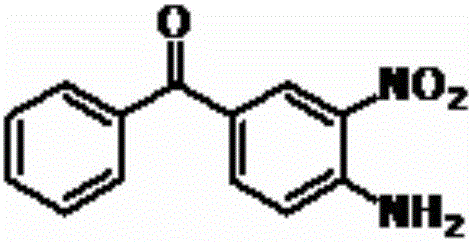
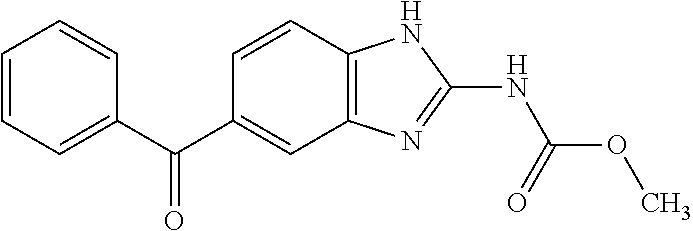
A preparing method of a mebendazole intermediate (3,4-diaminophenyl)phenyl methanone
ActiveCN106083622AMild reaction conditionsEasy to operateOrganic chemistryOrganic compound preparationReduction rateHydrogen
A preparing method of a mebendazole intermediate (3,4-diaminophenyl)phenyl methanone is disclosed. The method includes adding 4-amino-3-nitro diphenylmethanone and a catalyst into an organic solvent, adding a hydrogen donor, and performing transfer hydrogenation reduction to obtain the (3,4-diaminophenyl)phenyl methanone, wherein the catalyst is a Pd-X / C catalyst, and the X is one of Ni, Ru, La, Ce, Co, Li, K, Mg, Ti, Cu or Mo. The method has advantages of mild conditions, simple operation, a high reduction rate, a low cost, high environment protection performance, easy industrial production, and the like.
Owner:CHANGZHOU YABANG QH PHARMACHEM +1
A kind of synthetic method of 3,4-diamino-benzophenone
ActiveCN109467512BPrecise control of reaction conditionsHigh purityOrganic chemistryOrganic compound preparationHydrogenation processCombinatorial chemistry
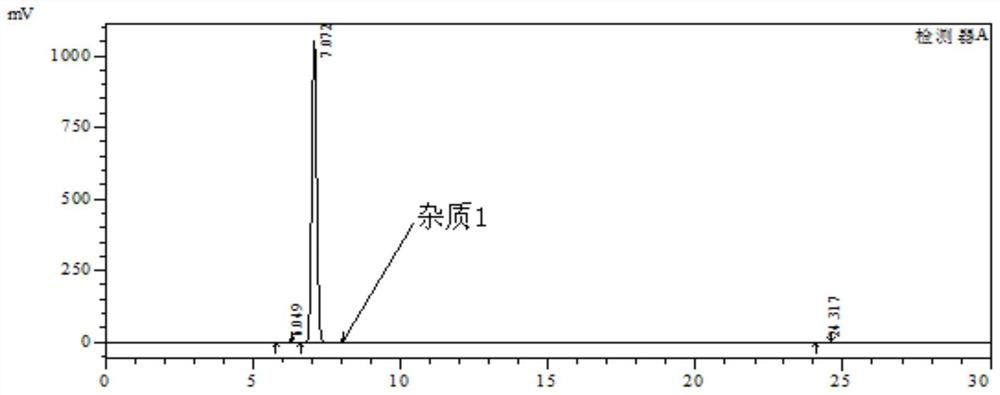
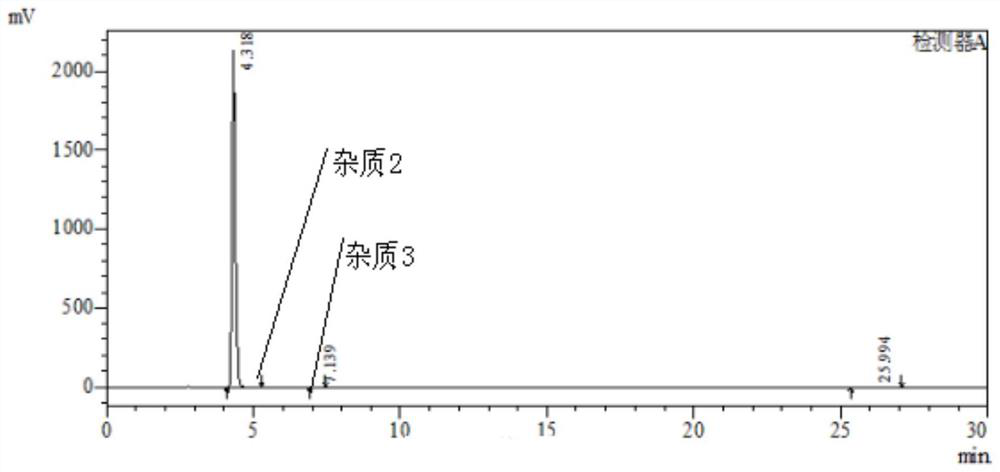
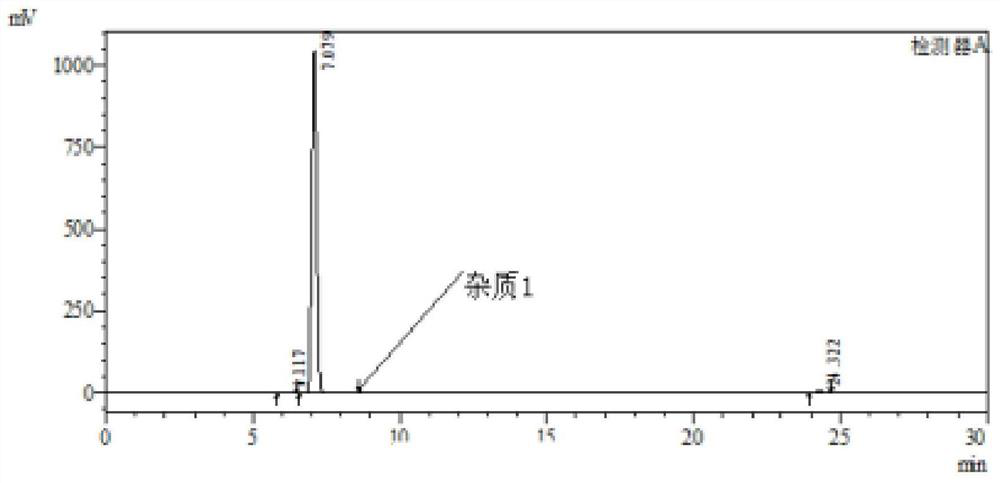
The invention discloses a method for synthesizing a mebendazole intermediate 3,4-diamino-benzophenone. By strictly controlling the parameters of the ammoniation process and the hydrogenation process, two impurities in the process: 3-amino- The contents of 4-hydroxy-benzophenone and 3-amino-4-chloro-benzophenone are both reduced to less than 0.05%, which ensures that the product quality is qualified and the total yield of the two-step reaction reaches 90%, which has a very high yield. Industrial production value.
Owner:SUZHOU KAIYUAN MINSHENG SCI & TECH CORP
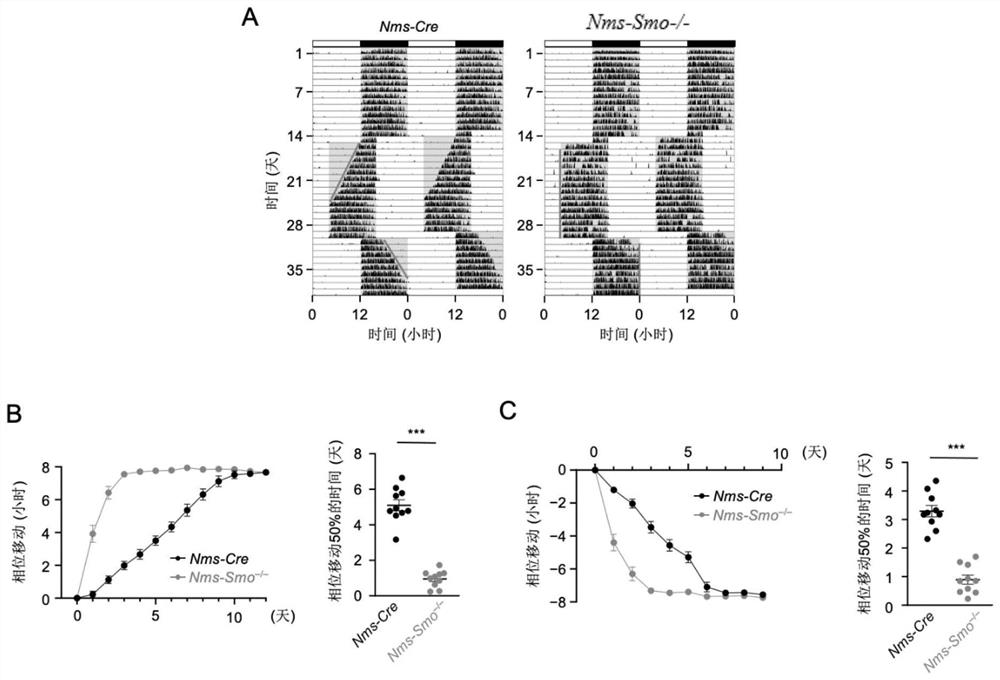
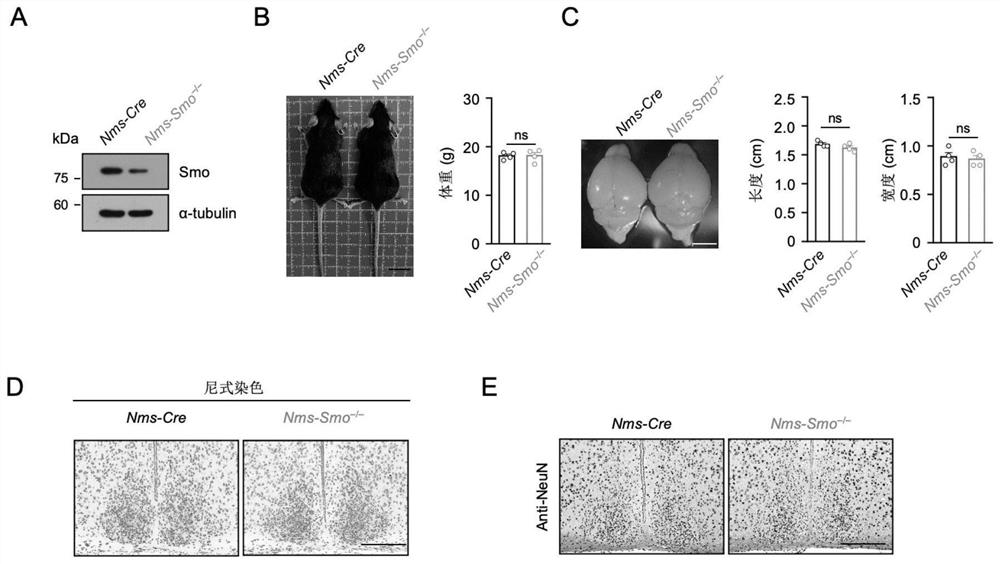
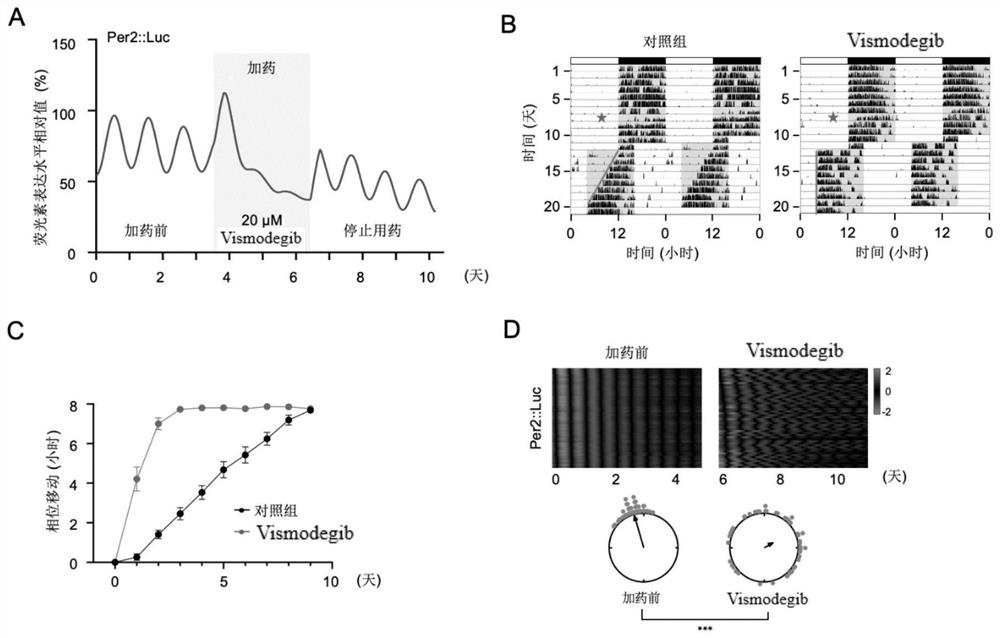
Shh pathway regulation and control biological rhythm and related application thereof
The invention relates to the technical field of biology, in particular to Shh pathway regulation and control biological rhythm and related application thereof. Specifically, the invention provides application of a Hedgehog pathway inhibitor and an SMO inhibitor in regulating the biological rhythm and treating diseases related to the biological rhythm. Preferably, the Hedgehog pathway inhibitor comprises the following components: PF-5274857, Mebendazole, HPI-4, SANT-1, Taladegib, Grasdegib, Cyclopamine, Itracazole, GANT61, JK184, Robotnikinin, Vismodegib, Purmorphamine, and Sonidegib Phoshate, and the Hedgehog pathway inhibitor comprises the following components: PF-5274857, the Mebendazole, the HPI-4, the SANT-1, the Taladegib, the Grasdegib, the Cyclopamine, the Itracazole, the GANT61, the JK184, the Robotnikinin, the Vismodegib, the
Owner:ACADEMY OF MILITARY MEDICAL SCI
Medicine composition for treating gastrointestinal psychoneurosis
InactiveCN103961391ASimple recipeShort treatment timeOrganic active ingredientsDigestive systemSide effectCodonopsis pilosula
The invention discloses a medicine composition for treating gastrointestinal psychoneurosis. The medicine composition is prepared from the following raw materials in parts by weight: 9-15 g of codonopsis pilosula, 3-12 g of fried atractylodes ovate, 3-10 g of radices saussureae and 90-110 mg of mebendazole. The dosage form of the medicine composition for treating the gastrointestinal psychoneurosis is capsule. The medicine composition for treating the gastrointestinal psychoneurosis is obtained via continuous scientific grope and researching, and specific to the pathogeny of stomach and intestine nerve function chaos and the development and recovery process of the disease. The medicine composition for treating the gastrointestinal psychoneurosis has the advantages of treating both manifestation and root cause of disease, the actovoty ratio of the medicine composition is up to over 95 percent, and the cure ratio of the medicine composition is up to over 80 percent. In addition, the medicine composition has the advantages of direct target for the pathogeny, simple formula, short cure time, low probability of recrudescence, less side effect, low cost and the like.
Owner:陈新灵
Imidaclothiz compound insecticide for shielding and protecting bees and preparation method thereof
The invention discloses an imidaclothiz compound insecticide for shielding and protecting bees and a preparation method thereof. The dosage form of the insecticide is water-dispersible granules, an emulsifiable concentrate or a suspending agent, and the insecticide is composed of the following raw materials in percentage by mass: 0.6-70% of main agent and 30-99.4% of adjuvant, wherein the main agent is composed of the following components: mebendazole and imidaclothiz in a mass ratio of (0.1-20):(0.5-50). The preparation method comprises the following steps: weighing the main agent and the adjuvant according to the ratio, and conducting mixing and granulating to obtain the water-dispersible granules of the insecticide; after heating, conducting even mixing under stirring to obtain the emulsifiable concentrate of the insecticide; weighing the main agent and the adjuvant according to the ratio, uniformly mixing the main agent with a dispersing agent, a wetting agent, an emulsifier, an antifreezing agent and a solvent, conducting sand grinding, adding a thickener, and then conducting uniform mixing again to obtain the suspending agent of the insecticide. According to the invention, the compound preparation of mebendazole and imidaclothiz is prepared, thus mebendazole marks the compound preparation, when the compound preparation is applied to, mistakenly applied to or used to conduct drift treatment on flowers, bees are repelled, and the effect of shielding and protecting bees is achieved, so that the insecticide is low-toxic or even non-toxic to bees.
Owner:SOUTH CHINA AGRI UNIV
Composition of photodynamic combination drug as well as preparation method and application thereof
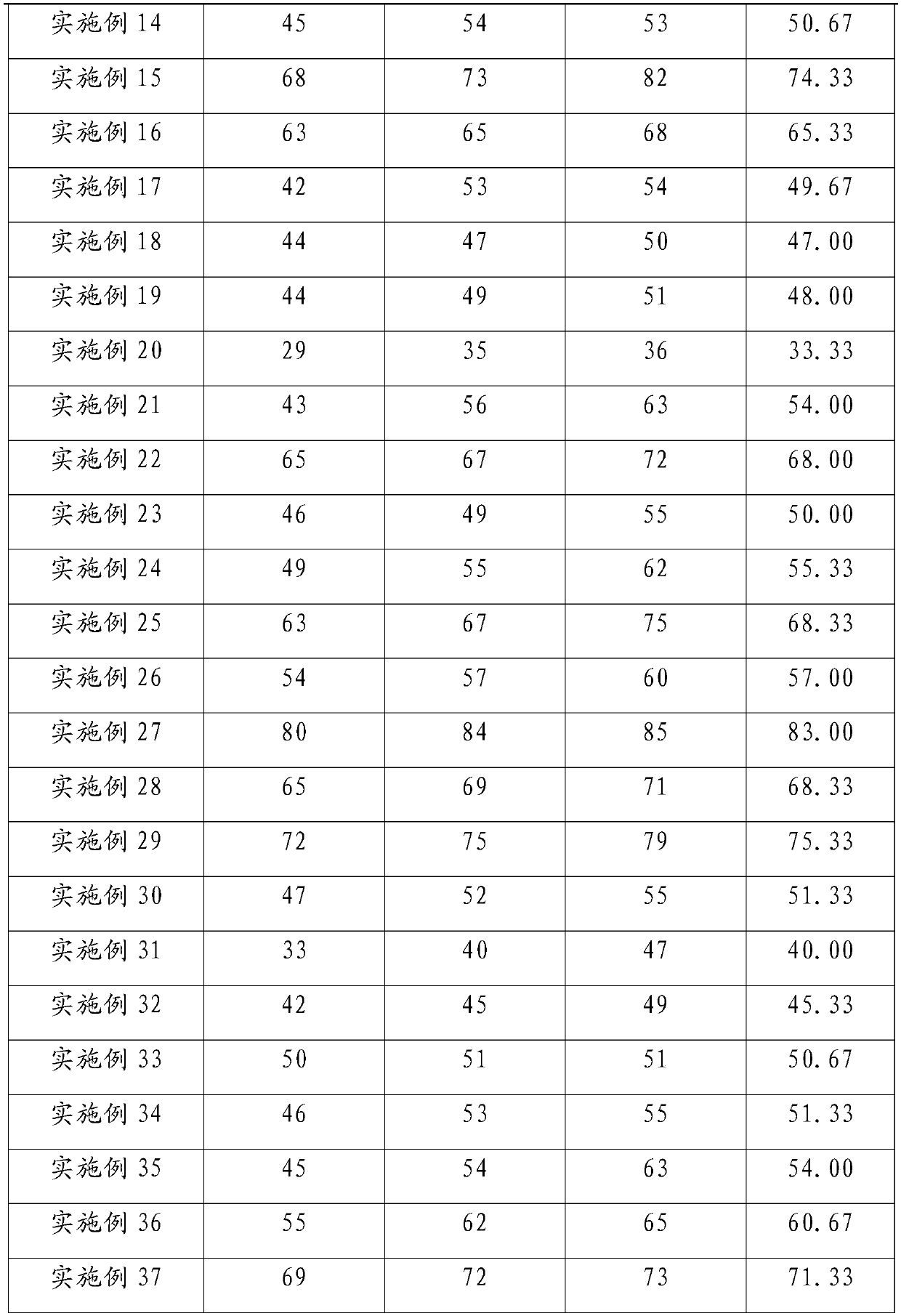
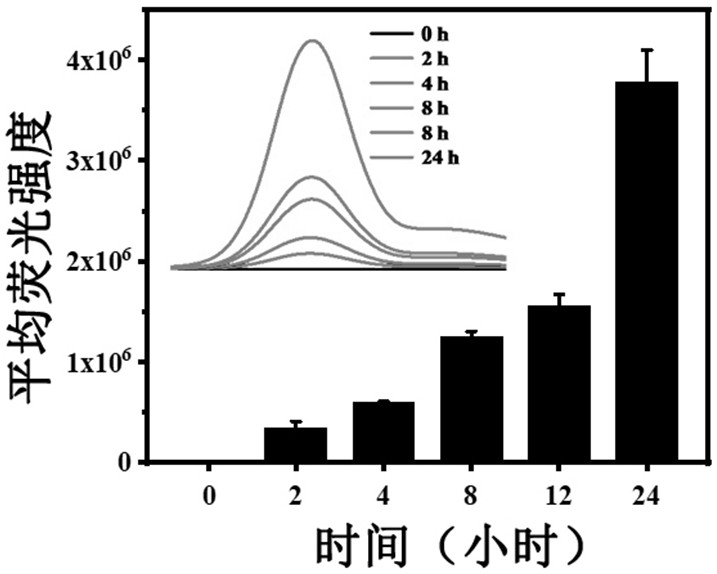
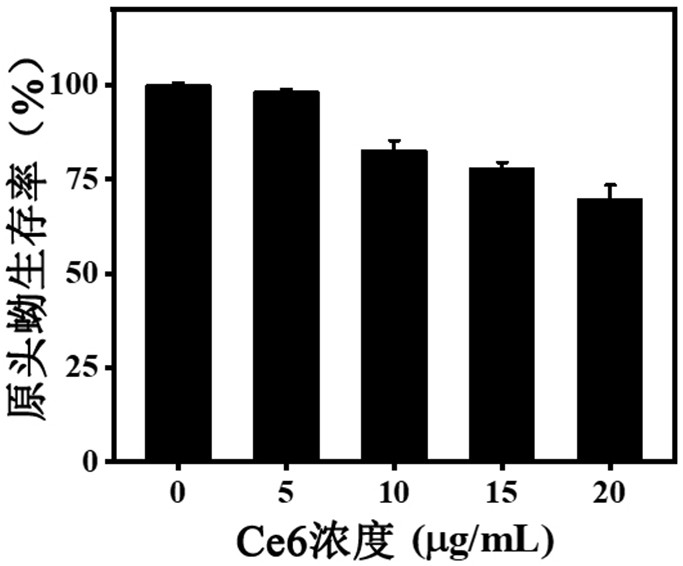
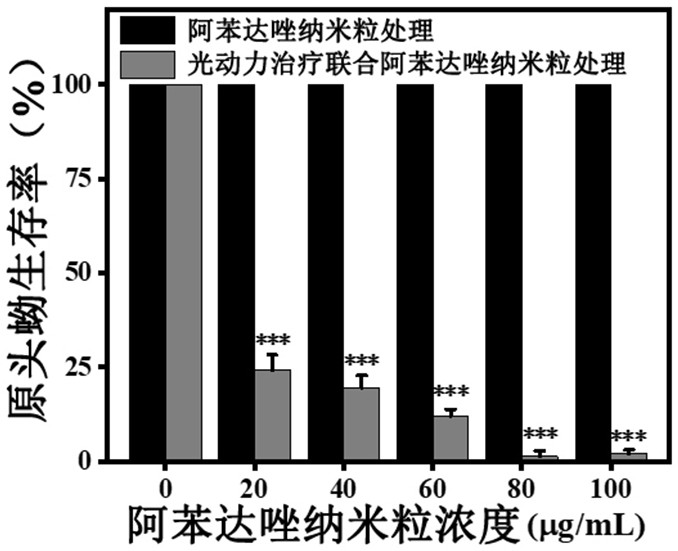
ActiveCN111744014ASmall toxicityReduce resistancePowder deliveryOrganic active ingredientsCystTetrandrine
The invention relates to the technical field of preparation of medicines for treating echinococcosis, and relates to a composition of a photodynamic combination drug and a preparation method and an application thereof. The composition comprises a photosensitizer or / and a chemical drug; wherein the photosensitizer is one or more of chlorin e6, viltipofen, indocyanine green, porphin sodium, 5-aminolevulinic acid, temopofen and talapofen, and the chemical drug is one or more of albendazole, albendazole sulfoxide, mebendazole, flubendazole, oxfendazole, artesunate, peganum harmala, tetrandrine andsophora japonica. According to the invention, through photodynamic therapy, the permeability of the hydatid and the hydatid cyst wall is increased; according to the invention, the target accumulationof chemotherapeutic drugs in the echinococcosis and the cyst wall is realized, the synergistic treatment effect is achieved, the resistance of the echinococcosis and the cyst to external drugs is reduced, the curative effect of the anti-echinococcosis drugs is improved, the administration dosage and the toxic and side effects of the drugs can be greatly reduced, and the application prospect in the preparation of echinococcosis treatment drugs is excellent.
Owner:XINJIANG MEDICAL UNIV +1
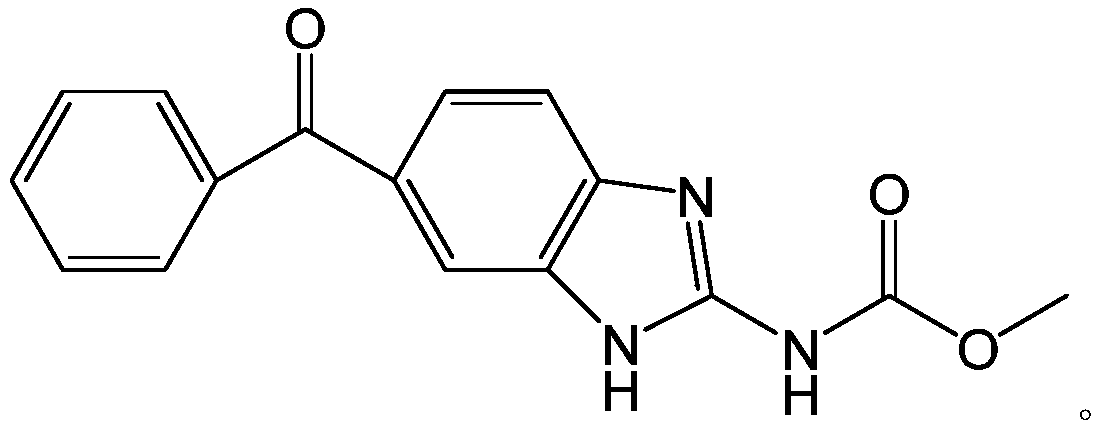
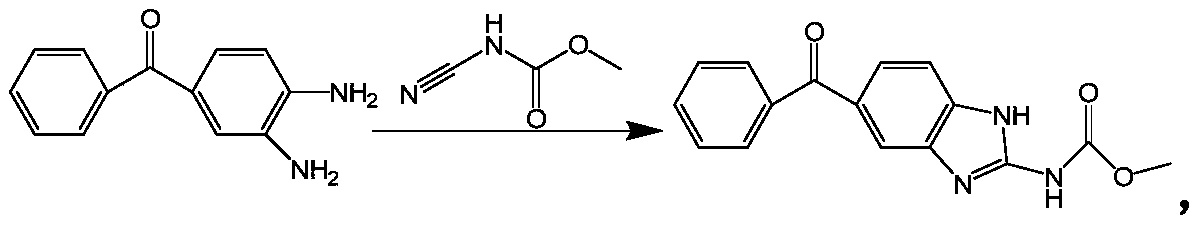
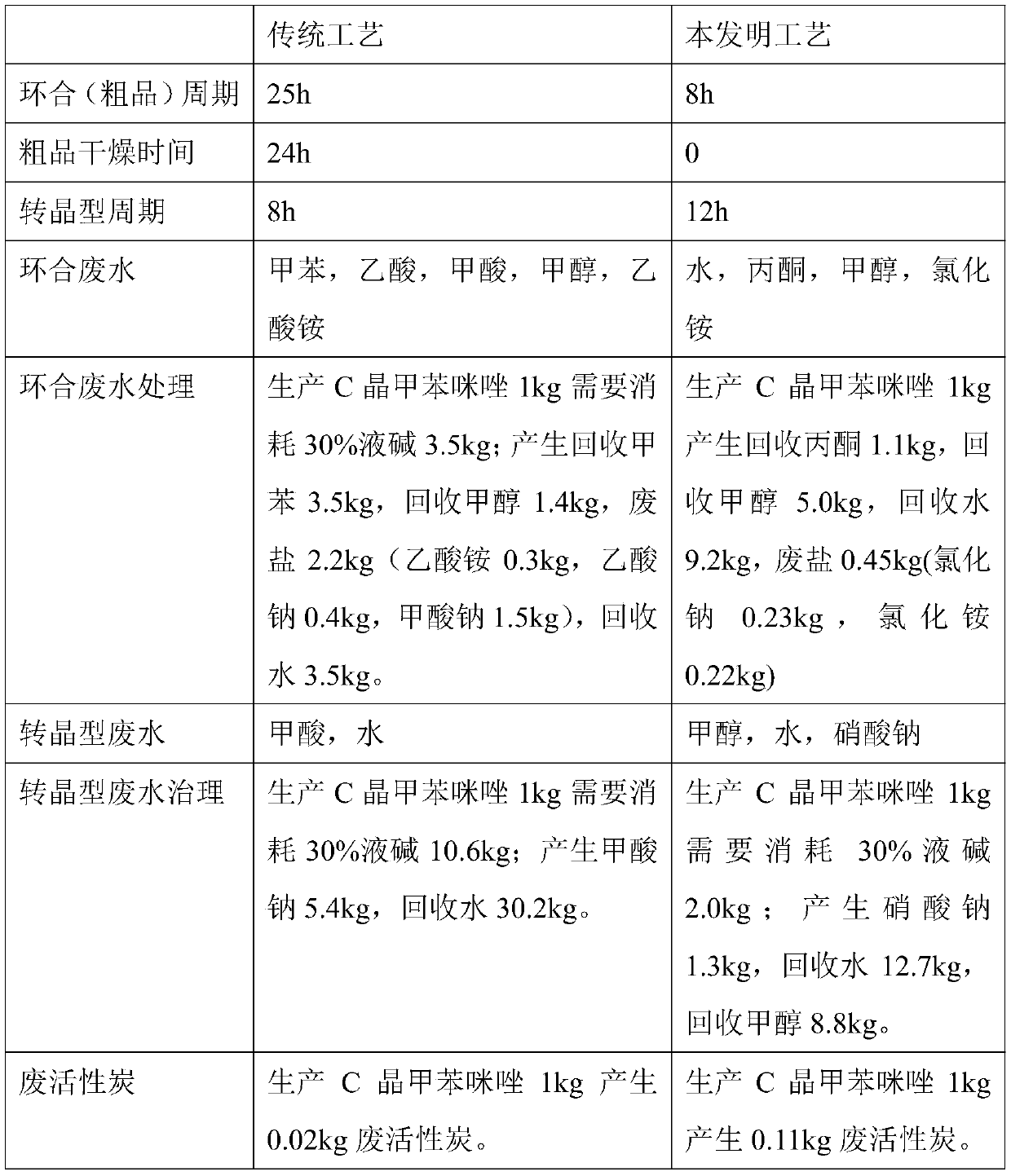
Method for synthesizing mebendazole by means of methyl cyanocarbamate
The invention discloses a method for synthesizing mebendazole by means of methyl cyanocarbamate. The method comprises the steps that 3,4-diaminobenzophenone and methyl cyanocarbamate react in acetone and hydrochloric acid to prepare a crude mebendazole, the crude mebendazole forms salt in methyl alcohol-nitric acid to obtain mebendazole nitrate, and finally the mebendazole nitrate is subjected to crystal transformation in methyl alcohol-nitric acid to obtain C crystal type mebendazole. The cheap and easily-obtained 3,4-diaminobenzophenone and methyl cyanocarbamate are adopted as raw materials, a large quantity of waste salts generated in the O-methyl isourea methyl formate synthesis process are avoided, the cyclization time is short and only needs 4-6 h, the production period is remarkably shortened, high-salt wastewater by-products can be treated through simple distillation, and the good industrial prospect is achieved.
Owner:CHANGZHOU YABANG QH PHARMACHEM +2
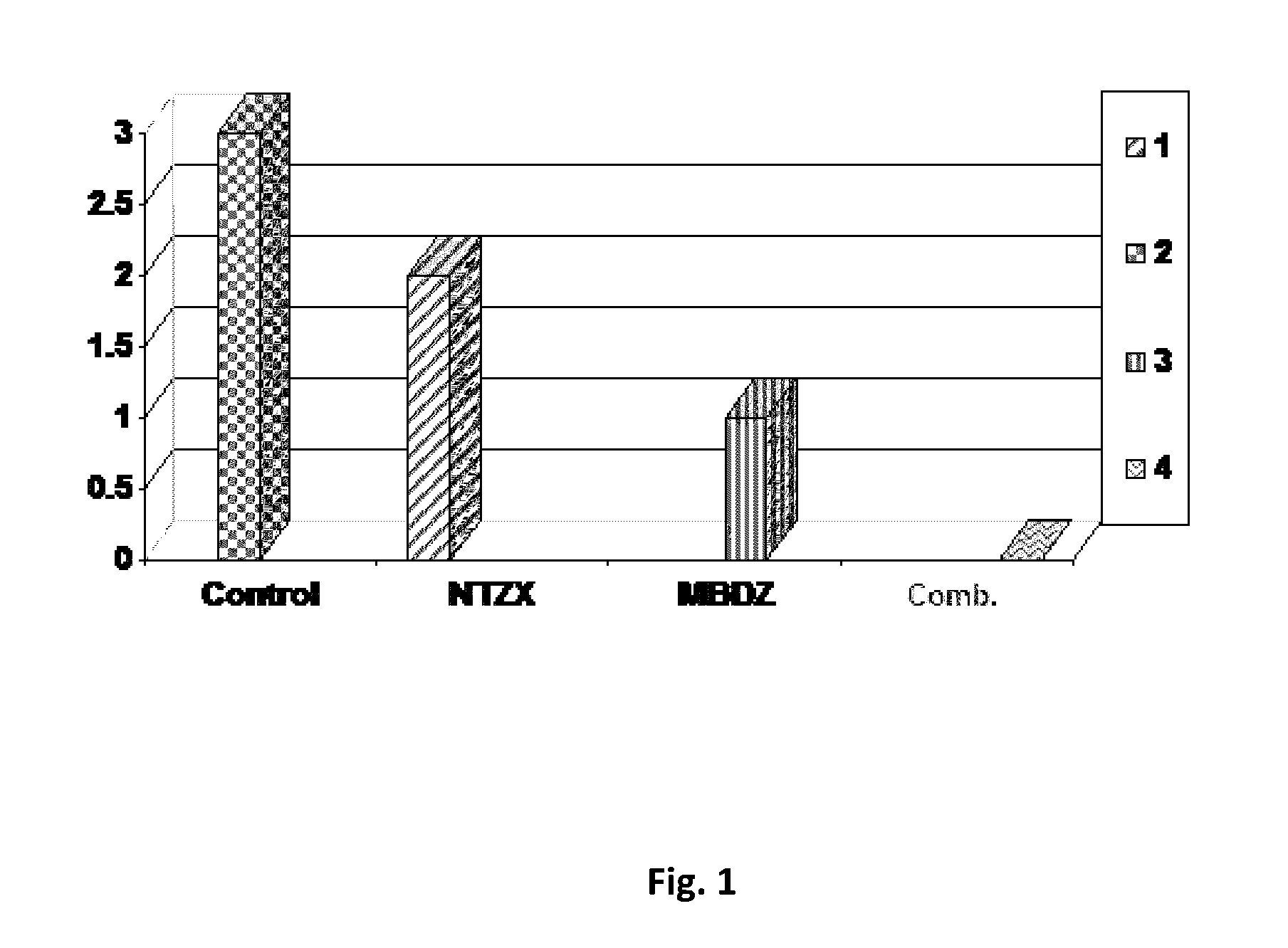
Nitazoxanide and mebendazole synergic composition, processes for the preparation thereof, and use of said composition for the treatment of human parasitosis
The invention relates to a synergic pharmaceutical composition of mebendazole (MBDZ) with nitazoxanide (NTZX) for the treatment of human parasitosis, which significantly increases the spectrum of MBDZ against protozoa and reinforces the anthelmintic action of NTZX. Furthermore, when said pharmaceutical combination of MBDZ and NTZX is used, the same effects of the individual active substances are maintained in the systemic action of the active metabolite of NTZX, tizoxanide, and in the treatment of some systemic forms of parasitosis. In addition, the synergic pharmaceutical combination of MBDZ with NTZX achieves a larger anti-parasite spectrum, while maintaining the efficiency and safety profiles of both active substances independently. All of these effects enable the differentiation of the pharmaceutical combination from the rest of the conventional anti-parasite treatments that have more limited spectra. The invention therefore relates principally to the synergic effects of the pharmaceutical combination of MBDZ and NTZX.
Owner:SIEGFRIED RHEIN DE C V
Composition for preventing or treating degenerative brain diseases including compound downregulating expression of BACE1 proteins
ActiveUS9549942B2Suppress generationBiocideHydroxy compound active ingredientsRaloxifene HydrochlorideDisease injury
Owner:RES & BUSINESS FOUND SUNGKYUNKWAN UNIV
Pharmaceutical combinations comprising mebendazole and strong or moderate cyp1a2 inhibitor
The invention relates to a pharmaceutical composition comprising mebendazole and a strong or moderate cytochrome P4501A2(CYP1A2) inhibitor, preferably fluvoxamine, thiabendazole or furafylline.
Owner:제파팜리미티드
Aquatic livestock helminth pesticides
InactiveCN101301288AHigh kill rateLower drug costsAntiparasitic agentsAnhydride/acid/halide active ingredientsAquatic productMebendazole
The invention relates to the aquatic livestock pesticide field, in particular to an aquatic livestock worm pesticide which is used to kill dactylogyruses and gyrodactyluses parasitized on aquatic livestock branchiae and has a liquid dosage form. The pesticide is characterized by comprising the compositions and the content of the pesticide in portion by weight: 5 to 15 portions of mebendazole and 85 to 95 portions of methanoic acid, the mebendazole is slowly added into the methanoic acid according to the proportion, the stirring is performed when the mebendazole is added until the mebendazole is completely dissolved, and then the aquatic livestock worm pesticide is prepared. The pesticide has simple preparation technology, low cost, good drug effect, and long dosing interval.
Owner:TIANJIN SHENGJI GRP CO LTD
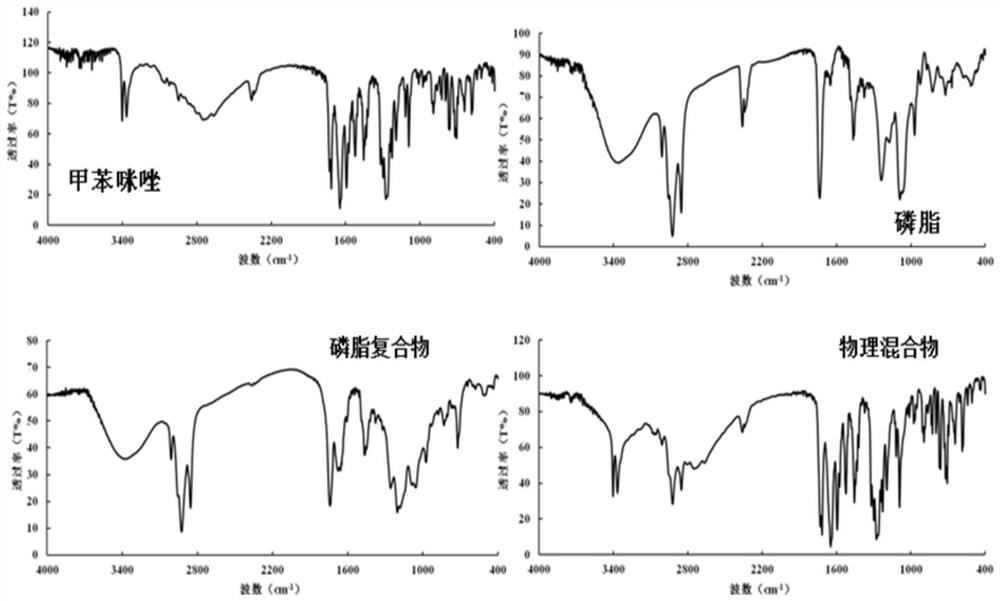
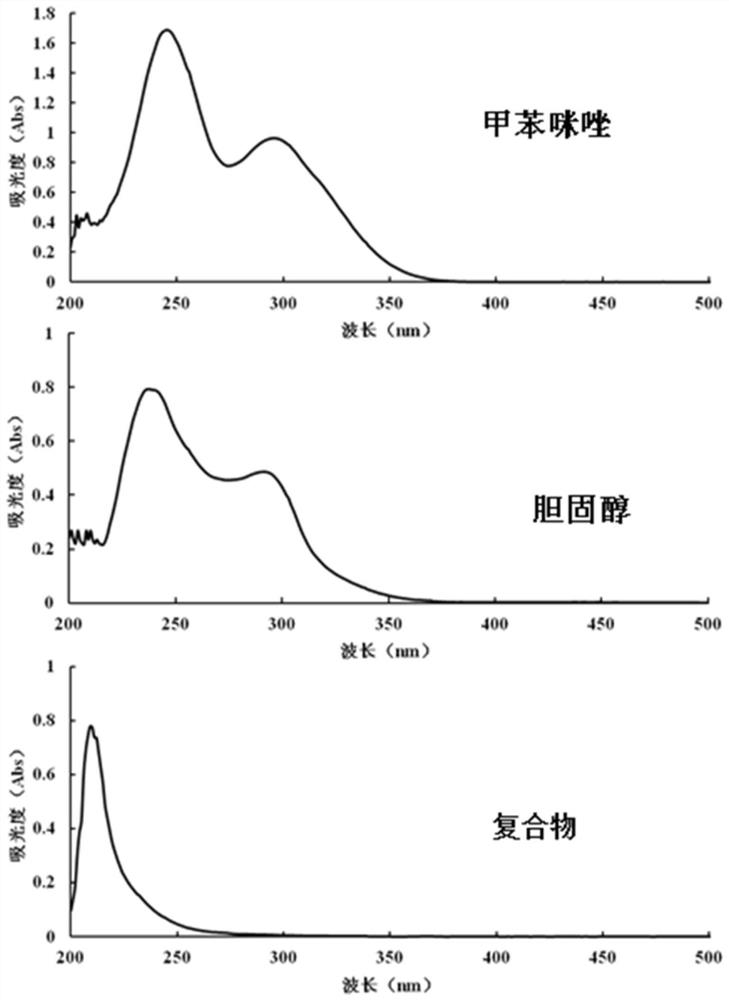
Mebendazole lipid compound, preparation method and application
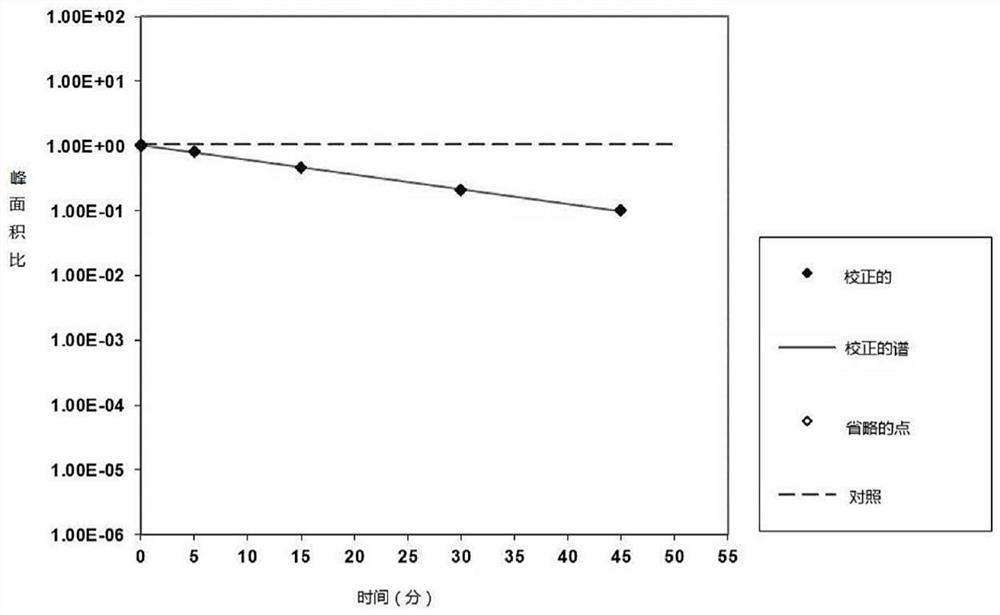
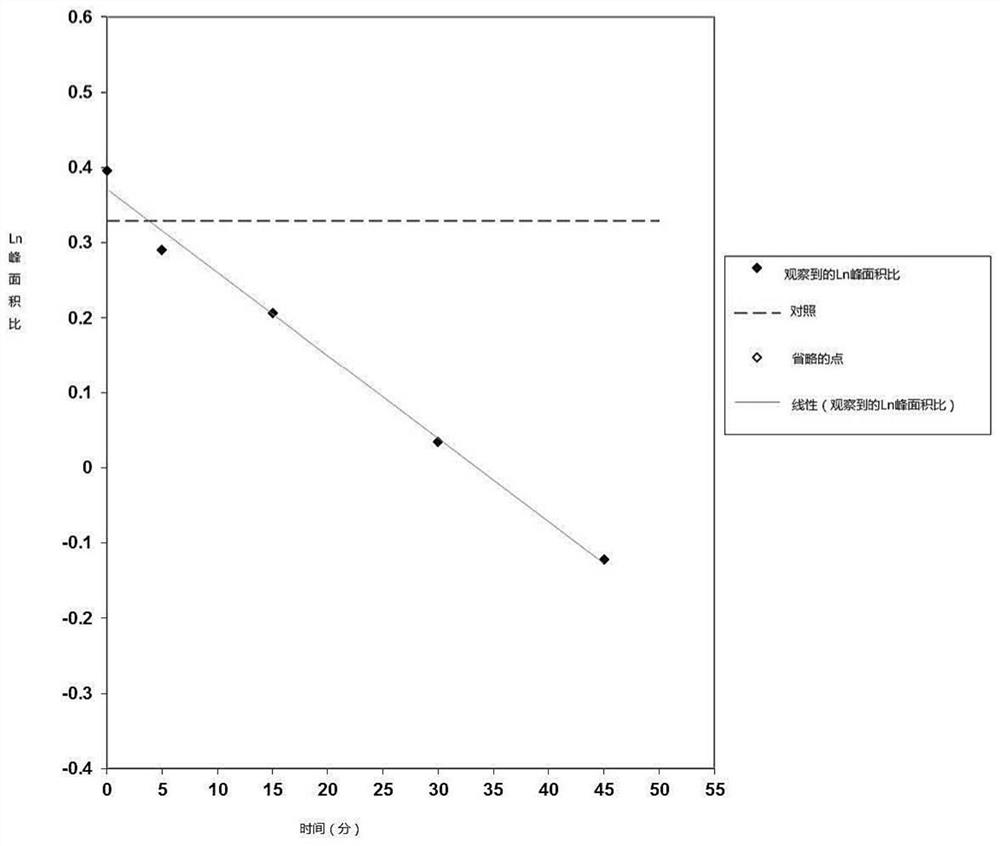
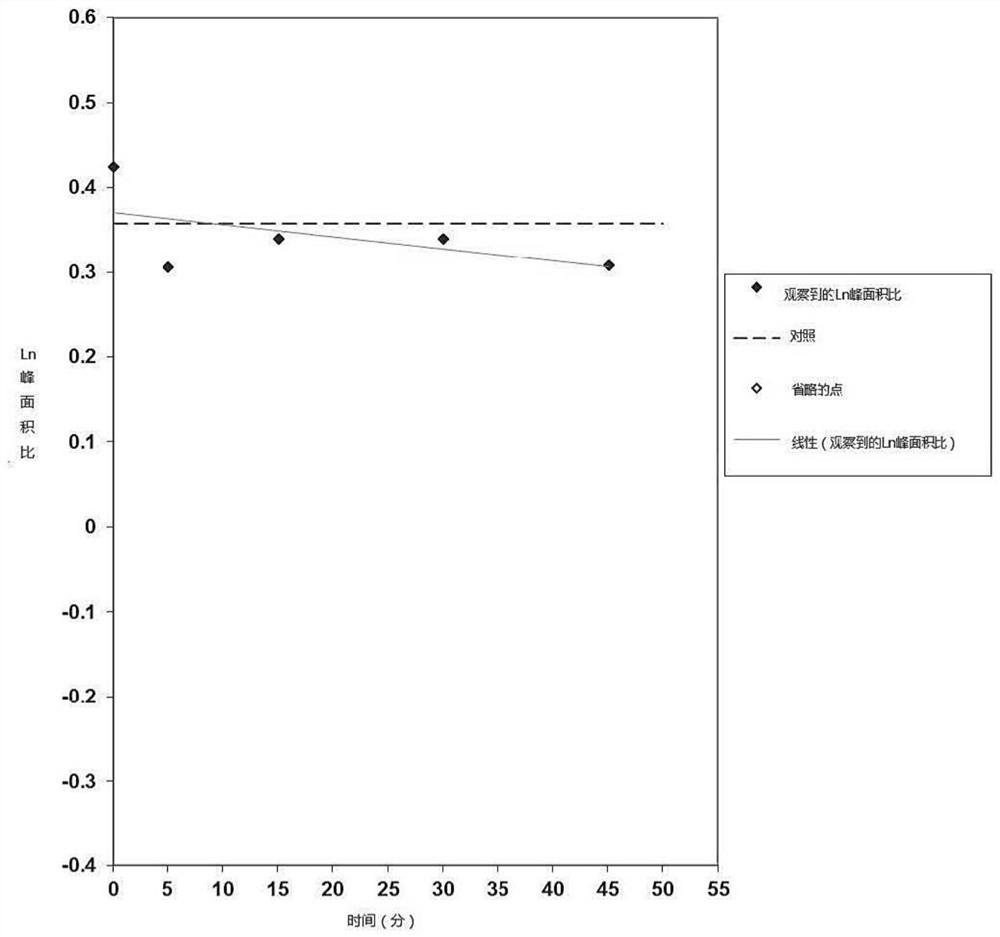
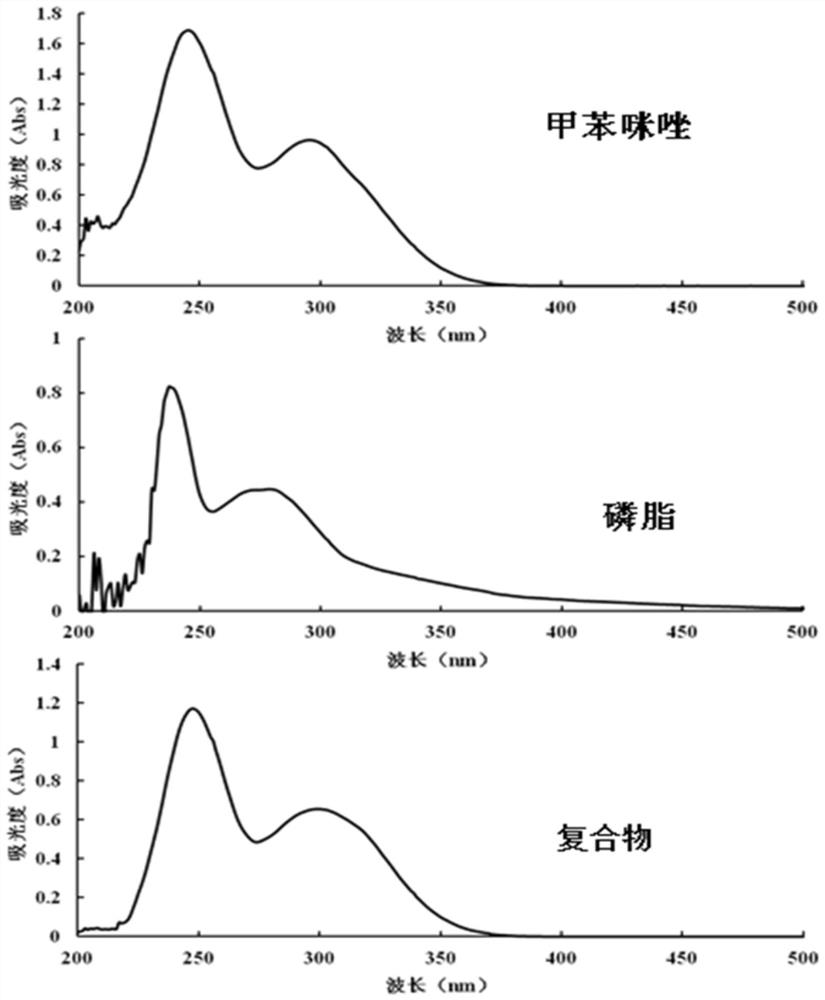
PendingCN113616798AIncrease fat solubilityGood intermediate carrierOrganic active ingredientsPharmaceutical non-active ingredientsCombinatorial chemistryMebendazole
The invention discloses a mebendazole lipid compound. The compound is formed by compounding mebendazole and a lipid material through intermolecular non-covalent bonds, wherein the molar ratio of the mebendazole to the lipid material is 1: (0.1-15). The compound provided by the invention effectively improves the lipid solubility of the mebendazole, and overcomes the limitation of solubility on mebendazole liquid preparation research. The compound provided by the invention provides a good intermediate carrier for preparation research of high-efficiency delivery of the mebendazole.
Owner:TIANJIN AGRICULTURE COLLEGE
Chinese and western medicine formula for treatment of capillariasis in yellow-margined box turtles
InactiveCN102973651AHigh cure rateHeal fastAntiparasitic agentsPlant ingredientsCapillariasisPlant disease
The invention belongs to a disease prevention and treatment formula for a rare and endangered animal. A traditional Chinese medicine is prepared by using cyrtomium, meliae cortex, sophora root, wormseed, perilla stem, and licorice, which are in a ratio of 4:2:2:1:1:1 (50g medicine per kg body weight of a yellow-margined box turtle), adding water with an amount of three times of the total medicine dose, and decocting until the water amount to be one-third of the original water amount, pouring the medicinal concoction out, decocting for a second time according to the method above, mixing the two medicinal concoctions obtained above, decocting on slow fire till the remaining concoction amount is one third of the original amount, and sealing for storage. The obtained remaining concoction can be used for seven days. When the excrement is gruel-like or watery stool, the traditional Chinese medicine is used for treatment first; and western medicine is used if symptoms improve. The dosage of western medicine can be calculated based on the body weight of the yellow-margined box turtle, wherein mebendazole or albendazole is selected for oral administration, with a dosage of 20-25mg per kg body weight of the yellow-margined box turtle; ivermectin is selected for injection, with a dosage of 0.2 mg per kg body weight of the yellow-margined box turtle; and the above-described oral administration and injection are repeated every 7-9 days. Or Shuangwei suspension with a dosage of 1ml per 10 kg body weight of the yellow-margined box turtle is used for oral administration, or Shuangwei tablets with a dosage of one tablet per 10 kg body weight of the yellow-margined box turtle is used for oral administration, or 90% crystal trichlorfon with a dosage of 150mg per kg body weight of the yellow-margined box turtle is used for oral administration.
Owner:赵林斌
Mebendazole polymorph for treatment and prevention of tumors
ActiveUS11110079B2Reduce riskOrganic active ingredientsNervous disorderGlioblastomaPharmaceutical drug
Mebendazole is an antiparasitic drug with over 40 years of safe use. Recently mebendazole was repurposed for glioblastoma therapy. Three polymorphs of mebendazole exist, but the relative polymorph content for existing drugs varies, and the therapeutic anti-cancer relevance of the different polymorphs was unknown. As an oral drug mebendazole polymorph C is a superior form, and it reaches the brain and brain tumors in effective concentrations. Efficacy is further improved by combining mebendazole with a P-glycoprotein inhibitor. Mebendazole may also be used for therapy of other cancers, as well as a chemo-preventative agent.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
Treatment For Inflammatory Disease
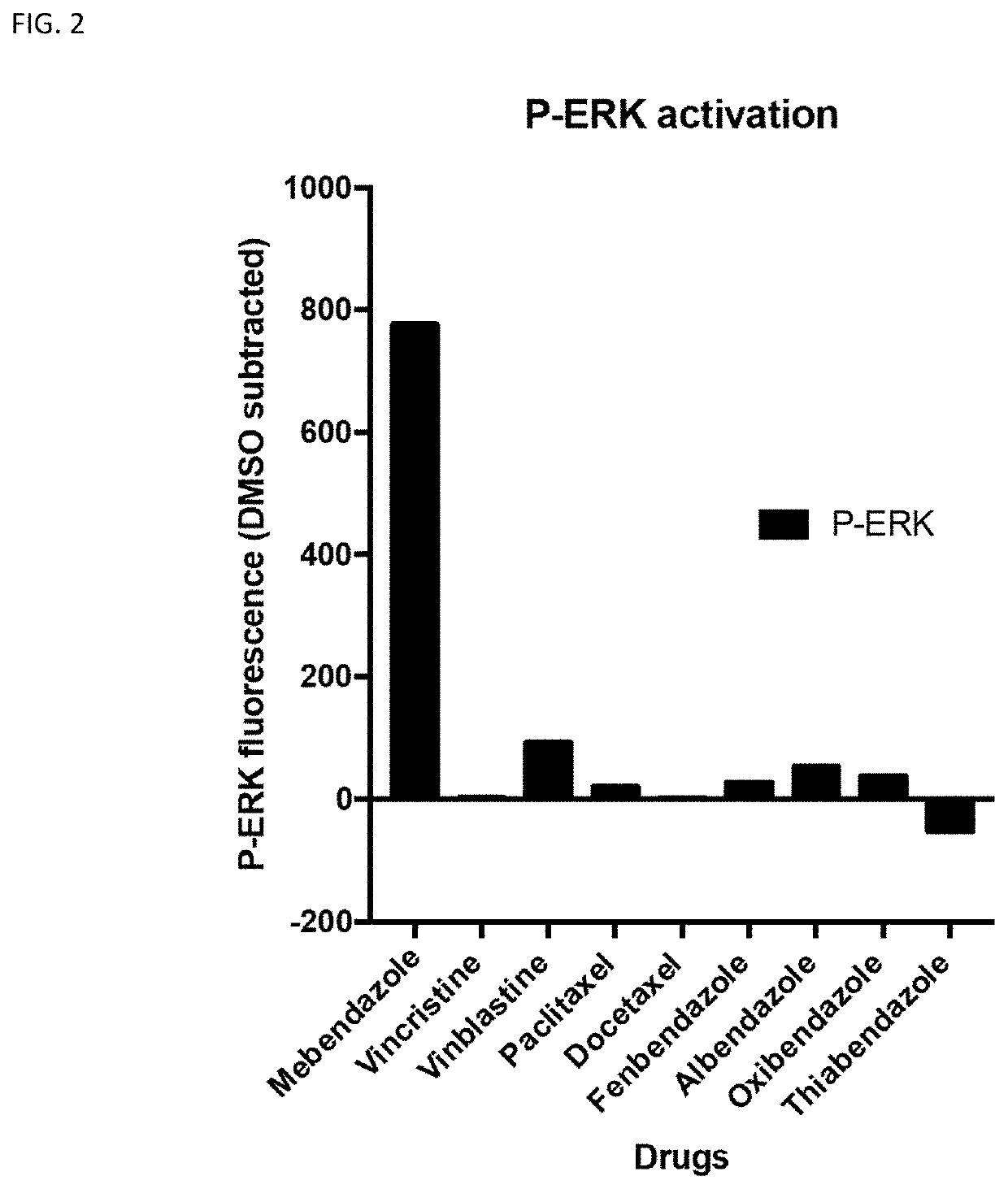
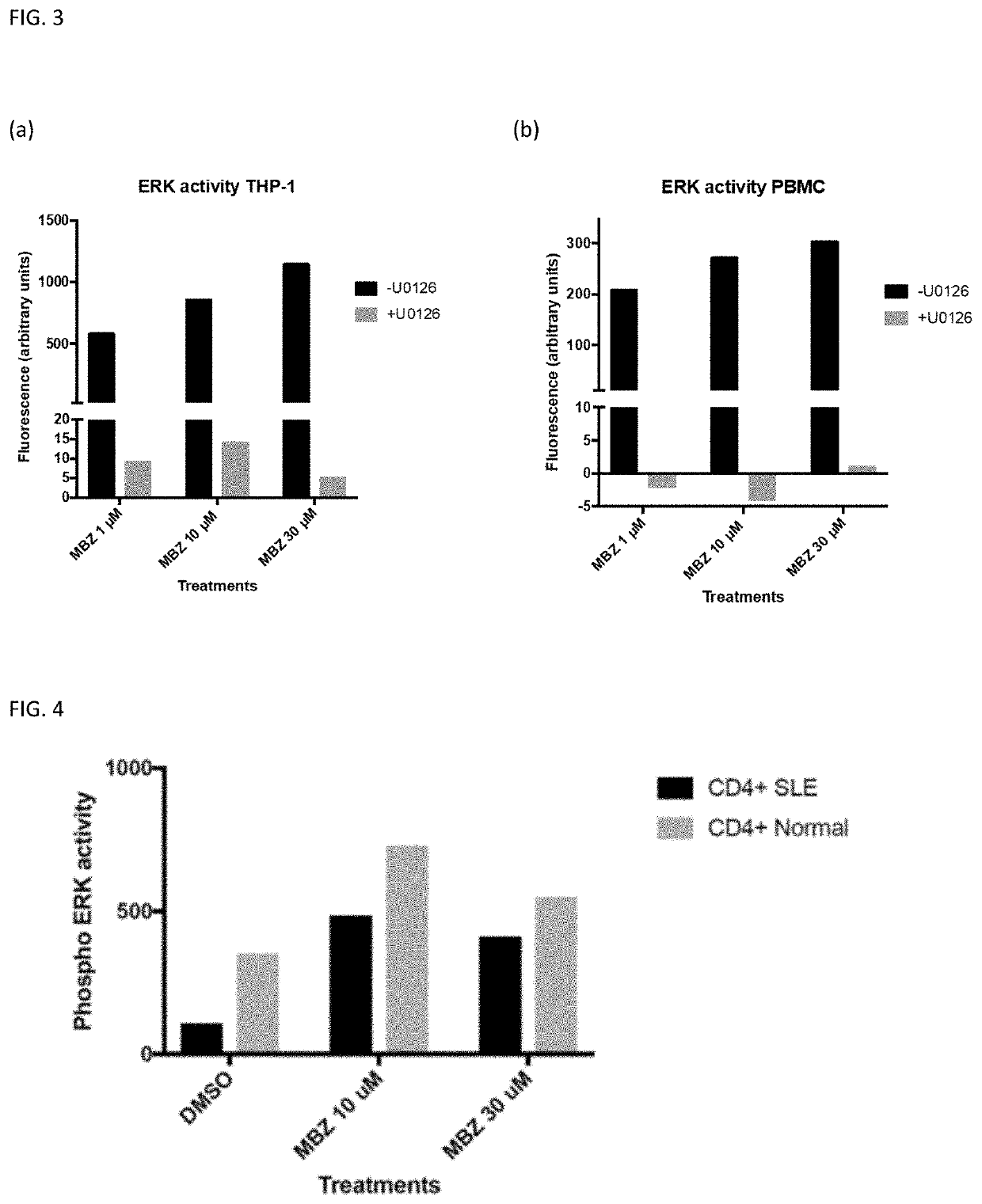
The invention provides mebendazole for use in the treatment or prophylaxis of a chronic inflammatory disease, and in particular wherein the chronic inflammatory disease is an autoimmune disease, for example sarcoidosis, systemic lupus erythematosus (SLE), Huntington's disease, end stage renal disease, systemic sclerosis (also called scleroderma), myositis, diabetes type 1, multiple sclerosis, Sjögren's syndrome, rheumatoid arthritis, psoriasis, primary biliary cirrhosis, autoimmune hepatitis, Graves' disease, Addison's disease, tuberculosis, Crohn's disease, ulcerative colitis, inflammatory bowel disease, Alzheimer's disease and coeliac disease. A method for the treatment or prophylaxis of a chronic inflammatory disease, comprising administering an effective amount of a mebendazole or a pharmaceutical composition of mebendazole is also provided. The use of mebendazole for the manufacture of a medicament for the treatment of a chronic inflammatory disease is also provided.
Owner:REPOS PHARMA AB
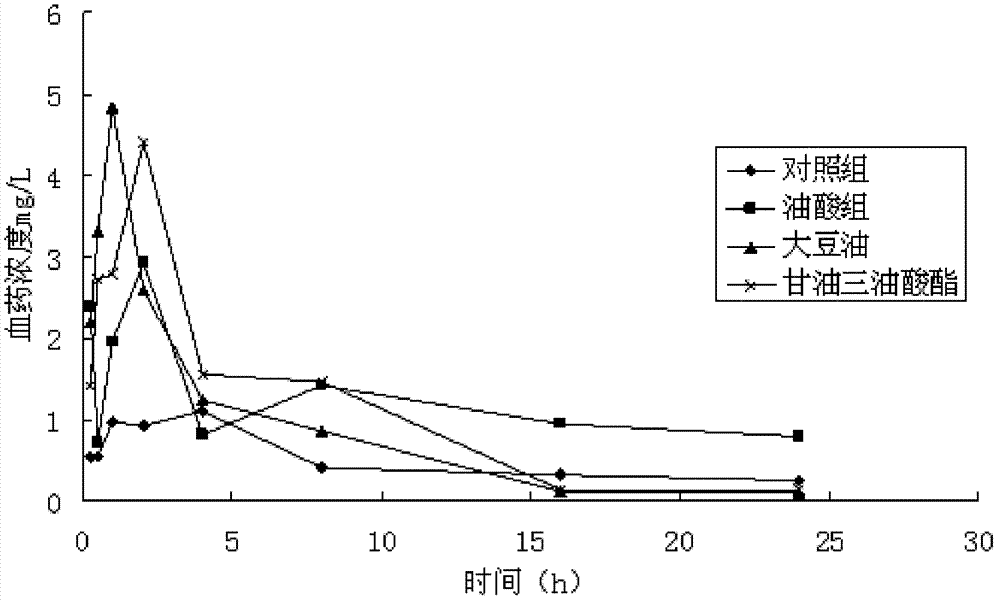
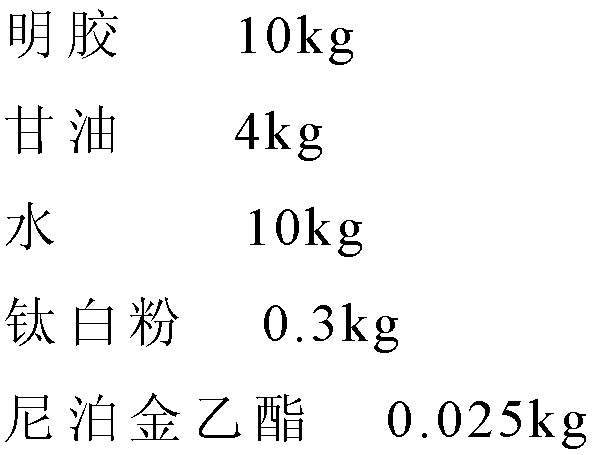
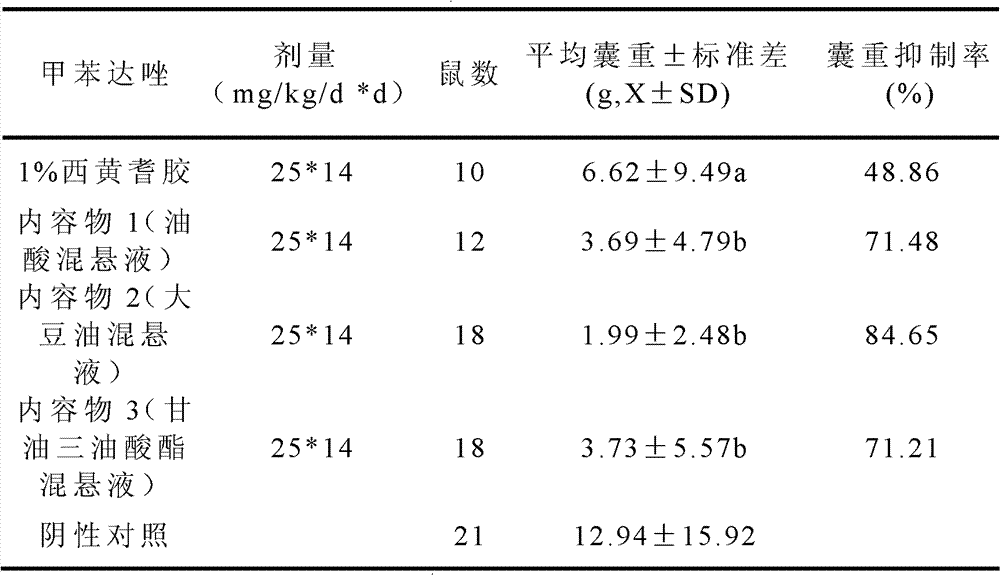
Mebendazole soft capsule
ActiveCN102813639AImprove bioavailabilityImprove complianceOrganic active ingredientsAntiparasitic agentsPlasticizerPreservative
The invention provides a novel preparation (soft capsule) for an antiparasitic drug-mebendazole. The content of the soft capsule comprises mebendazole, a suspending agent and liquid fat; and a capsule shell is mainly composed of gelatin, water, a plasticizer, a shielding agent and an antiseptic agent. The mebendazole soft capsule can increase plasma concentration and improve curative effect when being used for treating echinococcosis and other parasitic diseases. The soft capsule is simple in preparation method, has good stability and is easy to popularize.
Owner:STATION OF VIRUS PREVENTION & CONTROL CHINA DISEASES PREVENTION & CONTROL CENT +1
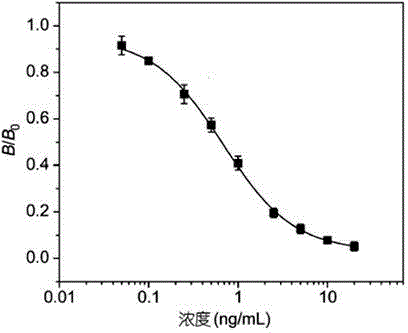
Time-resolved fluoroimmunoassy kit for detecting race-quantity mebendazole residual
InactiveCN105572359ASmall intra-batch errorHigh sensitivityFluorescence/phosphorescenceImmune profilingAntigen
The invention provides a time-resolved fluoroimmunoassy kit for detecting race-quantity mebendazole residual, and relates to a time-resolved fluorescence technology and an enzyme-linked immunoassay technology. The kit comprises a mebendazole drug antigen coated ELISA plate, an Eu<3+>-labeled mebendazole monoclonal antibody, a mebendazole standard substance, a washing buffer solution, a standard dilution solution and a fluorescence enhancement solution. The time-resolved fluoroimmunoassy kit for detecting race-quantity mebendazole residual is produced through comprehensively adopting the time-resolved fluorescence technology, a protein coupling technology and a biochemical preparation technology. A fluorescence antibody is prepared through coupling an anti-mebendazole monoclonal antibody with Eu<3+>, and the race-quantity mebendazole residual is detected through an enzyme linked immunosorbent assay completing method. The time-resolved fluoroimmunoassy kit has the advantages of simple structure, use convenience, low price, portability, high sensitivity, and suitableness for onsite massive screening. Compared with kits adopting the ELISA method, the kit provided by the invention is sensitive and efficient, and avoids interferences of some matrix substances.
Owner:JIANGSU WISE SCI & TECH DEV
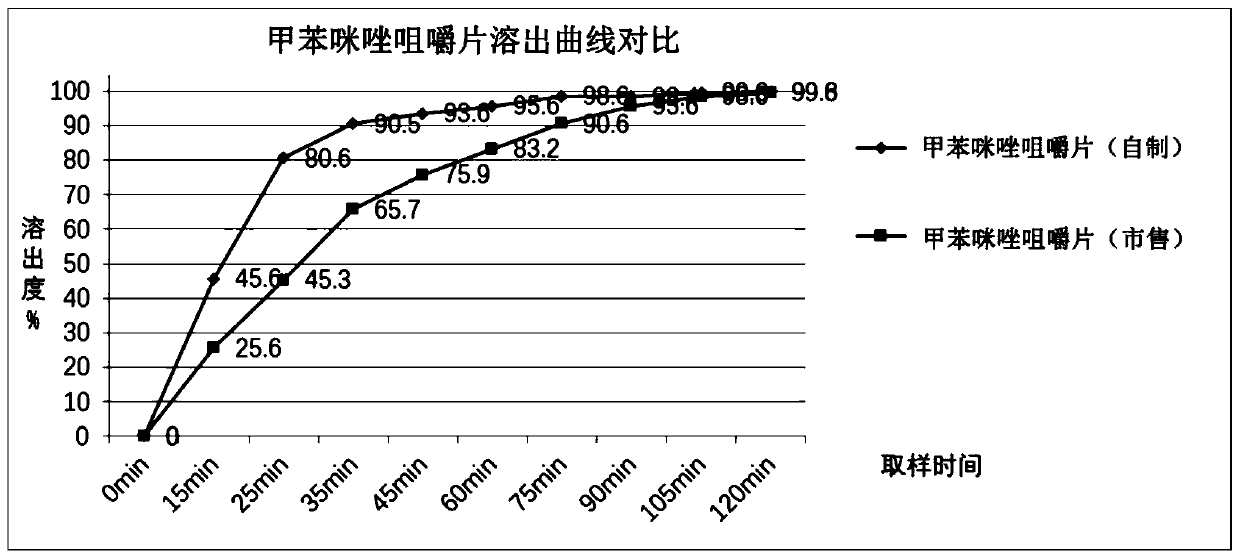
Preparation method of mebendazole chewable tablets
ActiveCN111494327AGreat tasteAvoid side effectsOrganic active ingredientsPill deliveryFluidized bedMebendazole
The invention belongs to the technical field of medicines, and particularly relates to a preparation method of mebendazole chewable tablets. The preparation method comprises the following steps: (1) adding mebendazole and auxiliary materials into a wet granulator according to a prescription amount, and premixing the components; (2) adding a prescription amount of adhesive, sunset yellow and lemonyellow to prepare a soft material; (3) granulating the prepared soft material by using a wet granulating machine; (4) pumping the wet granules into a fluidized bed for drying by using vacuum, and straightening the granules by using a crushing and straightening machine; and (5) adding essence and a lubricant into the granulated granules, mixing all the components, and tabletting the mixture to obtain the mebendazole chewable tablet. The preparation method disclosed by the invention is simple in process and good in batch-to-batch reproducibility, the production process meets the requirements ofGMP, and the preparation method is suitable for industrial large-scale production; the chewable tablet prepared by the method is stable in quality and good in taste.
Owner:REYOUNG PHARMA
Insecticide special for plutella xylostella
InactiveCN105850990ANon-toxicIncrease incomeBiocideDead animal preservationAlcoholHydrogen phosphate
The invention relates to the technical field of insecticide for plants, particularly to an insecticide special for plutella xylostella. The insecticide is prepared from the following raw materials in parts by weight: 6 to 8 parts of mebendazole, 2 to 5 parts of palmitic acid, 12 to 15 parts of calcium hydrogen phosphate, 15 to 18 parts of citric acid, 20 to 25 parts of ethyl alcohol and 500 to 600 parts of water. The insecticide special for the plutella xylostella is nontoxic; the conventional pest killing method is replaced by pest prevention; the pests can be reused after falling from leaf surfaces, so that the benefit of a farmer is increased, and the insecticide is extremely suitable for green organic planting.
Owner:梁文荣
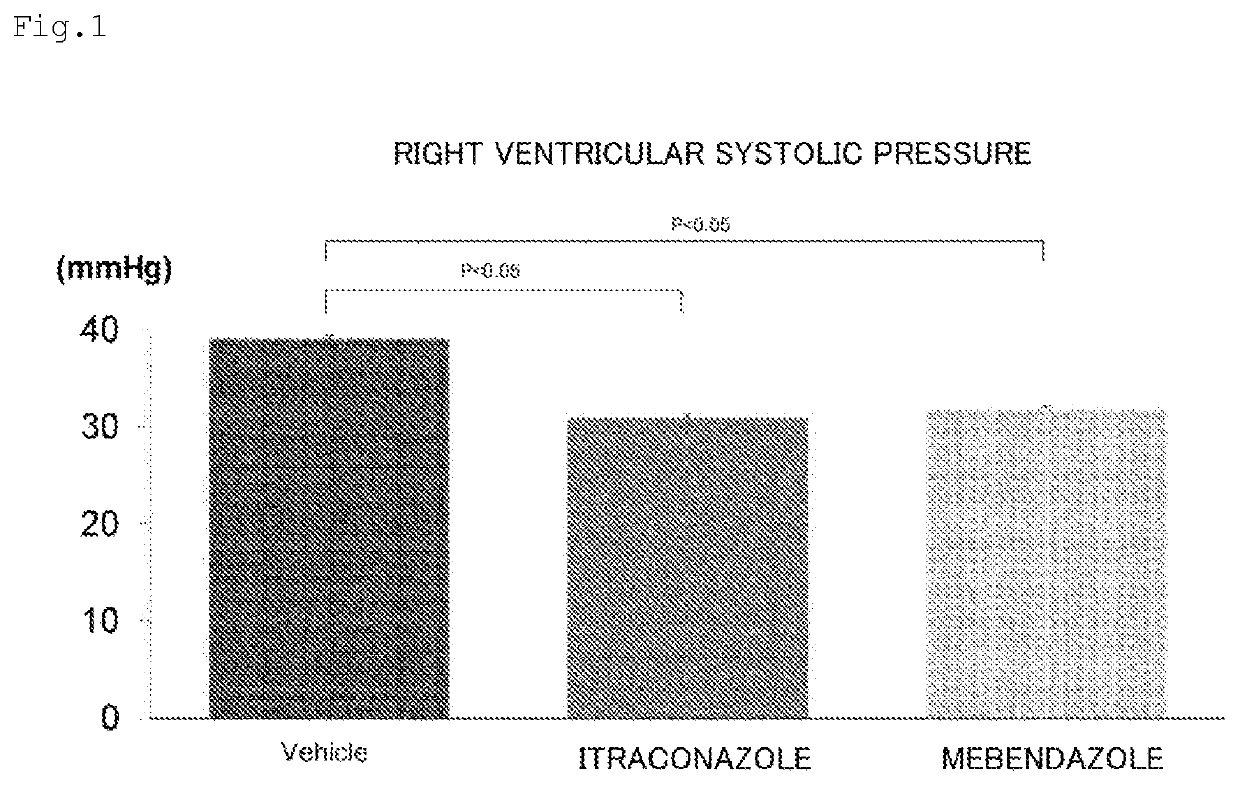
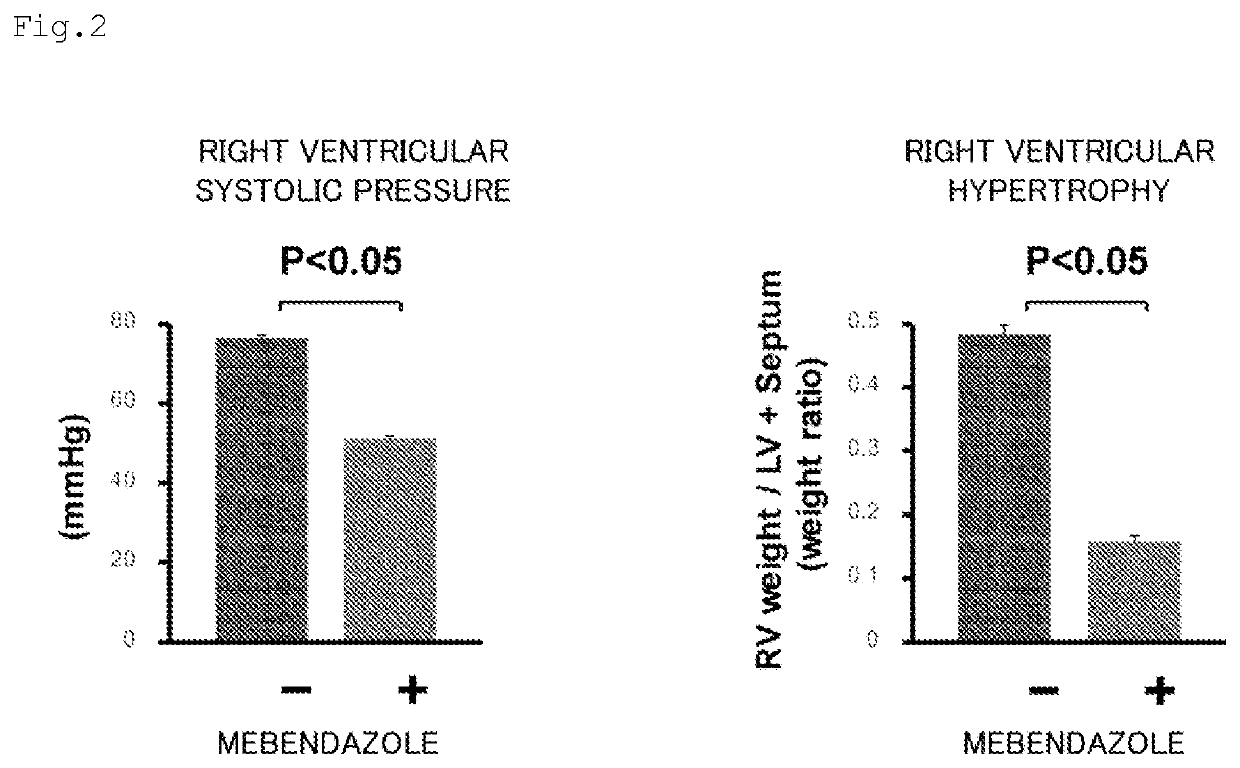
Prophylactic or therapeutic agent for pulmonary hypertension comprising mebendazole and/or itraconazole or salt thereof
ActiveUS11369601B2Organic active ingredientsPharmaceutical delivery mechanismItraconazoleTherapeutic effect
Owner:TOHOKU UNIV
A kind of synthetic method of mebendazole medicine intermediate o-chloroaniline
ActiveCN105968017BShort reaction timeHigh yieldOrganic compound preparationAmino compound preparationO-nitrochlorobenzeneSynthesis methods
The invention discloses a mebendazole drug intermediate o-chloroaniline synthesis method. The method is free of an additive. The method comprises that a sodium bromide solution and neodymium chloride undergo a reaction, a 2, 2, 2-trifluoroethylmethyl ether solution and o-nitrochlorobenzene are added into the reaction product and undergo a reflux reaction, an oxalic acid solution is added into the reaction product and undergo a reaction, the reaction product is cooled, filtered and repeatedly washed, and the washed product is subjected to reduced pressure distillation in a nitrogen protective atmosphere and re-crystallization so that o-chloroaniline crystals are produced. The whole reaction time can be controlled in 8h and a yield is high. The invention provides a novel synthesis route and lays a good basis for further improving a reaction yield.
Owner:湖北进创博生物科技有限公司
Insecticide special for cabbage caterpillars
The invention relates to the technical field of insecticides for plants, in particular to an insecticide special for cabbage caterpillars .The insecticide is prepared from, by weight, 8-10 parts of mebendazole, 2-5 parts of palmitic acid, 15-18 parts of calcium hydrophosphate, 15-20 parts of citric acid, 20-30 parts of ethyl alcohol and 500-600 parts of water .The insecticide is free of toxic properties, a traditional injurious insect killing mode is changed for insect prevention, injurious insects can be reused after falling from leaves, the income of farmer households is increased, and the insecticide is quite suitable for green organic plantation.
Owner:梁文荣
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com