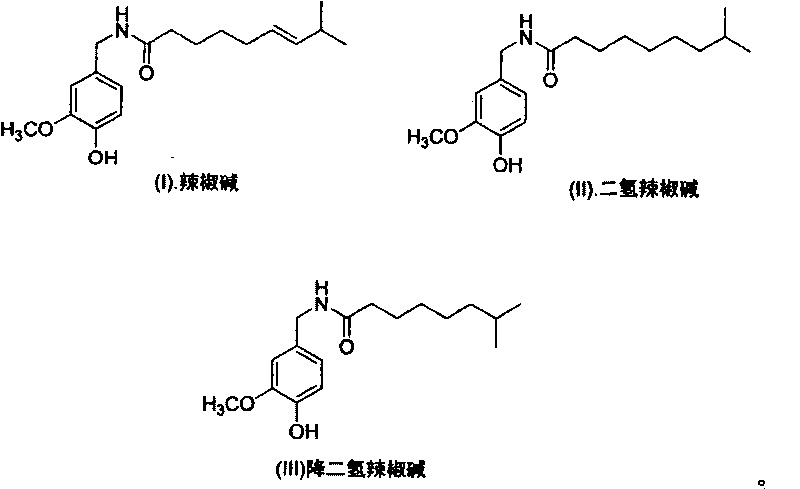
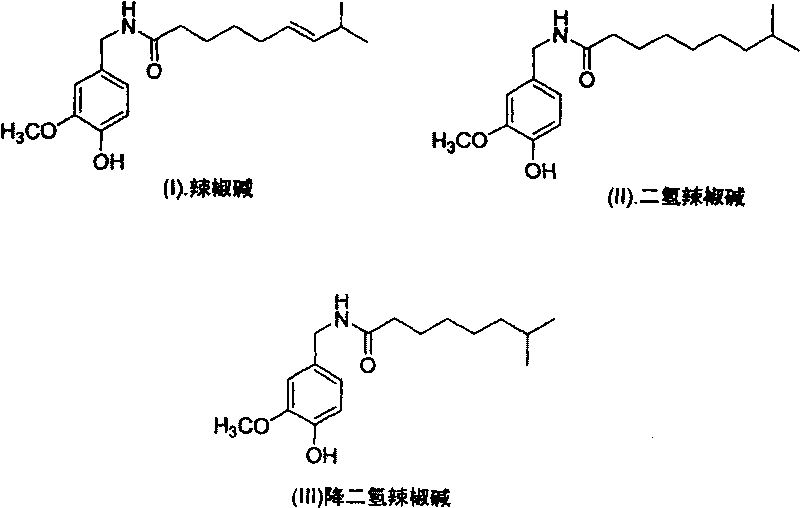
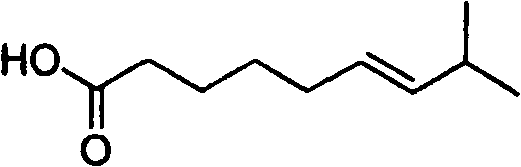
Artificial synthesis method of capsaicin homologue
A technology of artificial synthesis and capsaicin, which is applied in the field of artificial synthesis of capsaicin homologues, can solve the problems of unfriendly environment, difficult to apply to industrial production process, cumbersome steps, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Example 1. Artificial synthesis of capsaicin
[0041] (1) 4-Methyl-3-hydroxy-1-pentene
[0042] A reflux condenser, dropping funnel and stirrer were installed on a two-liter round bottom flask, and then 24.3 grams of magnesium bar (1.0 mol) and 1 liter of dry ether were added. Under the protection of nitrogen, 100 ml of 2-bromopropane (131 g, 1.06 mol) was added dropwise, and the dropping rate was controlled to maintain reflux. After the addition is completed, heat to reflux for 20 minutes, and add 70 ml of dry acrolein in 100 ml of ether under cooling with an ice water bath, and control the dropping rate to maintain the reaction temperature below 15°C. After the addition, the reaction was continued for one hour at room temperature. Slowly add 500 ml of 2N HCl under ice-water bath cooling, the ether solution is separated, the aqueous phase is extracted with ether to obtain the ether extract, the ether solution and the ether extract are combined and dried with sodium sulfat...
Embodiment 2
[0057] Example 2. Synthesis of dihydrocapsaicin
[0058] (1) 6-Methyl-3-hydroxy-1-heptene
[0059] A reflux condenser, dropping funnel and stirrer were installed on a two-liter round bottom flask, and then 24.3 grams of magnesium bar (1.0 mol) and 1 liter of dry ether were added. 126 ml of 1-bromo-3-methylbutane (159 g 1.05 mol) was added dropwise under the protection of nitrogen. Control the drip rate to maintain the reflux. After the addition is completed, heat to reflux for 20 minutes, and add 70 ml of dry acrolein in 100 ml of ether under cooling with an ice water bath, and control the dropping rate to maintain the reaction temperature below 15°C. After the addition, the reaction was continued for one hour at room temperature. Slowly add 500 ml of 2N HCl under ice-water bath cooling, the ether solution is separated, the aqueous phase is extracted with ether to obtain the ether extract, the ether solution and the ether extract are combined and dried with sodium sulfate and fi...
Embodiment 3
[0068] Example 3. Synthesis of nordihydrocapsaicin
[0069] (1) 5-Methyl-3-hydroxy-1-hexene
[0070] A reflux condenser, dropping funnel and stirrer were installed on a two-liter round bottom flask, and then 24.3 grams of magnesium bar (1.0 mol) and 1.0 liter of dry ether were added. Under nitrogen protection, 115 ml of isobutyl bromide (140 g, 1.02 mol) was added dropwise. Control the drip rate to maintain the reflux. After the addition is completed, heat to reflux for 20 minutes, and add 70 ml of dry acrolein in 100 ml of ether under cooling with an ice water bath, and control the dropping rate to maintain the reaction temperature below 15°C. After the addition, the reaction was continued for one hour at room temperature. Slowly add 500 ml of 2N HCl under ice-water bath cooling, the ether solution is separated, the aqueous phase is extracted with ether to obtain the ether extract, the ether solution and the ether extract are combined and dried with sodium sulfate and filtered,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com