Patents
Literature
32 results about "Benzylamine hydrochloride" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
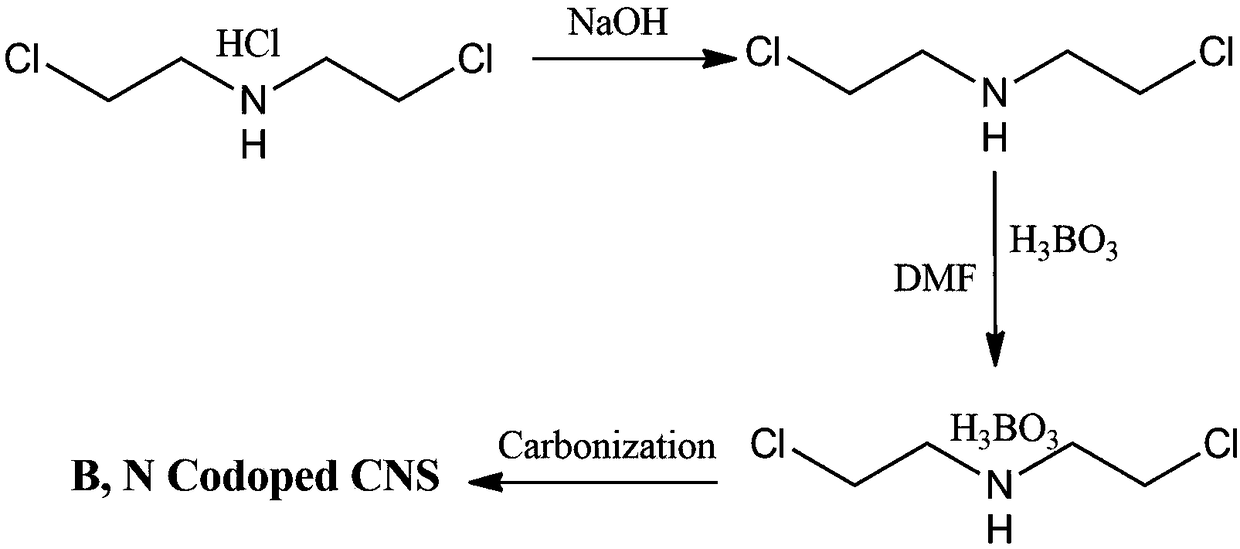
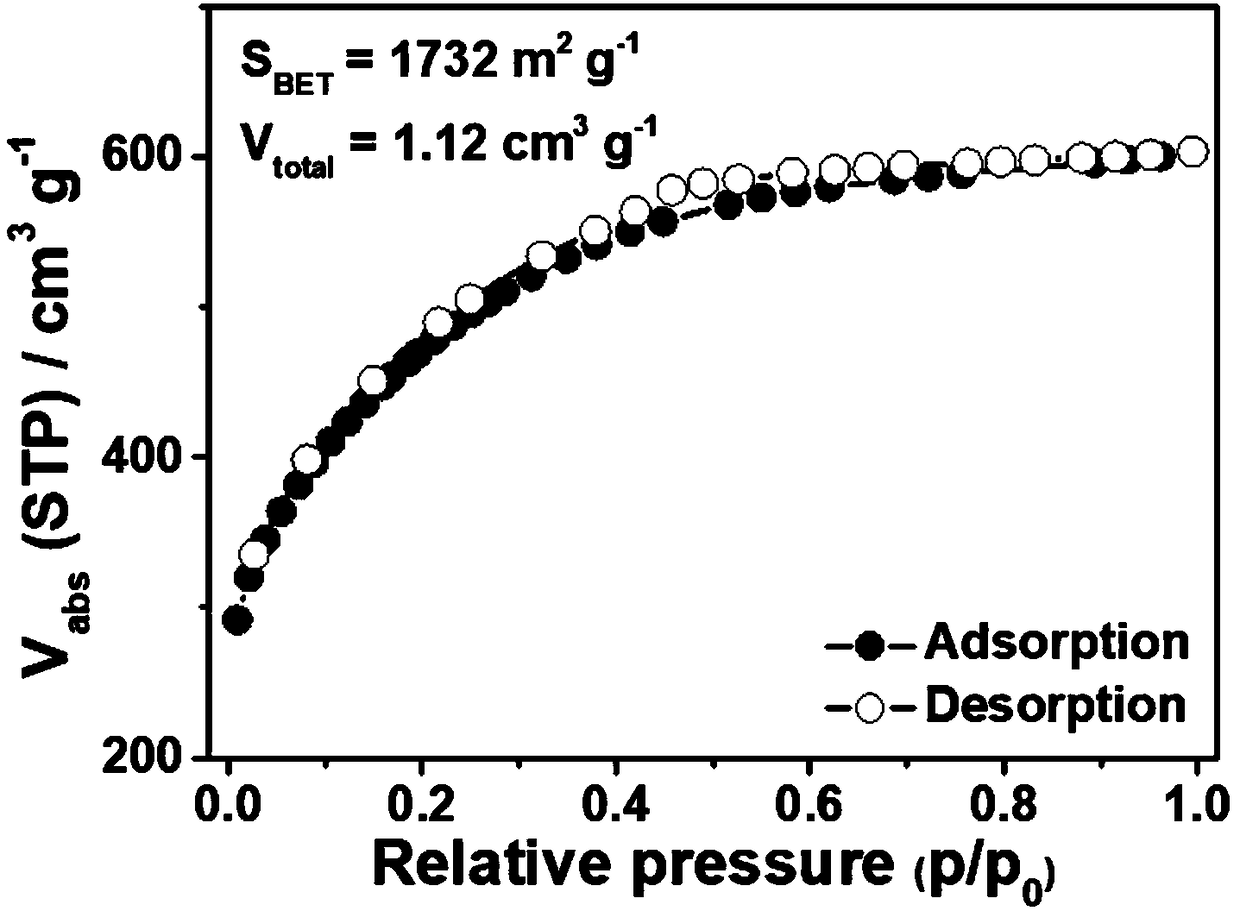
B,N-codoped porous carbon nanosheet and preparation method and usage thereof
InactiveCN108529591ASimple processLow costHybrid capacitor electrodesNano-carbonPorous carbonNitrogen
The invention discloses a B,N-codoped porous carbon nanosheet and a preparation method. The preparation method comprises the following steps: using bi-(2-chloroethyl)benzylamine hydrochloride as a rawmaterial, mixing with alkali liquor to prepare bi-(2-chloroethyl)amine; then, enabling the bi-(2-chloroethyl)amine to react with boric acid to obtain bi-(2-chloroethyl)amido boric acid; and under theprotection of nitrogen, pyrolyzing the bi-(2-chloroethyl)amido boric acid, successfully preparing the B,N-codoped porous carbon nanosheet. The prepared porous carbon nanosheet is high in B,N content,and large in specific surface area. While the porous carbon nanosheet is used as a super capacitor electrode material, the capacitive performance is high, the rate capability is good, and the cycle life is long. The preparation method is simple in operation, and efficient in economy, and capable of realizing the industrial production of the B,N-codoped porous carbon nanosheet.
Owner:XIANGTAN UNIV
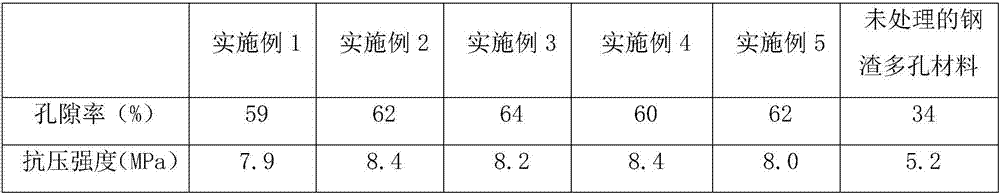
Porous material based on modified steel slag and preparation method of porous material
InactiveCN107117940APromote dissolutionImprove responseGrain treatmentsCeramic materials productionPorositySlag
The invention provides a porous material based on modified steel slag and a preparation method of the porous material. The porous material is prepared by mixing and sintering grinded and refined modified steel slag with coal ash, a pore-forming agent and clay, specifically, the preparation method comprises the following steps: dissolving calcium salt or sodium salt, polyhydric alcohol benzylamine hydrochloride into water so as to form a solution, mixing and stirring dihydric alcohol and an organic alkylol amine compound in sequence, finally adding glacial acetic acid, and uniformly mixing so as to obtain a grinding assisting activating agent; adding the grinding assisting activating agent into the steel slag, and performing high-speed ball milling in a ball mill so as to obtain grinded activated steel slag powder; uniformly mixing the grinded activated steel slag powder with the coal ash, moistening and aging with a small amount of water, adding sufficiently moistened polystyrene foam granules, further adding clay, uniformly mixing, pressing into round blanks, and performing high-temperature sintering, thereby obtaining the porous material based on the modified steel slag. As the modified steel slag which is small in granularity and high in activity is adopted as a raw material, the prepared porous material is moderate in porosity, high in mechanical strength and good in comprehensive property.
Owner:DONGGUAN JIAQIAN NEW MATERIAL TECH CO LTD
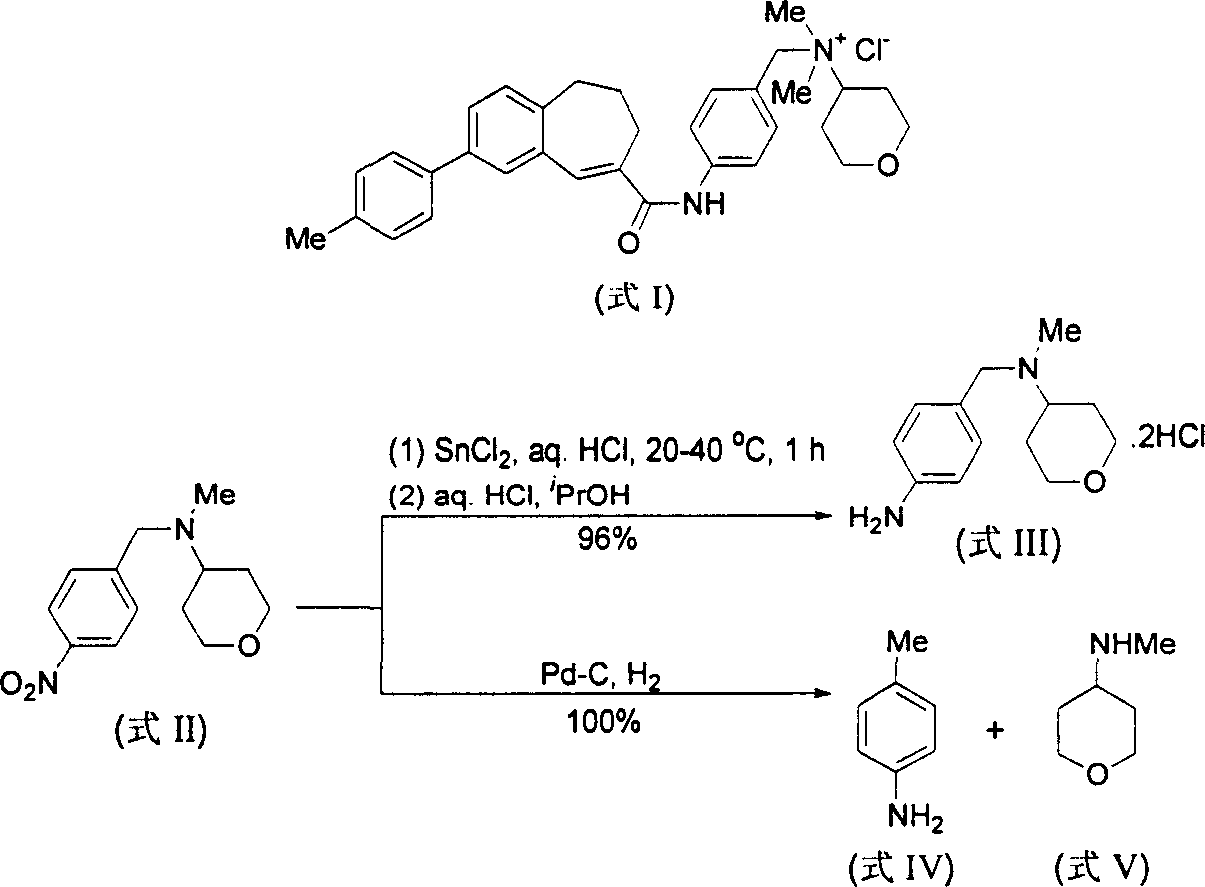
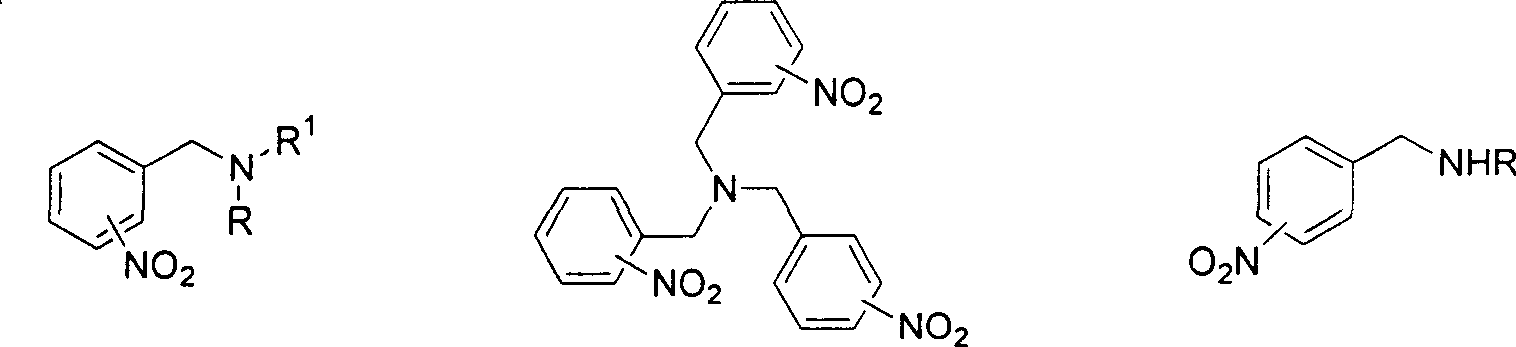
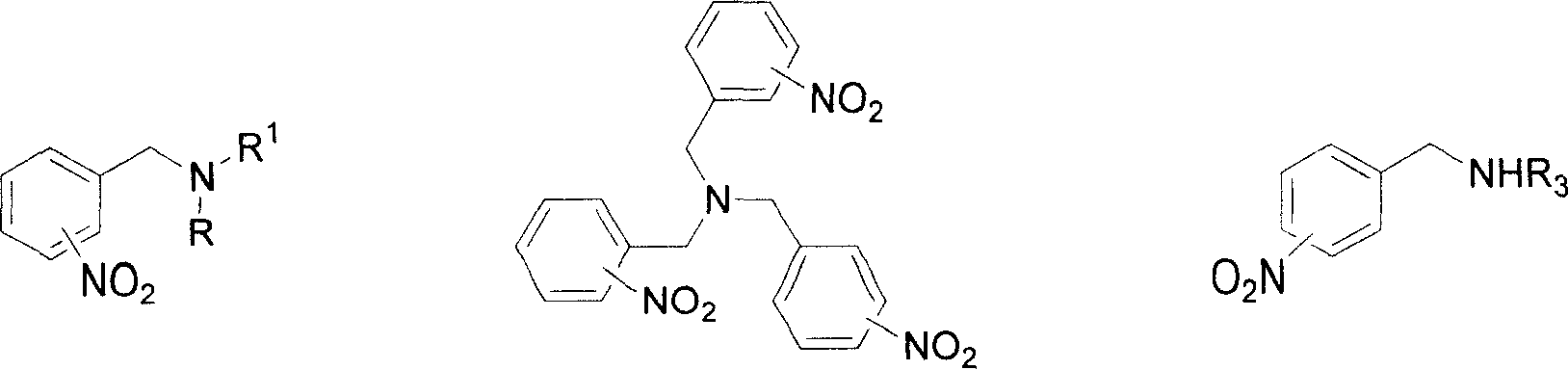
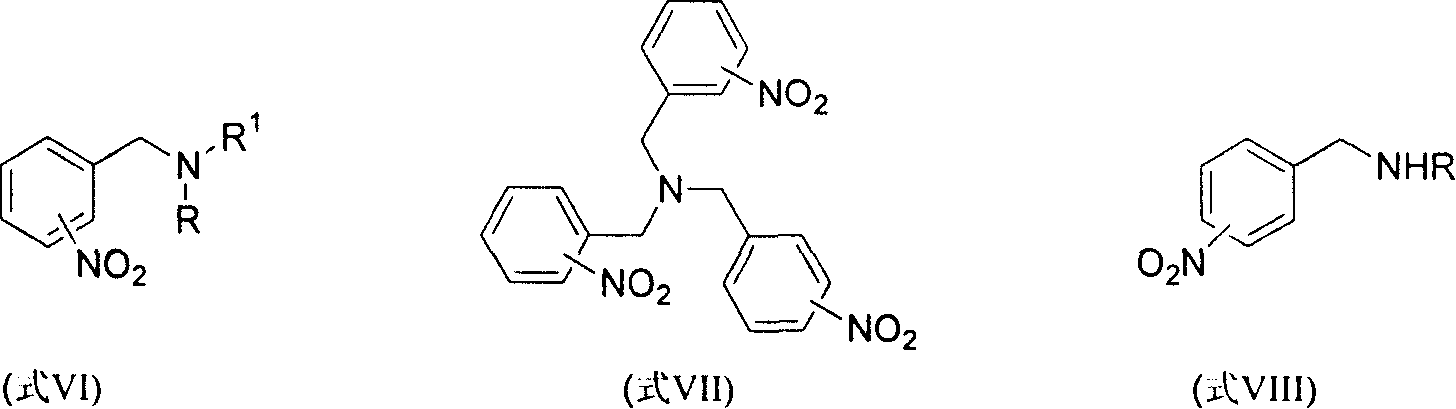
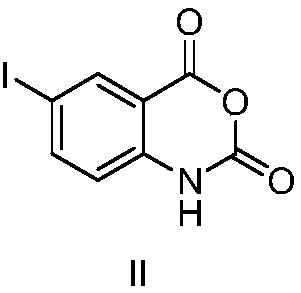
Method for reducing nitroxylbenzyl amine compound to amino-benzylamine hydrochloride
InactiveCN1865221AReduce reactivityEasy post-processingOrganic compound preparationAmino compound preparationHydrogenAmidol
This invention discloses a method for reducing the nitrabenzylamine compound to be nitrabenzylamine hydrochlorate whichreduces the nitrabenzylamine compound under hydrogen gas atmosphere by catalytic hydrogenation, using Pd-C / HCl catalytic system. This invention reduces the reaction activity of Pd-C catalyst by adding HCl or hydrochloric acid or CHCl3 which can produce HCl during the reaction to the reaction system, to entitle the Pd-C catalyst with the activity of catalyzing the nitro group of hydrogenated nitrobenzylamine compound to be amidol, without benzyl hydrogenolysis. This invention is characterized of simple operation, mild reaction condition, convenient treatment of post-production material, and broad application future.
Owner:TSINGHUA UNIV
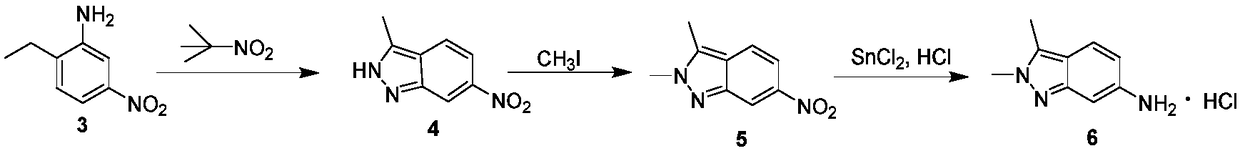
Method for preparing 2, 3-dimethyl-2H-indazole-6-benzylamine hydrochloride
InactiveCN108299304APrecise control of reaction conditionsLess side effectsOrganic chemistryChemical/physical/physico-chemical microreactorsBenzylamine hydrochlorideSide reaction
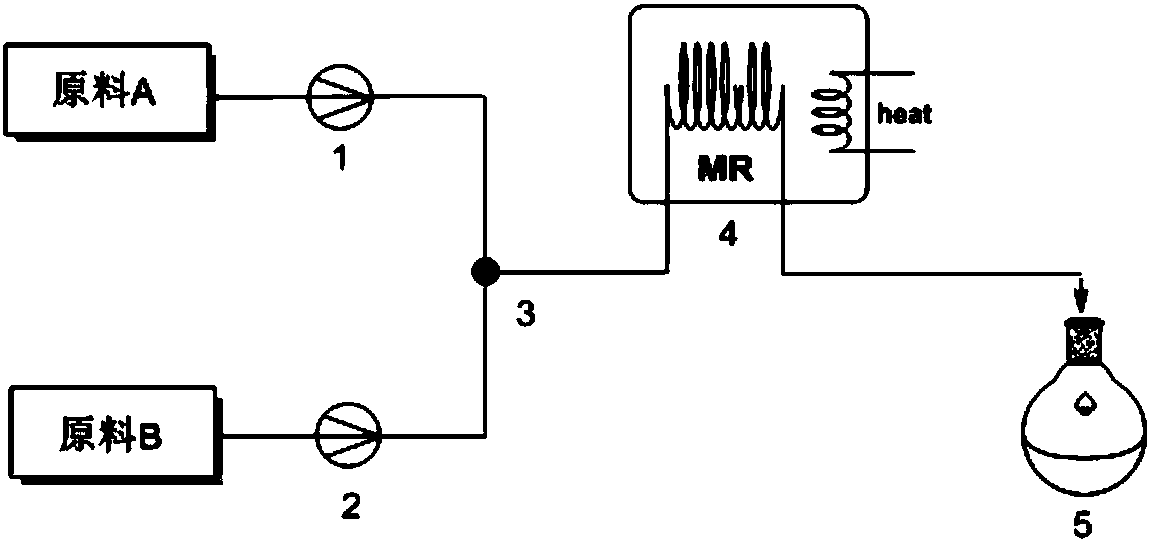
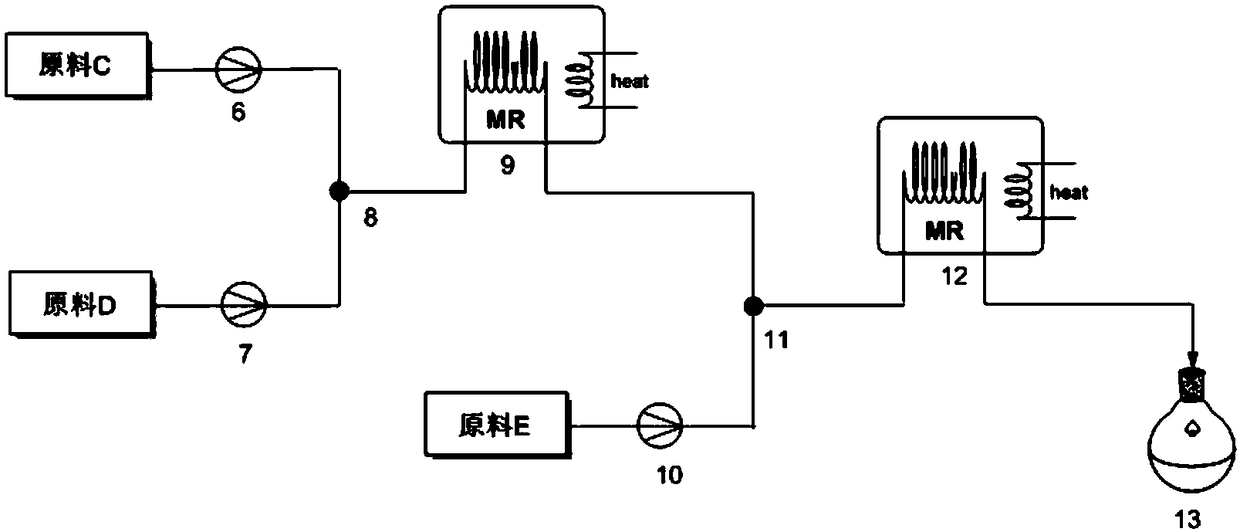
The invention discloses a method for preparing 2, 3-dimethyl-2H-indazole-6-benzylamine hydrochloride. The method comprises the steps of reacting a glacial acetic acid solution of tert-butyl nitrite and a glacial acetic acid solution of 5-nitro-2-ethylaniline in a first microreactor to generate 3-methyl-6-nitro-1H-indazole; reacting a homogeneous solution formed by mixing the 3-methyl-6-nitro-1H-indazole and a dimethyl sulfoxide solution of methyl iodide and a dimethyl sulfoxide solution of sodium ethoxide in a second microreactor to generate 2,3-dimethyl-6-nitro-2H-indazole; then reacting withmixed liquor formed by stirring a concentrated hydrochloric acid solution of stannous chloride and ethyl alcohol in a third microreactor to generate the 2, 3-dimethyl-2H-indazole-6-benzylamine hydrochloride. The method provided by the invention has the advantages of less side reaction, high yield, simplification of a complicated multi-step synthesis process, low toxicity and pollution, low production cost, good product quality, environment friendliness, energy saving and high efficiency, and is suitable for industrialized application.
Owner:CHINA PHARM UNIV
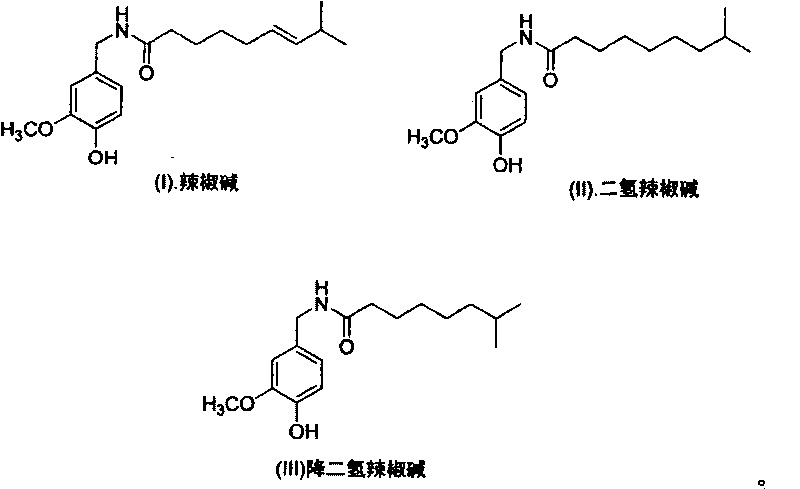
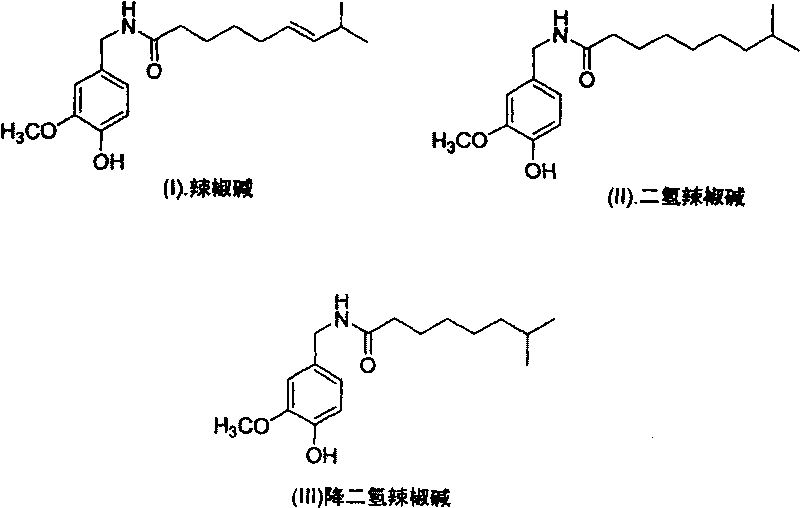
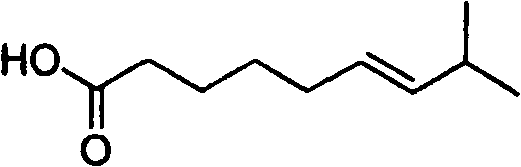
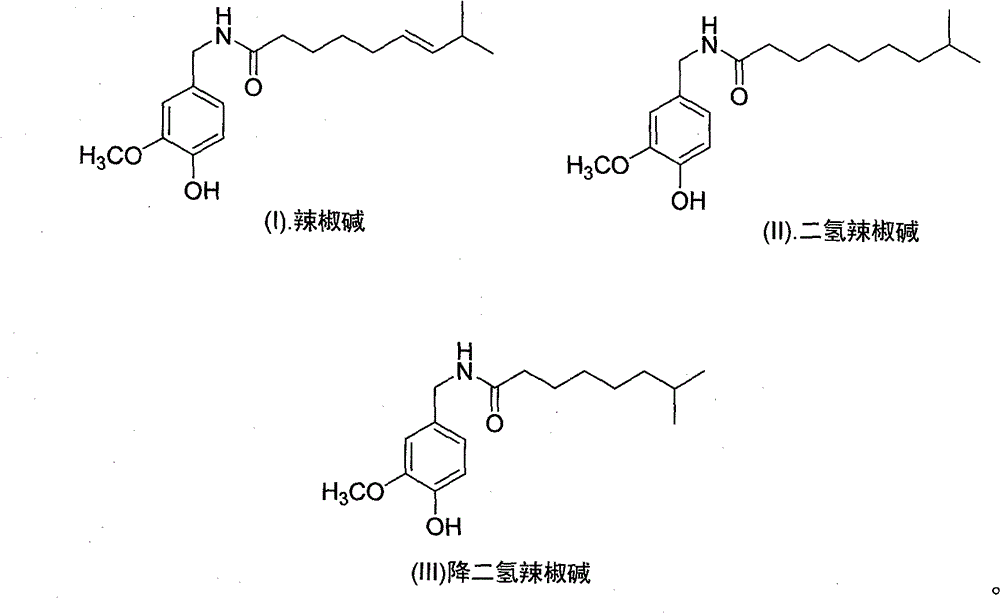
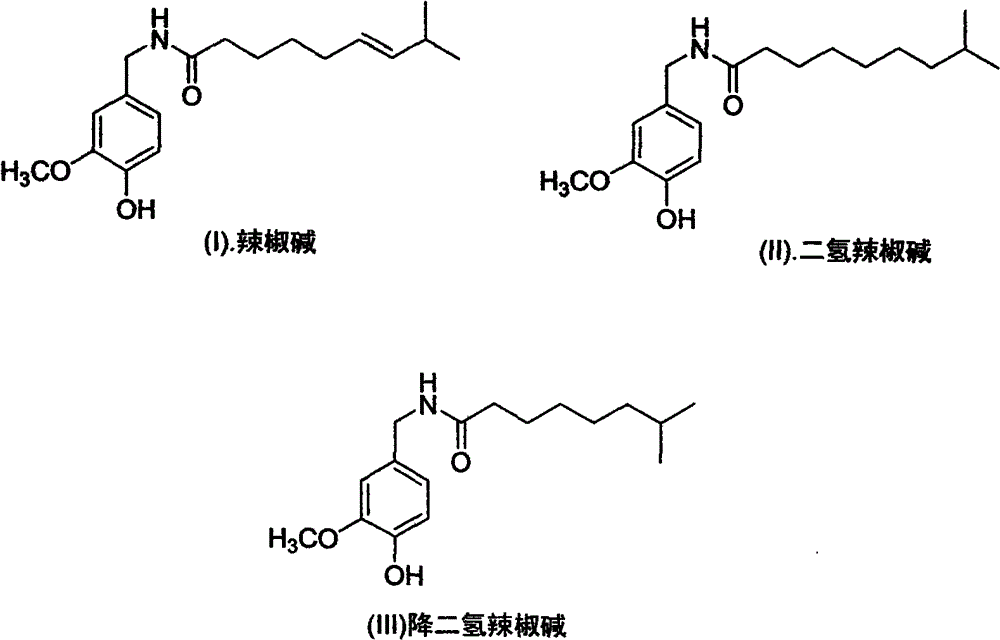
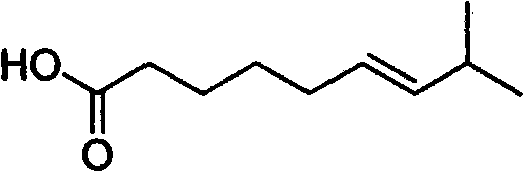
Artificial synthesis method of capsaicin homologue
ActiveCN101717346ASuitable for large-scale industrial productionOrganic compound preparationPreparation from carboxylic acid esters/lactonesNonanoic acidDihydrocapsaicin
The invention belongs to the field of artificial synthesis of natural products and relates to an artificial synthesis method of a capsaicin homologue. The artificial synthesis method of the capsaicin homologue is to lead 4-hydroxy-3-methoxy-benzylamine hydrochloride to be reacted with the corresponding long-chain acid (E)-8-methyl-6-nonenoic acid and 8-methyl nonanoic acid or 7-methyl octanoic acid for preparing the capsaicin homologue. The long-chain acid synthesis method is characterized in that the method can avoid the use of triphenyl phosphine and other toxic substances which are commonly used in the existing patents and toxic substance-triphenyl phosphineoxide in byproducts, thereby being relatively environment-friendly. For different capsaicin homologues, the required starting raw materials are different. In particular, dihydrocapsaicin is applicable to large-scale industrial production.
Owner:SUZHOU HUADAO BIOLOGICAL PHARMA
New preparation process of medicinal raw material duloxetine hydrochloride of antidepressant drug
InactiveCN103360365AMild reaction conditionsClear workmanshipOrganic chemistryTreatment effectMannich reaction
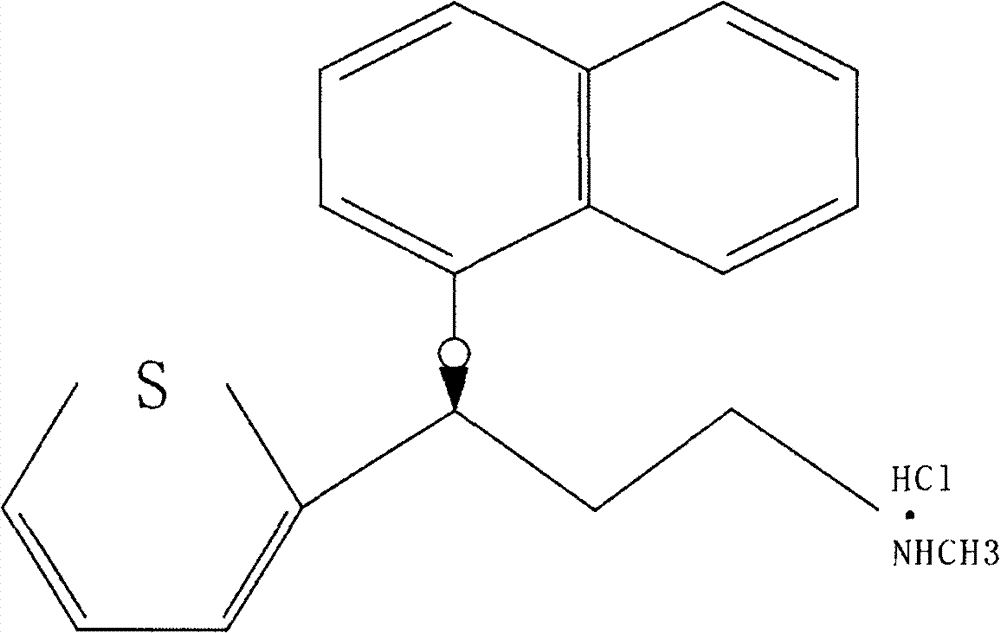
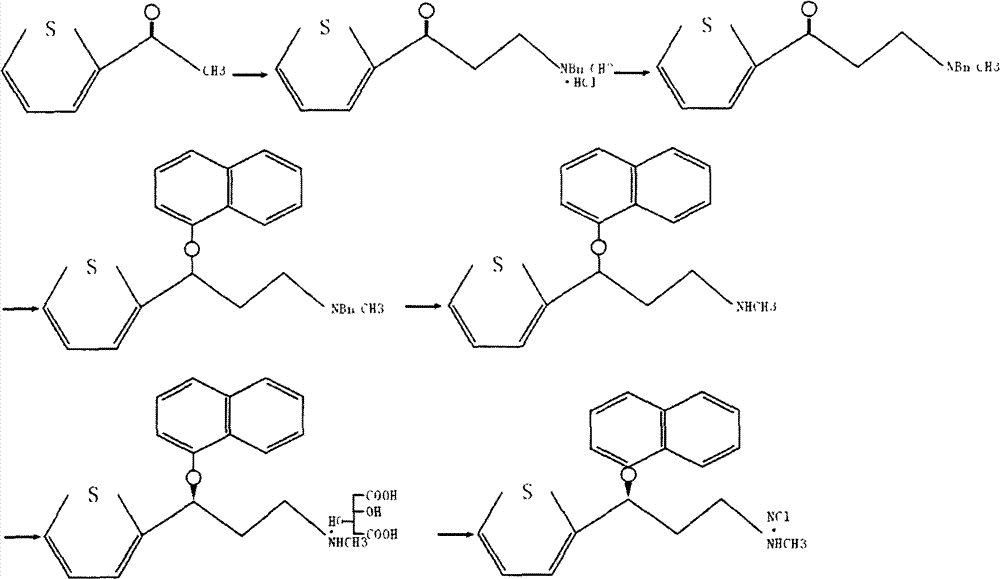
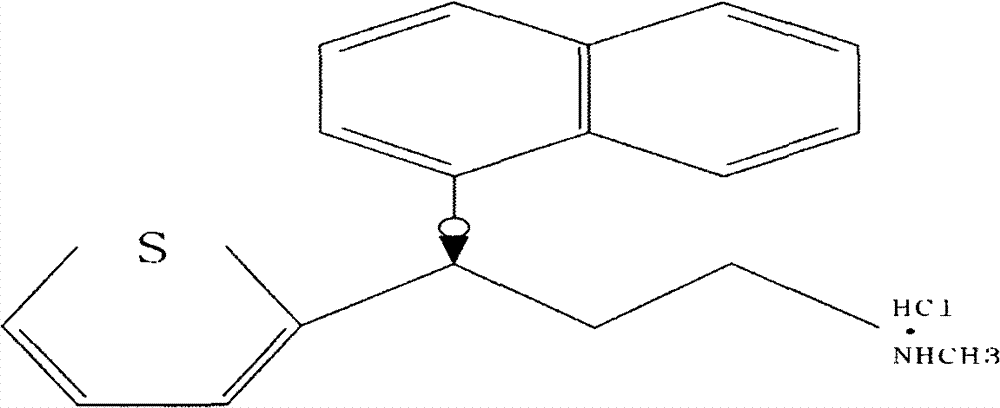
The invention relates to a new preparation process of the medicinal raw material duloxetine hydrochloride of an antidepressant drug and belongs to the technical field of drug synthesis. The new preparation process is characterized in that N-methyl benzylamine hydrochloride but not dimethylamine hydrochloride is used in Mannich reaction; because benzyl is easier to remove as compared with methyl, dealkylation in a subsequent step has better effect and higher yield; an expensive chiral catalyst or a phase-transfer catalyst is not used; a better solvent crystallizing and removing method is adopted, so that the harm of a residual crystallizing solvent to a human body is prevented; the splitting of a chiral compound is carried out after dealkylation, and a mixture obtained after splitting is separated by adopting a unique recrystallization technology to prepare (S)-N-methyl-3-(1-naphthoxy)-3-(2-thienyl) propylamine / tartrate with high purity and high optical activity, so that a finally obtained duloxetine hydrochloride product can achieve better treatment effect. The new preparation process disclosed by the invention has the advantages of mildness in reaction condition, clear process, good easiness and convenience in operation and low production cost and is extremely favorable for industrial production.
Owner:李晓红
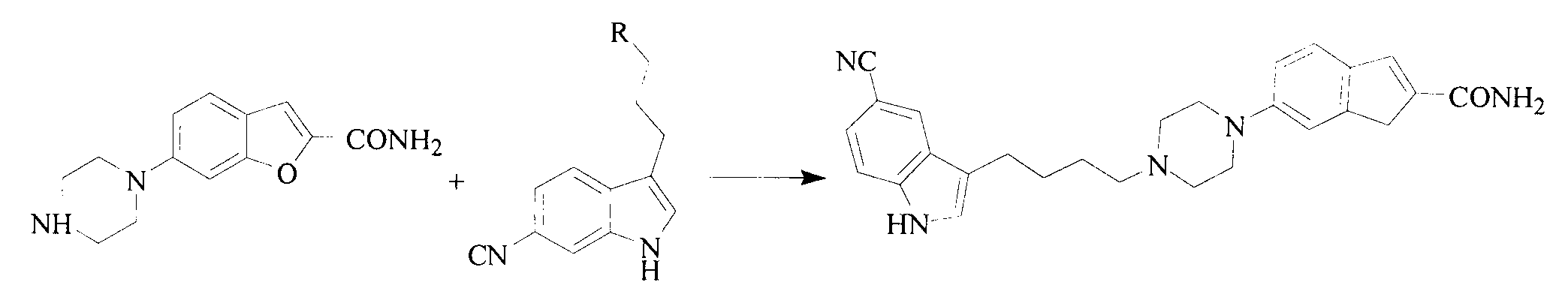
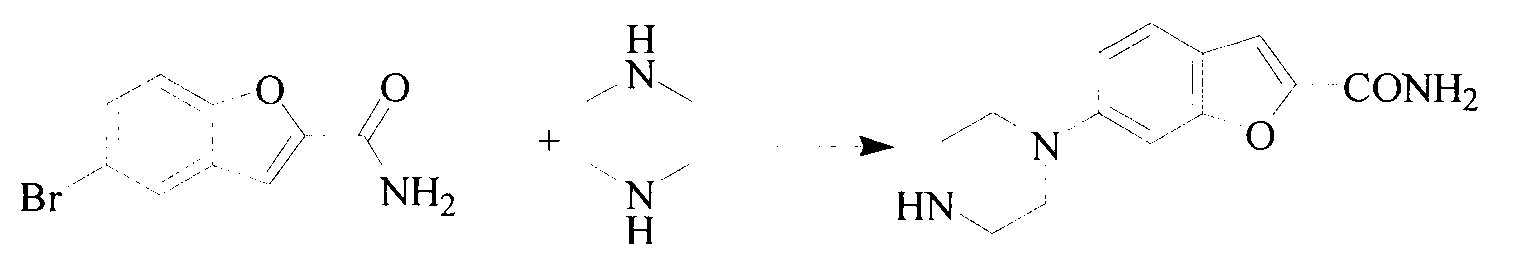
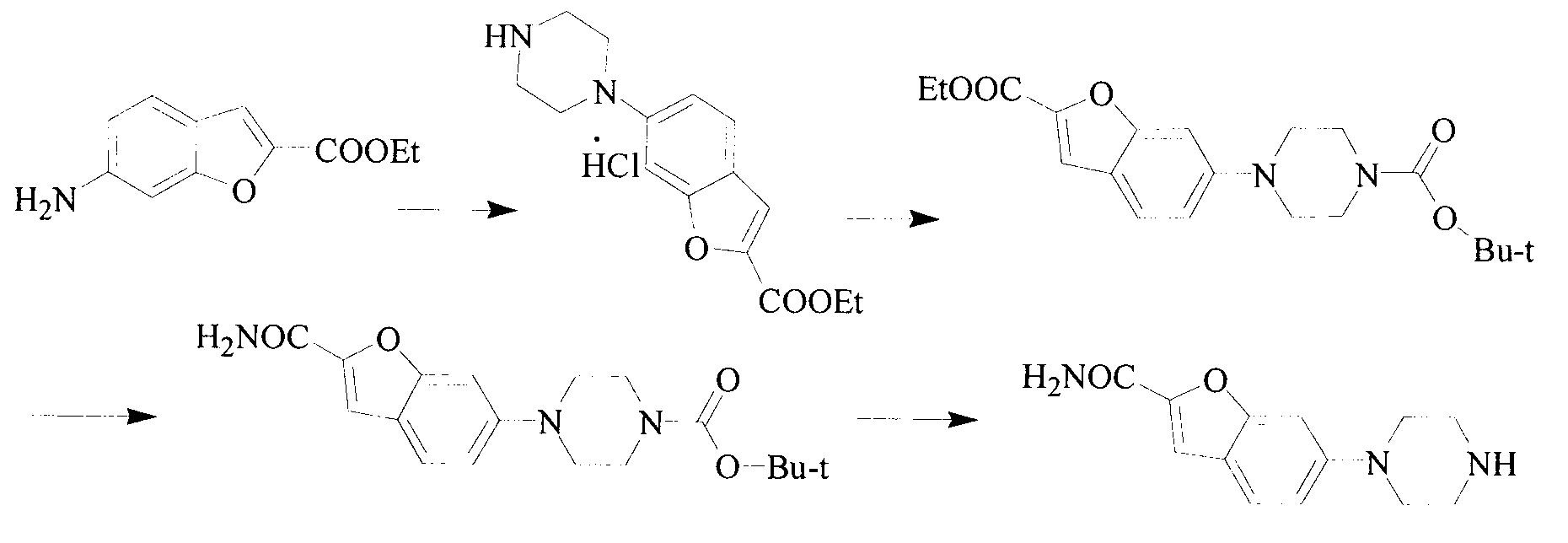
Method for preparing vilazodone hydrochloride midbody
The invention provides a method for preparing vilazodone hydrochloride midbody. The method comprises the steps of: based on 5-amino coumarone ethyl formate as an initial raw material, reacting with bi(2-chloroethy) benzylamine hydrochloride under the effect of tetrabutylammonium bromide to generate compound II, and reacting with ammonia to prepare compound I 5-(1-piperazinyl)-benzofuran-2-formamide. The used method is easy in raw material obtaining, low in cost, high in reaction yield, simple to operate and suitable for industrial production.
Owner:NANJING HUAWE MEDICINE TECH DEV
Preparation method of O-benzylhydroxylamine hydrochloride
The invention discloses a preparation method of O-benzylhydroxylamine hydrochloride, and relates to the technical field of organic synthesis. The method comprises the following steps: synchronously dropwise adding alcoholic solutions of benzyl halide and alkoxide into oxime to perform a reaction; when no benzyl halide exists in the reaction system, carrying out solid-liquid separation, dropwise adding dilute hydrochloric acid for carrying out hydrolytic rectification, and distilling while dropwise adding the dilute hydrochloric acid to separate out ketone; concentrating the reaction solution and separating out O-benzylhydroxylamine hydrochloride; and drying the benzylamine hydrochloride to obtain an O-benzylhydroxylamine hydrochloride pure product. Oxime is used as a raw material, and thefinally generated ketone or aldehyde can also be recycled to synthesize corresponding oxime. According to the method, the atom utilization rate is improved, the production cost of the product is reduced, the emission of three wastes is reduced, and the goal of green production is achieved.
Owner:ZHEJIANG SAINON CHEM
Preparation method of tetraimidazole free alkali
ActiveCN111377949AHigh activityShort synthetic stepsOrganic chemistryThioureaBenzylamine hydrochloride
The invention discloses a preparation method of tetraimidazole free alkali. Alpha-[[(2-hydroxyethyl)amino]methyl]benzyl alcohol reacts with thionyl chloride, then water is added for heating dissolution, after activated carbon thermal filtration, N-(2-chloroethyl)-alpha-(chloromethyl)-benzylamine hydrochloride is obtained through cooling crystallization, then N-(2-chloroethyl)-alpha-(chloromethyl)-benzylamine hydrochloride and thiourea are subjected to direct cyclization, and tetraimidazole free alkali is generated. The method is simple in production process, mild in reaction condition and highin total yield, the production cost is reduced, the generation amount of three wastes is small, the double contradiction between economy and environment in the development process of modern enterprises is well solved, and the production process has great competitiveness and good industrial prospects.
Owner:SHANDONG GUOBANG PHARMA +1
Artificial synthesis method of capsaicin homologue
ActiveCN101717346BSuitable for large-scale industrial productionOrganic compound preparationPreparation from carboxylic acid esters/lactonesNonanoic acidOctanoic Acids
The invention belongs to the field of artificial synthesis of natural products and relates to an artificial synthesis method of a capsaicin homologue. The artificial synthesis method of the capsaicin homologue is to lead 4-hydroxy-3-methoxy-benzylamine hydrochloride to be reacted with the corresponding long-chain acid (E)-8-methyl-6-nonenoic acid and 8-methyl nonanoic acid or 7-methyl octanoic acid for preparing the capsaicin homologue. The long-chain acid synthesis method is characterized in that the method can avoid the use of triphenyl phosphine and other toxic substances which are commonly used in the existing patents and toxic substance-triphenyl phosphineoxide in byproducts, thereby being relatively environment-friendly. For different capsaicin homologues, the required starting raw materials are different. In particular, dihydrocapsaicin is applicable to large-scale industrial production.
Owner:SUZHOU HUADAO BIOLOGICAL PHARMA
Method for reducing nitroxylbenzyl amine compound to amino-benzylamine hydrochloride
InactiveCN100383112CReduce reactivityEasy post-processingOrganic compound preparationAmino compound preparationHydrogenAmidol
This invention discloses a method for reducing the nitrabenzylamine compound to be nitrabenzylamine hydrochlorate whichreduces the nitrabenzylamine compound under hydrogen gas atmosphere by catalytic hydrogenation, using Pd-C / HCl catalytic system. This invention reduces the reaction activity of Pd-C catalyst by adding HCl or hydrochloric acid or CHCl3 which can produce HCl during the reaction to the reaction system, to entitle the Pd-C catalyst with the activity of catalyzing the nitro group of hydrogenated nitrobenzylamine compound to be amidol, without benzyl hydrogenolysis. This invention is characterized of simple operation, mild reaction condition, convenient treatment of post-production material, and broad application future.
Owner:TSINGHUA UNIV
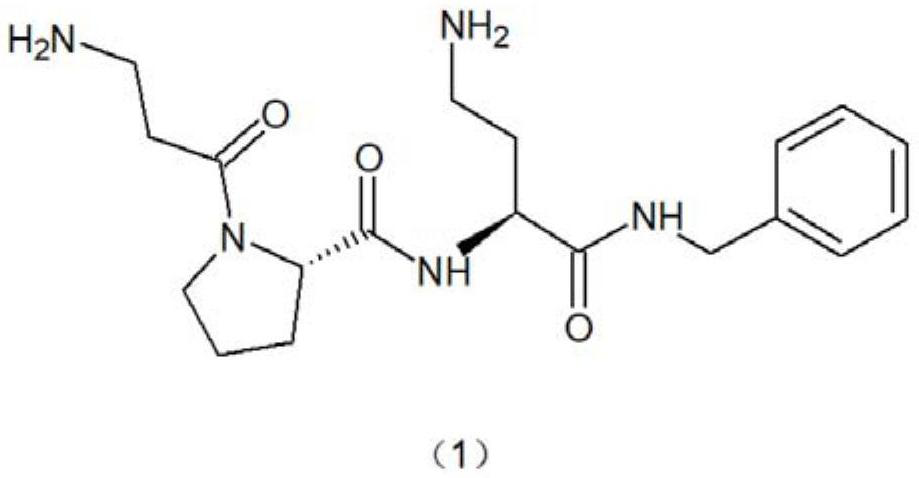
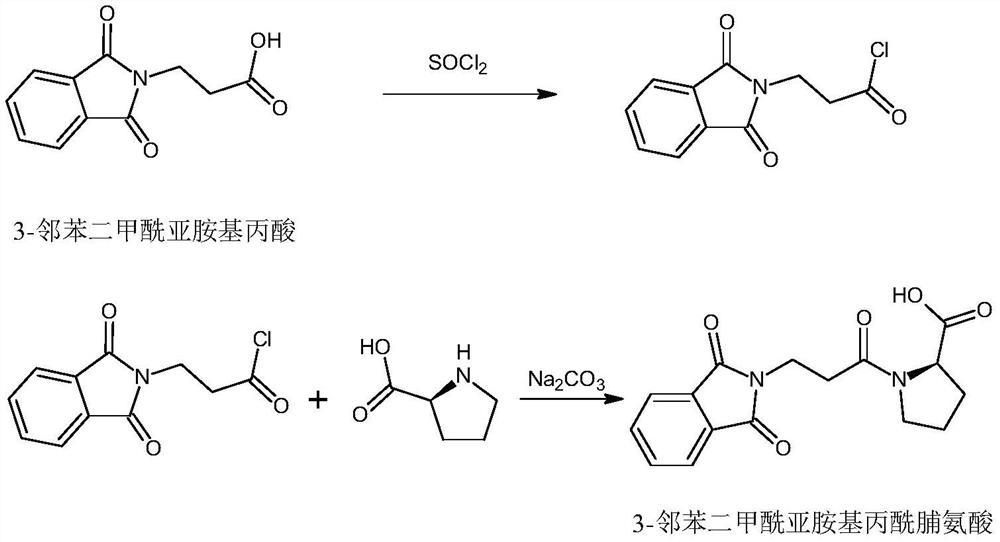
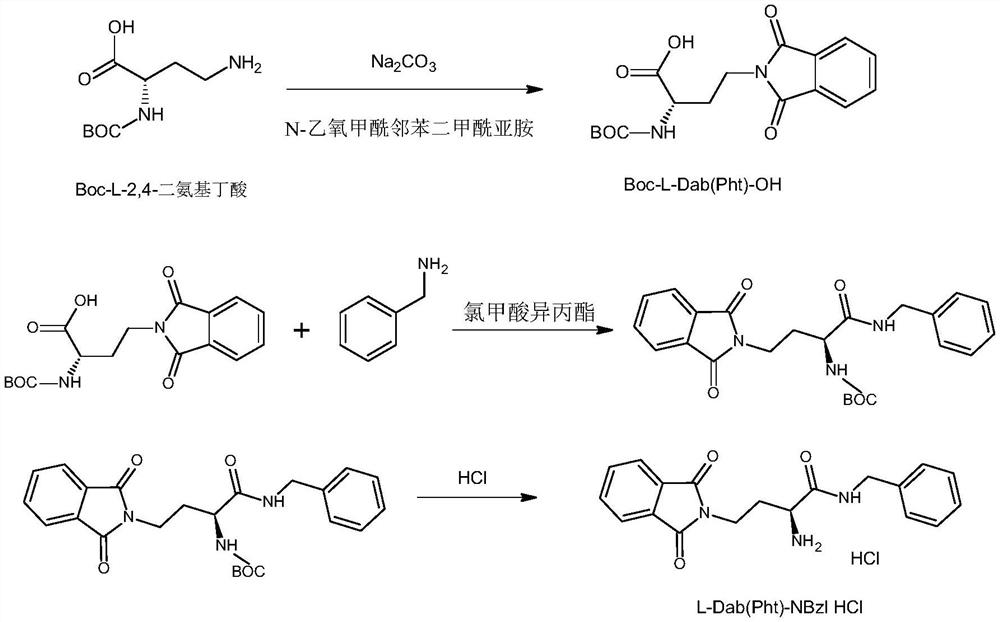
Synthesis method of snake venom-like tripeptide
PendingCN114213503AThe synthesis process steps are simpleSmooth responsePeptide preparation methodsBulk chemical productionPropanoic acidSide chain
The invention relates to a synthetic method of snake venom-like tripeptide, which comprises the following steps: by taking 3-phthalimidopropionic acid as an initial raw material, performing acylating chlorination, condensing with proline, and condensing an obtained fragment with L-2, 4-diaminobutyryl benzylamine hydrochloride with a side chain protected by phthaloyl, thereby obtaining the phthaloyl-protected snake venom-like tripeptide. Finally, a phthaloyl protecting group is removed through hydrazine, and then the free snake venom-like tripeptide is obtained. The free snake venom-like tripeptide is obtained after a phthaloyl protecting group is removed by hydrazine, and can be conveniently prepared into acetate and hydrochloride products. Compared with the prior art, the method has the advantages of easily available raw materials, high product purity, simplicity and convenience in operation and suitability for industrial production.
Owner:上海予利生物科技股份有限公司 +1
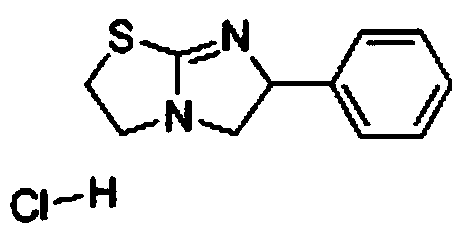
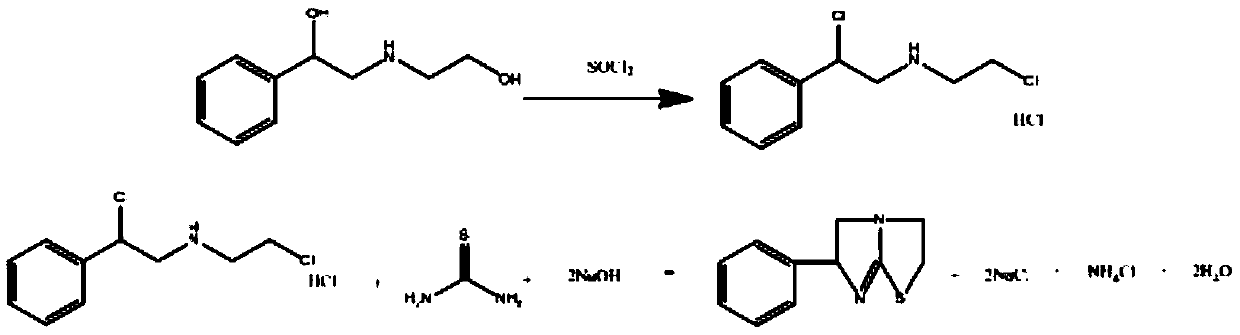
Preparation method of tetraimidazole hydrochloride
The invention provides a preparation method of tetraimidazole hydrochloride, which comprises the following steps: preparing tetraimidazole; the preparation method comprises the following step: reacting tetraimidazole, 1, 2-dibromoethyl benzene and 2-aminothiazoline hydrochloride to generate tetraimidazole. The synthetic route is shorter, the comprehensive yield is higher than that of other processroutes, styrene is used as an initiator, tetra-imidazole free alkali is used as a final product, the yield of the hydroxysalt process route is about 65%, the yield of the N (2chloroethyl) alpha (chloromethyl) benzylamine hydrochloride process route is about 72%, and the yield can reach 85% or above when the patent route is used. Meanwhile, the problem of large amount of waste water and waste saltgenerated by multi-step reactions such as chlorination, hydrolysis and cyclization is avoided, the investment of reaction equipment such as an autoclave is avoided, the green chemical engineering requirement is met, the economic benefit is remarkable, and the industrial prospect is good.
Owner:SHANDONG GUOBANG PHARMA +1
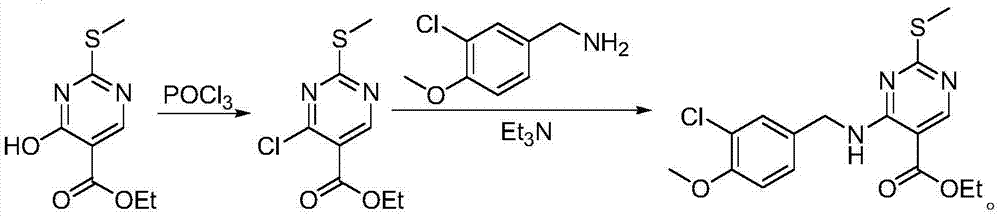
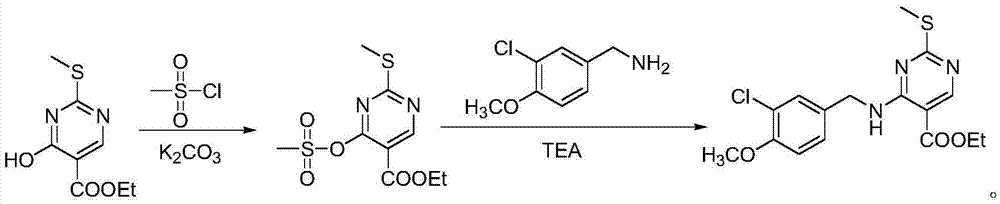
Preparation method of Avanafil intermediate
InactiveCN105439964AReduce dosageSafe post-processingOrganic chemistryEthyl esterMethanesulfonyl chloride
The invention provides a preparation method of an Avanafil intermediate. The preparation method comprises the following steps: dissolving 4-hydroxy-2-methylthio-ethyl 5-pyrimidinecarboxylate and potassium carbonate in DMF to obtain a mixed solution, adding methanesulfonyl chloride into the mixed solution under the stirring condition, continuously stirring after addition until a reaction is ended, adding water and ethyl acetate into a reaction solution, carrying out extraction and liquid separation, carrying out vacuum concentration on an organic phase to prepare 4-methanesulfonic sulfonic ester-2-methylthio-ethyl 5-pyrimidinecarboxylate; dissolving the obtained 4-methanesulfonic sulfonic ester-2-methylthio-ethyl 5-pyrimidinecarboxylate into DMF, adding a (3-chloro-4-methoxy)Benzylamine hydrochloride to obtain mixed liquor, adding triethylamine into the mixed liquor under the stirring condition, continuously stirring after addition until a reaction is ended, adding water and ethyl acetate, carrying out extraction and liquid separation, carrying out vacuum concentration on an organic phase, and recrystallizing to prepare 4-(3-chloro-4-methoxybenzylamino)-5- ethyloxycarbonyl-2-methylthiopyrimidine. The invention has advantages of less environmental pollution, high product yield, safe post-processing and simple operation.
Owner:HEBEI UNIVERSITY
Rectoscope washing agent
InactiveCN104031779AKeep clean and safeGood stain removalSurface-active non-soap compounds and soap mixture detergentsPropanoic acidBenzylamine hydrochloride
The invention relates to a gastroendoscope cleaning product and in particular relates to a rectoscope washing agent. The rectoscope washing agent comprises the following raw materials: water, 2-hydracrylic acid, methacrolein, (2-hydroxyethyl)diisopropyl methylammonium bromide xanthene-9-carboxylic ester, 2,3,4,5,6-pentahydroxy-2-hexenoic acid-4-lactone, cetyl trimethyl ammonium bromide, bicyclo[2.2.1]heptyl-5-allyl-2-carbonitrile, N-(1-methyl-2-phenoxyethyl)-N-(2-chloroethyl)benzylamine hydrochloride, 3-[4,5-dihydro-1H-imidazole-2-ylmethyl-(4-methylphenyl)amino]phenol. The washing agent has a good and remarkable dirt removal effect, especially has a relatively good sterilizing effect while removing dirt generated in a rectal examination process and especially has a remarkable removal effect on three types of bacteria existent in rectums, so that the cleanness and the safety of a rectoscope are ensured on aspects of dirt removal and sterilization.
Owner:安徽斯迈特新材料股份有限公司
A kind of b, n co-doped porous carbon nanosheet and its preparation method and application
InactiveCN108529591BSimple processLow costHybrid capacitor electrodesNano-carbonCapacitancePorous carbon
The invention discloses a B,N-codoped porous carbon nanosheet and a preparation method. The preparation method comprises the following steps: using bi-(2-chloroethyl)benzylamine hydrochloride as a rawmaterial, mixing with alkali liquor to prepare bi-(2-chloroethyl)amine; then, enabling the bi-(2-chloroethyl)amine to react with boric acid to obtain bi-(2-chloroethyl)amido boric acid; and under theprotection of nitrogen, pyrolyzing the bi-(2-chloroethyl)amido boric acid, successfully preparing the B,N-codoped porous carbon nanosheet. The prepared porous carbon nanosheet is high in B,N content,and large in specific surface area. While the porous carbon nanosheet is used as a super capacitor electrode material, the capacitive performance is high, the rate capability is good, and the cycle life is long. The preparation method is simple in operation, and efficient in economy, and capable of realizing the industrial production of the B,N-codoped porous carbon nanosheet.
Owner:XIANGTAN UNIV
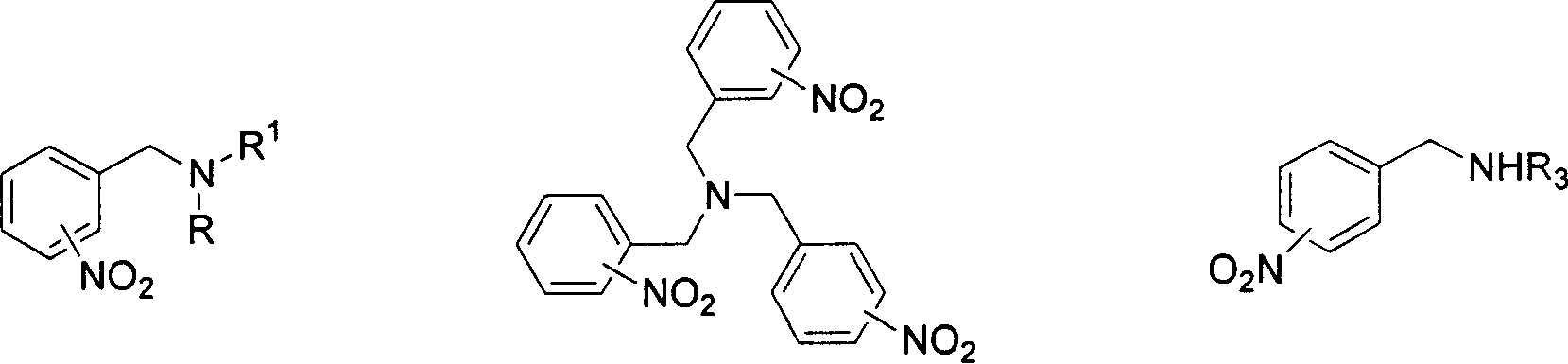
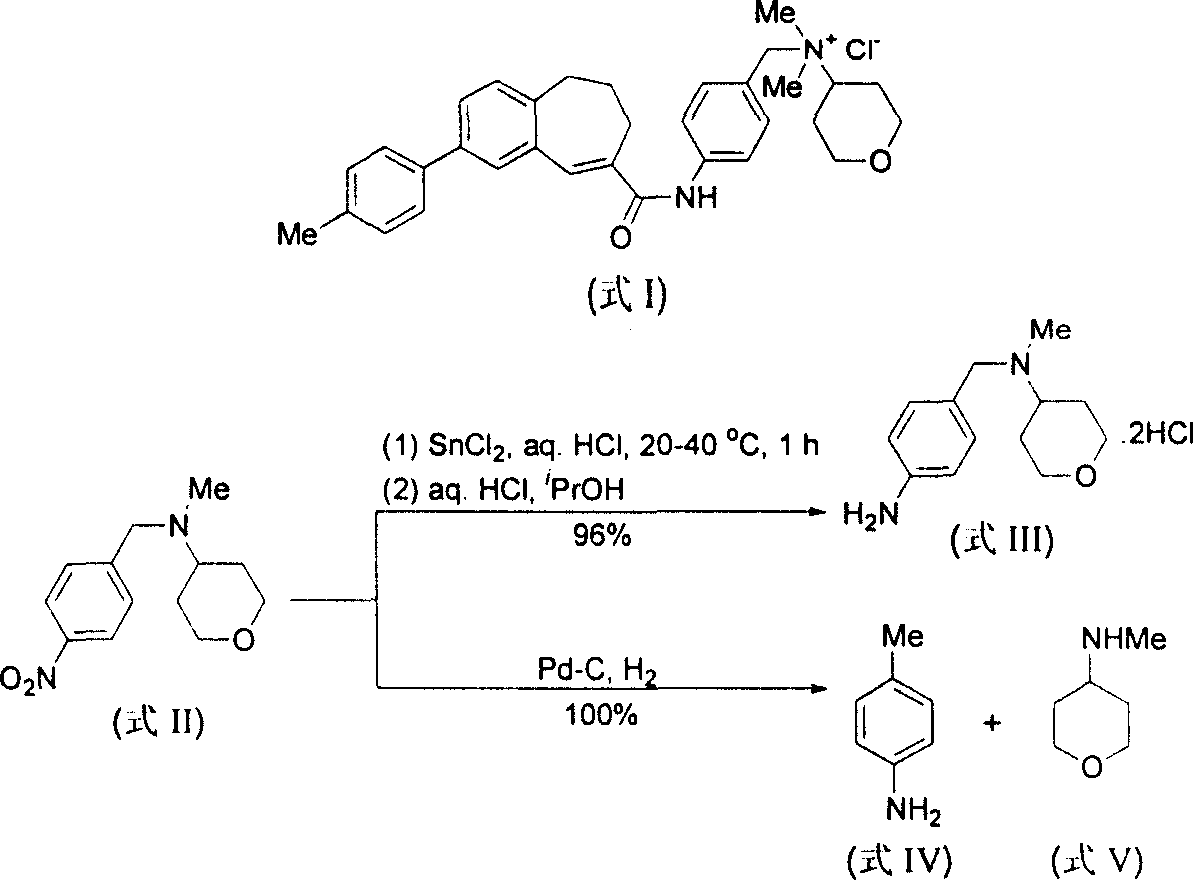
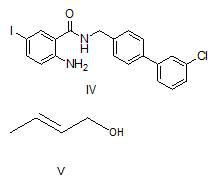
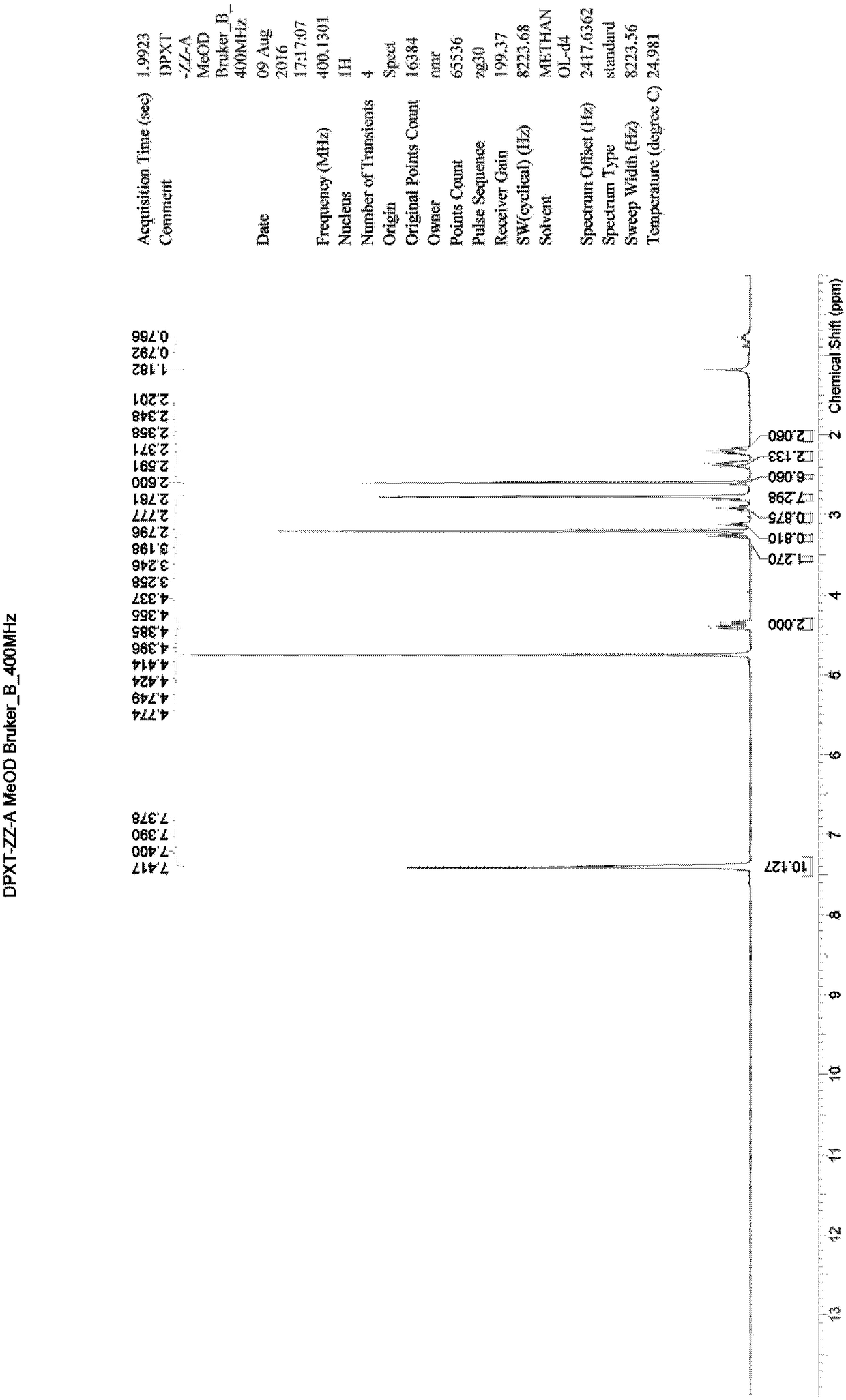
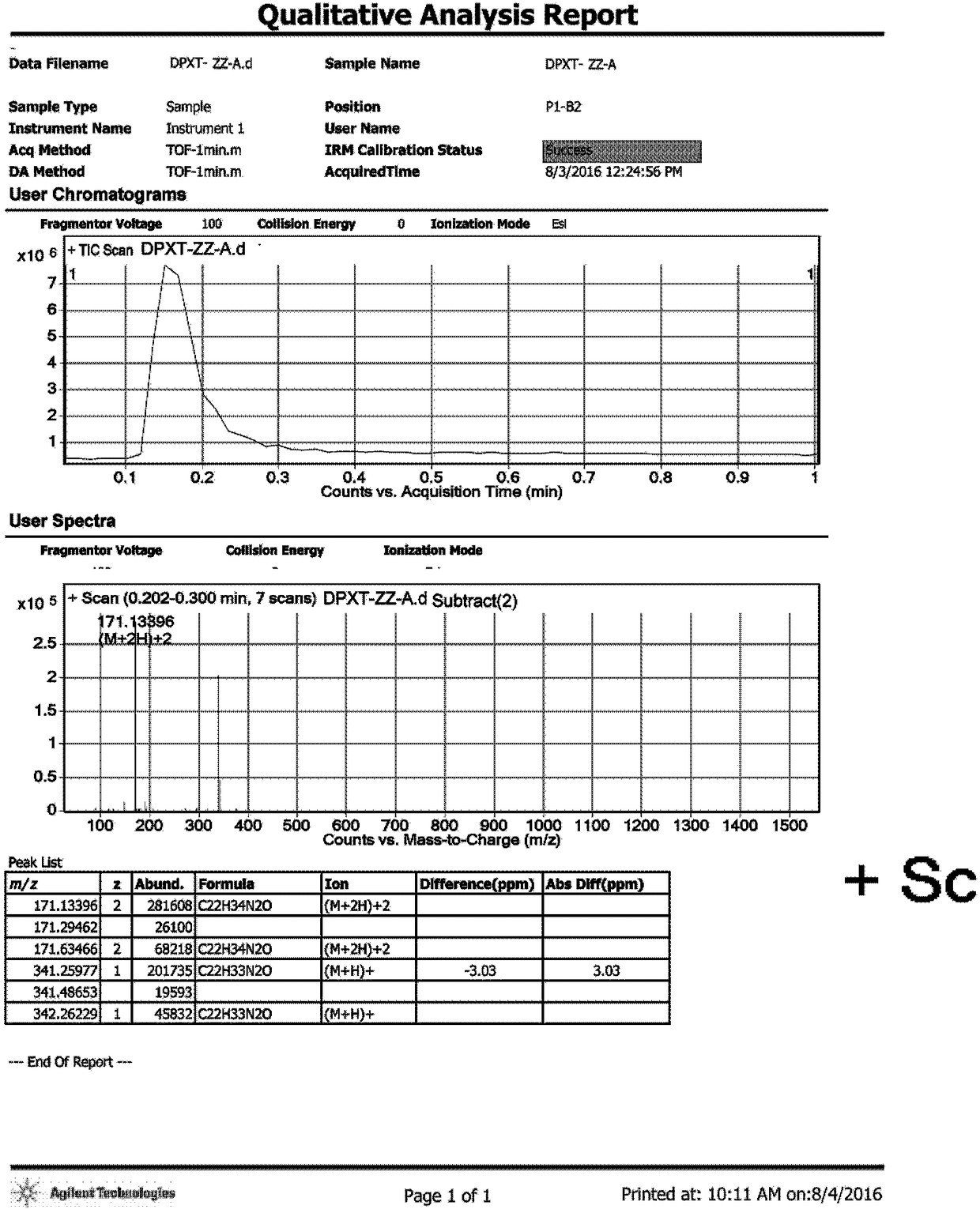
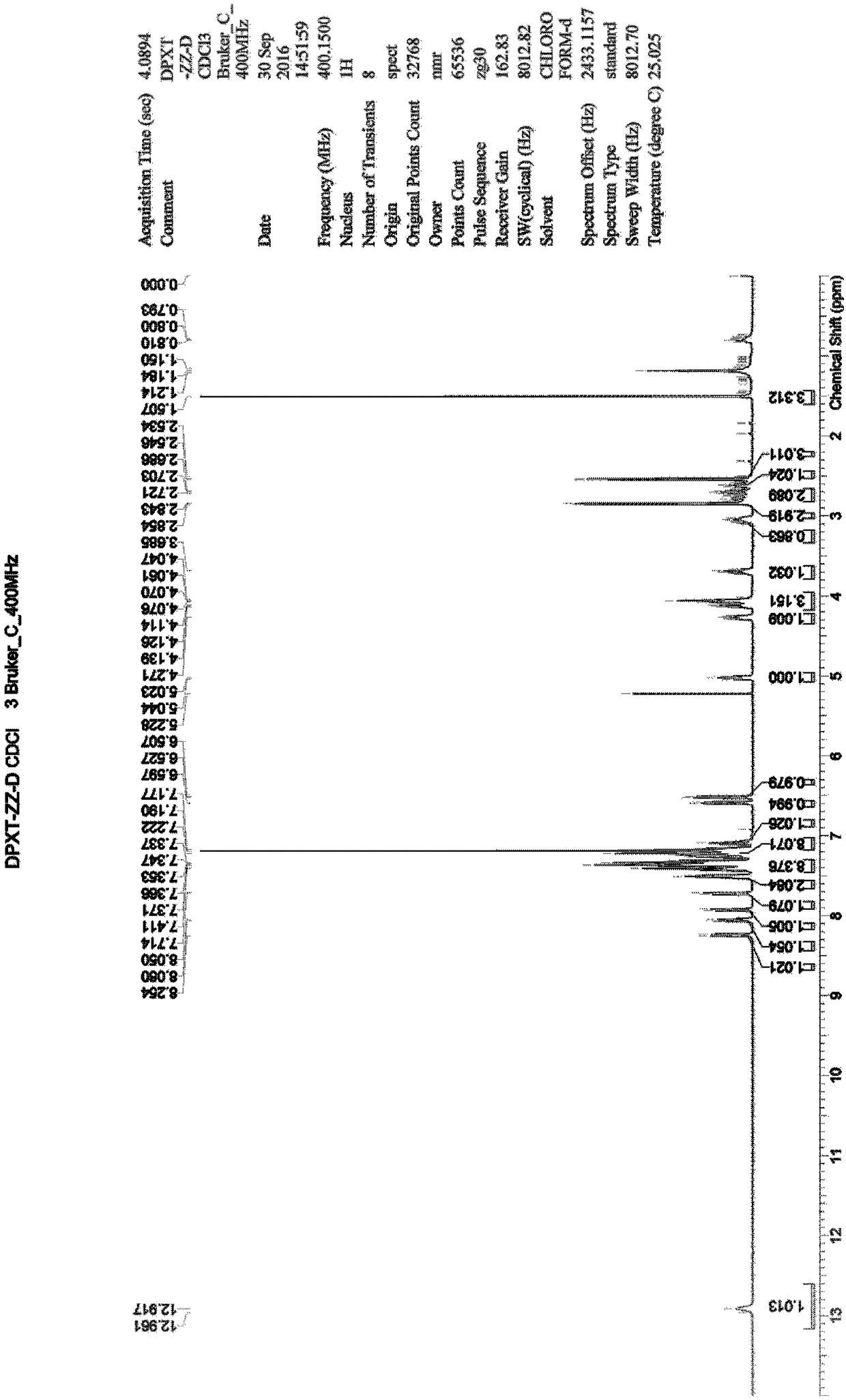
Method for preparing N-[4-[1-hydroxy-2-[(1-methylethyl)amino]ethyl]methylsulfonyl benzylamine hydrochloride
ActiveCN102329254AEasy to manufactureShort stepsSulfonic acid amide preparationIsopropamideSodium borohydride
Owner:SHANGHAI AOBO PHARMTECH INC LTD
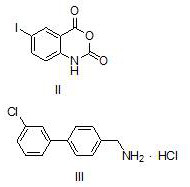
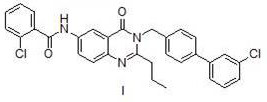
Method for preparing microsomal prostaglandin E2 synthase-1 inhibitor
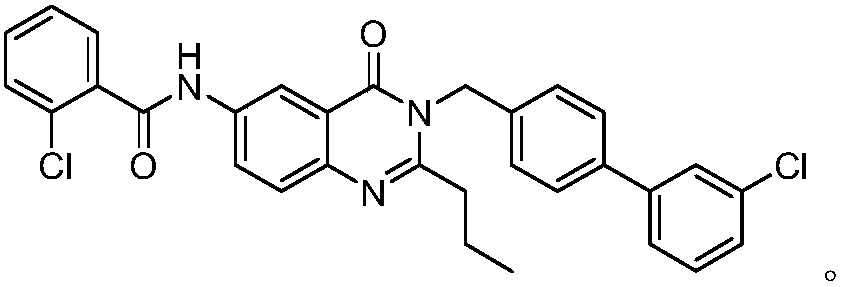
The invention discloses a method for preparing a microsomal prostaglandin E2 synthase-1 inhibitor. The method comprises the following three steps: 1) preparing N-substituted amide from 5-iodoisatoic anhydride and 4-(3-chlorophenyl)benzylamine hydrochloride; 2) reacting N-substituted amide with crotonyl alcohol to produce 3-[(3'-chloro[1,1'-diphenyl]-4-yl)methyl]-2-propyl-6-iodoquinazolinone; and 3) subjecting a reaction product of the previous step and the o-chlorobenzamide to a Goldberg amination reaction to obtain the target product. The preparation method is simple in steps, avoids the useof alkali and toxic chemical reagents such as triphenyl phosphite, uses cheap and readily-available crotonyl alcohol as a raw material, realizes automatic transferring of hydrogen, does not use alkaliduring the reaction, achieves one-pot reaction, only by-produces water, has high atom economy in the reaction, meets the requirements of green chemistry and shows good development prospects.
Owner:NANJING UNIV OF SCI & TECH
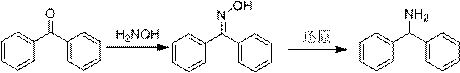
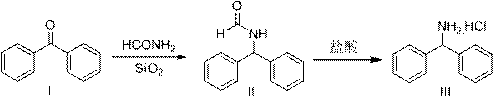
A kind of preparation method of diphenylmethylamine hydrochloride
ActiveCN107089918BShort reaction timeHigh yieldOrganic compound preparationCarboxylic acid amides preparationPtru catalystBenzole
The invention provides a preparation method of azelnidipine starting material ethylamine benzhydrylamine. The method comprises the step of using benzophenone and formamide as raw materials to carry out aLeuckart reaction. The catalyst of silicon dioxide is added into a reaction system, and thus the reaction time is drastically reduced, wherein the time is decreased to 3-4 h from 8 h, and therefore energy consumption is significantly reduced; the rough product yield of a compound II is dramatically increased and is up to 96-98%. The purity of HPLC is not lower than 96.5%, so that it is ensured that after the ethylamine benzhydrylamine obtained by hydrochloric acid hydrolysis is purified once, the yield can reach 80%, the purity is not lower than 99.9%, and the ethylamine benzhydrylamine is quite suitable for industrial production.
Owner:迪嘉药业集团股份有限公司
Synthesis method of 2-fluoro-3-methyl-4-(trifluoromethyl) benzylamine hydrochloride
ActiveCN112194558AReasonable designEasy to operateCarbamic acid derivatives preparationOrganic compound preparationCompound aMethyl benzene
The invention belongs to the technical field of medical intermediates, and particularly relates to a synthesis method of 2-fluoro-3-methyl-4-(trifluoromethyl) benzylamine hydrochloride. A compound A and a compound E are used as initial raw materials for the first time to form a compound B, then the compound B is subjected to a reaction to form a compound C, and the compound C is used for preparingthe 2-fluoro-3-methyl-4-(trifluoromethyl) benzylamine hydrochloride.
Owner:阿里生物新材料(常州)有限公司
A kind of preparation method of tetraimidazole hydrochloride
The invention provides a kind of preparation method of tetramisole hydrochloride, described preparation method, comprises preparation tetramisole; Described preparation tetramisole, 1,2-dibromoethylbenzene and 2-aminothiazoline hydrochloride react to generate tetramisole . The synthetic route of the present invention is shorter, and comprehensive yield is higher than other operational routes, with styrene as starting material, tetramisole free base is final product, and the yield of hydroxyl salt technical route is about 65%, N-(2- Chloroethyl)-α-(chloromethyl)-benzylamine hydrochloride process route has a yield of about 72%, while using this patented route, the yield can reach more than 85%. At the same time, it avoids the problems of multi-step reactions, such as chlorination, hydrolysis, cyclization, etc., resulting in a large amount of waste water and waste salt, and avoids the investment in reaction equipment such as autoclaves. It meets the requirements of green chemical industry, has significant economic benefits, and has a good industrialization prospect.
Owner:SHANDONG GUOBANG PHARMA +1
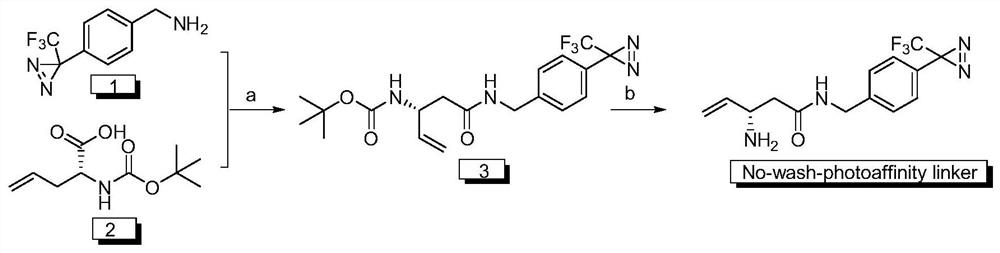
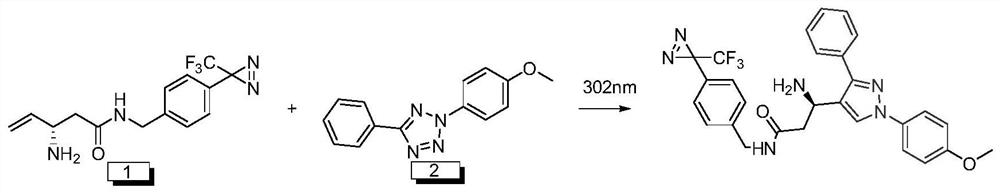
A kind of disposable photoaffinity linker and preparation method and application
ActiveCN111187212BEasy to prepareEasy to implementOrganic chemistry methodsFluorescence/phosphorescenceFluorescenceDouble bond
A kind of wash-free photoaffinity linker and its preparation method and application, 4-[3-(trifluoromethyl)-3H-diaziridine-3-yl]benzylamine hydrochloride and (R)-2 ‑((tert-butoxycarbonyl) amino)‑4‑pentenoic acid is condensed under EDC HCl to obtain a photoaffinity linker intermediate containing a carbon-carbon double bond with a Boc protecting group; the Boc protecting group will be The photoaffinity linker intermediate containing a carbon-carbon double bond was deprotected under the action of trifluoroacetic acid to obtain a wash-free photoaffinity linker. The preparation method of the free-washing photoaffinity linker molecule of the invention is simple, easy to realize, and has a high yield. The wash-free photoaffinity linker molecule in the present invention can chemically covalently modify the target recognition molecule to construct a photoaffinity probe molecule, and then carry out specific covalent labeling of its target molecule by photocrosslinking technology, and finally The fluorescence is illuminated through a bioorthogonal reaction, so as to realize the tracking of the target molecule.
Owner:XI AN JIAOTONG UNIV
Polymorphic form of N-[2-(6-fluoro-1H-indol-3-yl)ethyl]-3-(2,2,3,3-tetrafluoropropoxy)benzylamine hydrochloride for the treatment of Alzheimer's disease
Owner:H LUNDBECK AS
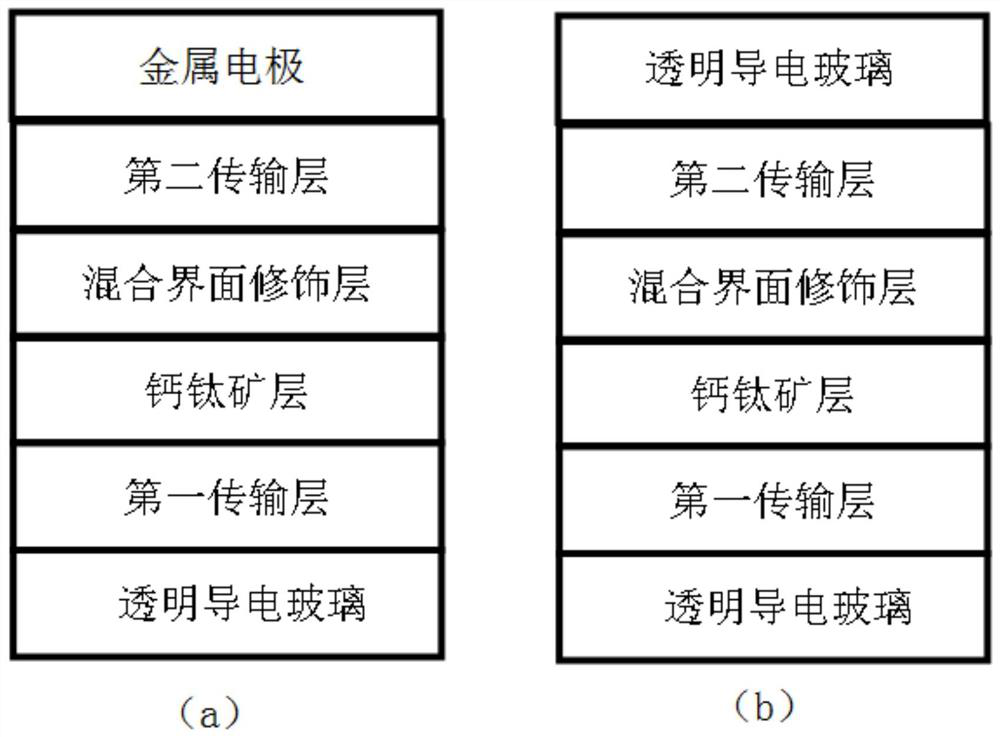
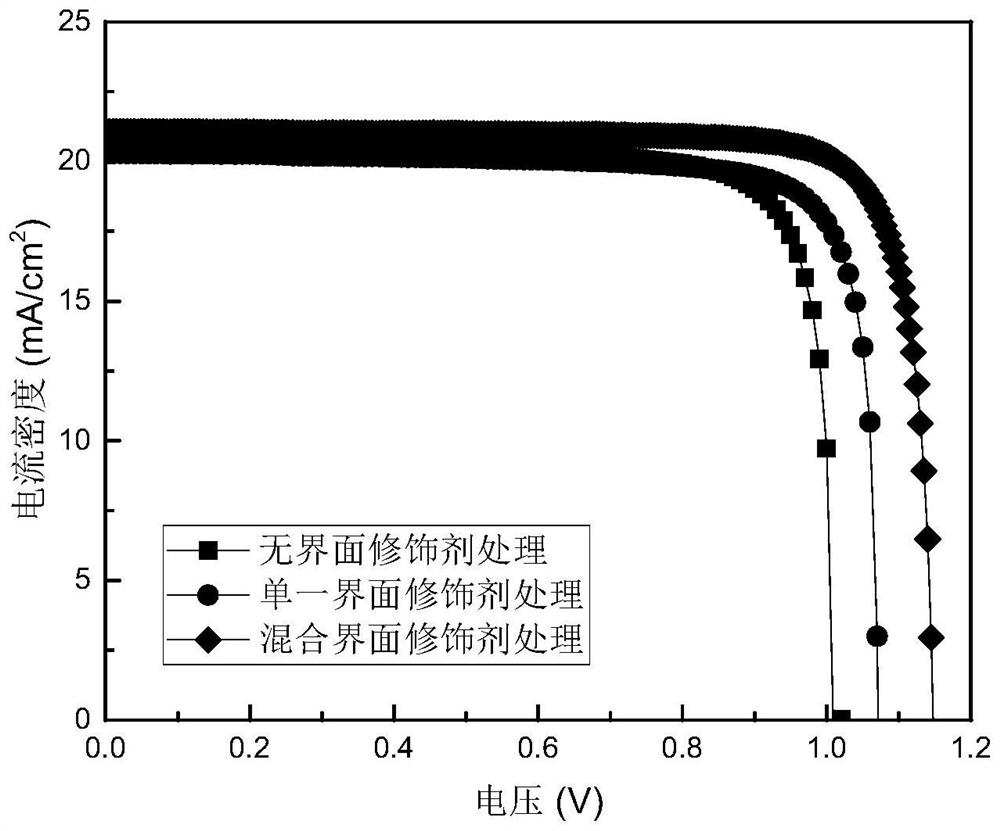
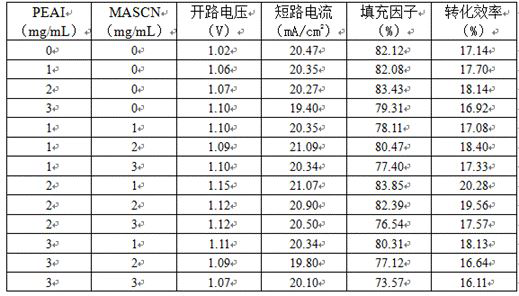
Perovskite solar cell
PendingCN114420847AOptimize secondary crystallizationEasy transferSolid-state devicesSemiconductor/solid-state device manufacturingFormateEthylic acid
The invention discloses a perovskite solar cell, and belongs to the technical field of solar cells. The perovskite solar cell comprises a perovskite layer, an interface modification layer is arranged on the surface of the perovskite layer, and the interface modification layer is made of a passivator and a modifier. The passivating agent is selected from one or more of 2-phenylethylamine hydriodate, 2-phenylethylamine hydrochloride, benzylamine hydrochloride, benzylamine hydriodate, butylamine hydrobromide, ethylenediamine hydrochloride, ammonium halide, guanidine halide, tetramethyl guanidine fluoborate or alkali metal halide salt; the modifier is selected from one or more of ammonium thiocyanate, formamidine thiocyanate, halogenated methylamine, halogenated formamidine, methylamine acetate, formamidine acetate, formamidine formate, methylamine formate, methylamine cyanate or formamidine cyanate. By using the mixed passivator composed of the passivator and the modifier, the crystallinity of perovskite can be effectively improved, surface defects are passivated, the performance of the perovskite solar cell is improved, and the stability of a device is improved.
Owner:仁烁光能(苏州)有限公司
Novel Polymorphic Form of N-[2-(6-fluoro-lH-indol-3-yl)ethyl]-3-2,2,3,3-tetrafluoropropoxy)benzylamine hydrochoride for the treatment of Alzheimer's
The present invention relates to a novel polymorphic form of N-[2-(6-fluoro-1H-indol-3-yl)ethyl]-3-(2,2,3,3-tetrafluoropropoxy)benzylamine hydrochloride.
Owner:H LUNDBECK AS
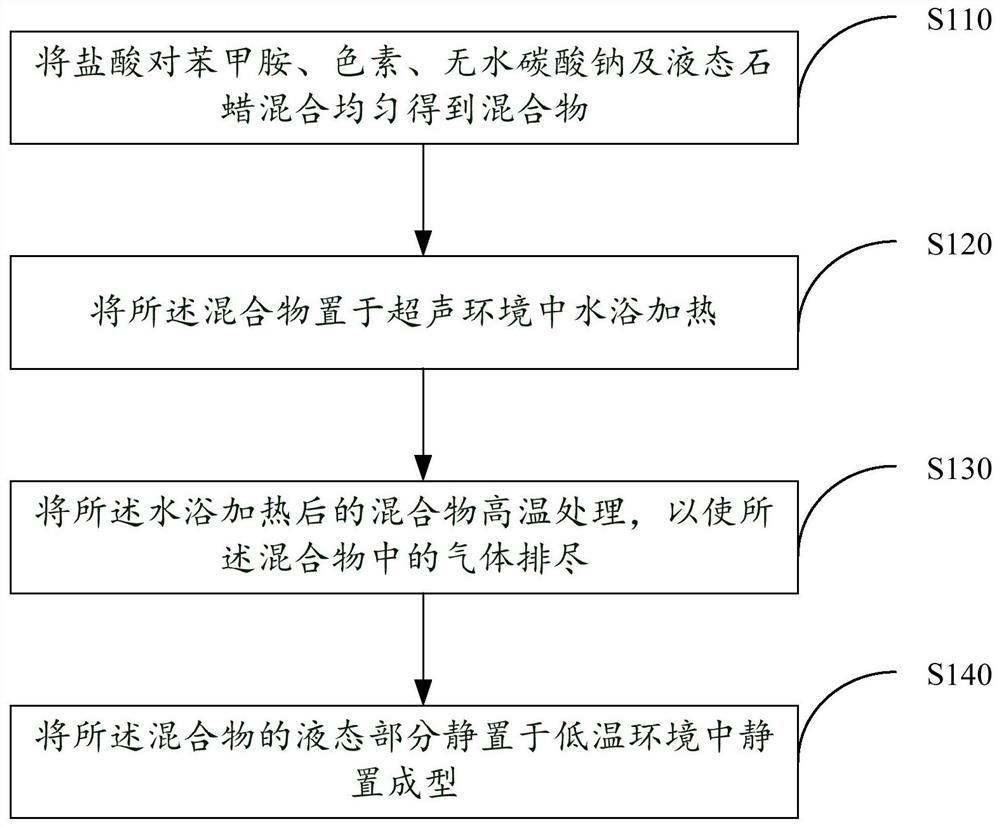
A preparation method of a drug detection equipment calibration product and a drug detection equipment calibration product
ActiveCN110261194BIncrease relative volatilityRealize Gas Phase CalibrationPreparing sample for investigationGeological measurementsParaffin waxBenzylamine hydrochloride
The preparation method of the drug detection equipment calibration product provided by the present invention comprises uniformly mixing p-benzylamine hydrochloride, pigment, anhydrous sodium carbonate and liquid paraffin to obtain a mixture, placing the mixture in an ultrasonic environment and heating it in a water bath, and The mixture heated in a water bath is treated at high temperature to exhaust the gas in the mixture, and the liquid part of the mixture is placed in a low-temperature environment for static molding to obtain the verification product of the drug detection equipment, which is provided by the present invention The preparation method of the calibration product of drug detection equipment is to mix the chemical calibration product into solid paraffin after deacidification treatment, and use the paraffin to slowly melt at high temperature to realize the slow volatilization of the calibration product, and then realize the gas phase calibration, which is easy to use and low in cost , easy to store and carry.
Owner:深圳砺剑防卫技术有限公司
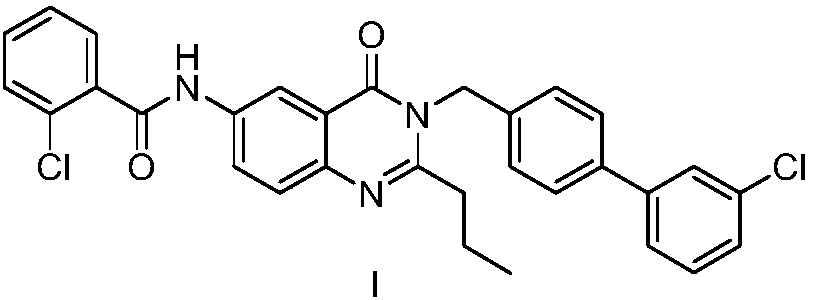
Method for preparing microsomal prostaglandin e2 synthase-1 inhibitor
The invention discloses a method for preparing microsomal prostaglandin E2 synthase-1 inhibitor. Contains three steps: 1) 5‑iodoisatoic anhydride and 4‑(3‑chlorophenyl) benzylamine hydrochloride to prepare N‑substituted amide; 2) N‑substituted amide and crotyl alcohol to generate 3‑[( 3'-chloro[1,1'-diphenyl]-4-yl)methyl]-2-propyl-6-iodoquinazolone; 3) The product of the previous step reacts Goldberg with o-chlorobenzamide The amination reaction yields the target product. The preparation method has relatively simple steps, avoids the use of alkali and toxic chemical reagents, such as triphenyl phosphite, uses cheap and easy-to-obtain crotyl alcohol as a raw material, automatically transfers hydrogen, does not add alkali in the reaction process, and "one-pot" reaction Complete, the by-product is only water, the reaction atom economy is high, meets the requirements of green chemistry, and has broad development prospects.
Owner:NANJING UNIV OF SCI & TECH
A kind of preparation method of avanafil intermediate
InactiveCN105439964BReduce dosageSafe post-processingOrganic chemistryEthyl esterMethanesulfonyl chloride
The invention provides a preparation method of an Avanafil intermediate. The preparation method comprises the following steps: dissolving 4-hydroxy-2-methylthio-ethyl 5-pyrimidinecarboxylate and potassium carbonate in DMF to obtain a mixed solution, adding methanesulfonyl chloride into the mixed solution under the stirring condition, continuously stirring after addition until a reaction is ended, adding water and ethyl acetate into a reaction solution, carrying out extraction and liquid separation, carrying out vacuum concentration on an organic phase to prepare 4-methanesulfonic sulfonic ester-2-methylthio-ethyl 5-pyrimidinecarboxylate; dissolving the obtained 4-methanesulfonic sulfonic ester-2-methylthio-ethyl 5-pyrimidinecarboxylate into DMF, adding a (3-chloro-4-methoxy)Benzylamine hydrochloride to obtain mixed liquor, adding triethylamine into the mixed liquor under the stirring condition, continuously stirring after addition until a reaction is ended, adding water and ethyl acetate, carrying out extraction and liquid separation, carrying out vacuum concentration on an organic phase, and recrystallizing to prepare 4-(3-chloro-4-methoxybenzylamino)-5- ethyloxycarbonyl-2-methylthiopyrimidine. The invention has advantages of less environmental pollution, high product yield, safe post-processing and simple operation.
Owner:HEBEI UNIVERSITY
Hydrochloric acid dapoxetine technology impurities, preparation and use thereof
The invention belongs to the field of drug synthesis, and relates to impurities in a raw material medicine production process and a preparation method thereof, in particular to a hydrochloric acid dapoxetine: the technology impurities of (+)-(S)-N,N-dimethyl-(alpha)-[2-(1-naphthyloxy) ethyl] benzylamine hydrochloride and the preparation method thereof. The impurities are selected from a compound A(3,3'-oxybis(N,N-dimethyl-1-amphetamine) hydrochloride), a compound D (N,N-dimethyl-3-(1-naphthyloxy)-N-(3-(1-naphthyloxy)-1-phenylpropyl)-1-phenylpropylammonium chloride).
Owner:扬子江药业集团江苏紫龙药业有限公司
Method for preparing N-[4-[1-hydroxy-2-[(1-methylethyl)amino]ethyl]methylsulfonyl benzylamine hydrochloride
ActiveCN102329254BEasy to manufactureShort stepsSulfonic acid amide preparationIsopropamideBenzylamine hydrochloride
The invention discloses a novel method for preparing N-[4-[1-hydroxy-2-[(1-methylethyl)amino]ethyl]methylsulfonyl benzylamine hydrochloride (I), which comprises: reaction of phenylamine and methanesulfonyl chloride to produce N-phenyl methanesulfonamide (II), reaction of the obtained compound (II) and chloroacetyl chloride to produce N-[4-[2-chloroacetyl phenyl]methanesulfonamide (III), reaction of the obtained compound (III) and isopropamide to produce N-[4-[2-(1-methylethyl)amino]acetyl]methylsulfonyl benzylamine hydrochloride (VI), and reduction of the obtained compound (VI) by sodium borohydride to produce the target product N-[4-[1-hydroxy-2-[(1-methylethyl)amino]ethyl]methylsulfonyl benzylamine hydrochloride (I). The method has the advantages of low cost, short reaction procedures, simple operation, high yield, high product purity and the like, and is suitable for industrial production.
Owner:SHANGHAI AOBO PHARMTECH INC LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com


![Method for preparing N-[4-[1-hydroxy-2-[(1-methylethyl)amino]ethyl]methylsulfonyl benzylamine hydrochloride Method for preparing N-[4-[1-hydroxy-2-[(1-methylethyl)amino]ethyl]methylsulfonyl benzylamine hydrochloride](https://images-eureka.patsnap.com/patent_img/068abe0a-bf82-4c82-8f81-2f82b6e85878/BSA00000184793200011.PNG)
![Method for preparing N-[4-[1-hydroxy-2-[(1-methylethyl)amino]ethyl]methylsulfonyl benzylamine hydrochloride Method for preparing N-[4-[1-hydroxy-2-[(1-methylethyl)amino]ethyl]methylsulfonyl benzylamine hydrochloride](https://images-eureka.patsnap.com/patent_img/068abe0a-bf82-4c82-8f81-2f82b6e85878/BSA00000184793200021.PNG)
![Method for preparing N-[4-[1-hydroxy-2-[(1-methylethyl)amino]ethyl]methylsulfonyl benzylamine hydrochloride Method for preparing N-[4-[1-hydroxy-2-[(1-methylethyl)amino]ethyl]methylsulfonyl benzylamine hydrochloride](https://images-eureka.patsnap.com/patent_img/068abe0a-bf82-4c82-8f81-2f82b6e85878/BSA00000184793200022.PNG)

![Polymorphic form of N-[2-(6-fluoro-1H-indol-3-yl)ethyl]-3-(2,2,3,3-tetrafluoropropoxy)benzylamine hydrochloride for the treatment of Alzheimer's disease Polymorphic form of N-[2-(6-fluoro-1H-indol-3-yl)ethyl]-3-(2,2,3,3-tetrafluoropropoxy)benzylamine hydrochloride for the treatment of Alzheimer's disease](https://images-eureka.patsnap.com/patent_img/80a0d55f-ba90-42f2-8ac4-5d13ff83ee5a/US10071963-20180911-D00001.png)
![Polymorphic form of N-[2-(6-fluoro-1H-indol-3-yl)ethyl]-3-(2,2,3,3-tetrafluoropropoxy)benzylamine hydrochloride for the treatment of Alzheimer's disease Polymorphic form of N-[2-(6-fluoro-1H-indol-3-yl)ethyl]-3-(2,2,3,3-tetrafluoropropoxy)benzylamine hydrochloride for the treatment of Alzheimer's disease](https://images-eureka.patsnap.com/patent_img/80a0d55f-ba90-42f2-8ac4-5d13ff83ee5a/US10071963-20180911-D00002.png)
![Polymorphic form of N-[2-(6-fluoro-1H-indol-3-yl)ethyl]-3-(2,2,3,3-tetrafluoropropoxy)benzylamine hydrochloride for the treatment of Alzheimer's disease Polymorphic form of N-[2-(6-fluoro-1H-indol-3-yl)ethyl]-3-(2,2,3,3-tetrafluoropropoxy)benzylamine hydrochloride for the treatment of Alzheimer's disease](https://images-eureka.patsnap.com/patent_img/80a0d55f-ba90-42f2-8ac4-5d13ff83ee5a/US10071963-20180911-D00003.png)
![Novel Polymorphic Form of N-[2-(6-fluoro-lH-indol-3-yl)ethyl]-3-2,2,3,3-tetrafluoropropoxy)benzylamine hydrochoride for the treatment of Alzheimer's Novel Polymorphic Form of N-[2-(6-fluoro-lH-indol-3-yl)ethyl]-3-2,2,3,3-tetrafluoropropoxy)benzylamine hydrochoride for the treatment of Alzheimer's](https://images-eureka.patsnap.com/patent_img/8820eb7a-06a6-49c6-b8df-da550b4db97c/US20170152227A1-20170601-D00000.png)
![Novel Polymorphic Form of N-[2-(6-fluoro-lH-indol-3-yl)ethyl]-3-2,2,3,3-tetrafluoropropoxy)benzylamine hydrochoride for the treatment of Alzheimer's Novel Polymorphic Form of N-[2-(6-fluoro-lH-indol-3-yl)ethyl]-3-2,2,3,3-tetrafluoropropoxy)benzylamine hydrochoride for the treatment of Alzheimer's](https://images-eureka.patsnap.com/patent_img/8820eb7a-06a6-49c6-b8df-da550b4db97c/US20170152227A1-20170601-D00001.png)
![Novel Polymorphic Form of N-[2-(6-fluoro-lH-indol-3-yl)ethyl]-3-2,2,3,3-tetrafluoropropoxy)benzylamine hydrochoride for the treatment of Alzheimer's Novel Polymorphic Form of N-[2-(6-fluoro-lH-indol-3-yl)ethyl]-3-2,2,3,3-tetrafluoropropoxy)benzylamine hydrochoride for the treatment of Alzheimer's](https://images-eureka.patsnap.com/patent_img/8820eb7a-06a6-49c6-b8df-da550b4db97c/US20170152227A1-20170601-D00002.png)
![Method for preparing N-[4-[1-hydroxy-2-[(1-methylethyl)amino]ethyl]methylsulfonyl benzylamine hydrochloride Method for preparing N-[4-[1-hydroxy-2-[(1-methylethyl)amino]ethyl]methylsulfonyl benzylamine hydrochloride](https://images-eureka.patsnap.com/patent_img/bc21df8e-771e-49d2-b23d-a6682d848618/BSA00000184793200011.png)
![Method for preparing N-[4-[1-hydroxy-2-[(1-methylethyl)amino]ethyl]methylsulfonyl benzylamine hydrochloride Method for preparing N-[4-[1-hydroxy-2-[(1-methylethyl)amino]ethyl]methylsulfonyl benzylamine hydrochloride](https://images-eureka.patsnap.com/patent_img/bc21df8e-771e-49d2-b23d-a6682d848618/BSA00000184793200021.png)
![Method for preparing N-[4-[1-hydroxy-2-[(1-methylethyl)amino]ethyl]methylsulfonyl benzylamine hydrochloride Method for preparing N-[4-[1-hydroxy-2-[(1-methylethyl)amino]ethyl]methylsulfonyl benzylamine hydrochloride](https://images-eureka.patsnap.com/patent_img/bc21df8e-771e-49d2-b23d-a6682d848618/BSA00000184793200022.png)