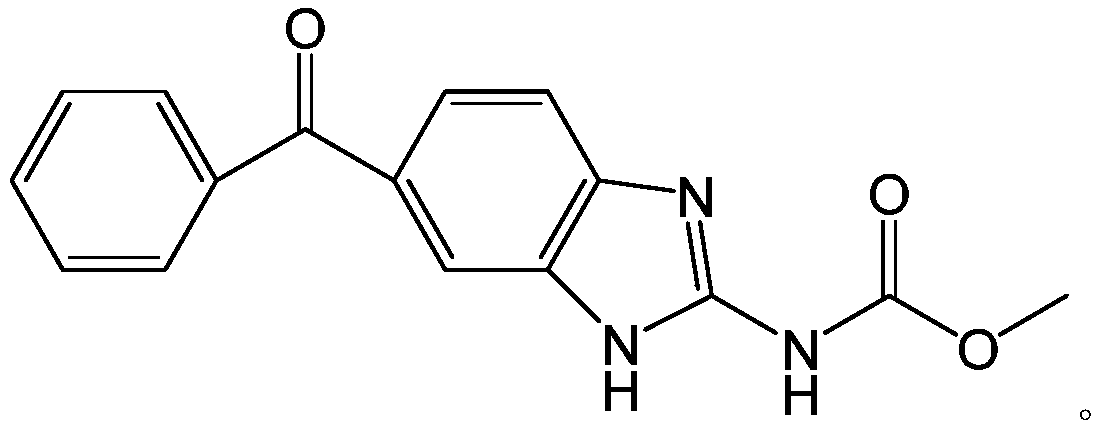
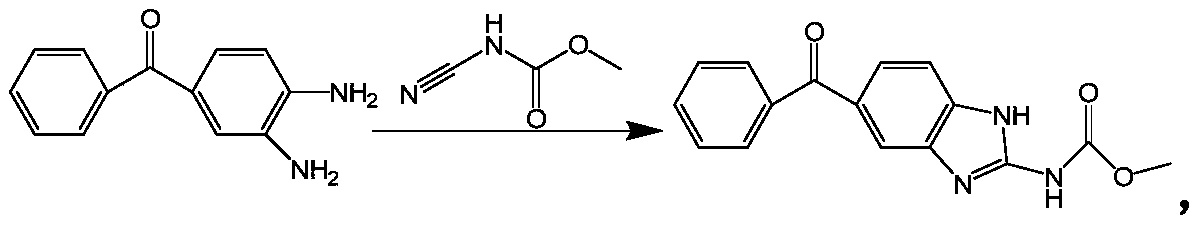
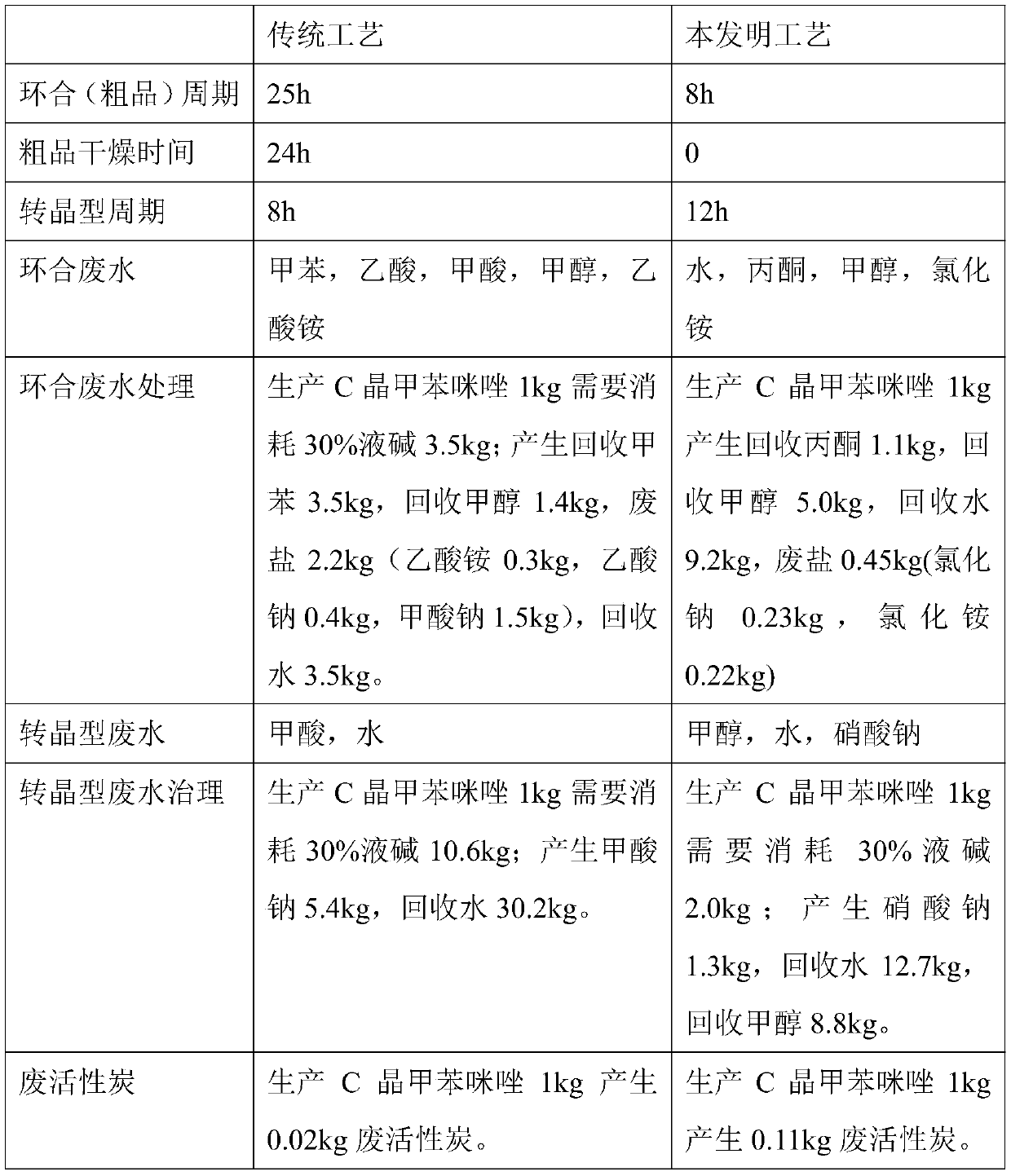
Method for synthesizing mebendazole by means of methyl cyanocarbamate
A technology of methyl cyanocarbamate and mebendazole, which is applied in the field of drug synthesis, can solve the problems of difficult handling, long cyclization time and high cost of raw materials, and achieves the effects of short production cycle, short cyclization time and shortened production cycle.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Add 42.6g of 3,4-diaminobenzophenone, 65ml of acetone, and 48g of hydrochloric acid into a 500ml four-neck flask, heat to dissolve, add 242g of 12.5% methyl cyanocarbamate, and reflux for 10min. Distill acetone to 97°C, reflux for 5 hours, cool down to 60°C and filter with suction, wash with 200ml of water and 300ml of methanol, the filter cake is crude mebendazole, wet weight 89.0g, dry weight 54.73g, yield: 92.3%.
[0027] Add 25g of crude mebendazole (dried) and 125ml of methanol into a 500ml reaction bottle, stir, heat to 60°C, add 20.4g of 65% nitric acid, after dissolving, add 2.5g of activated carbon at 60°C, heat, reflux for 55min, and heat Suction filtration, the filtrate was cooled slowly and stirred to crystallize, kept at 20°C for 1h, and suction filtration to obtain mebendazole nitrate.
[0028] Return mebendazole nitrate to the bottle, add 100ml of methanol, heat to 60°C, add about 11.5g of 65% nitric acid, dissolve it, cool down to 54°C, add 0.5g of meb...
Embodiment 2
[0030] Add 42.6g of 3,4-diaminobenzophenone, 18ml of new acetone to a 500ml four-neck flask, recover 47ml of acetone, 49g of hydrochloric acid, heat to dissolve, add 242g of 12.5% methyl cyanocarbamate, continue heating, and reflux for 10min . Distill acetone to 95°C, keep at 95°C for 5 hours, cool down to 60°C and filter with suction, wash with 200ml of water and 300ml of methanol. The filter cake is crude mebendazole, with a wet weight of 87.5g and a dry weight of 54.61g. Yield: 92.09%.
[0031] Add 25g of crude mebendazole (dried), 125ml of methanol into a 500ml reaction bottle, stir, add 25g of nitric acid, heat, add 2.5g of activated carbon at 60°C after dissolving, heat to reflux for 55min, suction filter while hot, and slowly cool down and stir the filtrate Crystallize, keep warm at 20°C for 1h, and filter with suction to obtain mebendazole nitrate.
[0032] Return mebendazole nitrate to the bottle, add 100ml of methanol, add 12g of nitric acid, heat to dissolve, coo...
Embodiment 3
[0034] In a 500ml four-necked flask, add 42.6g of 3,4-diaminobenzophenone, 20ml of new acetone, recover 51ml of acetone, 48.5g of hydrochloric acid, heat to dissolve, add 242g of 12.5% methyl cyanocarbamate, continue heating, and reflux 10min. Distill acetone to 96°C, keep at 96°C for 5 hours, cool down to 60°C and filter with suction, wash with 200ml of water and 300ml of methanol. The filter cake is crude mebendazole, with a wet weight of 86.28g and a dry weight of 55.0g, with a yield of 92.75%.
[0035] Add 39.3g of the wet crude product of mebendazole (25g after drying), 120ml of methanol into a 500ml reaction flask, stir, add 25g of nitric acid, heat, after dissolving, add 2.5g of activated carbon at 60°C, heat, reflux for 55min, and suction filter while hot, the filtrate Slowly lower the temperature and stir to crystallize, keep warm at 20°C for 1 hour, and filter with suction to obtain mebendazole nitrate.
[0036] Return mebendazole nitrate to the bottle, add 100ml ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com