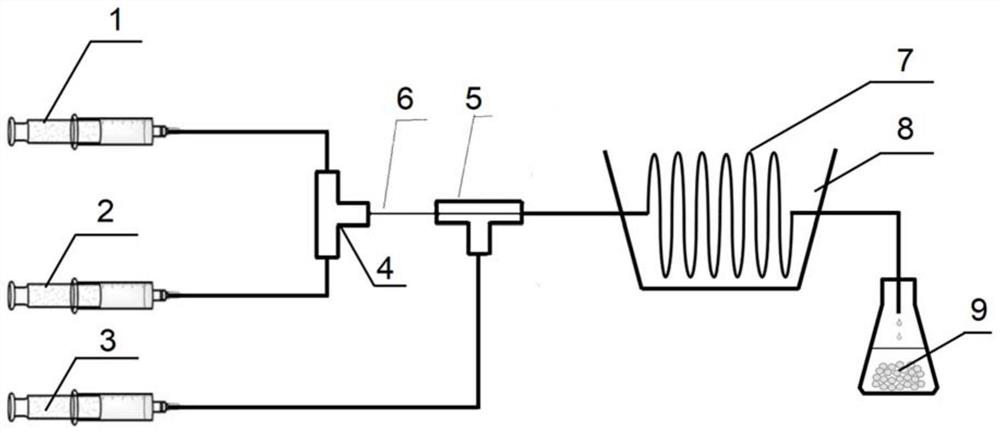
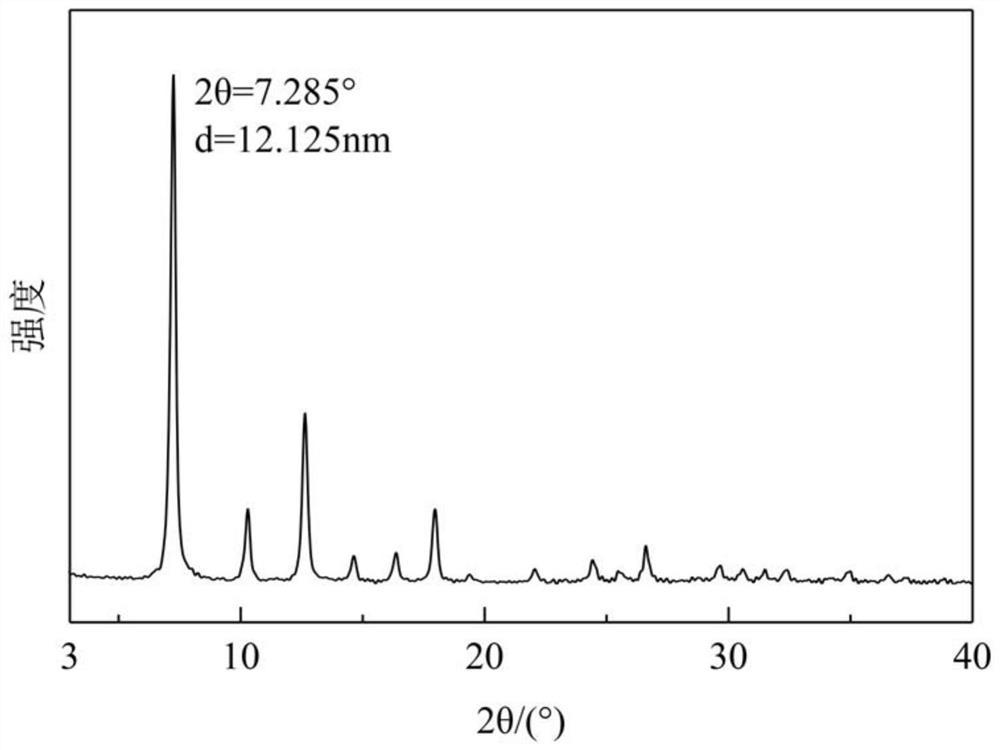
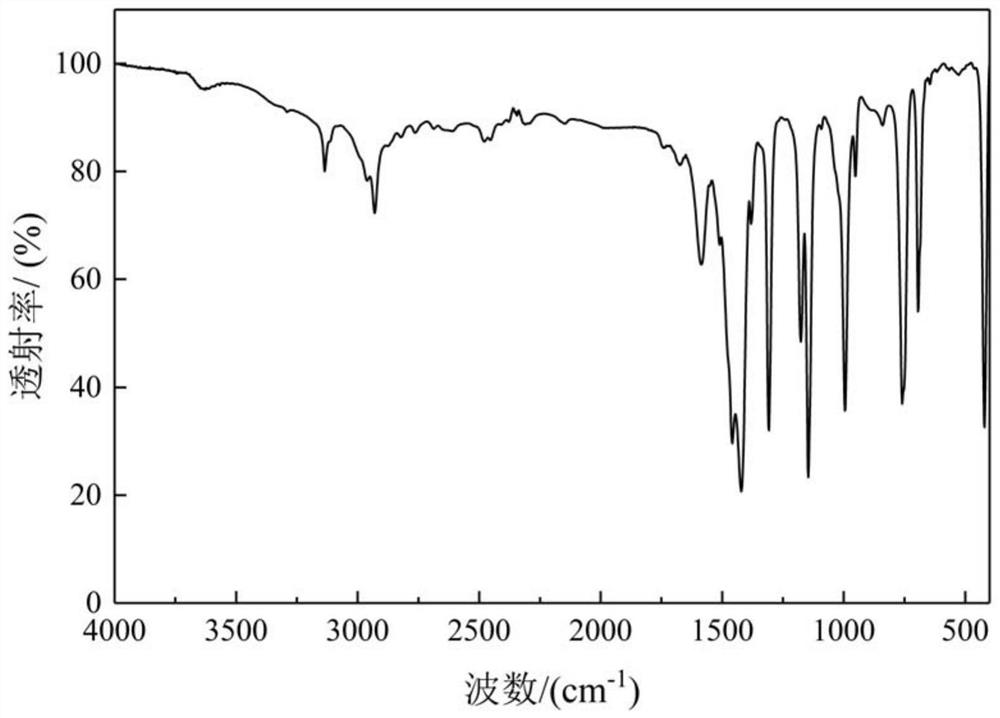
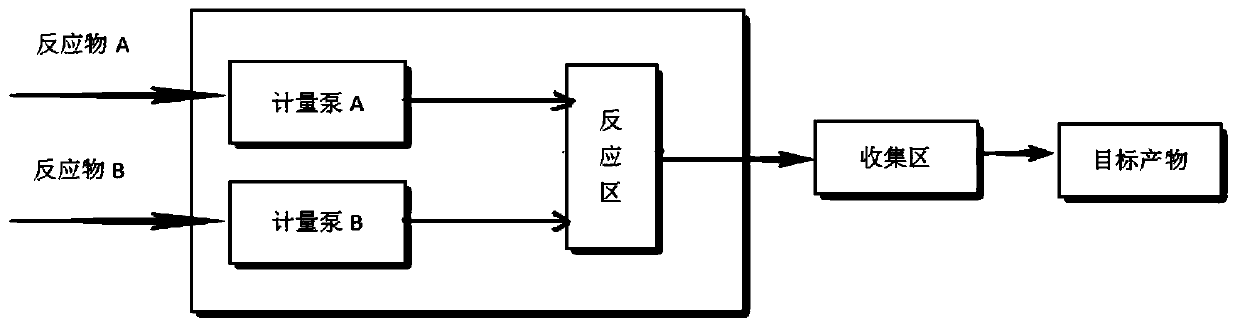
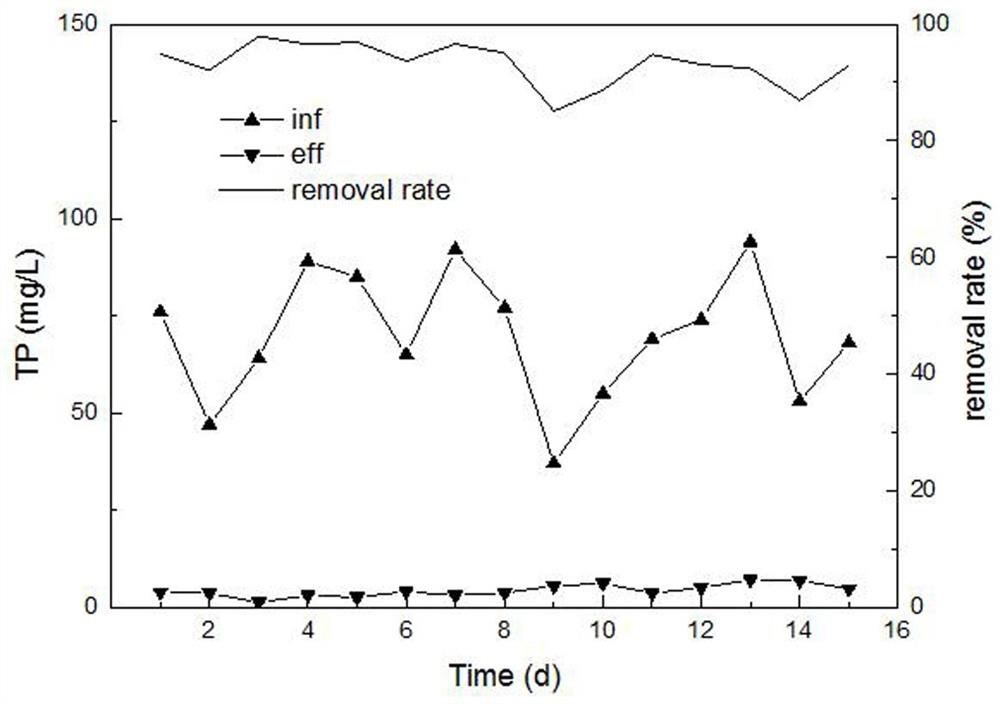
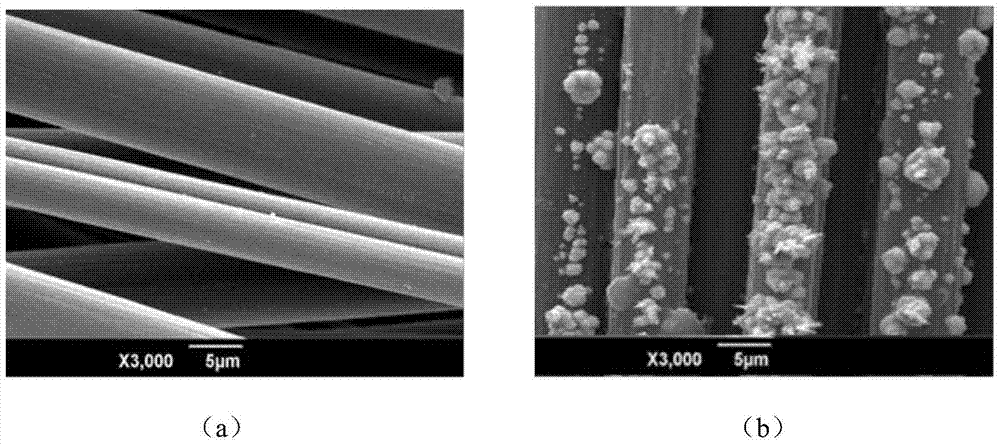
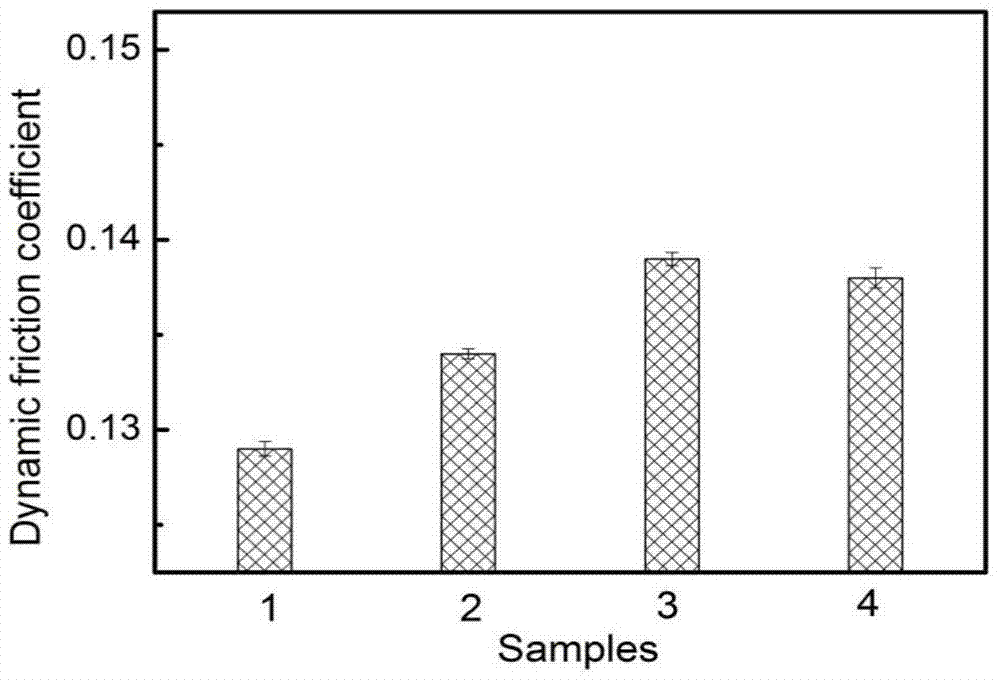
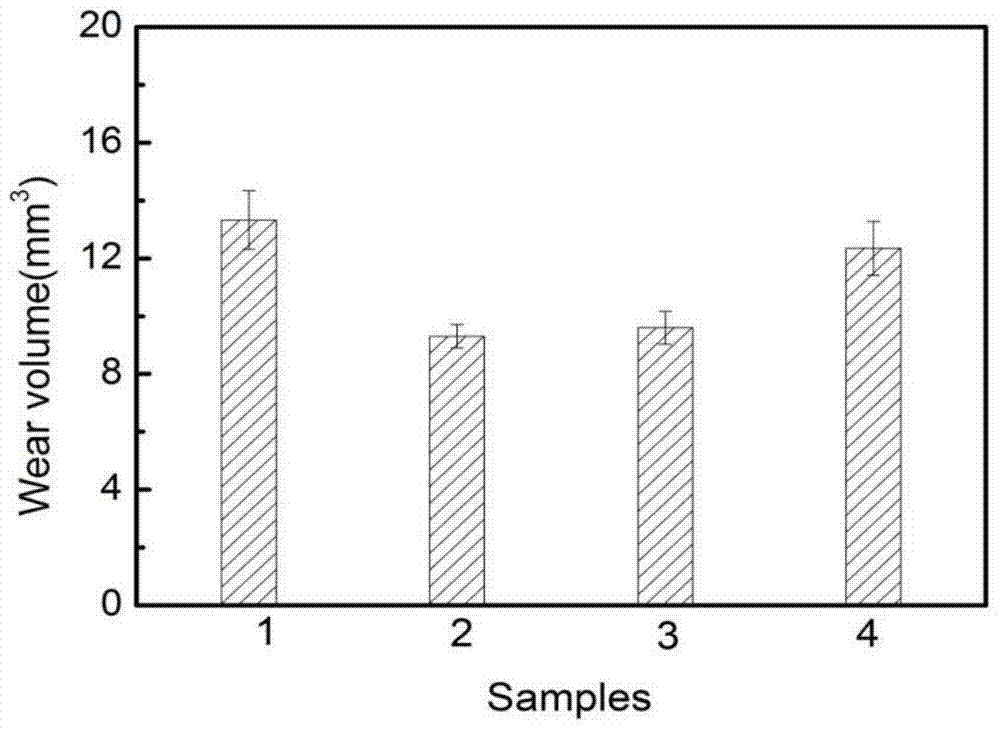
Patents
Literature
42results about How to "Precise control of reaction conditions" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
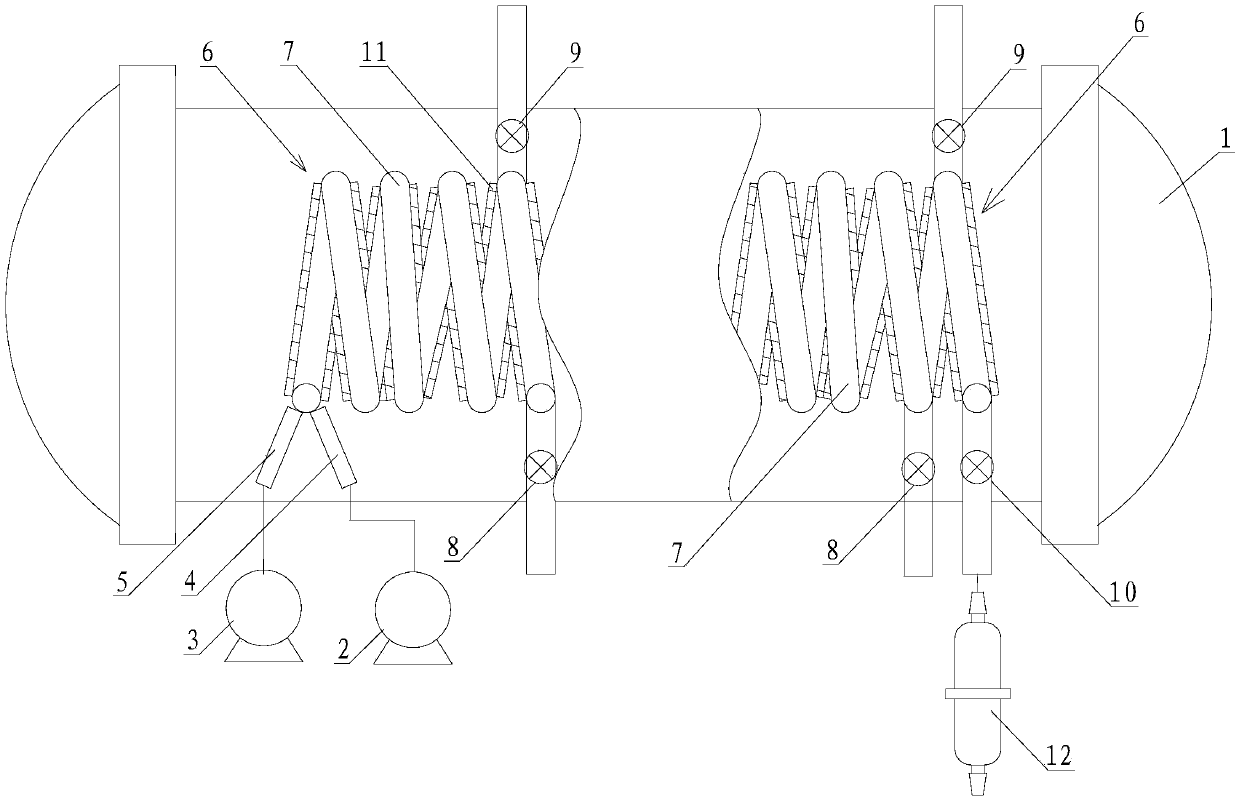
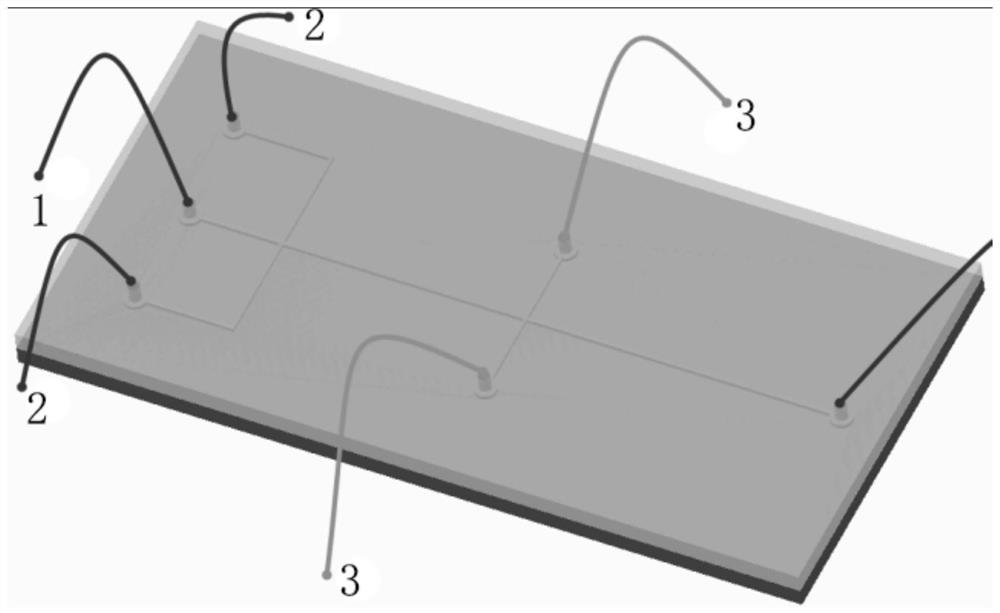
System and process for denitration by spraying ammonia gas in large-sized boiler high-temperature flue gas area
ActiveCN105509081AGuarantee the effect of denitrification onceGuaranteed denitrification effectGas treatmentEmission preventionFlue gasEngineering
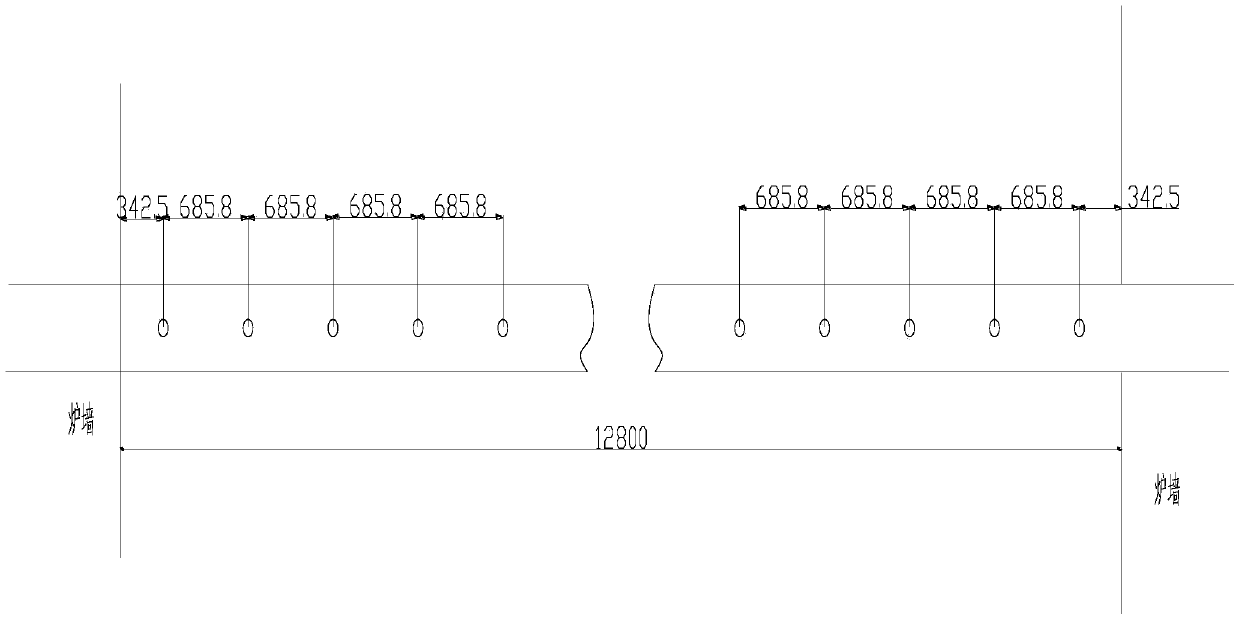
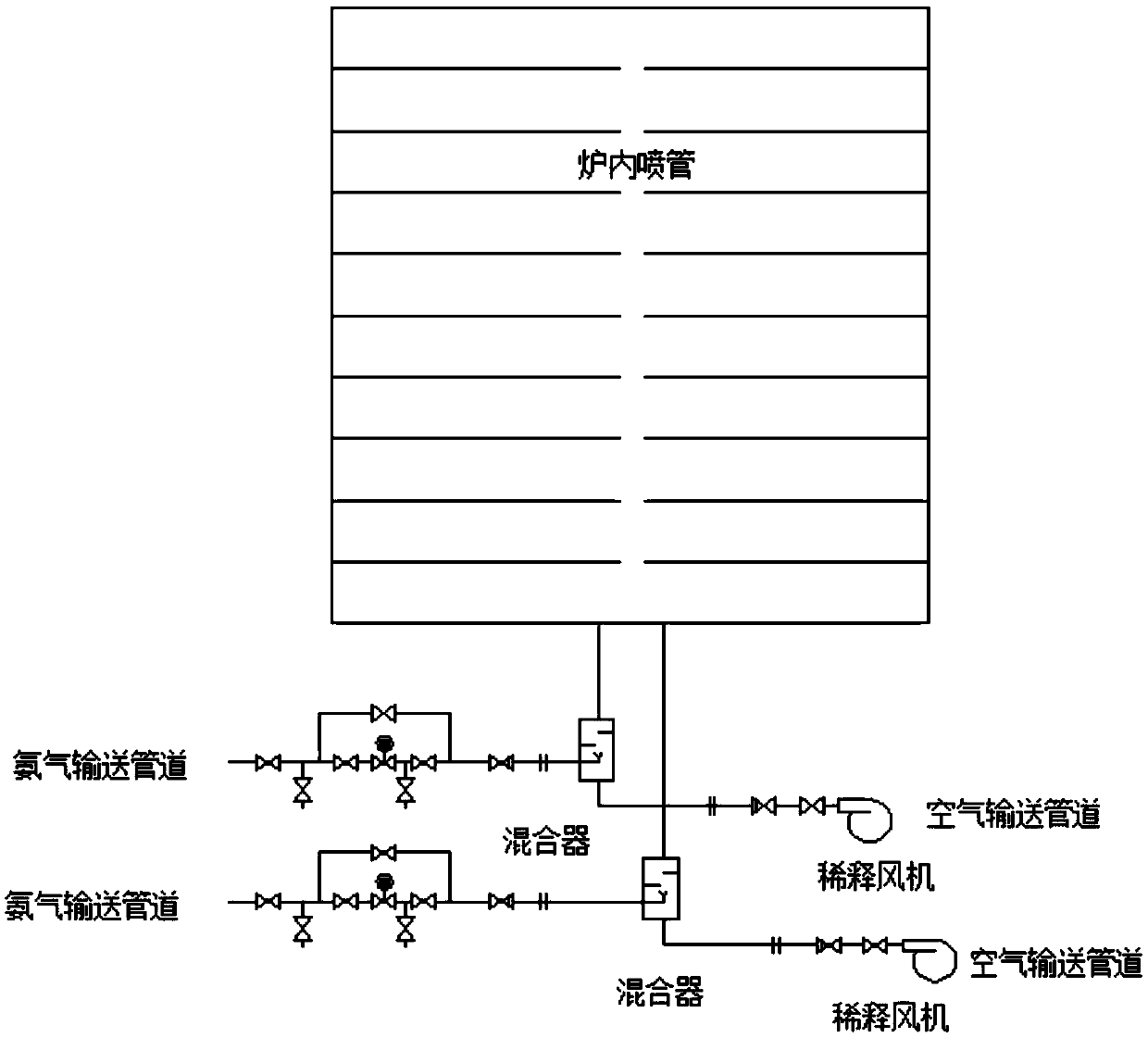
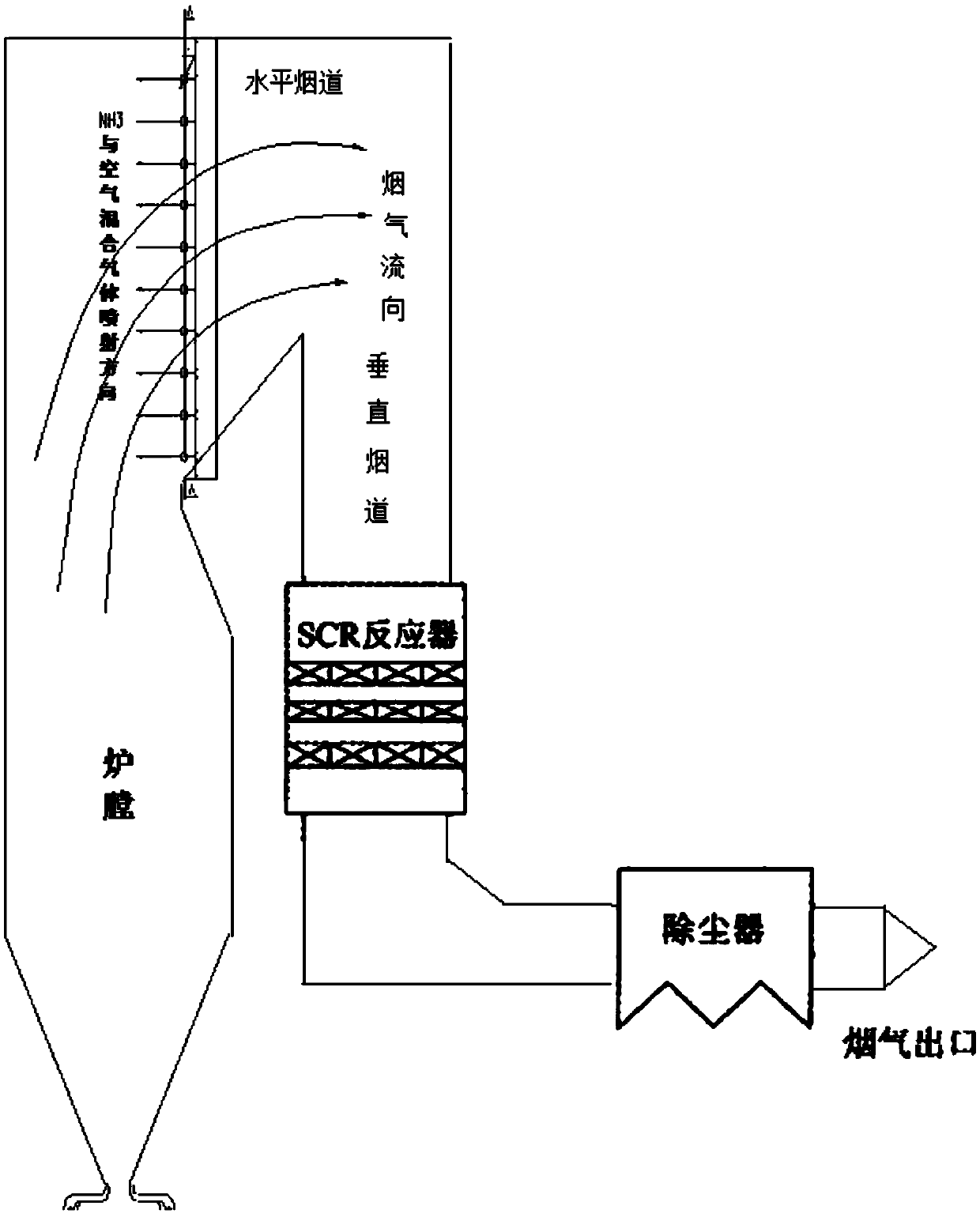
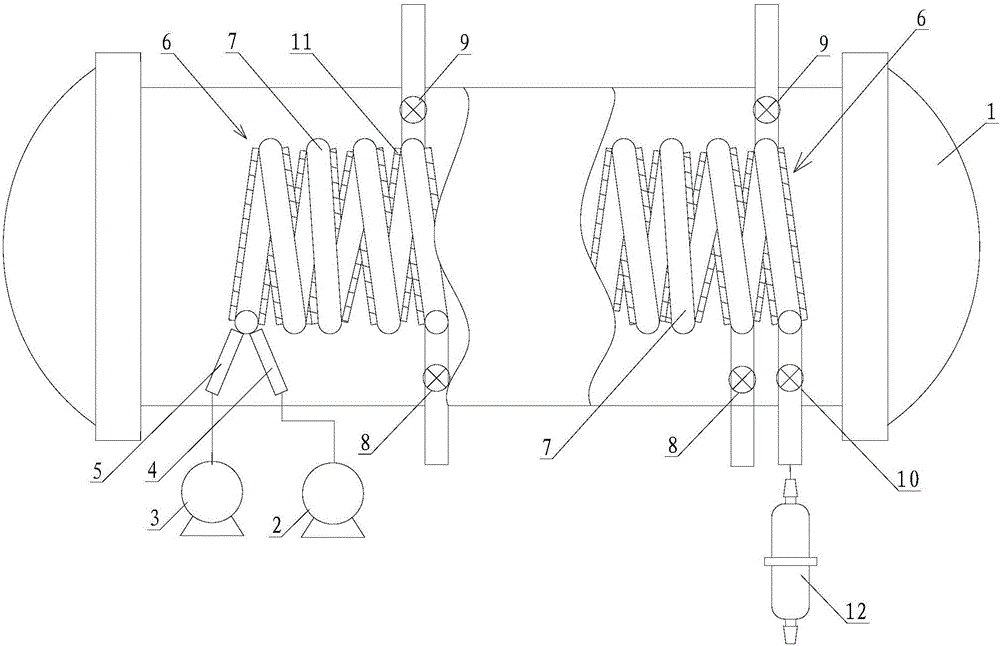
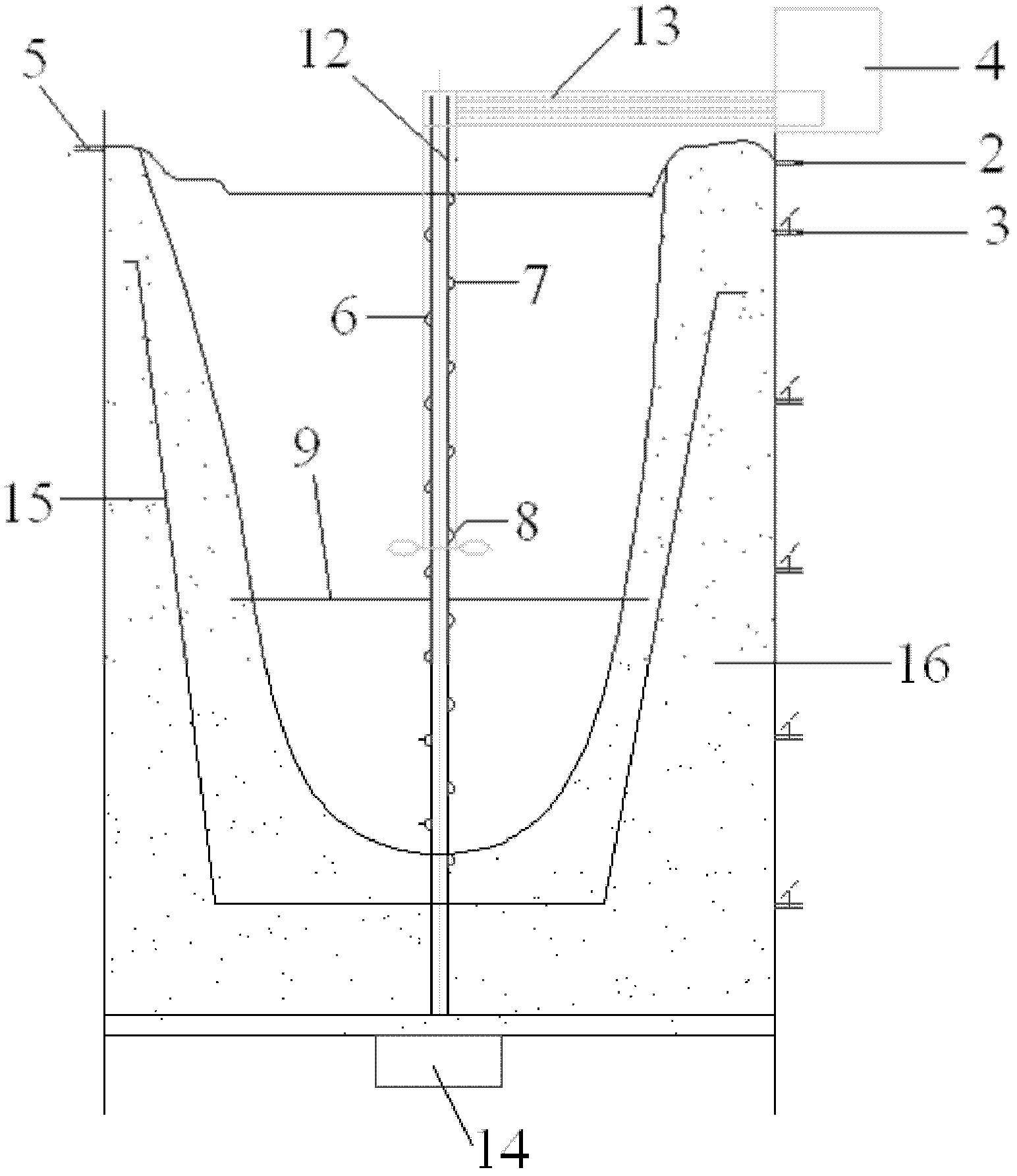
The invention belongs to the technical field of flue gas denitration and relates to a system for denitration by spraying ammonia gas in a large-sized boiler high-temperature flue gas area. A boiler comprises a horizontal flue and a vertical flue. The system comprises an SNCR (selective non-catalytic reduction) system provided with a reducing agent injector, the reducing agent injector is a spray pipe disposed on the transverse section of the horizontal flue, an input end of the spray pipe is connected with a reducing agent conveyer, the other end of the spray pipe is closed, and the wall of the spray pipe opposite to flue gas is provided with a spray hole. The invention also discloses a denitration process for denitration treatment using the system, the denitration treatment with the system gives high denitration rate and low ammonia escape quantity as well as full flue gas purification, this product is applicable to present coal-consuming boilers for thermal power generation, iron and steel, chemicals, cements and the like, and satisfactory NOx emission index of these boilers can be ensured.
Owner:杜梦凡
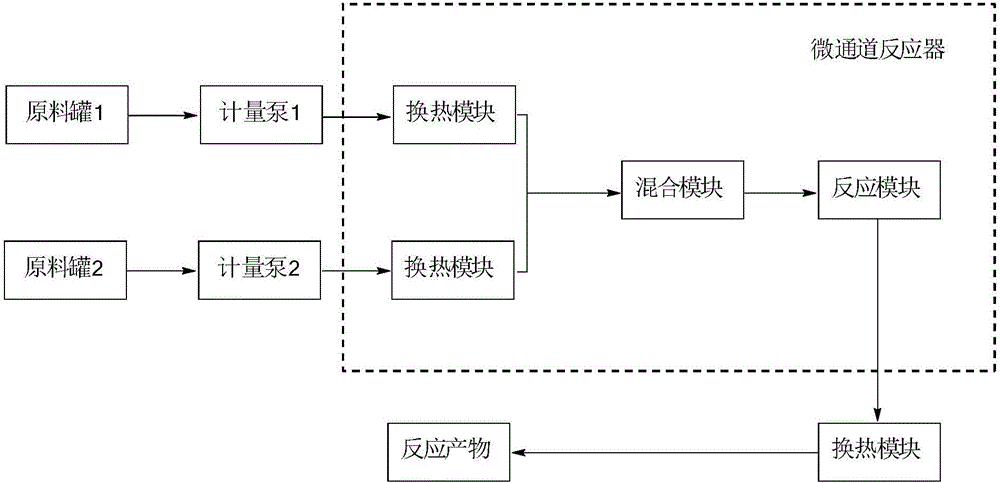
Method for preparing vinylene carbonate through micro channel reaction
ActiveCN106749155AFully contactedHigh reaction mass transfer and heat release efficiencyOrganic chemistrySecondary cellsEngineeringEthyl Chloride
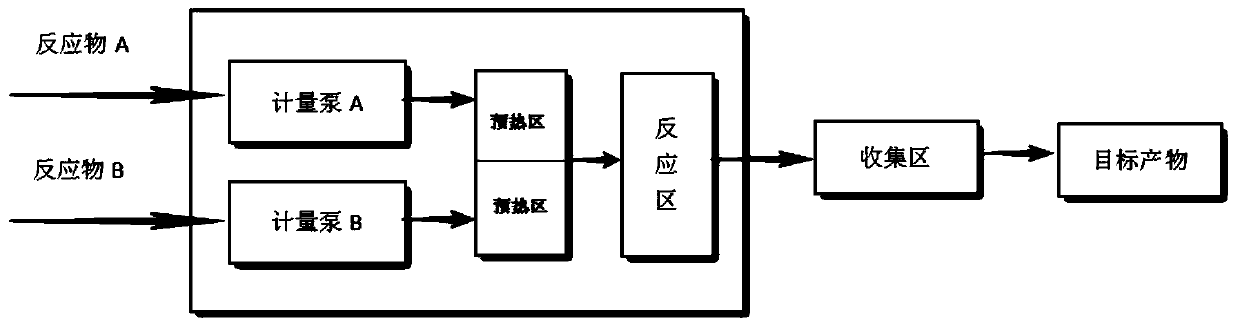
The invention provides a method for preparing vinylene carbonate through micro channel reaction. The method comprises the following steps: (1) preparing equipment, including an enhanced mass transfer micro channel reactor, a metering pump I, a metering pump II and a micro filter, wherein the enhanced mass transfer micro channel reactor comprises a preheater I, a preheater II, an ultrasonic device and a micro channel module; (2) uniformly mixing chloroethylene carbonate with an ester solvent so as to obtain a mixed liquid, synchronously inputting the preheated mixed liquid and triethylamine into a micro channel, heating, mixing, performing reaction, discharging a product obtained after the reaction is completed from a discharge valve, filtering so as to obtain a crude product, and rectifying the crude product into a rectifying still, so as to obtain the vinylene carbonate, wherein the mole ratio of the chloroethylene carbonate to the triethylamine is 1:(1.5-2.0). The method is simple, convenient and safe to operate, relatively small in quantity of byproducts, small in single solvent consumption, low in moisture content, relatively high in product purity and yield, small in environment pollution and applicable to industrial large-scale production, and continuous production can be achieved.
Owner:江苏瀚康新材料有限公司
Process and equipment for preparing anisotropic rare-earth permanent-magnet powder and product prepared thereby
ActiveCN102107277APrecise control of reaction conditionsWeak performance impactMagnetic materialsRare-earth elementControl system
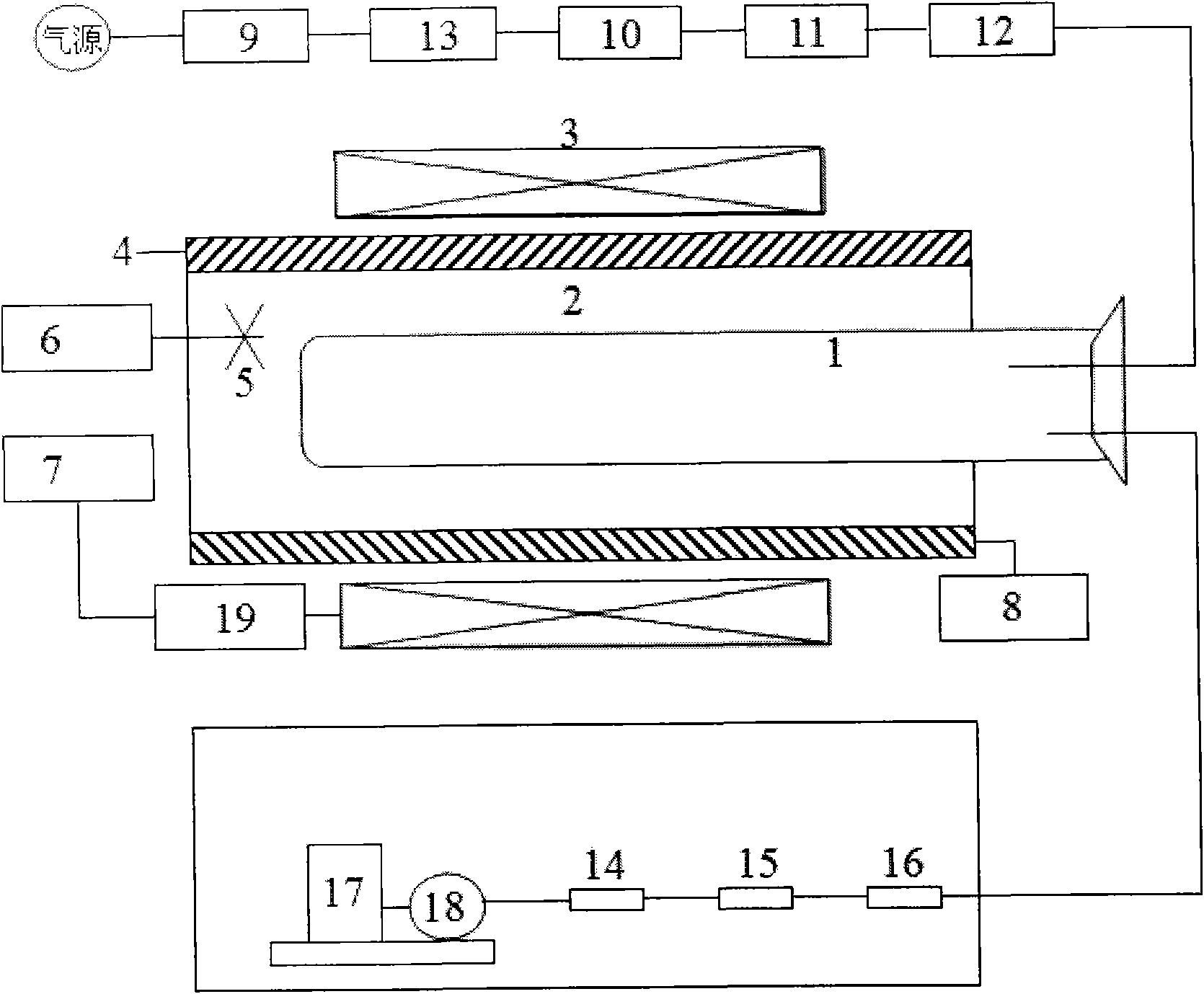
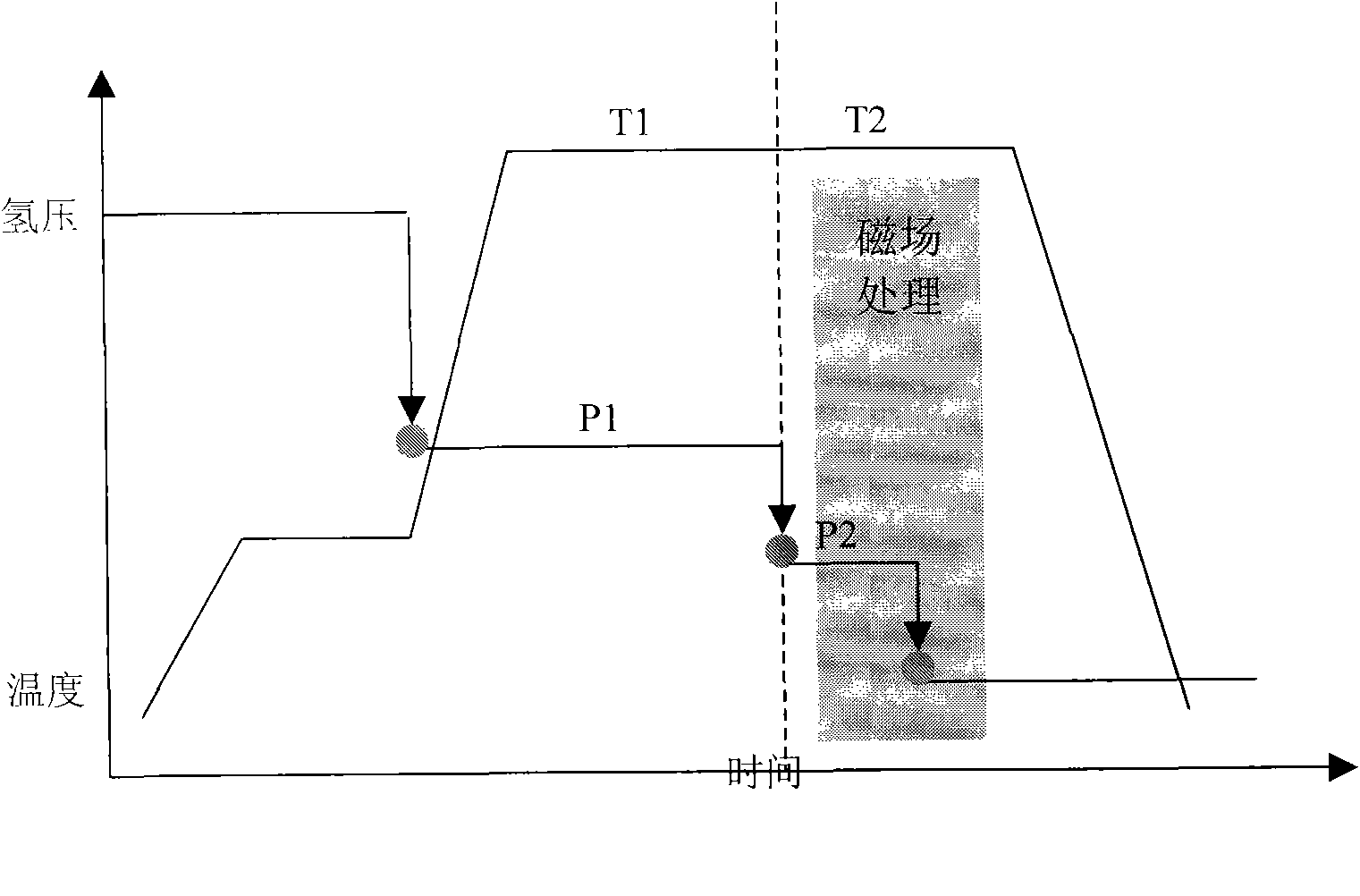
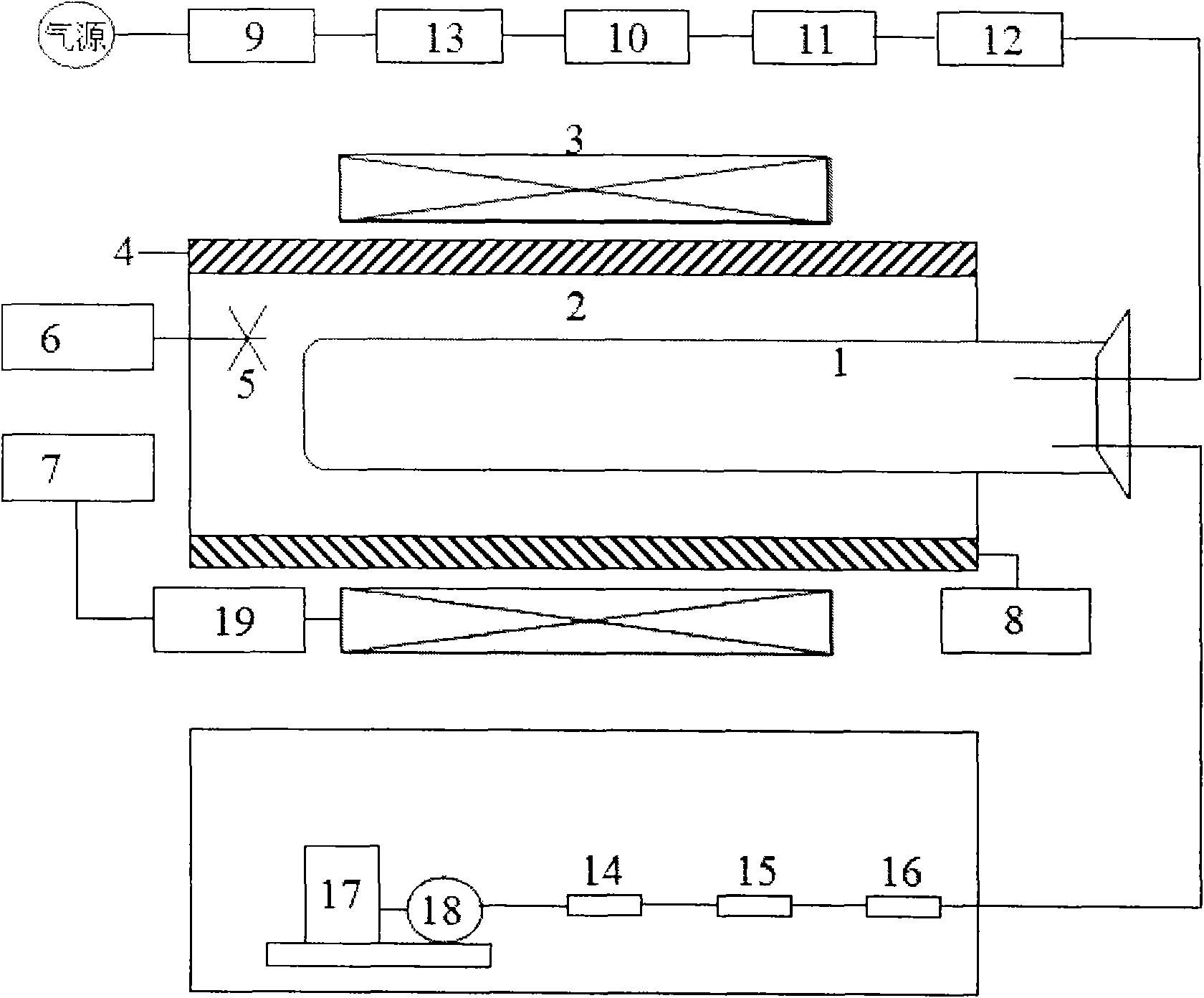
The invention relates to a process and equipment for preparing anisotropic rare-earth permanent-magnet powder and a product prepared thereby. The equipment for preparing the magnetic powder comprises three parts, namely a heating and magnetic field orientation system, a flow control system and a vacuum system. The preparation process comprises the following steps of: putting rare earth elements containing Sc and Y and rare earth alloy which takes boron and iron as principal components into the preparation equipment, and performing high-temperature hydrogen treatment at the temperature of between 650 and 900 DEG C under the hydrogen partial pressure of 10 to 90 kPa; and performing multi-step dehydrogenation at the temperature of between 750 and 950 DEG C under a certain magnetic field. Through the method, magnetic powder grains can be refined, and anisotropy and magnetic property are improved.
Owner:GRIREM ADVANCED MATERIALS CO LTD
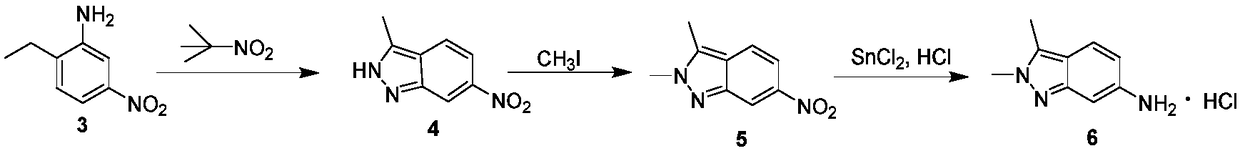
Method for preparing 2, 3-dimethyl-2H-indazole-6-benzylamine hydrochloride
InactiveCN108299304APrecise control of reaction conditionsLess side effectsOrganic chemistryChemical/physical/physico-chemical microreactorsBenzylamine hydrochlorideSide reaction
The invention discloses a method for preparing 2, 3-dimethyl-2H-indazole-6-benzylamine hydrochloride. The method comprises the steps of reacting a glacial acetic acid solution of tert-butyl nitrite and a glacial acetic acid solution of 5-nitro-2-ethylaniline in a first microreactor to generate 3-methyl-6-nitro-1H-indazole; reacting a homogeneous solution formed by mixing the 3-methyl-6-nitro-1H-indazole and a dimethyl sulfoxide solution of methyl iodide and a dimethyl sulfoxide solution of sodium ethoxide in a second microreactor to generate 2,3-dimethyl-6-nitro-2H-indazole; then reacting withmixed liquor formed by stirring a concentrated hydrochloric acid solution of stannous chloride and ethyl alcohol in a third microreactor to generate the 2, 3-dimethyl-2H-indazole-6-benzylamine hydrochloride. The method provided by the invention has the advantages of less side reaction, high yield, simplification of a complicated multi-step synthesis process, low toxicity and pollution, low production cost, good product quality, environment friendliness, energy saving and high efficiency, and is suitable for industrialized application.
Owner:CHINA PHARM UNIV
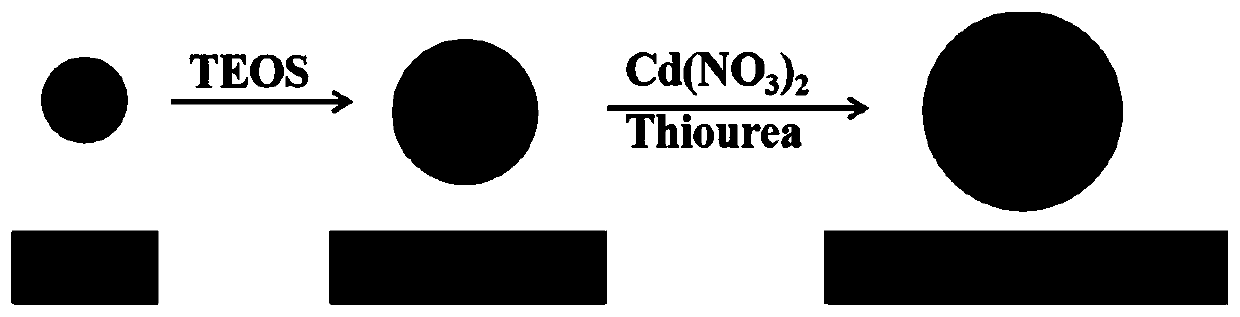
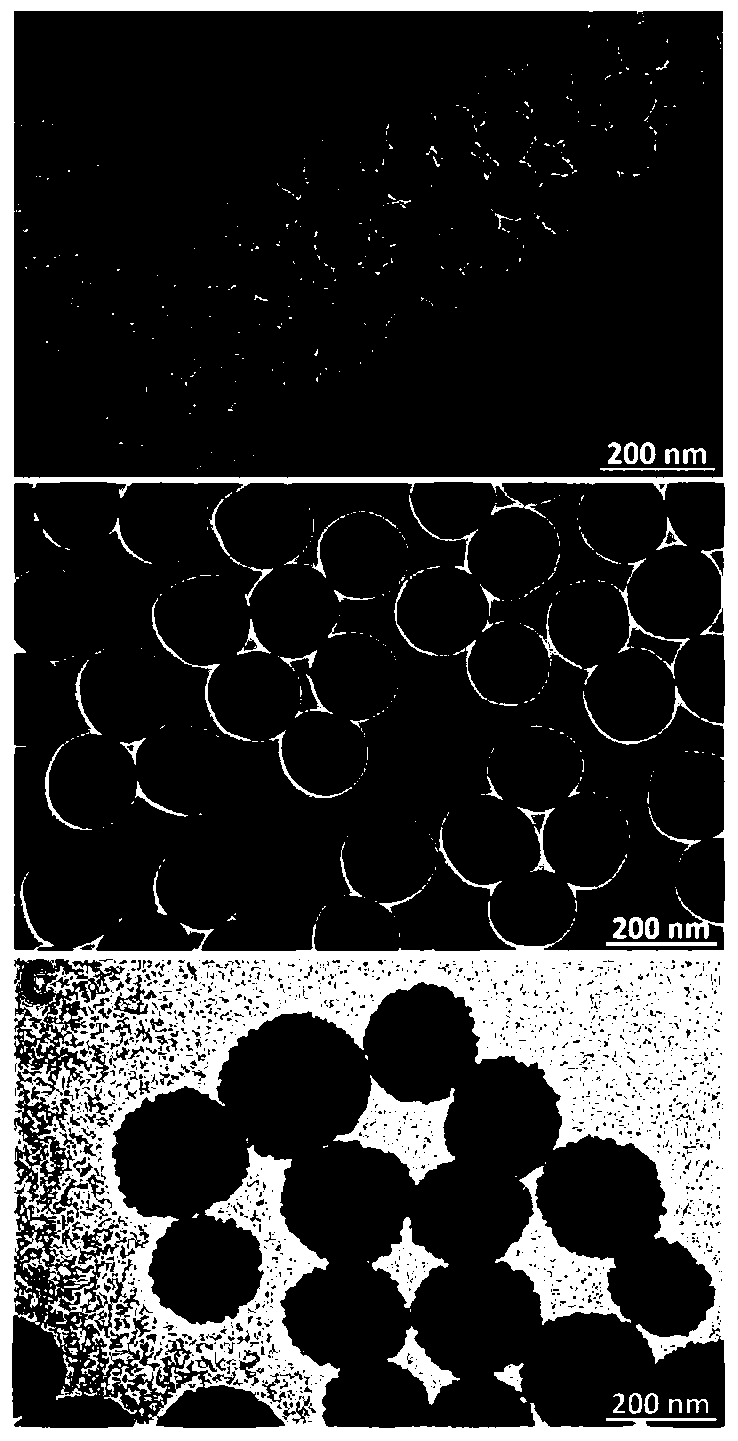
Preparation method and application of superparamagnetic ferroferric oxide@silicon dioxide@cadmium sulfide core-shell nano-structure material
InactiveCN110560090APreparation of uniform sizePrecise control of reaction conditionsWater/sewage treatment by irradiationWater treatment compoundsNano structuringHigh pressure
The invention belongs to the field of functional materials and discloses a preparation method and application of a superparamagnetic ferroferric oxide@silicon dioxide@cadmium sulfide core-shell nano-structure material. The Fe3O4@SiO2@CdS double core-shell structure nano-material with uniform size and even dispersion is successfully prepared by linking SiO2 as an interface transition medium with asuperparamagnetic Fe3O4 nano-cluster and a CdS nano-semiconductor shell layer. The double core-shell structure nano-material has excellent optical performance, and can be reclaimed and separated in acomplicated reaction through a simple magnetic operation. The synthesizing method is simple in operation, can avoid high-temperature high-pressure strict reacting conditions, and can effectively eliminate crystal face mismatching of Fe3O4 and CdS, so that the form and dispersibility of the double core-shell structure can be well guaranteed. Furthermore, the Fe3O4@SiO2@CdS core-shell structure nano-material prepared by the method can be successfully applied to photocatalytic degradation of rhodamine B.
Owner:JIANGSU UNIV
Electrochemical upgrading method for bio-oil
ActiveCN111909736AKeep carbonaceous componentsSimple processLiquid carbonaceous fuelsSupporting electrolyteElectrochemical response
The invention belongs to the field of biomass energy utilization, and discloses a bio-oil electrochemical upgrading method which comprises the following steps: (a) mixing bio-oil, an organic solvent and a supporting electrolyte to obtain a catholyte; (b) preparing an acid solution as an anolyte; (c) constructing an electrochemical reactor by adopting the catholyte and the anolyte and separating the catholyte from the anolyte through one or two ion exchange membranes, thus forming a current loop; and (d) introducing a protective gas into one side of the catholyte, and introducing current through the working electrode and the anode electrode to carry out electrochemical reaction, thereby realizing the electrochemical upgrading of the bio-oil. Compared with the prior art, the problem that carbon deposition is easily formed when the bio-oil is upgraded by a thermochemical method can be effectively solved, the bio-oil is subjected to electrochemical upgrading under mild conditions, and thecontent of bio-oleic acid, the content of aromatic components and the content of heavy components can be reduced by upgrading, so that the bio-oil is suitable for transportation and storage; and meanwhile, carbon deposition is avoided in the upgrading process.
Owner:HUAZHONG UNIV OF SCI & TECH
Method for preparing o-nitro anisole by using micro-channel reaction apparatus
InactiveCN105503610APrecise control of reaction conditionsEasy to operateOrganic chemistryOrganic compound preparationO-NitroanisoleReaction rate
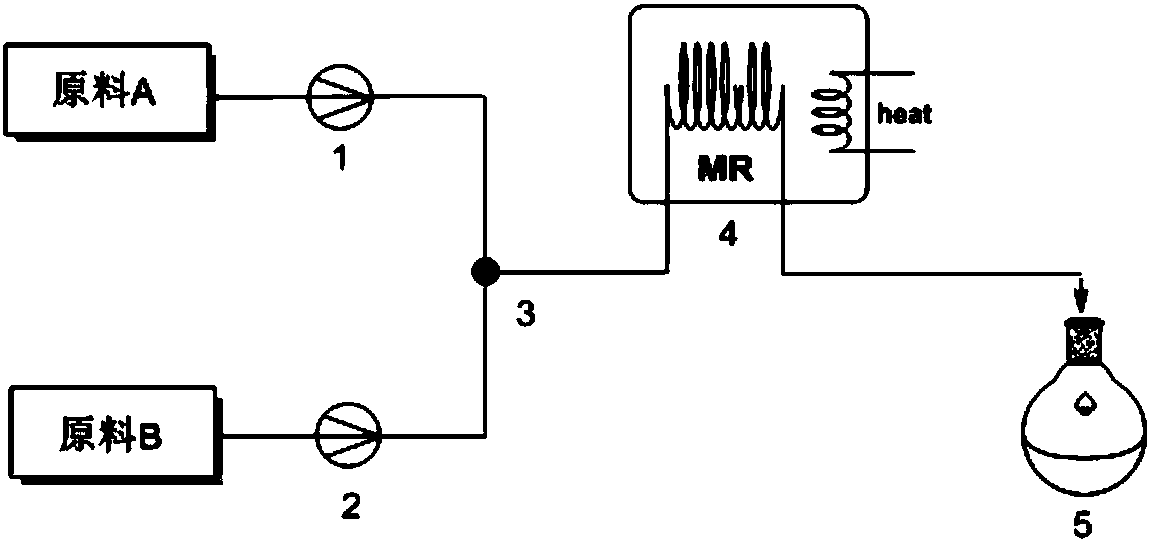
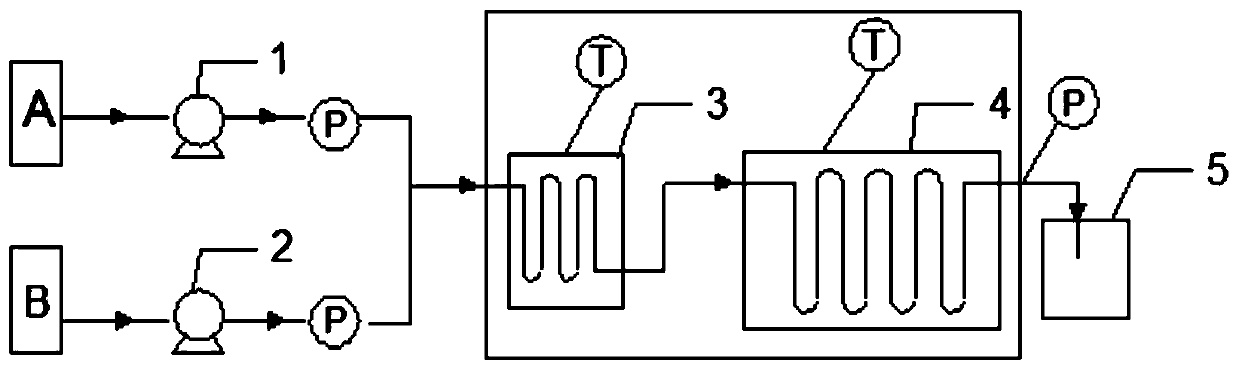
The present invention provides a new method for preparing o-nitro anisole by using a micro-channel reactor, particularly application of a micro-channel reactor with an enhanced mass transfer unit structure to carry out a continuous flow o-nitro chloro benzene etherification reaction. According to the present invention, the mass transfer and the heat transfer can be completed dependent on the kinetic energy of the fluid without the mechanical stirring process under the reactor operation condition higher than the normal temperature, the space time reaction rate of the micro-channel reactor can be substantially improved, the temperature fluctuation and the concentration fluctuation during the reaction process can be avoided, the phenomena such as temperature runaway and overheating do not exist, and the reaction process is safe.
Owner:CHINA PETROLEUM & CHEM CORP +1
Preparation method of gold nano-octahedrons
InactiveCN108161020AGive full play to the selective adsorptionPrecise control of reaction conditionsMaterial nanotechnologyTransportation and packagingOctahedronSolvent
The invention provides a preparation method of gold nano-octahedrons and belongs to the technical field of nanometer materials. The preparation method is provided for overcoming the defects in the prior art. The preparation method of the gold nano-octahedrons, provided by the invention, comprises the steps of selecting a cationic surfactant PDDA as a protective agent and a morphological control agent; synthesizing the gold nano-octahedrons from chloroauric acid under high temperature through one-time reduction. The preparation method has the characteristics of adopting the cationic surfactantand not introducing any new chemical components and foreign ions into the system. The preparation method is easy and convenient to operate and good in repeatability and controllability. The prepared and synthesized gold nano-octahedrons are good in monodispersity and uniform in particle size distribution. Moreover, various sizes of high-purity gold nano-octahedrons.
Owner:INST OF OPTICS & ELECTRONICS - CHINESE ACAD OF SCI
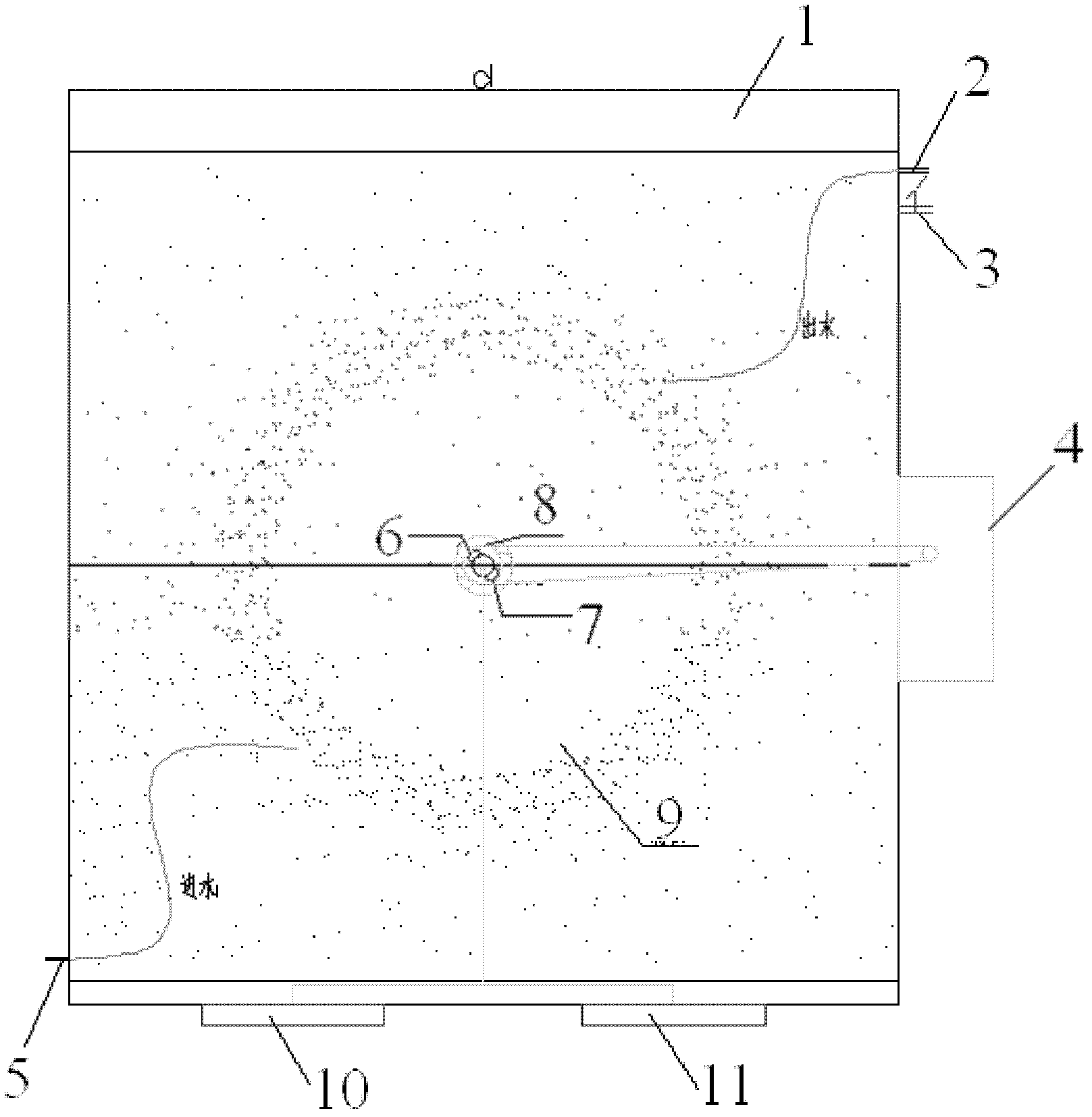
Water environment simulation reactor based on water-soil interface
InactiveCN103157424APrecise control of reaction conditionsEasy to collectChemical/physical/physico-chemical stationary reactorsEutrophicationControl zone
The invention relates to a water environment simulation reactor based on a water-soil interface. The invention belongs to the technical field of water environment research. When a simulator hydraulic retention time of the device is the same with that of a natural water body, precise controls over different hydraulic conditions and environmental factors (temperature, DO or ORP, etc.) of the simulator are realized. Water passes through a water inlet port, a water inlet area A, a control zone, a water outlet area B, and a water outlet, and contacts soil or sediments in the device, such that natural water body environment can be simulated. Compared with prior arts, the reactor provided by the invention has the advantages of simple structure, easy operation, and low running cost. The reactor can be used in laboratory researches of water-soil contaminant migration, degradation mechanism, eutrophication process, heavy metal migration, and the like, and has wide application prospect.
Owner:TONGJI UNIV
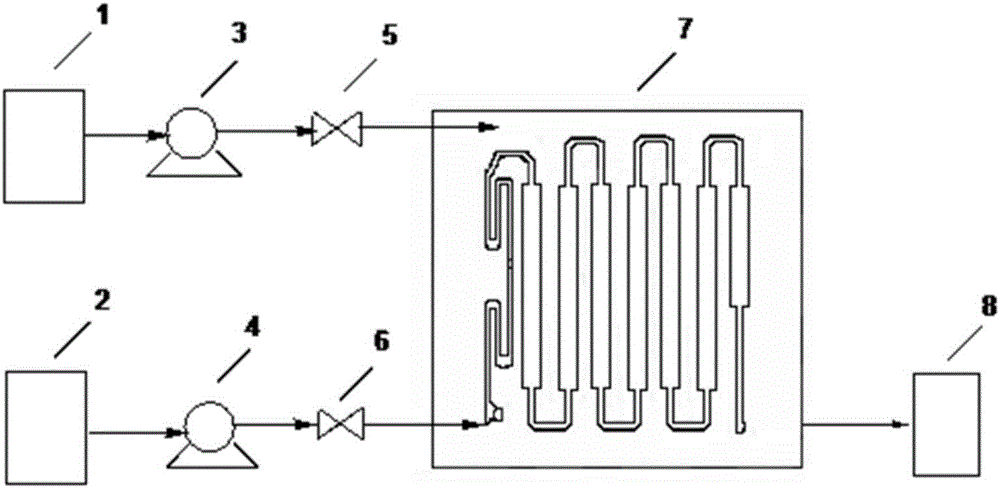
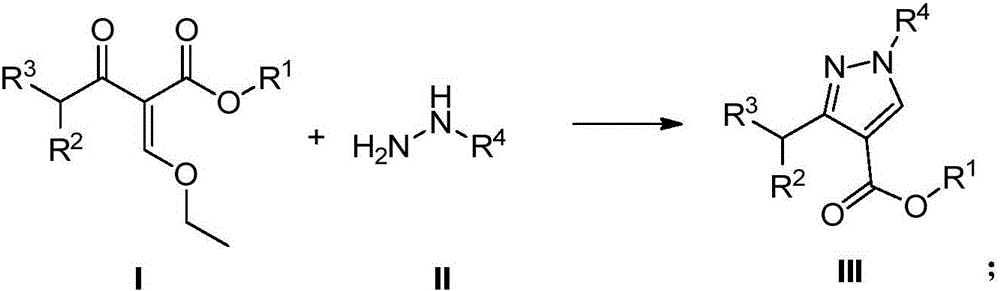
Pyrazole compound preparation method
InactiveCN106800535AEnhance heat and mass transferWell mixedOrganic chemistryHalogenRegioselectivity
The invention discloses a preparation method of a pyrazole compound which is shown as a formula III. The preparation method disclosed by the invention comprises the following step of introducing a compound which is shown as a formula I and a compound which is shown as a formula II into a micro-channel reactor through different pipelines to react to obtain the pyrazole compound, wherein reaction temperature is 10 to 60 DEG C, R1 is C1-C4 alkyl, R2 and R3 are halogen independently, and R4 is methyl or phenyl. The preparation method disclosed by the invention is performed through the micro-channel reactor, has extremely-short reaction time, accurate reaction condition control and high safety, is suitable for quickly preparing products, achieves continuous production and has low cost; furthermore, the micro-channel reactor is utilized to prepare the pyrazole compound, so that reaction region selectivity is high, target compound purity is good, and the preparation method is more suitable for large-scale industrial production.
Owner:LIANHE CHEM TECH TAIZHOU
Method for preparing 2-ethylhexyl chloroformate through continuous flow of micro-channel reactor
InactiveCN111269122AImprove conversion rateTransfer in timeChemical/physical/physico-chemical microreactorsPreparation from phosgene or haloformatesEthyl groupProcess engineering
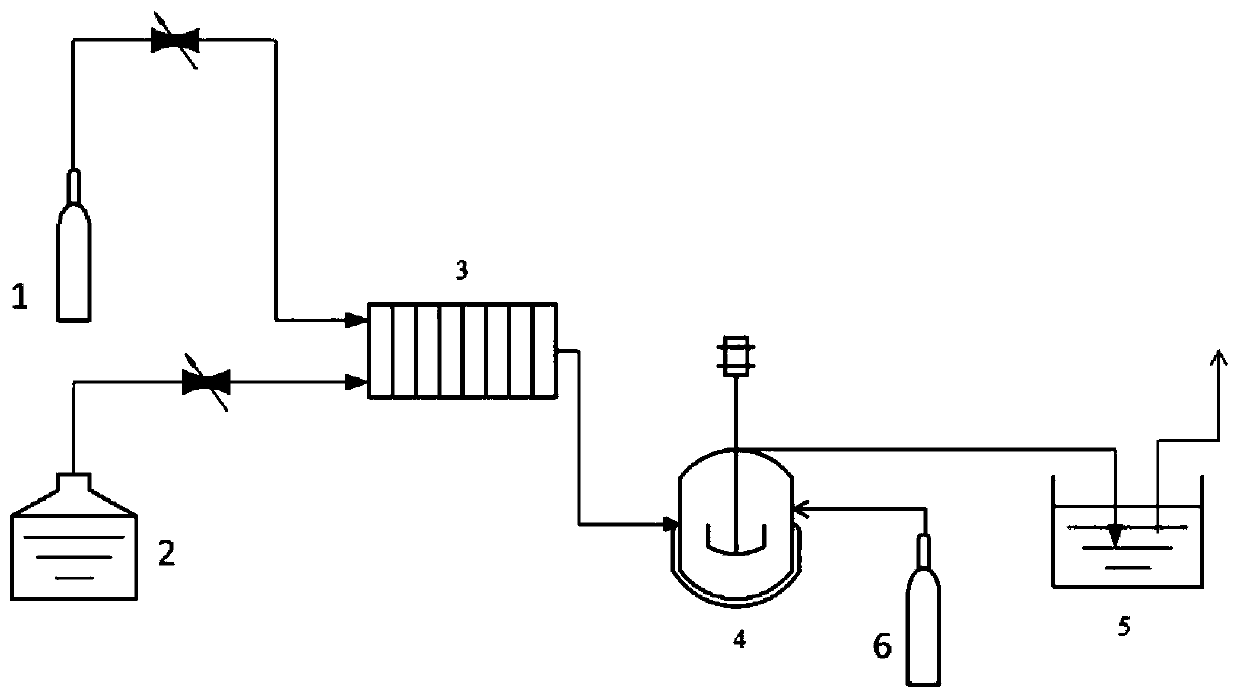
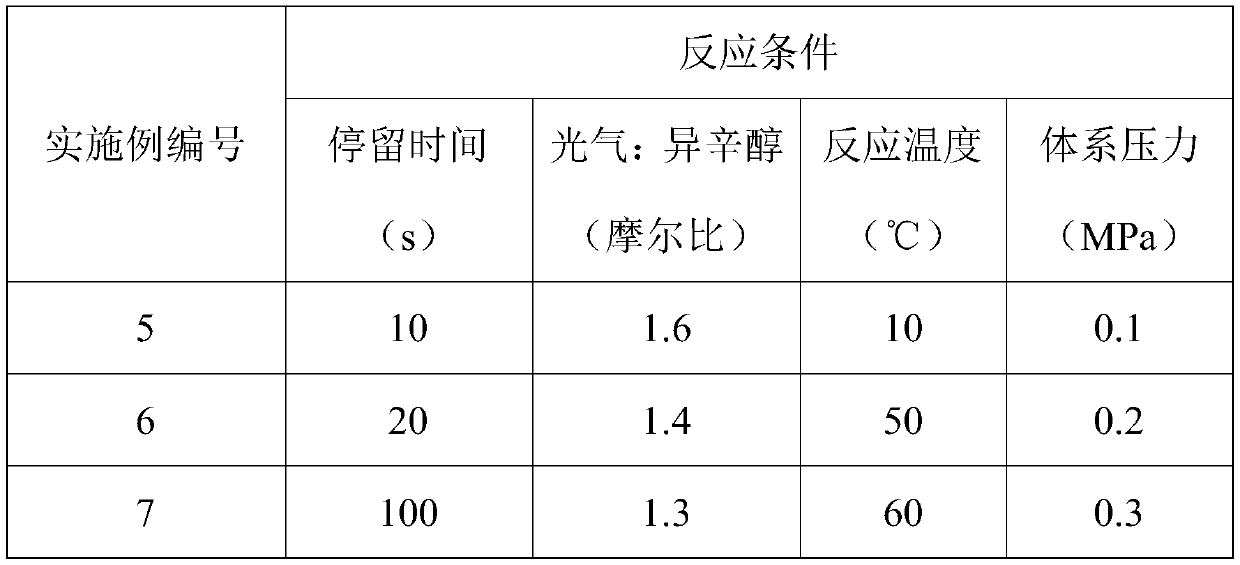
The invention provides a method for preparing 2-ethylhexyl chloroformate through continuous flow of a micro-channel reactor. According to the method, isooctanol and phosgene react by adopting the micro-channel reactor. The reaction conditions can be accurately controlled, the phosgene utilization rate is high, the reaction time is short, the yield and selectivity of 2-ethylhexyl chloroformate arehigh, and the industrial application value is relatively high.
Owner:JIANGSU YANGNONG CHEM GROUP +3
A kind of continuous production process of hexafluoropropylene oxide
ActiveCN104672177BEnhance heat and mass transferHigh reaction conversion rateOrganic chemistryTemperature controlHexafluoropropylene oxide
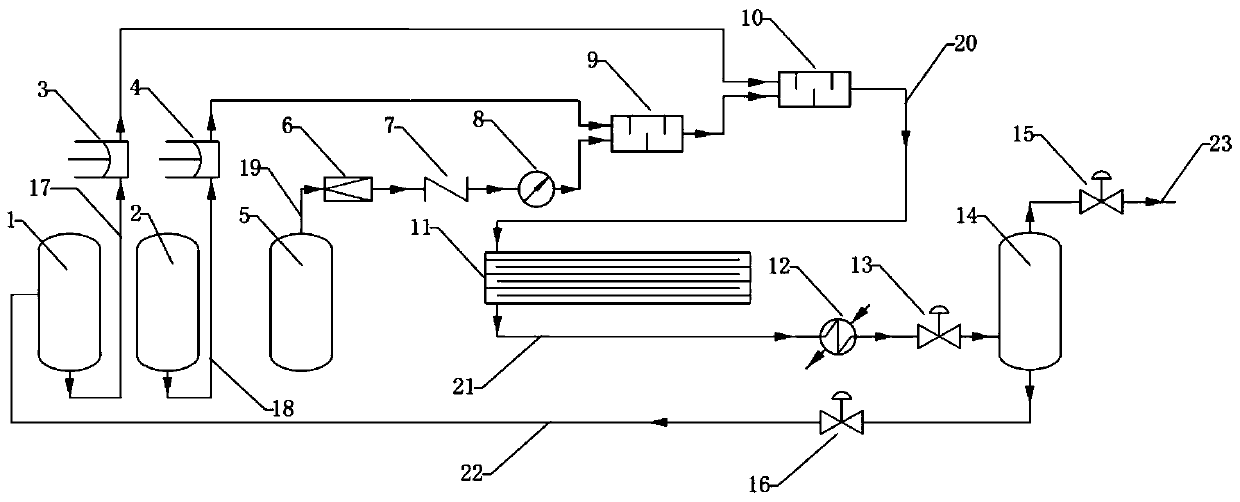
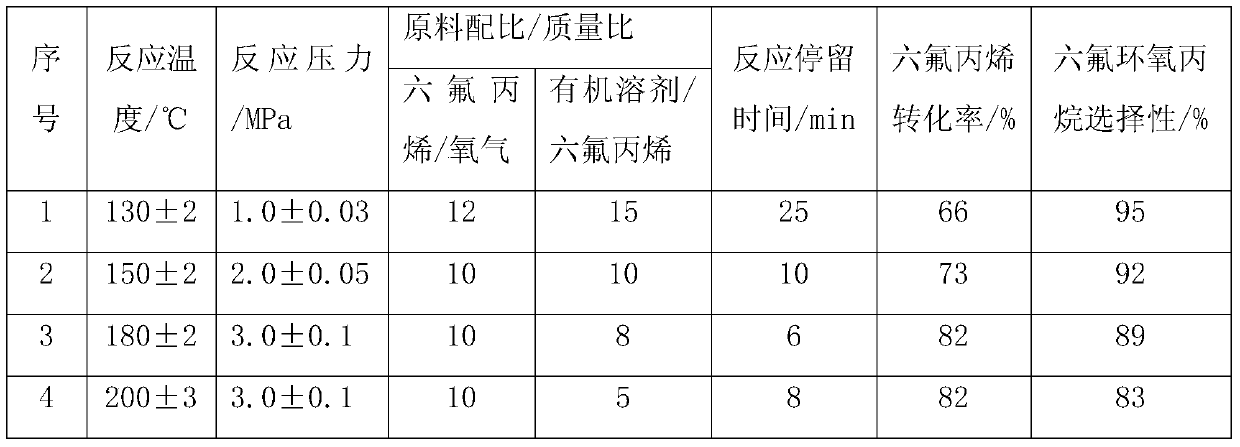
The invention discloses a hexafluropropylene oxide continuous production technique by using a microchannel reactor. The technique can avoid the phenomena of temperature runaway and oxidative burst, implements smooth temperature control, and has the advantages of high reaction conversion rate and favorable selectivity.
Owner:中化蓝天氟材料有限公司 +1
A kind of synthetic method of 3,4-diamino-benzophenone
ActiveCN109467512BPrecise control of reaction conditionsHigh purityOrganic chemistryOrganic compound preparationHydrogenation processCombinatorial chemistry
The invention discloses a method for synthesizing a mebendazole intermediate 3,4-diamino-benzophenone. By strictly controlling the parameters of the ammoniation process and the hydrogenation process, two impurities in the process: 3-amino- The contents of 4-hydroxy-benzophenone and 3-amino-4-chloro-benzophenone are both reduced to less than 0.05%, which ensures that the product quality is qualified and the total yield of the two-step reaction reaches 90%, which has a very high yield. Industrial production value.
Owner:SUZHOU KAIYUAN MINSHENG SCI & TECH CORP
A kind of method that microchannel reaction prepares vinylene carbonate
ActiveCN106749155BFully contactedHigh reaction mass transfer and heat release efficiencyOrganic chemistrySecondary cellsEngineeringSolvent
The invention provides a method for preparing vinylene carbonate through micro channel reaction. The method comprises the following steps: (1) preparing equipment, including an enhanced mass transfer micro channel reactor, a metering pump I, a metering pump II and a micro filter, wherein the enhanced mass transfer micro channel reactor comprises a preheater I, a preheater II, an ultrasonic device and a micro channel module; (2) uniformly mixing chloroethylene carbonate with an ester solvent so as to obtain a mixed liquid, synchronously inputting the preheated mixed liquid and triethylamine into a micro channel, heating, mixing, performing reaction, discharging a product obtained after the reaction is completed from a discharge valve, filtering so as to obtain a crude product, and rectifying the crude product into a rectifying still, so as to obtain the vinylene carbonate, wherein the mole ratio of the chloroethylene carbonate to the triethylamine is 1:(1.5-2.0). The method is simple, convenient and safe to operate, relatively small in quantity of byproducts, small in single solvent consumption, low in moisture content, relatively high in product purity and yield, small in environment pollution and applicable to industrial large-scale production, and continuous production can be achieved.
Owner:江苏瀚康新材料有限公司
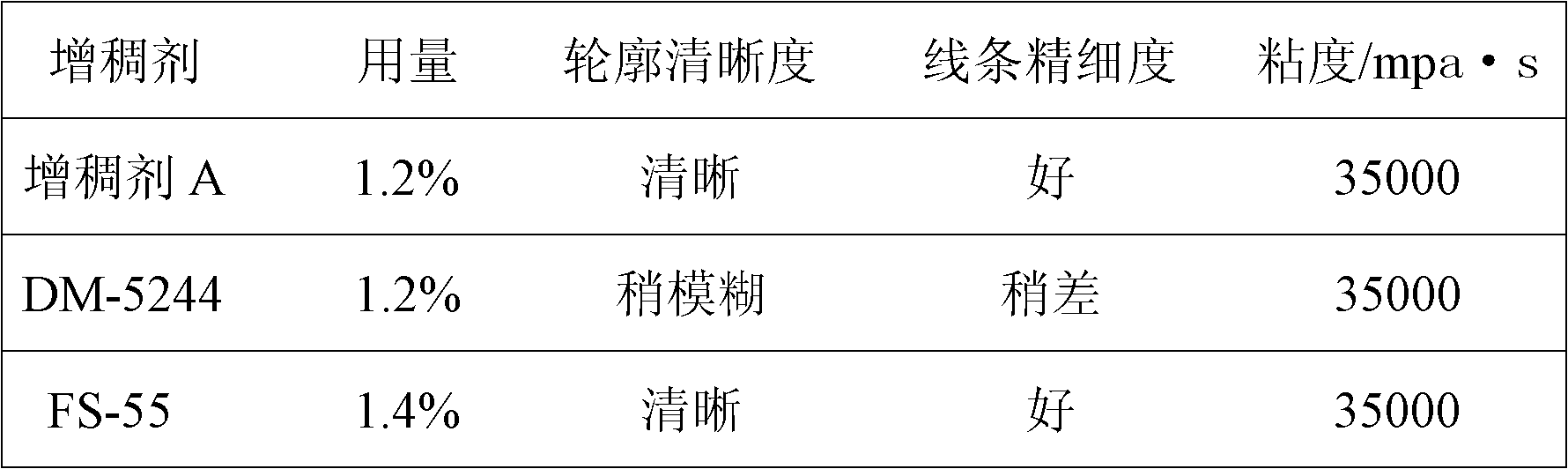
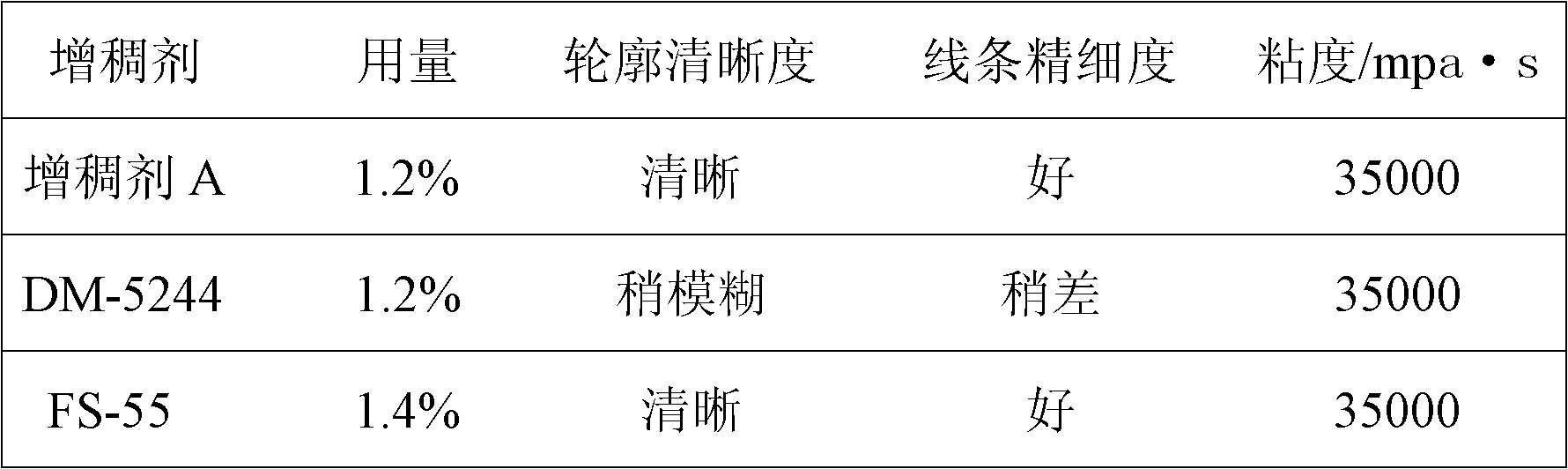
Method for synthesizing coating printing thickening agent
Owner:成都德美精英化工有限公司
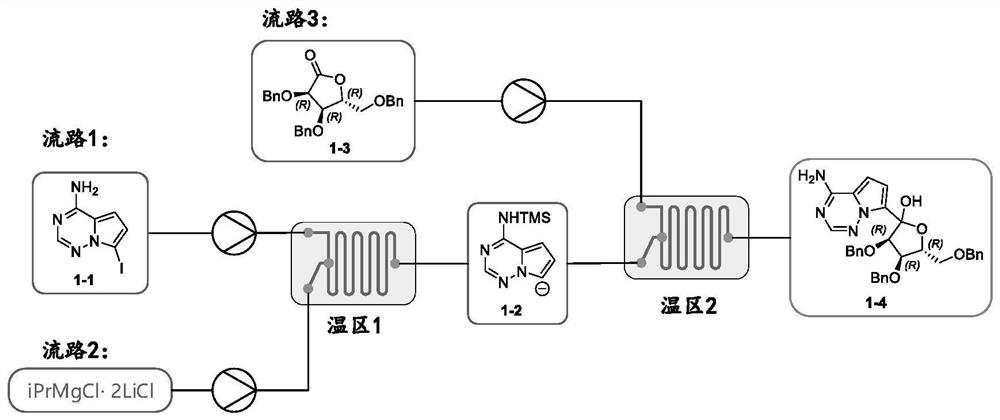
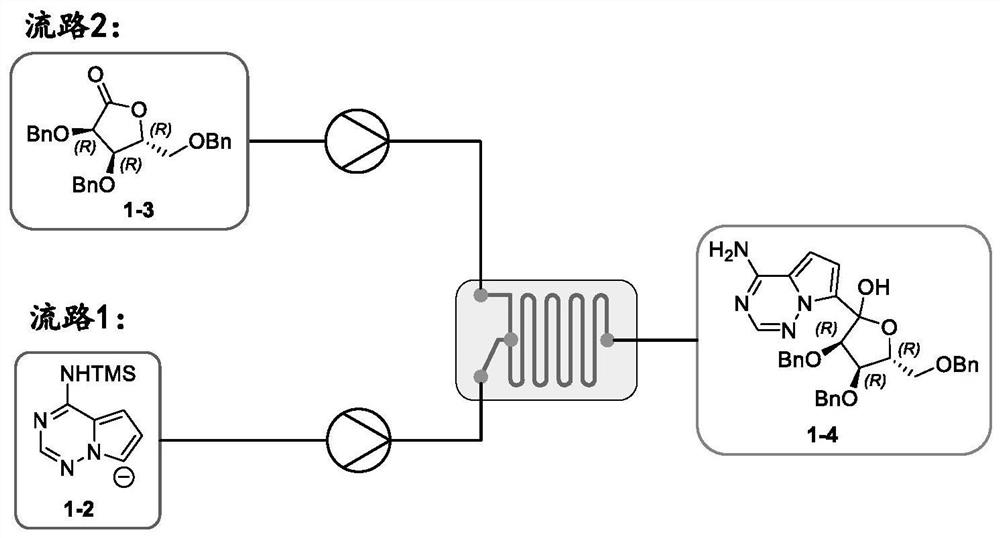
Method for preparing retegravir intermediate by using continuous flow reactor
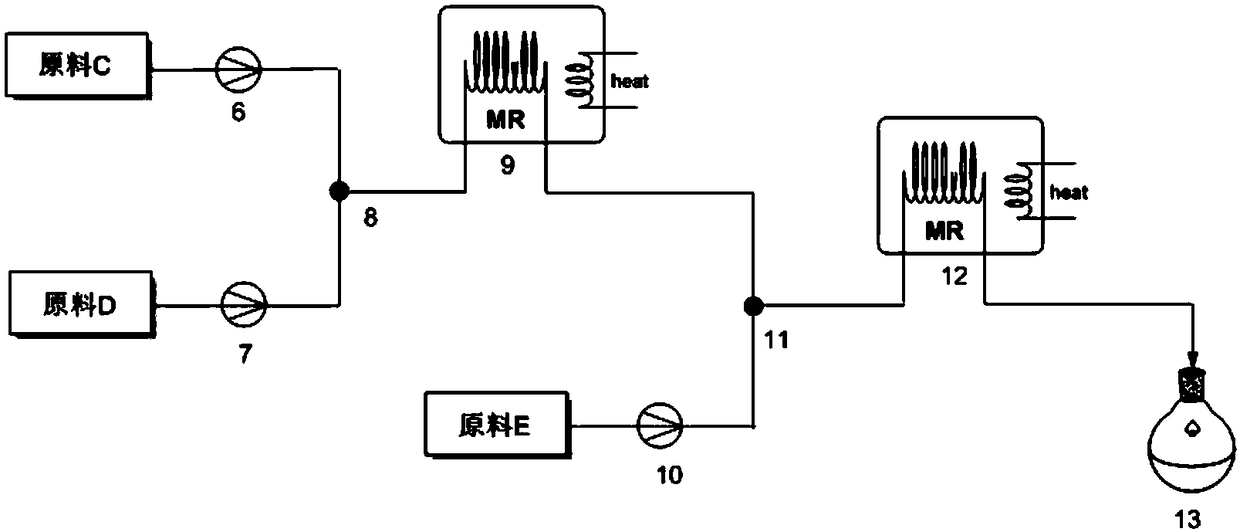
PendingCN112358513AReduce volumePrecise control of reaction conditionsSaccharide with heterocyclic radicalsGroup 4/14 element organic compoundsPyrroleKetone
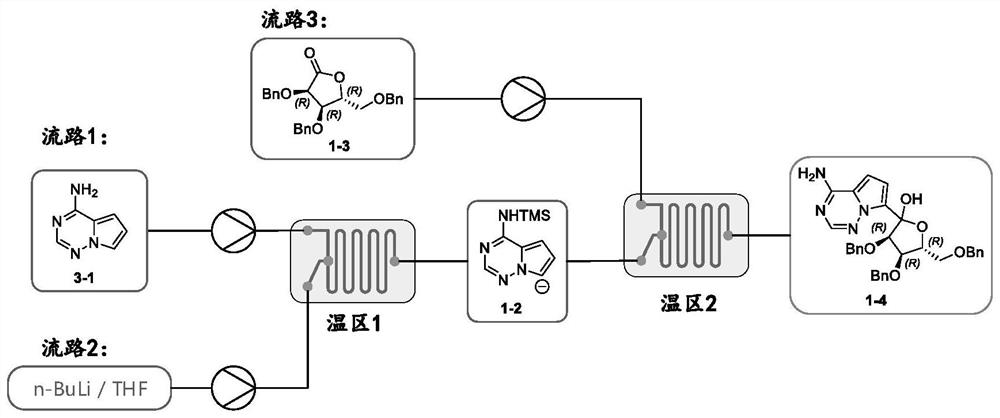
The invention provides a method for preparing a retegravir intermediate (3R, 4R, 5R) 2 (4aminopyrrole [2, 1f] [1, 2, 4] triaza7yl) 3, 4dibenzyl 5benzyl methyl) tetrahydrofuran diol (I) by using a continuous flow reactor. The method comprises the following steps: taking a prepared negative ion solution of an intermediate pyrrole [2,1-f] [1, 2, 4] triaza4amine (III) as a material 1, taking a mixed solution of (3R, 4R, 5R) 3, 4-dibenzyl-5-(benzyl methyl) dihydrofuran-2(3H)-ketone (II), a catalyst and a solvent as a material 2, and reacting through a continuous flow reactor to synthesize a compound (I) at -20 to 0 DEG C for 50-150 seconds. the negative ion solution of the intermediate pyrrole [2,1-f] [1, 2, 4] triaza-4amine (III) is prepared by taking 7halogenated pyrrole [2,1-f] [1, 2, 4] triaza-4-amine or pyrrole [2,1-f] [1, 2, 4] triaza-4amine (IV) as a raw material and enabling the raw material, a metal reagent and the like to pass through a kettle type or continuous flow reactor. Compared with an existing conventional tank reactor, the process is short in reaction time and small in liquid holding volume, the temperature of a low-temperature reaction is increased, energy consumption is reduced, the safety of the reaction is also improved, and continuous automatic control is facilitated.
Owner:SHANGHAI PUYI CHEM CO LTD
A kind of preparation method of lithium dioxalate borate
InactiveCN101602773BSimple processOvercome the disadvantages of complex processGroup 3/13 element organic compoundsOxalateSlurry
A kind of preparation method of lithium bisoxalate borate of the present invention relates to boron compound, and its process step is: the first step, raw material is mixed under the liquid phase condition and forms raw material slurry: analytically pure solid raw material ammonium oxalate, lithium iodide and The boron-containing oxygen compound is added to the aqueous ethanol solution, stirred and mixed evenly to form a raw material slurry; the second step, liquid phase reaction to prepare lithium bisoxalate borate slurry: the raw material slurry obtained in the first step is placed in an airtight container Inside, react to prepare lithium bisoxalate borate slurry; the third step, solid-phase burning to obtain lithium bisoxalate borate finished product: place the lithium bisoxalate borate slurry prepared in the second step in a vacuum oven , dried to powder, then placed in an electric furnace, heated to 200-240°C at a rate of 5-10°C / min, and then kept at this temperature for 1-6 hours to obtain the finished product of lithium bisoxalate borate. The process of the method of the invention is simple, and the obtained lithium bisoxalate borate product has high purity and high yield, and is suitable for large-scale production.
Owner:HEBEI UNIV OF TECH
Two-source controllable SiO production system and production method
ActiveCN106966398ASolve technical problems with low production efficiencyQuality improvementChemical industrySilicon oxidesThermodynamicsSilicon monoxide
Provided are a two-source controllable SiO production system and a production method. The technical problem of low production efficiency of a traditional SiO production system can be solved. The two-source controllable SiO production system comprises a double-crucible sending heating part and a static collecting device, wherein the double-crucible sending heating part is arranged in a stainless steel vacuum cavity and comprises double crucibles and two sensing heating coils, and the two sensing heating coils are arranged outside the double crucibles respectively. The static collecting device comprises a collecting pipe and a collecting rod, wherein the inside of the collecting pipe is a static metal collecting cavity, the collecting rod is arranged in the collecting pipe, and the collecting pipe is arranged above the stainless steel vacuum cavity and communicated with the inside of the stainless steel vacuum cavity. The two-source controllable SiO production system further comprises an electric control cabinet, wherein the electric control cabinet is connected with the sensing heating coils. The two-source controllable SiO production system adopts two-source control and can accurately control reaction conditions, a silicon monoxide production period can be shortened, energy consumption can be reduced, large-scale silicon monoxide production can be achieved, and the exploratory research requirements of the scientific research institutions can be also met.
Owner:合肥科晶材料技术有限公司
Phosphorus-doped ternary lithium ion positive electrode material, preparation method thereof and lithium ion battery
PendingCN112635750AUniform shapeNo aggregationCell electrodesSecondary cellsDischarge efficiencyPhysical chemistry
The invention provides a phosphorus-doped ternary lithium ion positive electrode material, a preparation method thereof and a lithium ion battery. The preparation method comprises the following steps: S1, dissolving a nickel source, a cobalt source, a manganese source and a phosphorus source into a solvent to obtain a mixed solution; S2, in an inert environment, enabling the mixed solution to be subjected to a staged reaction under different pH values to obtain a precursor; and S3, mixing the precursor with a lithium source, and performing sintering to obtain the phosphorus-doped ternary lithium ion positive electrode material. The phosphorus-doped ternary lithium ion positive electrode material prepared by the method is uniform in form, and no aggregation phenomenon exists among particles, so that the first charge-discharge efficiency of the lithium ion battery and the cycle performance of the battery are improved.
Owner:YINLONG ENERGY CO LTD
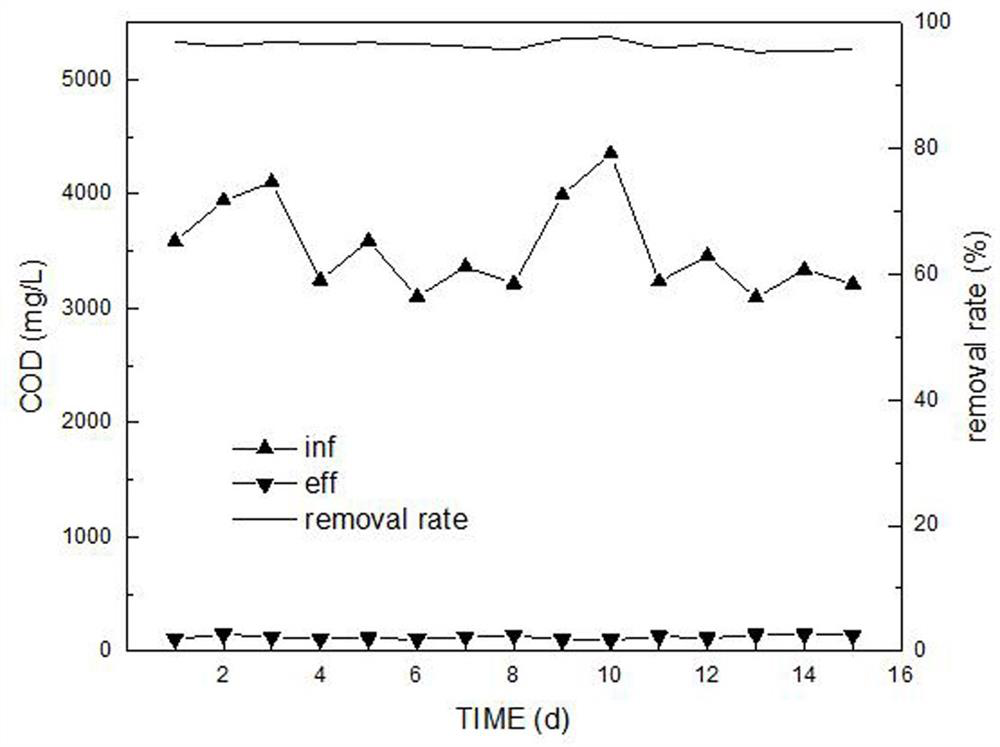
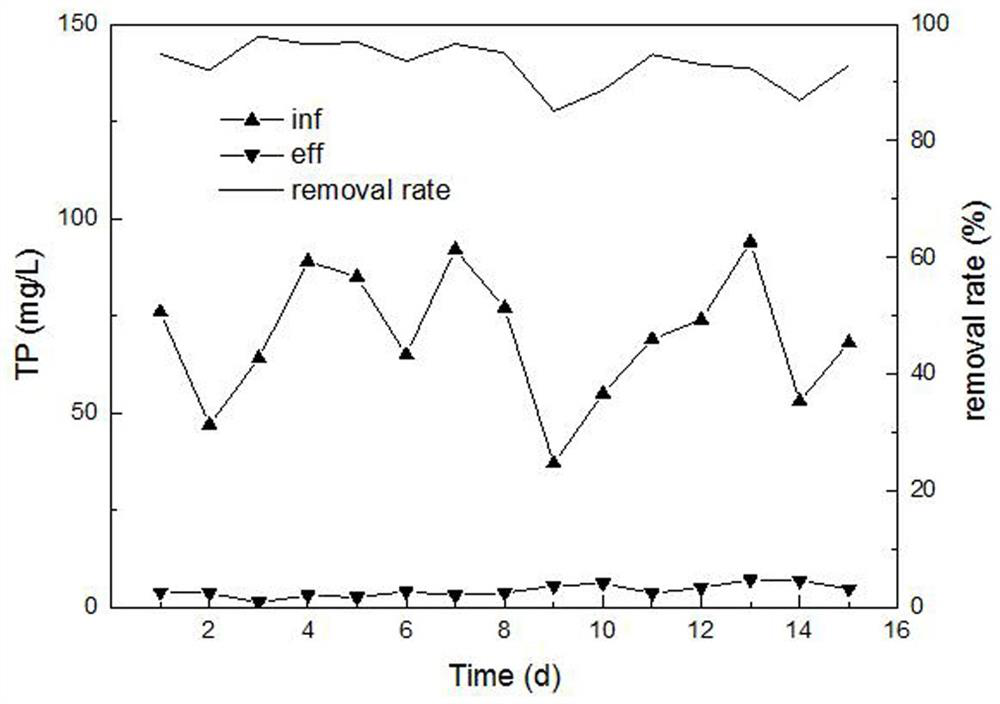
Livestock and poultry breeding wastewater CC-HBDP treatment process
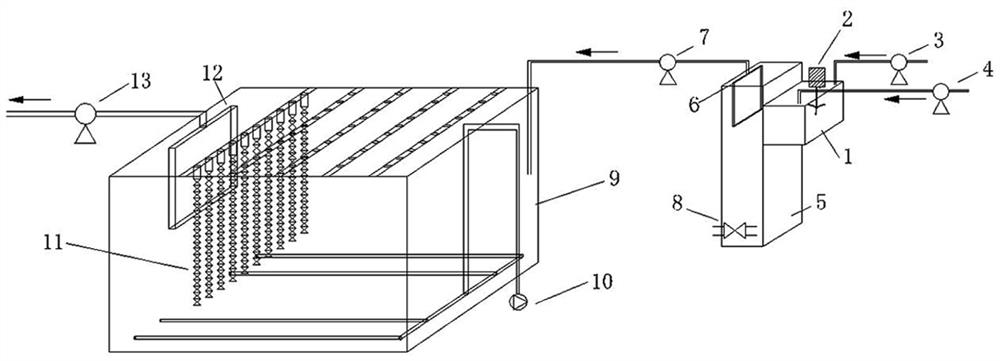
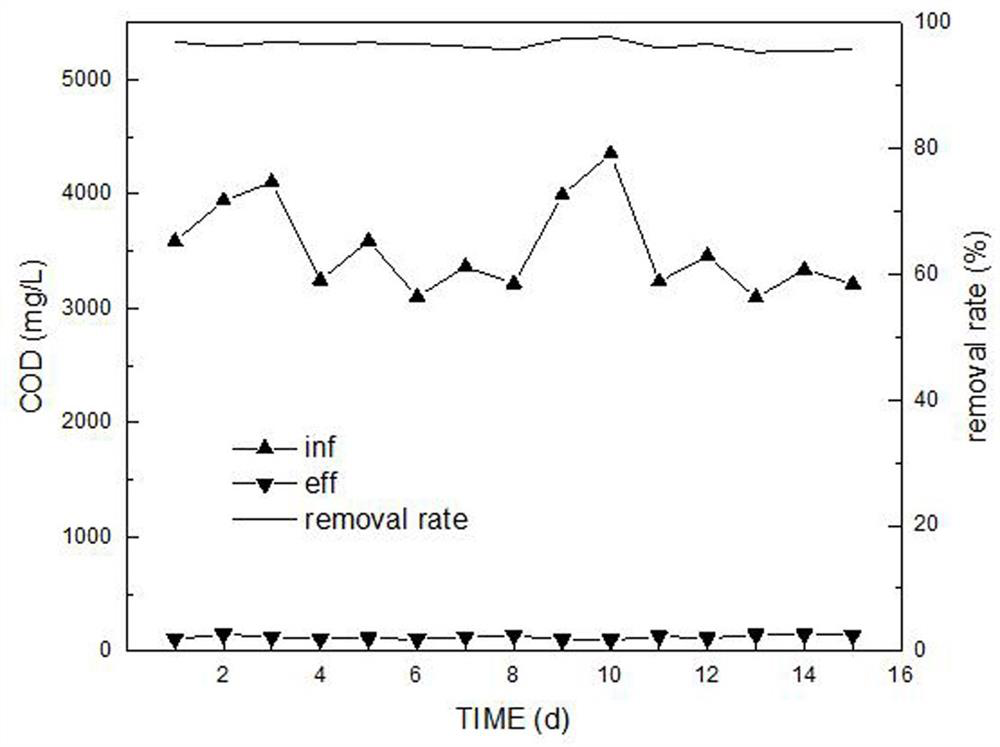
ActiveCN111807650AReduce outputSolve the problem of slow enrichmentSpecific water treatment objectivesWaste water treatment from animal husbandryAmmoniacal nitrogenNitrogen removal
The invention relates to a livestock and poultry breeding wastewater CC-HBDP treatment process, which comprises the following steps of: (1) feeding livestock and poultry breeding wastewater into a coagulating basin, adding a coagulant and performing stirring to generate flocs, and then feeding the mixed solution into a clarification basin; (2) conducting gravity precipitation on a large amount offlocs in the clarification basin, subjecting large-particle organic matters to net capture sweeping to form a particle floating bed, enabling the mixed solution to pass through the particle floating bed in an up-flow mode, then intercepting the flocs with low density by first solid-liquid separation equipment, and letting the separated supernatant with a low carbon-nitrogen ratio enter a mixed denitrification basin; and (3) ammonifying organic nitrogen in the mixed denitrification baisn, converting part of ammonia nitrogen into nitrite nitrogen, converting the nitrite nitrogen and residual ammonia nitrogen into nitrogen and nitrate nitrogen, converting the nitrate nitrogen and part of organic matters into nitrogen, thus finally realizing synergistic efficient denitrification, and discharging the treated water after passing through second solid-liquid separation equipment. The synergistic effect of autotrophic bacteria nitrogen removal and heterotrophic bacteria denitrification has theadvantages of high nitrogen removal efficiency, low energy consumption and low sludge yield.
Owner:FUJIAN HAIXIA ENVIRONMENTAL PROTECTION GRP +1
Method for synthesizing coating printing thickening agent
The invention discloses a method for synthesizing a coating printing thickening agent. The method comprises the steps of: preparation of polyurethane prepolymer, preparation of polyurethane-polyacrylic acid composite emulsion, dropwise addition of ammonia water into the composite emulsion for pH regulation, and the like. The high thickening capability of polyacrylic acid and the good diffusion resistant effect of polyurethane are utilized, an interpenetrating polymer network synthesis technology is adopted, and a novel coating printing thickening agent with high thickening capability and a good diffusion resistant effect is prepared by stepwise synthesis and accurate control over a reaction condition.
Owner:成都德美精英化工有限公司
Method for preparing zirconia hollow film by microfluidic control
ActiveCN112679221BPrecise thickness controlPrecise control of ingredientsCeramicwareMicrofluidicsThin membrane
Owner:THE UNIV OF NOTTINGHAM NINGBO CHINA
A preparation method of fluoro phosphate materials
ActiveCN102659835AOvercome acid hydrolysisEasy to refineGroup 5/15 element organic compoundsSecondary cellsPhosphoric Acid EstersPhosphate
The present invention provides a preparation method of fluoro phosphate materials, comprising the following steps: adding into a sealed polytetrafluoroethylene-lined anti-corrosion austenitic stainless steel container ethyl-phosphate and iron powder with the mass of the iron powder being 1-2% of that of the ethyl-phosphate, a step of pumping nitrogen into the container to displace air when the temperature of the container reaches 80-92 DEG C via heating in a water bath, a step of slowly adding hydrofluoric acid liquor in a dropwise manner with a molar ratio of HF and (CH3CH2O)PO4 being 2.5-3.5:1], a step of pumping nitrogen into the container again after the adding step until the inner pressure of the container reaches 2-3 atmospheres, a step of ending the reaction by relieving the inner pressure to atmospheric pressure after the container maintains 2-6 hours of constant temperature, a step of distilling the obtained product under reduced pressure, and a final step of dewatering and filtrating the obtained product and therefore the required product is gained. The gained product in the present invention is free from acidic material such as hydrochloric acid produced by the prior art and capable of overcoming the following acidolysis phenomenon while water is the only material left in the product and can be removed. The preparation method has advantages of simple technological process, low cost and suitability for mass production.
Owner:HEBEI UNIV OF TECH
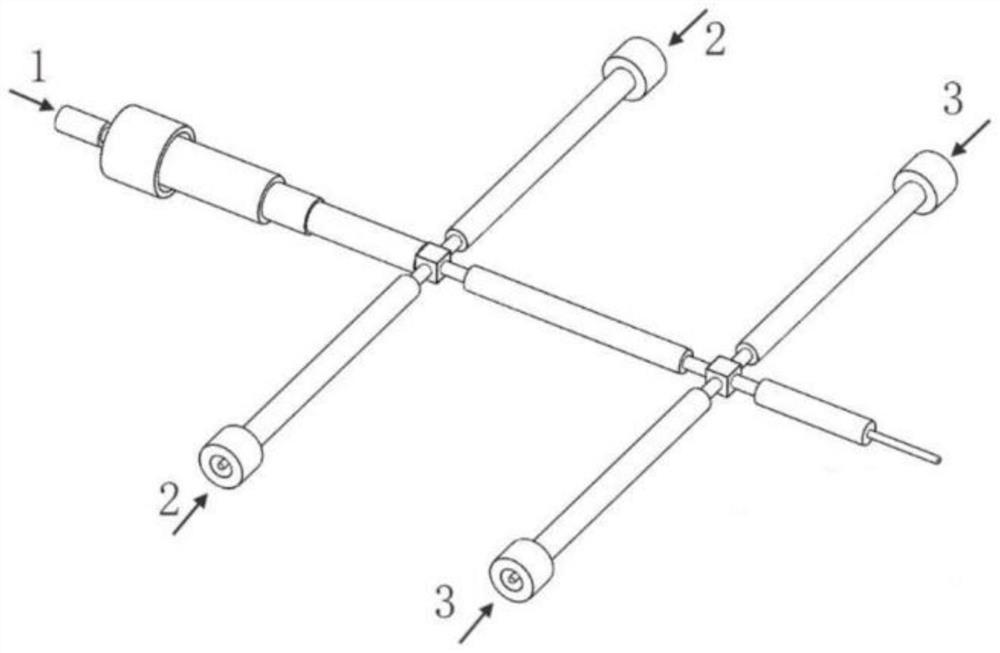
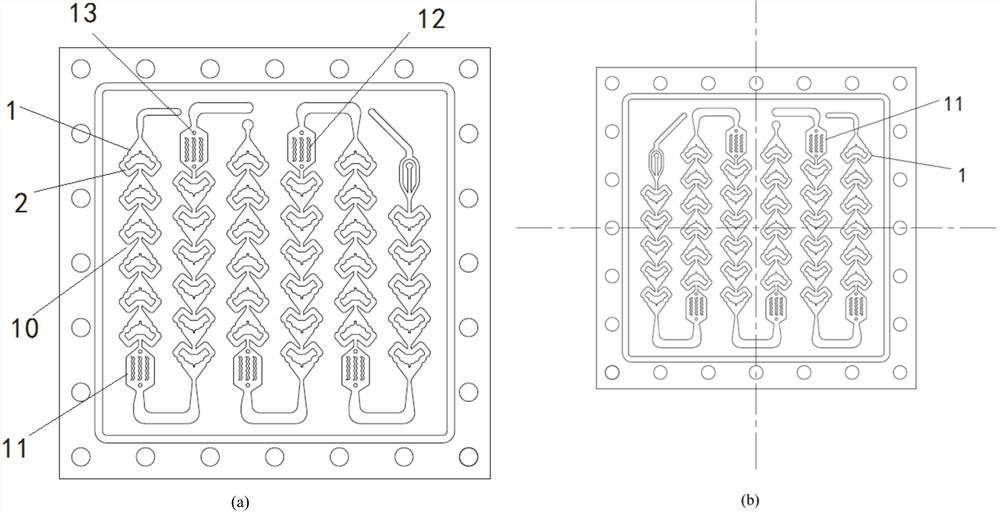
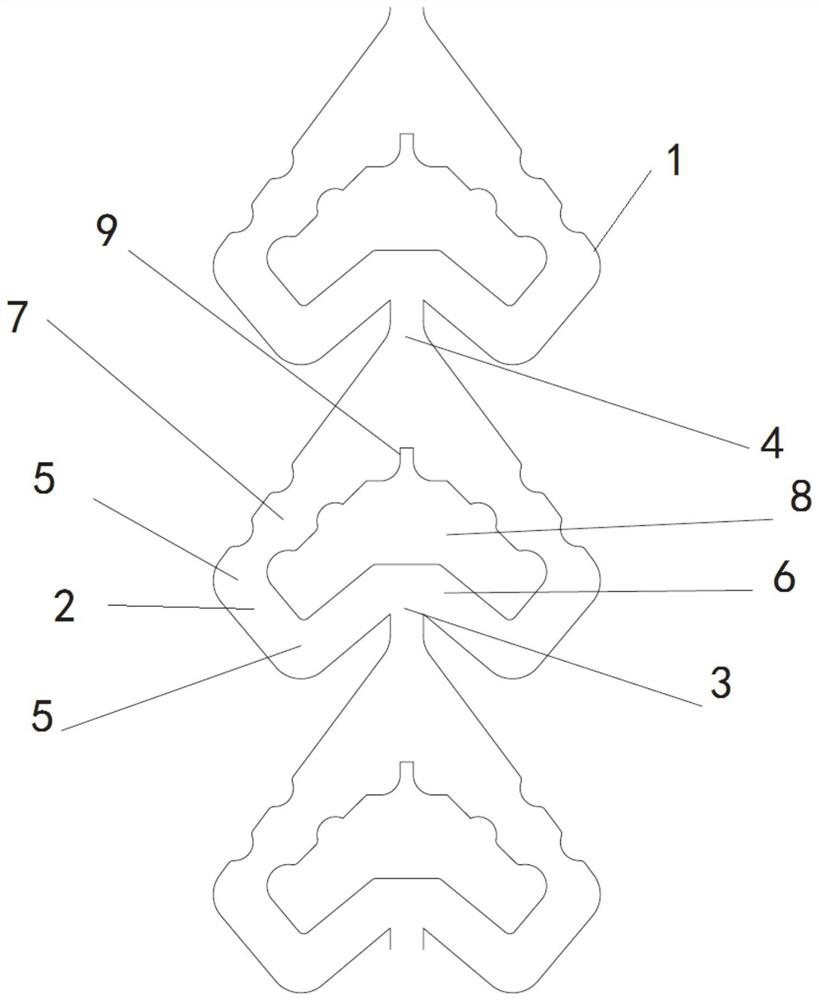
Micro-channel reactor
PendingCN114130326ALow costAdequate responseChemical/physical/physico-chemical microreactorsMicroreactorEngineering
The invention relates to a micro-channel reactor, which comprises a micro-reactor, an annular micro-channel is arranged in the micro-reactor, two opposite ends of the annular micro-channel are respectively a liquid inlet and a liquid outlet, the annular micro-channel has a straight corner structure between the liquid inlet and the liquid outlet, a liquid inlet included angle is formed between the liquid inlet direction of the liquid inlet and the annular micro-channel on two sides, and a liquid outlet included angle is formed between the liquid inlet direction of the liquid inlet and the annular micro-channel on two sides. The liquid inlet included angle is larger than 90 degrees. The mass and heat transfer process is enhanced, the process reaction conditions can be reduced, the reaction of the raw materials is more sufficient, the reaction time is shortened, byproducts in the reaction process can be reduced, the production efficiency of enterprises is improved, the enterprise cost is reduced, and the subsequent environmental protection pressure is reduced. Meanwhile, the research and development period is shortened, and the research and development efficiency is improved.
Owner:山东科加工业技术研究院有限公司
Method for syntheizing 3,4-diamido-benzophenone
ActiveCN109467512APrecise control of reaction conditionsHigh purityOrganic chemistryOrganic compound preparationHydrogenBenzophenone
The invention discloses a method for syntheizing 3,4-diamido-benzophenone. By strictly controlling ammoniation process parameters and hydrogen process parameters, the contents of two impurities in theprocess, namely 3-amino-4-hydroxyl-benzophenone and 3-amino-4-chlorine-benzophenone, are both reduced to 0.05% or less, so that the total yield of two steps of reactions is up to 90% while product quality is ensured, and very high industrial production values can be made.
Owner:SUZHOU KAIYUAN MINSHENG SCI & TECH CORP
A device, method and application for synthesizing drug-loaded metal organic framework materials based on microfluidic one-pot method
ActiveCN109662946BPrecise control of reaction conditionsGood reproducibilityOrganic active ingredientsPowder deliveryCapillary TubingMetal-organic framework
Owner:CHINA PHARM UNIV
A kind of method for preparing n-alkyl-4-nitrophthalimide
ActiveCN109305933BReduce dosageReduce the discharge of three wastesOrganic chemistryOrganic synthesisNitration
The invention belongs to the technical field of organic synthetic processes, and relates to a method for preparing N-alkyl-4-nitrophthalimide by adopting N-alkyl phthalimide as a raw material, nitricacid as a nitrating agent, and performing low-temperature nitration reaction by virtue of a continuous flow to prepare the series product of N-alkyl-4-nitrophthalimide. The method is short in reactiontime and short in production period, solves the problems produced by the accumulation of raw materials in an intermittent reaction kettle, is more stable in the reaction process, and can significantly improve the reaction efficiency. The mass transfer performance and heat conduction performance in a micro-channel reactor of different structures can be improved, the constant reaction temperature can be kept, the temperature runaway phenomenon can be avoided, the generation of byproducts can be reduced, and the safety of the reaction process can be improved. By adopting the high mass transfer effect in the micro-channel reactor, a liquid-liquid reaction solution is sufficiently mixed, in the reaction process, the consumption of concentrated sulfuric acid and nitric acid can be greatly reduced, and the generation of waste acid can be reduced.
Owner:ZHEJIANG WANFENG CHEM
A cc-hbdp treatment process for livestock and poultry breeding wastewater
ActiveCN111807650BReduce outputSolve the problem of slow enrichmentSpecific water treatment objectivesWaste water treatment from animal husbandryAmmoniacal nitrogenNitration
The invention relates to a CC-HBDP treatment process for livestock and poultry breeding wastewater, comprising the following steps: (1) livestock and poultry breeding wastewater enters a coagulation tank, adds a coagulant and stirs to form flocs, and then the mixed liquid enters a clarifier; ( 2) A large number of flocs are gravity-precipitated in the clarifier, and the large particles of organic matter are caught and swept by the net to form a particle floating bed. The flocs are intercepted, and the separated low-carbon nitrogen ratio supernatant enters the mixed denitrification tank; (3) In the mixed denitrification tank, the organic nitrogen is ammoniated, and part of the ammonia nitrogen is converted into nitrite nitrogen, nitrous nitrogen and The remaining ammonia nitrogen is converted into nitrogen and nitrate, and the nitrate and part of the organic matter are converted into nitrogen, so as to finally achieve synergistic and efficient denitrification, and the treated water is discharged through the second solid-liquid separation equipment. The synergistic effect of autotrophic bacteria denitrification and heterotrophic bacteria denitrification has the advantages of high denitrification efficiency, low energy consumption and low sludge yield.
Owner:FUJIAN HAIXIA ENVIRONMENTAL PROTECTION GRP +1
A kind of preparation method of fluorophosphate material
ActiveCN102659835BOvercome acid hydrolysisEasy to refineGroup 5/15 element organic compoundsSecondary cellsWater bathsPhosphate
The present invention provides a preparation method of fluoro phosphate materials, comprising the following steps: adding into a sealed polytetrafluoroethylene-lined anti-corrosion austenitic stainless steel container ethyl-phosphate and iron powder with the mass of the iron powder being 1-2% of that of the ethyl-phosphate, a step of pumping nitrogen into the container to displace air when the temperature of the container reaches 80-92 DEG C via heating in a water bath, a step of slowly adding hydrofluoric acid liquor in a dropwise manner with a molar ratio of HF and (CH3CH2O)PO4 being 2.5-3.5:1], a step of pumping nitrogen into the container again after the adding step until the inner pressure of the container reaches 2-3 atmospheres, a step of ending the reaction by relieving the inner pressure to atmospheric pressure after the container maintains 2-6 hours of constant temperature, a step of distilling the obtained product under reduced pressure, and a final step of dewatering and filtrating the obtained product and therefore the required product is gained. The gained product in the present invention is free from acidic material such as hydrochloric acid produced by the prior art and capable of overcoming the following acidolysis phenomenon while water is the only material left in the product and can be removed. The preparation method has advantages of simple technological process, low cost and suitability for mass production.
Owner:HEBEI UNIV OF TECH
A kind of electrochemically modified carbon cloth reinforced friction material and preparation method thereof
The invention provides an electrochemically modified carbon cloth reinforced friction material and a preparation method thereof. The method uses carbon fiber cloth and modified phenolic resin as raw materials, treats the carbon cloth under electrochemical conditions, selects a composite nickel source as the electrolyte, and adopts Carbon fibers are treated by anodic oxidation and cathodic deposition of metallic nickel. Then spray the modified phenolic resin solution on the treated carbon cloth, take it out and dry it naturally, and use a vulcanizing machine to form it by hot pressing. In the present invention, the carbon cloth is anodized and cathodically Deposition treatment increases the oxygen-containing functional groups on the surface of carbon cloth and uniformly deposits metallic nickel. It has the advantages of simple equipment and high efficiency. Metallic nickel has high hardness, wear resistance and catalytic activity, which greatly improves the bonding strength between carbon cloth and resin. , thereby improving the uniformity of the network polymer formed by the linear polymer through crosslinking during the vulcanization process, so that it has better tribological properties.
Owner:SHAANXI UNIV OF SCI & TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com