A kind of disposable photoaffinity linker and preparation method and application
A link body, wash-free technology, applied in the direction of chemical instruments and methods, organic chemical methods, luminescent materials, etc., can solve the problems of cumbersome operation, prone to false positive results, large background interference, etc., and achieve high tracer accuracy , avoid false positive results, low background interference effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
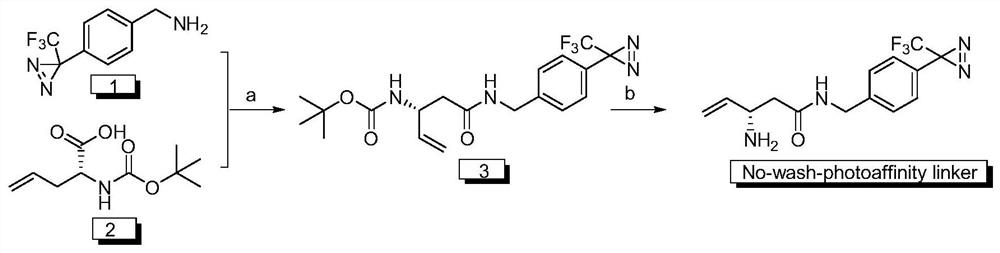
[0051] The preparation process in the wash-free photoaffinity linker is as follows: figure 1 as shown,
[0052] a) 4-[3-(trifluoromethyl)-3H-diaziridin-3-yl]benzylamine hydrochloride and (R)-2-((tert-butoxycarbonyl)amino)-4- Under the condensation of pentenoic acid with EDC·HCl, an intermediate containing a carbon-carbon double bond is obtained, and the specific process is as follows:
[0053] (R)-2-((tert-butoxycarbonyl)amino)-4-pentenoic acid (0.0430g, 0.2000mmol), EDC·HCl (0.0500g, 0.2400mmol), NHS (0.0300g, 0.2400mmol) Dissolve in 3mL of anhydrous dichloromethane, stir at 0°C for a period of time, after the system cools down, slowly add triethylamine (60.00μL, 0.4400mmol) dropwise, after reacting for 2h, add 4-[3-(trifluoromethyl )-3H-diaziridin-3-yl]benzylamine hydrochloride (0.05g, 0.2000mmol) was reacted to obtain a crude product containing a carbon-carbon double bond intermediate, washed three times with saturated sodium bicarbonate, saturated sodium chloride Washin...
Embodiment 2
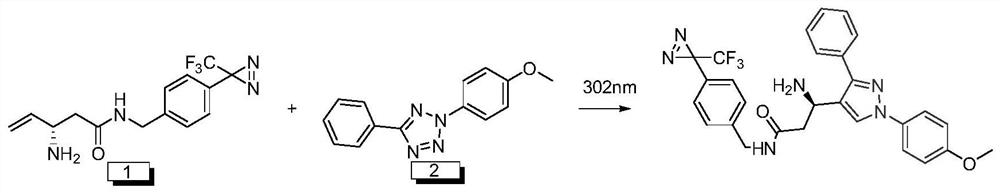
[0059] The principle of the probe of the present invention for cell tracer positioning or imaging is: bio-orthogonal reaction route such as figure 2 Shown, the specific process of reaction is as follows:
[0060] Dissolve 2-(4-methoxyphenyl)-5-phenyl-2H-tetrazole (0.0800g, 0.3200mmol), no-wash photoaffinity linker (0.0100g, 0.0320mmol) in 6ml of ethyl acetate , 302nm UV irradiation for 30min, the reactant was concentrated in vacuo, and separated by column chromatography (P:E=1:1), the product was about 0.04mg, and the yield was 23.4%.
[0061] LC-MS(ESI,m / z):535.10[M+H] + , 533.35[M-H] — .
Embodiment 3
[0063] Preliminary application of wash-free photoaffinity linkers containing carbon-carbon double bonds in photoaffinity labeling technology:
[0064] In the present invention, cytokines are used as targeted recognition molecules, and photoaffinity probe molecules are constructed by chemically modifying cytokines with wash-free photoaffinity linkers, and the constructed photoaffinity probe molecules are used to treat some highly expressed EGFR receptors. The cells of the body were tracked and positioned for imaging analysis.
[0065] Among them, the cytokines are transforming growth factor-β (transforming growth factor-β, TGF-β), vascular endothelial growth factor (VEGF), epidermal growth factor (Epidermal Growth Factor, EGF) or basic growth factor Fibroblast growth factor (bFGF).
[0066] Specifically, the construction process of the photoaffinity probe for EGF cytokines is as follows: (1) Prepare solution I: dissolve EGF cytokines in 1 mL triple-distilled water, store at 4°...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com