A kind of synthetic method of 3,4-diamino-benzophenone
A technology of benzophenone and synthesis method, which is applied in chemical instruments and methods, preparation of organic compounds, organic chemistry, etc., can solve the problems of qualified product purity, low yield, and deconstruction, and achieves improved product purity and improved The effect of reaction yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
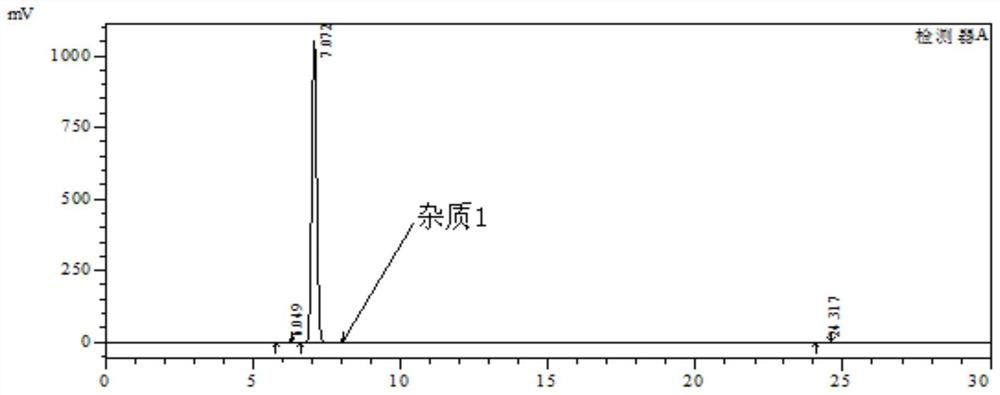
[0035] 1), put 3-nitro-4-chloro-benzophenone: 180g, ethanol: 200g and 15% (mass fraction) ammonia water: 300g into the autoclave, stir and heat up to 120-125°C, keep warm for 24h, At the same time, the pressure in the kettle reaches 0.55-0.60MPa. After the reaction, cool down to 20-25°C, depressurize, and filter with suction. The filter cake is rinsed with 80g of water, and dried to obtain the intermediate product I, 3-nitro-4-amino - Benzophenone: 160 g, purity 99.8%; impurity 1 purity 0.08%.
[0036] The HPLC chromatographic conditions are as follows: chromatographic column: Kromasil C18 250×4.6mm 5μm, wavelength: 254nm, mobile phase: methanol:0.1% phosphoric acid aqueous solution=60:40, column temperature: 30°C, flow rate: 1.0ml / min.
[0037] Chromatograms such as figure 1 As shown, the results are shown in Table 1 below.
[0038] Table 1
[0039] Intermediate I purity Impurity 1 Remark 99.8% 0.08% Other impurities 0.12%
[0040] 2), the interme...
Embodiment 2
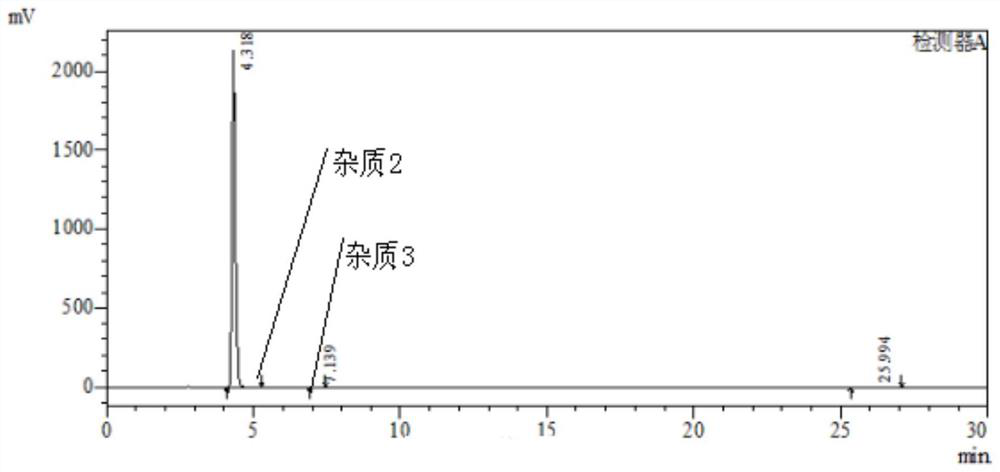
[0045] 1), put 3-nitro-4-chloro-benzophenone: 180g, isopropanol: 200g and 20% (mass fraction) ammonia water: 230g into the autoclave, stir and heat up to 70-75°C, heat preservation reaction After 24 hours, the pressure in the kettle reached 0.3-0.35MPa. After the reaction, the temperature was lowered to 20-25°C, depressurized, and suction filtered. The filter cake was rinsed with 80g of water, and dried to obtain the intermediate product I, 3-nitro-4 -Amino-benzophenone: 158 g, purity 98.8%; impurity 1 purity 0.54%.
[0046] HPLC chromatographic condition is the same as embodiment 1, and HPLC chromatogram is as follows image 3 As shown, the results are shown in Table 3.
[0047] table 3
[0048] Intermediate I purity Impurity 1 Remark 98.8% 0.54% Other impurities 0.66%
[0049]2), the intermediate product I: 158g and methanol: 500g were added to the beaker and stirred to dissolve, then added to the autoclave, and then added palladium carbon catalys...
Embodiment 3
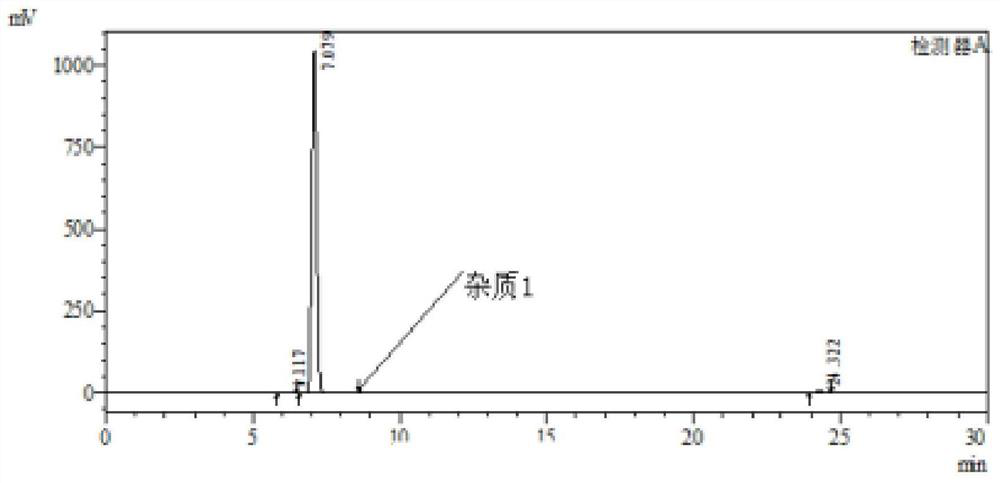
[0058] 1), put 3-nitro-4-chloro-benzophenone: 180g, N,N-dimethylformamide: 200g and 18% (mass fraction) ammonia water: 270g into the autoclave, stir and heat up to 110 -115°C, heat preservation reaction for 21h, while the pressure in the kettle reaches 0.50-0.55MPa, after the reaction, cool down to 20-25°C, release pressure, filter with suction, rinse the filter cake with 80g of water, and dry to obtain the intermediate product I. 3-nitro-4-amino-benzophenone: 158 g, purity 99.9%; impurity 1 purity 0.06%.
[0059] 2), the intermediate product I: 158g and methanol: 500g were added to the beaker and stirred to dissolve, then added to the autoclave, and then added palladium carbon catalyst: 3.5g, followed by nitrogen replacement 3 times, hydrogen replacement 3 times, and finally filled with hydrogen to 0.15MPa, stir and heat up to 40-45°C, keep warm for 4h. After the reaction, the temperature was lowered to 20-25°C, the pressure was released, the discharge was suction-filtered, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com