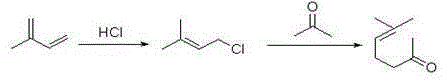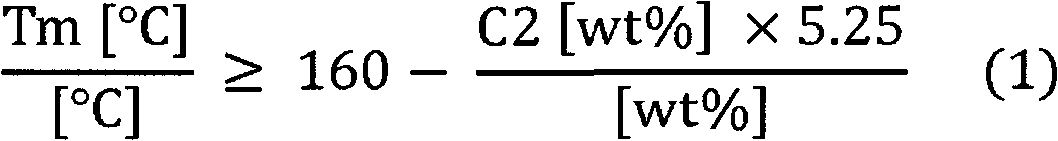Patents
Literature
321 results about "Heptene" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
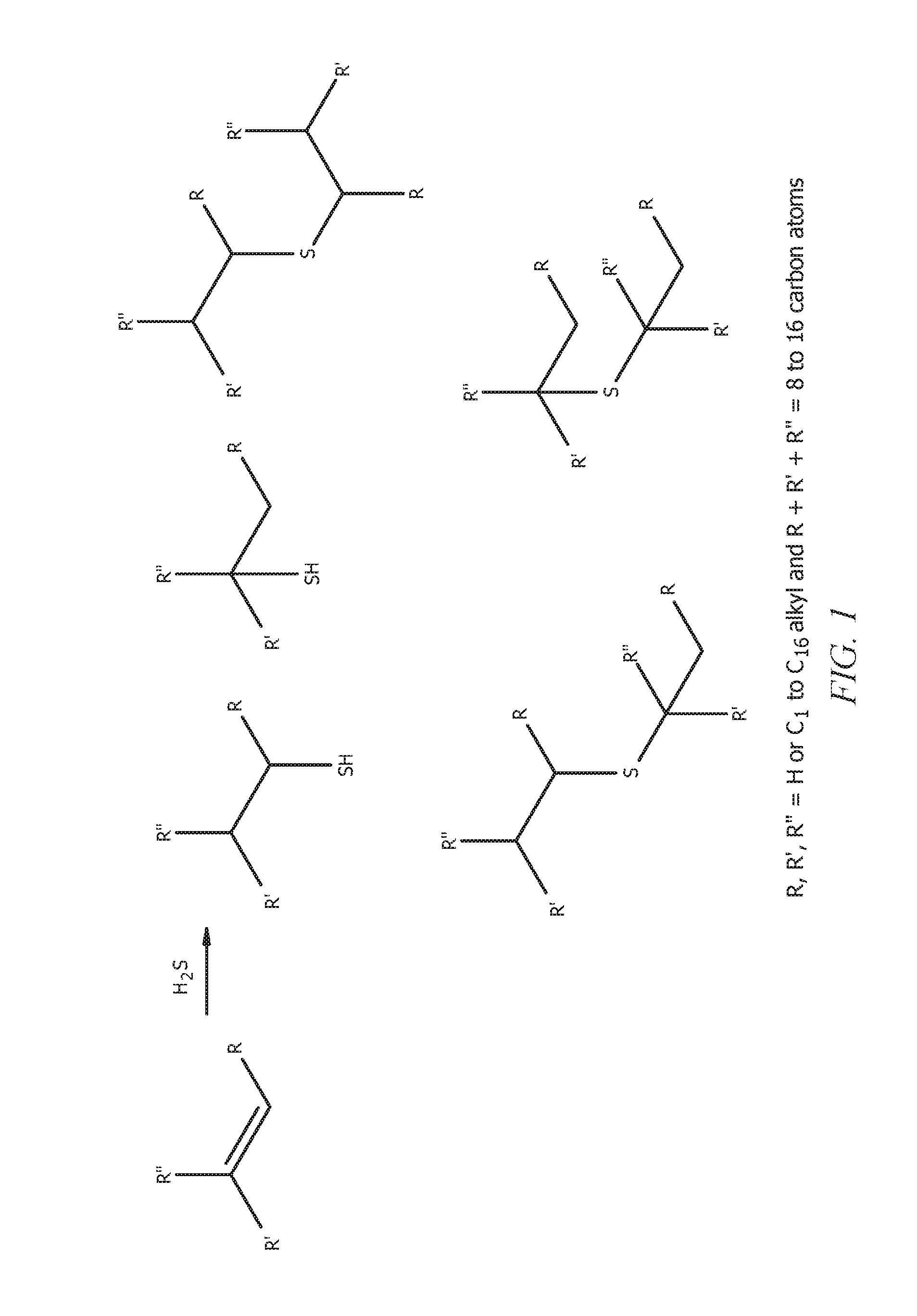
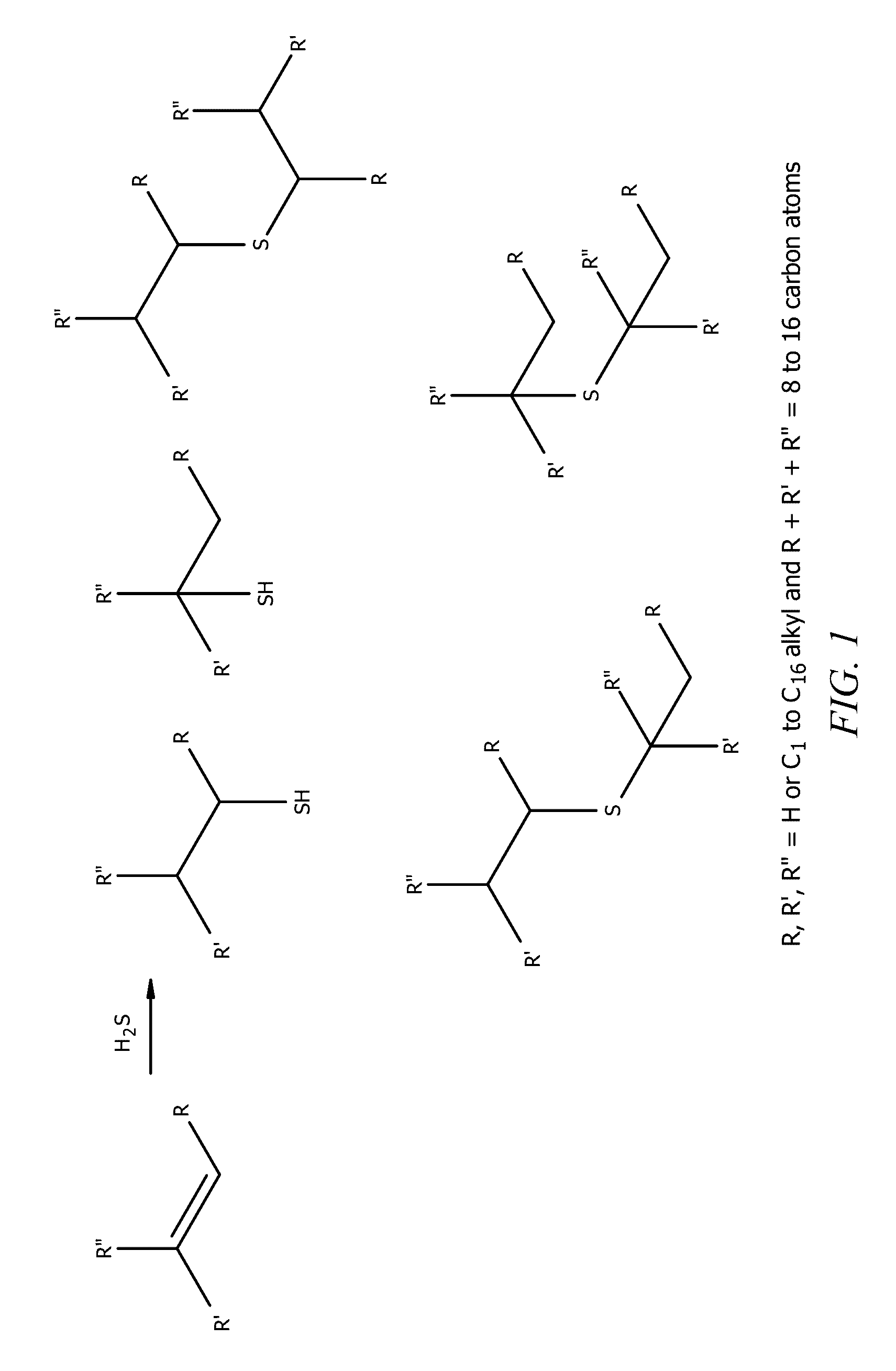
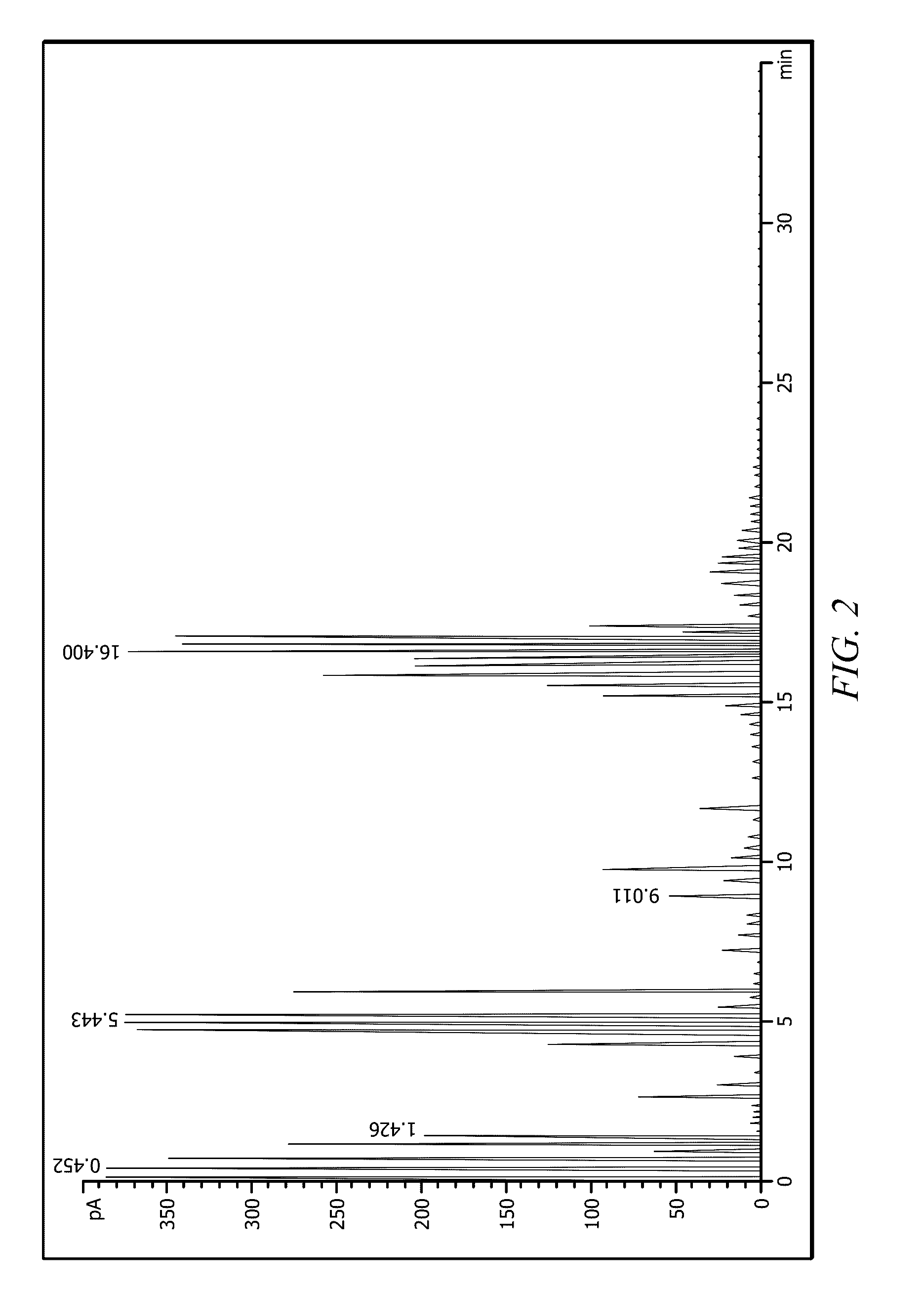
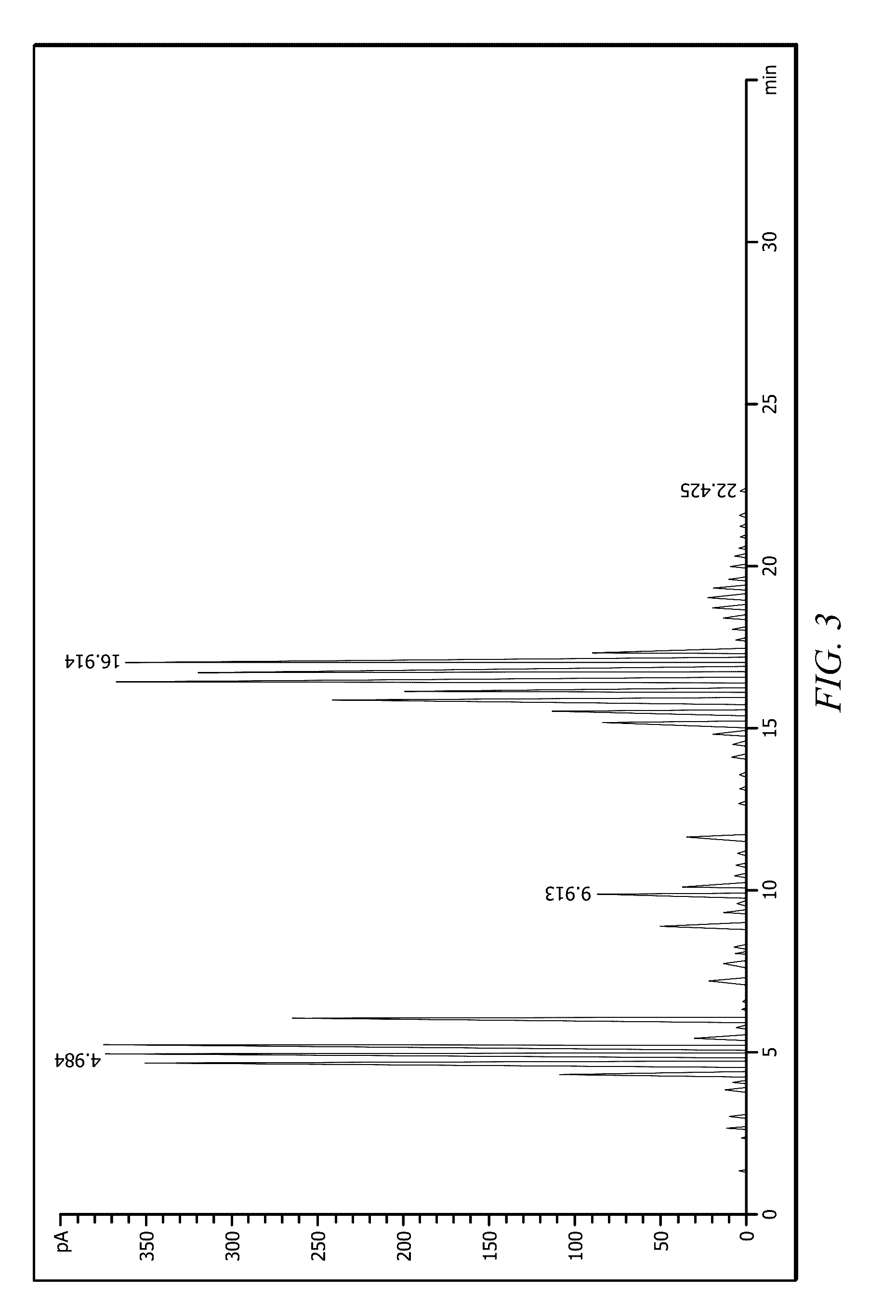
Heptene is a higher olefin, or alkene with the formula C₇H₁₄. The commercial product is a liquid that is a mixture of isomers. It is used as an additive in lubricants, as a catalyst, and as a surfactant. This chemical is also known as heptylene.
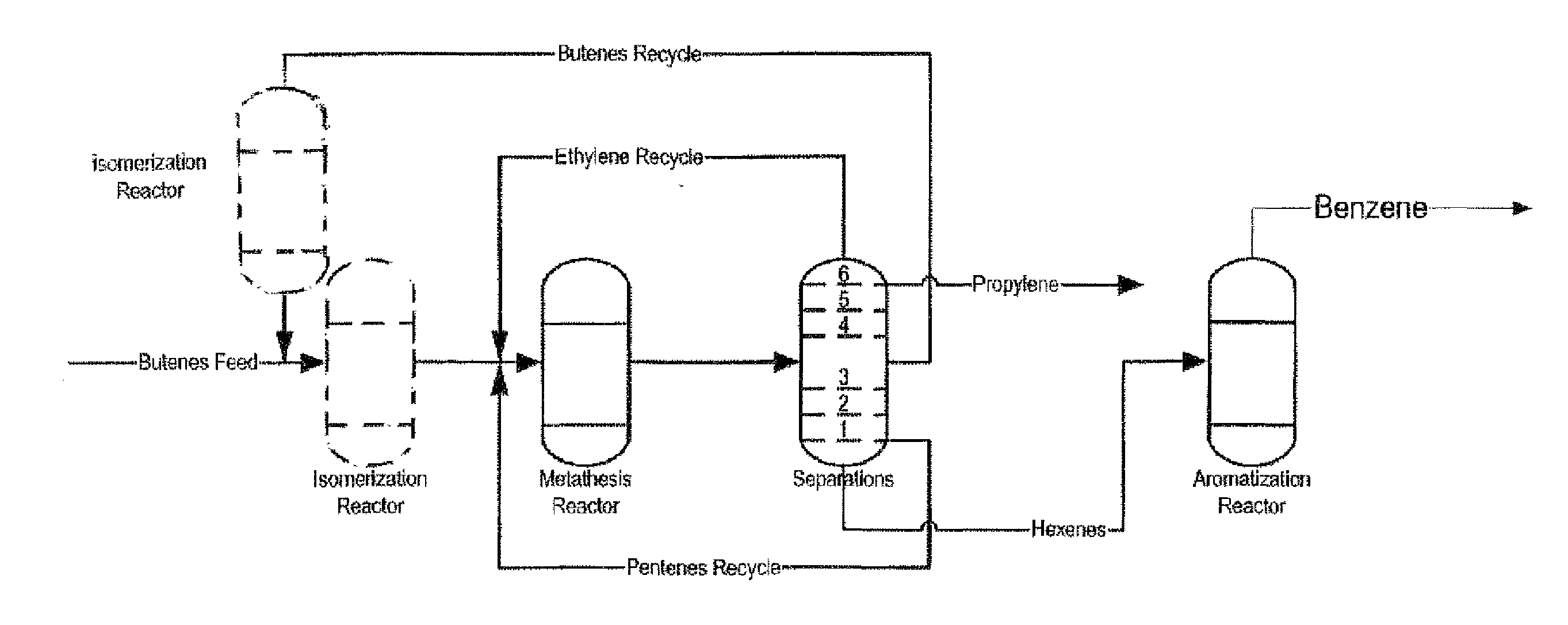
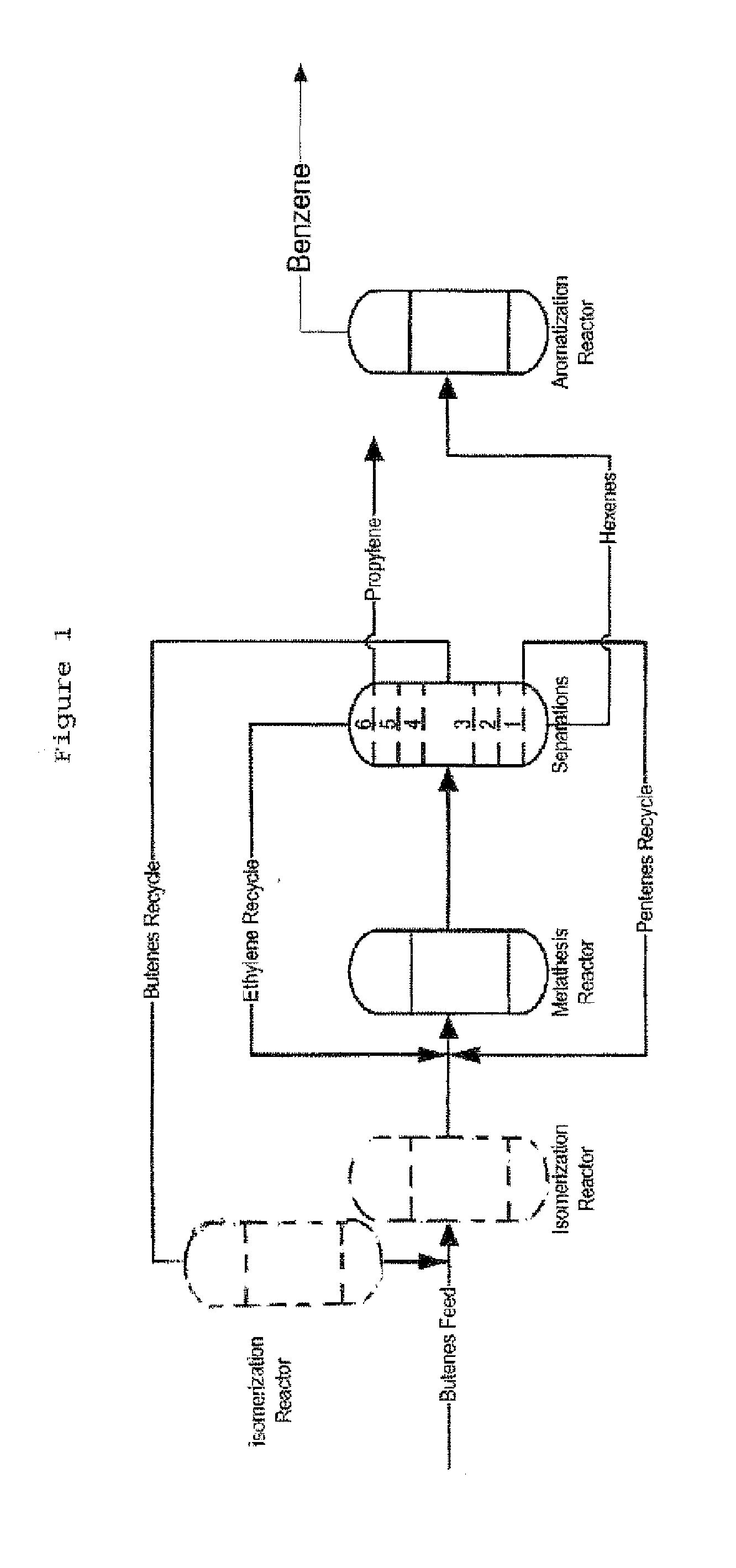
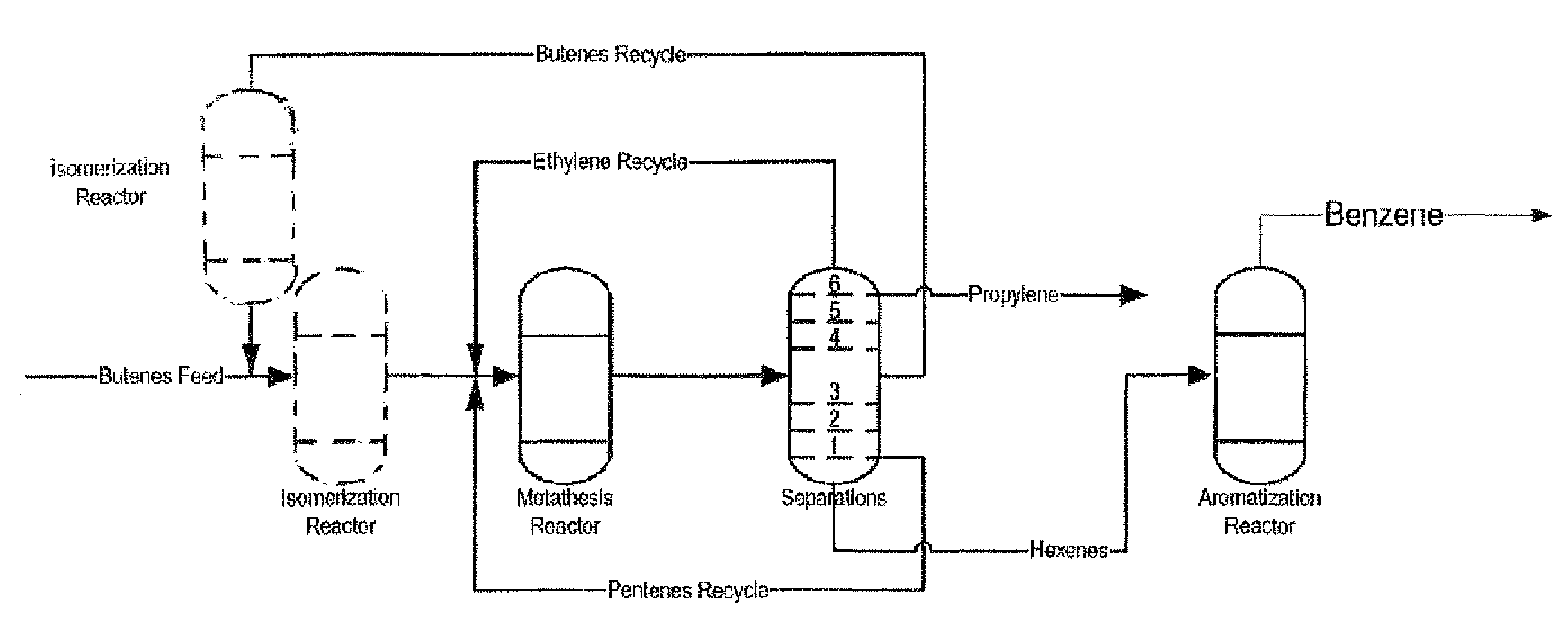
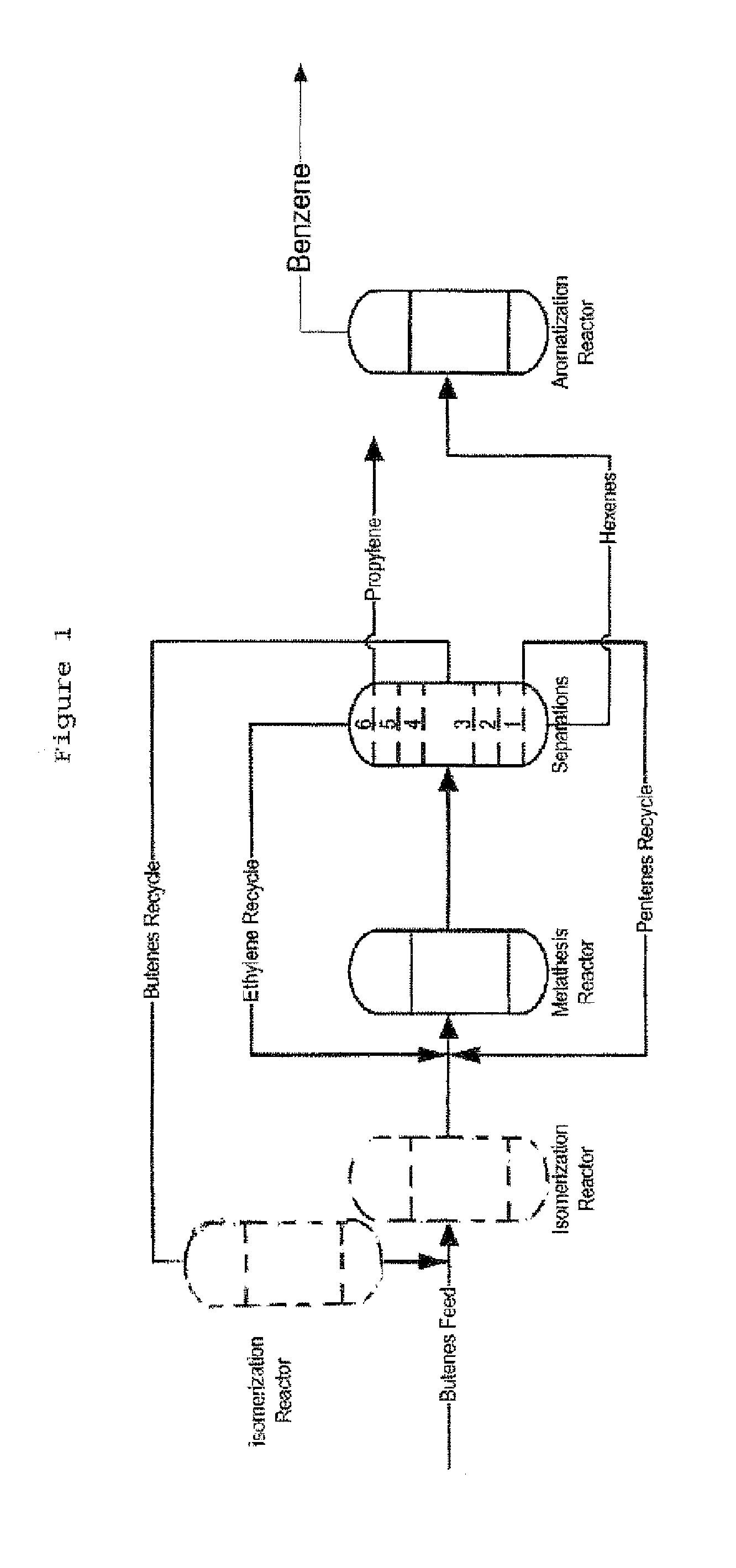
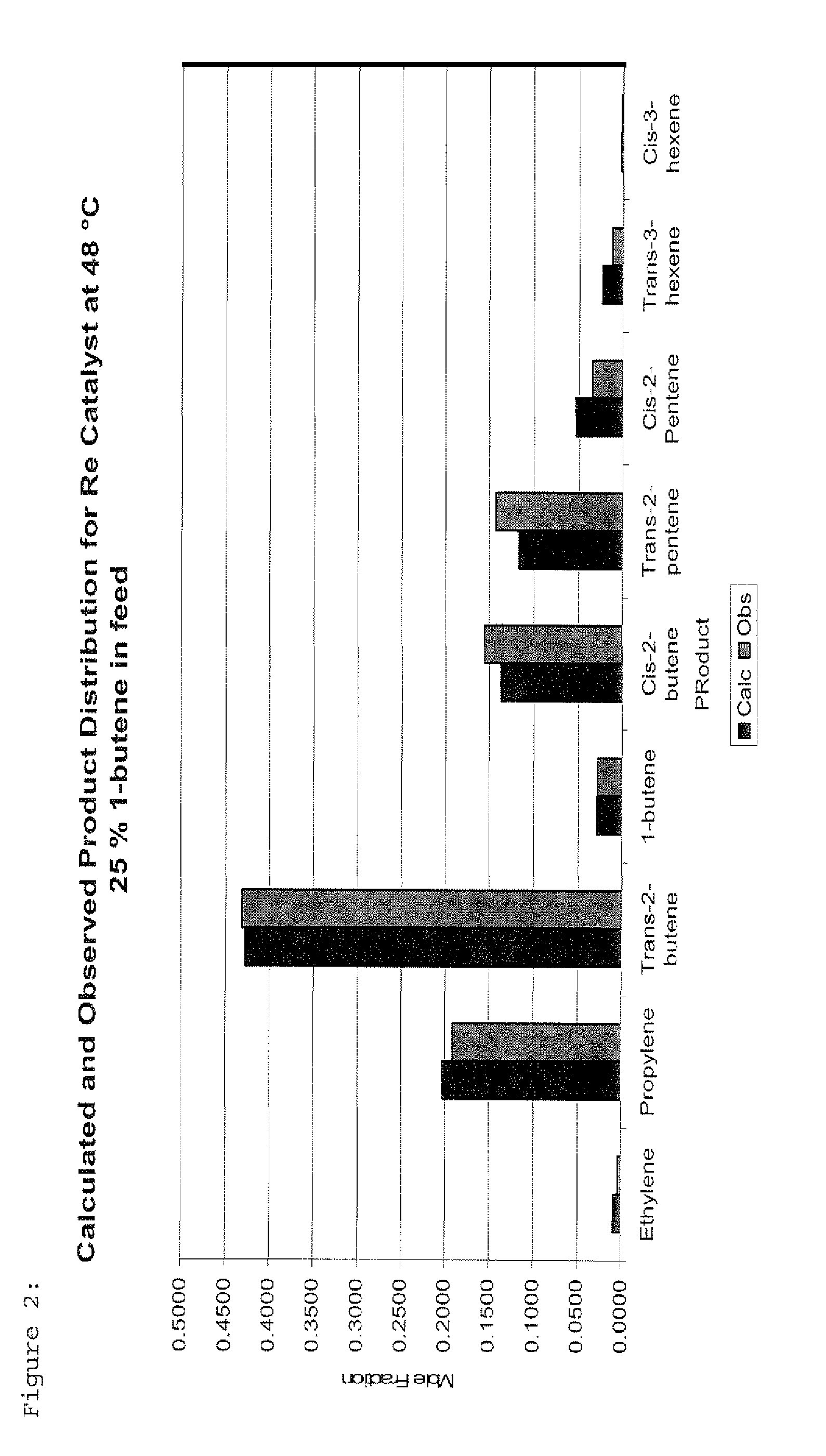
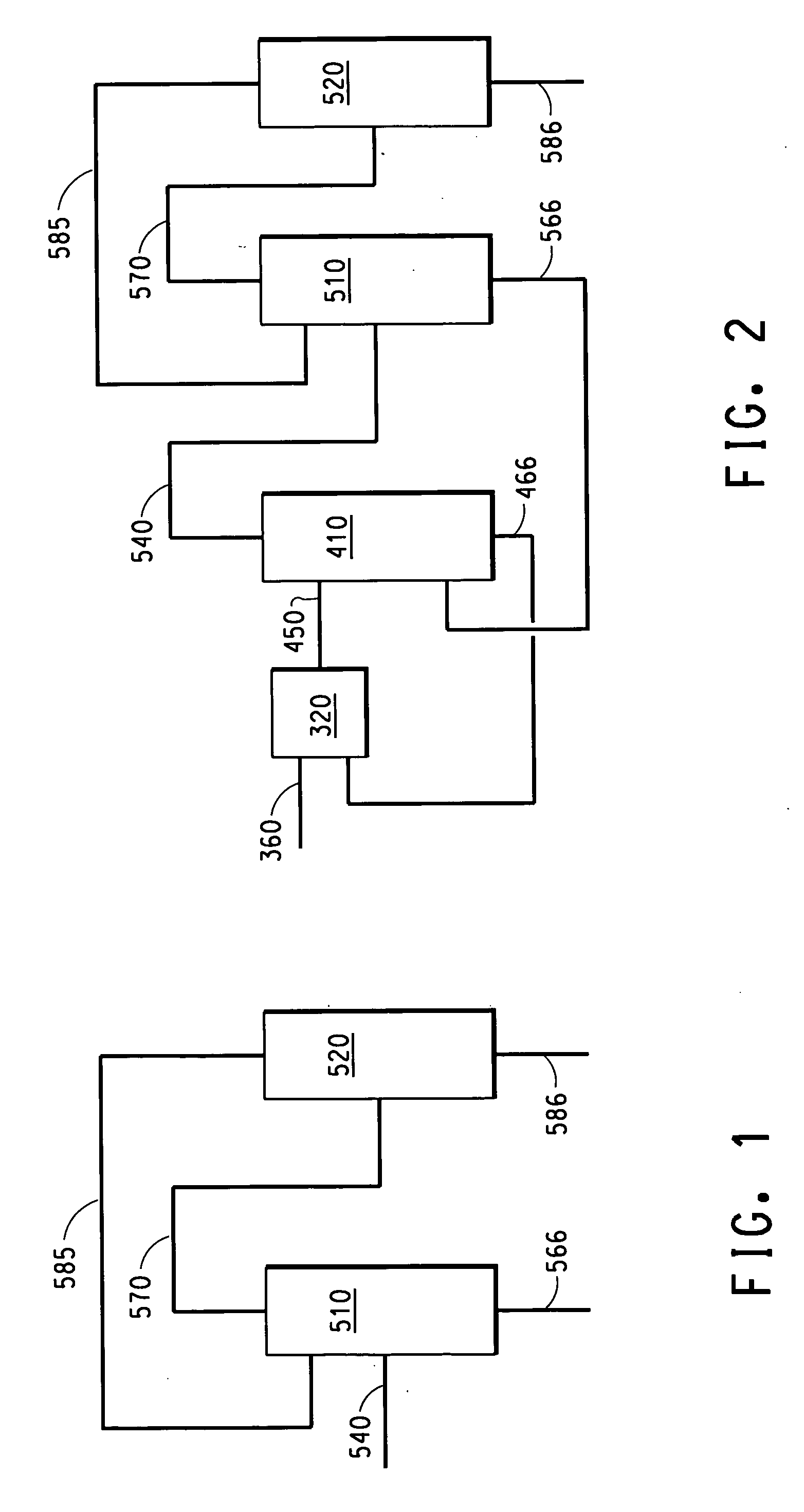
Process for Producing Propylene and Aromatics from Butenes by Metathesis and Aromatization
InactiveUS20110263917A1Molecular sieve catalystDistillation purification/separationIsomerizationOctene
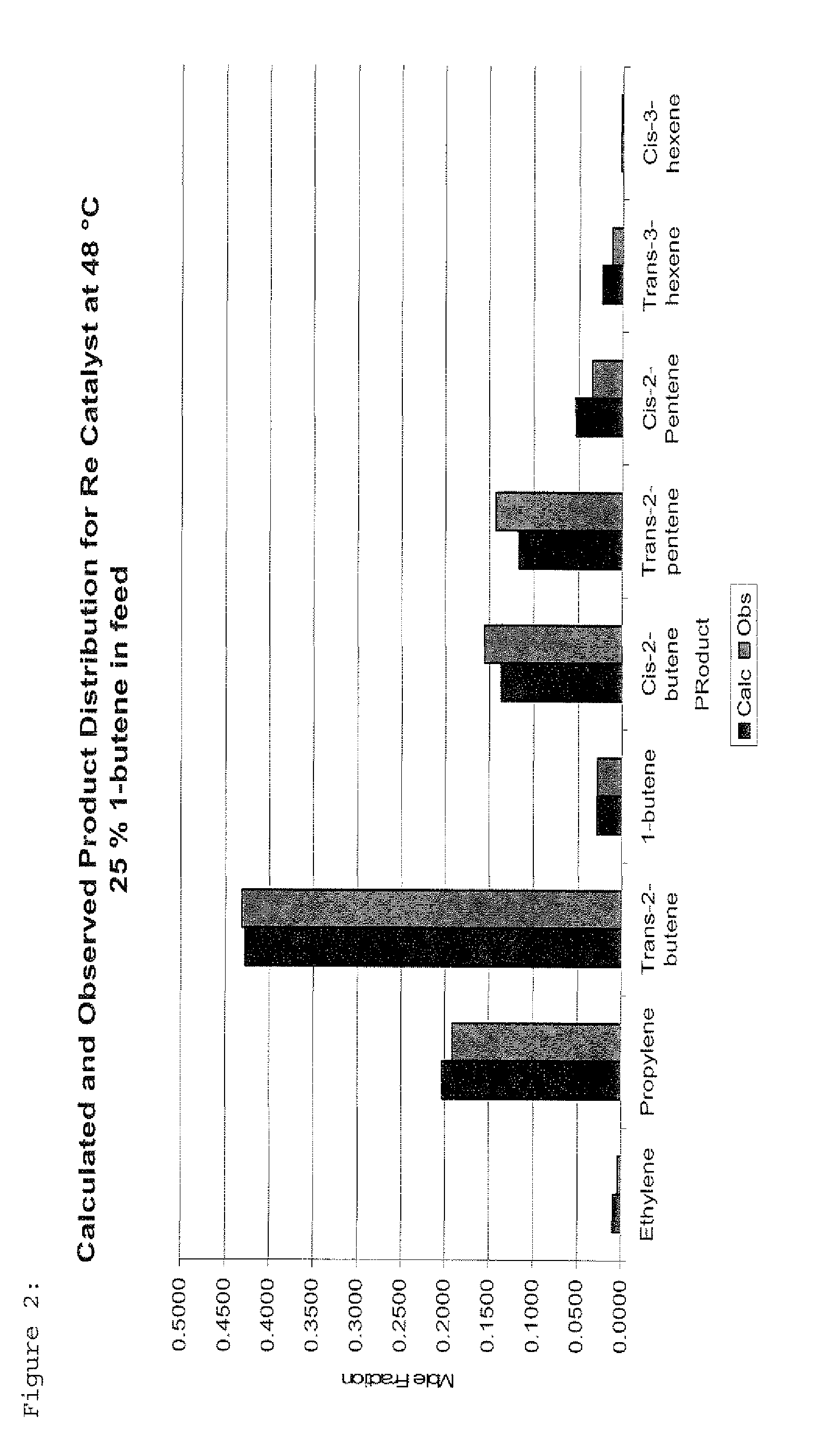
The invention is for a process for producing propylene and hexene (along with ethylene, pentenes, product butenes, heptenes and octenes) by metathesis from butenes (iso-, 1- and cis and trans 2-) and pentenes and then aromatizing the hexenes (along with higher olefins, such as heptenes and octenes) to benzene (along with toluene, xylenes, ethylbenzene and styrene). Since the desired products of the metathesis reaction are propylene and hexene, the feed to the metathesis reaction has a molar ratio for 1-butene:2-butene which favors production of propylene and 3-hexene with the concentration of hexenes and higher olefins in the metathesis product being up to 30 mole %. An isomerization reactor may be used to obtain the desired molar ratio of 1-butene:2-butene for the feed composition into the metathesis reactor. After the metathesis reaction, of hexene and higher olefins are separated for aromatization to benzene and other aromatics.
Owner:SAUDI BASIC IND CORP SA
Methods, systems and catalysts for the hydration of olefins
InactiveUS20040236158A1Low compressibilityOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsButenePolymer science
The present invention provides a system, method and catalyst for olefin hydration. The method includes hydrating the olefin using a base treated, sulfonated, halogenated and acid regenerated thermally stable catalyst. In several variants, the olefin hydration comprises butene hydration, propene hydration, hydration of cyclohexene, propylene hydration, pentene hydration, hexene hydration, and heptene hydration. The present invention also provides a method of making a catalyst for olefin hydration, and provides alcohols manufactured by the catalyst(s), systems and methods described herein.
Owner:COLLIN JENNIFER REICHI +1
Process for producing propylene and aromatics from butenes by metathesis and aromatization
The invention is for a process for producing propylene and hexene (along with ethylene, pentenes, product butenes, heptenes and octenes) by metathesis from butenes (iso-, 1- and cis and trans 2-) and pentenes and then aromatizing the hexenes (along with higher olefins, such as heptenes and octenes) to benzene (along with toluene, xylenes, ethylbenzene and styrene). Since the desired products of the metathesis reaction are propylene and hexene, the feed to the metathesis reaction has a molar ratio for 1-butene:2-butene which favors production of propylene and 3-hexene with the concentration of hexenes and higher olefins in the metathesis product being up to 30 mole %. An isomerization reactor may be used to obtain the desired molar ratio of 1-butene:2-butene for the feed composition into the metathesis reactor. After the metathesis reaction, of hexene and higher olefins are separated for aromatization to benzene and other aromatics.
Owner:SAUDI BASIC IND CORP SA
Azeotropic and azeotrope-like compositions of methyl perfluoroheptene ethers and trans-1,2-dichloroethylene and uses thereof
ActiveUS8410039B2Detergent mixture composition preparationChemical paints/ink removersEtherDichloroethylenes
Owner:THE CHEMOURS CO FC LLC
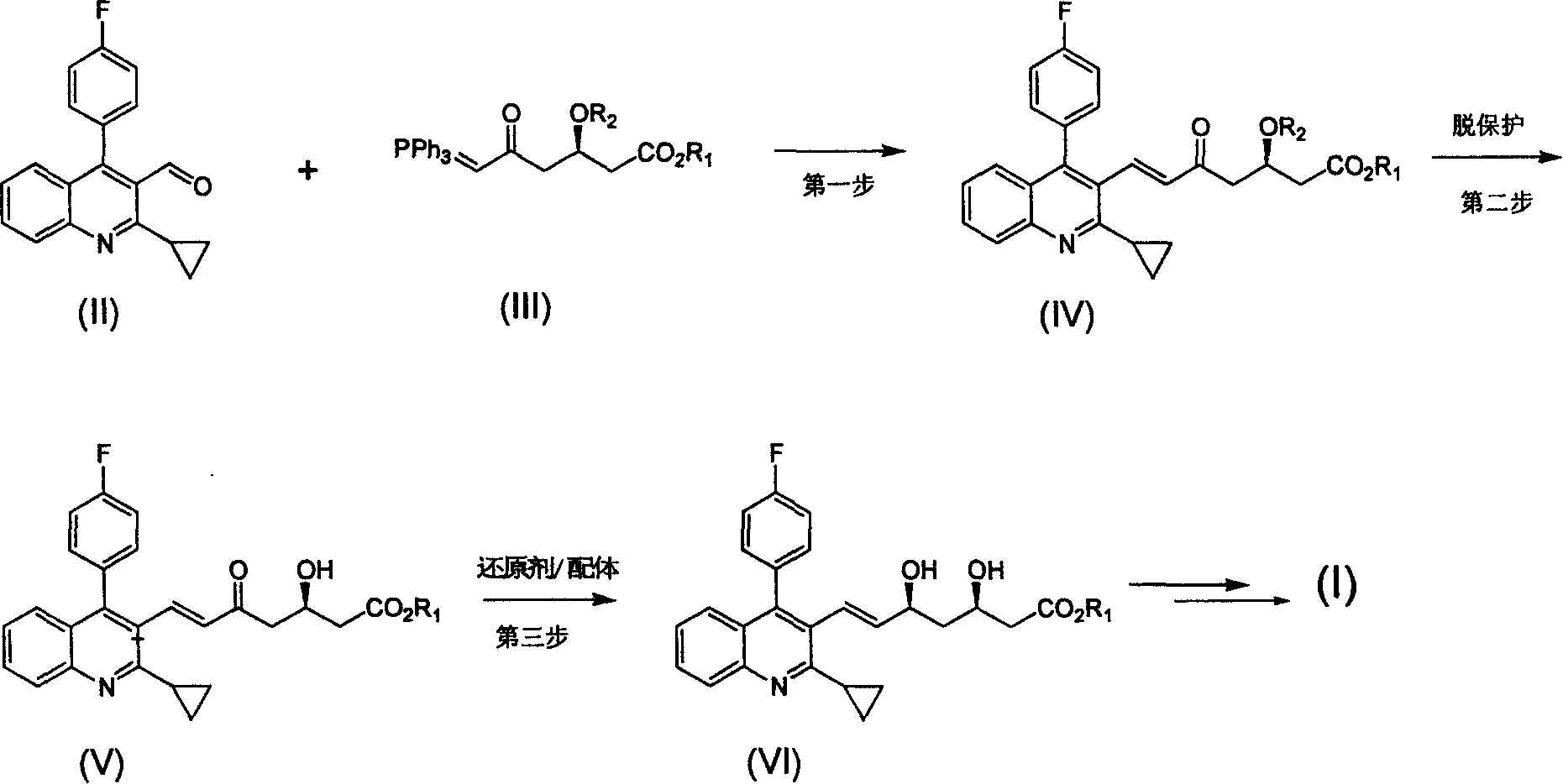
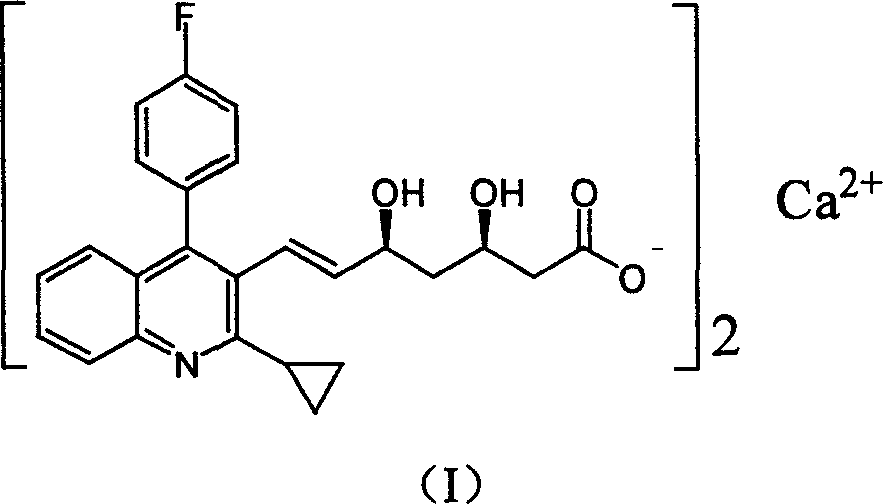
Method for preparing high optical purity pitavastatin calcium raw material drug
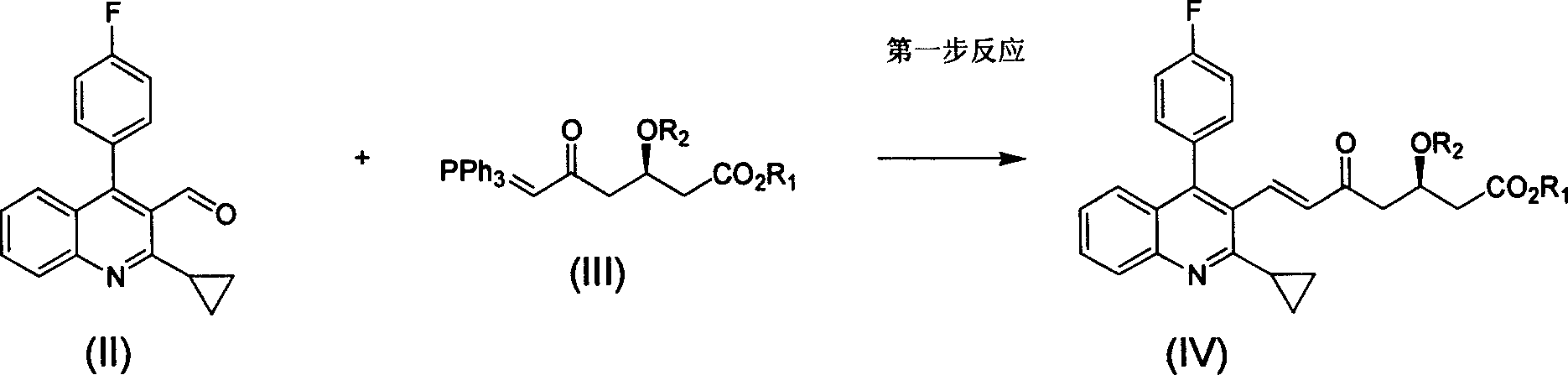
The invention relates the method of preparing the raw material of high optical purity pravastatin calcium. The method comprises the following steps: adding the 2- cyclopropyl-4-(4- fluorophenyl)-3-quinoline aldehyde II and (3R)-3- alkoxy silane-5- carbonyl-6- triphenyl phosphor heptene acid ester III in dissolvent, getting (E)-7- [2-2- cyclopropyl-4-(4- fluorophenyl)-3- chinoline]-5- carbonyl-(3R)-3- alkoxy silane-6- triphenyl phosphor heptene acid ester IV, removing the protection of IV, getting (E)-7- [2-2- cyclopropyl-4-(4- fluorophenyl)-3- chinoline]-5- carbonyl-(3R)- hydroxyl -6- triphenyl phosphor heptene acid ester V, deacidizing it in the mixture dissolvent of alcohol and ether with NaBH4 or KBH4 at -100-0Deg.C, getting (E)-7- [2-2- cyclopropyl-4-(4- fluorophenyl)-3- chinoline]-(3R, 5S)- dihydroxy -6- triphenyl phosphor heptene acid ester VI, hydrolyzing it with alkali, and getting pravastatin calcium. The material is used to prepare HMG-CoA reductase inhibiting agent.
Owner:CHINA RESOURCES DOUBLE CRANE PHARMA COMPANY
Bifunctional lactide monomer derivative and polymers and materials prepared using the same
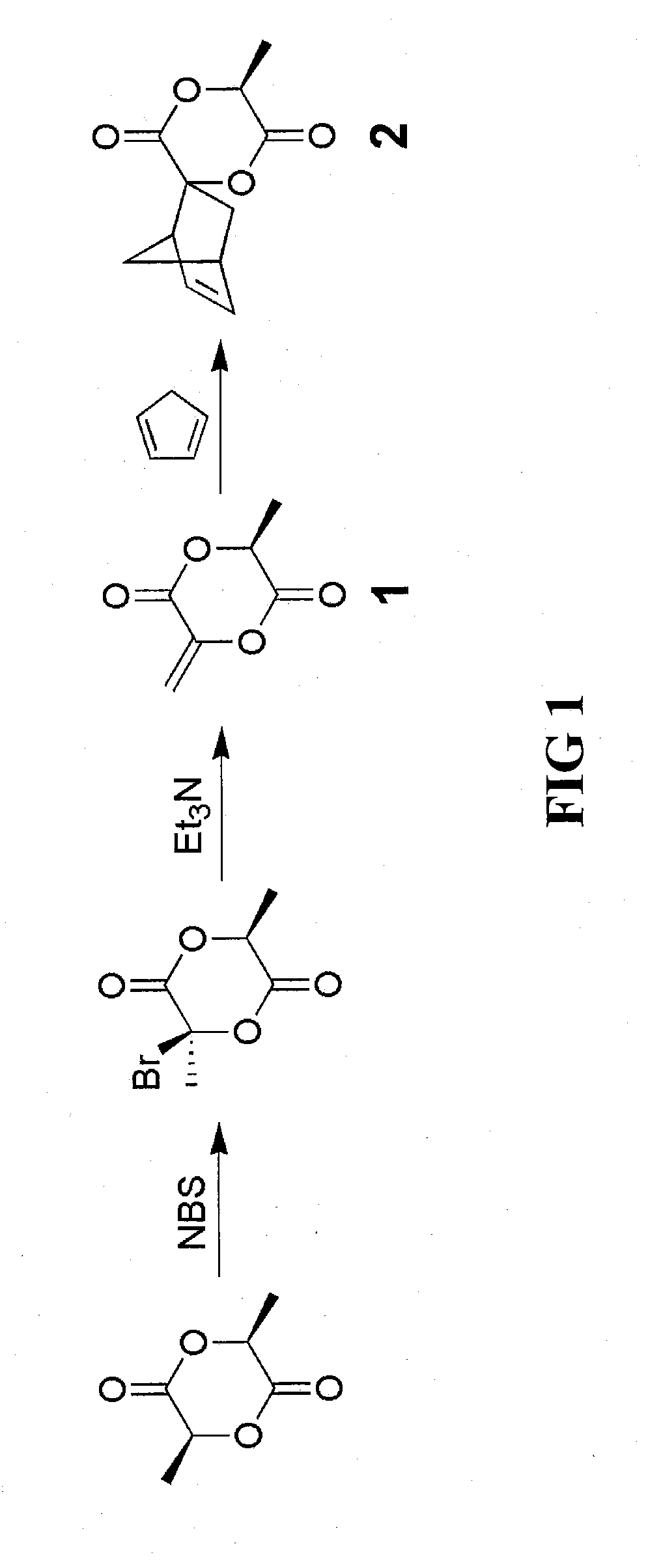
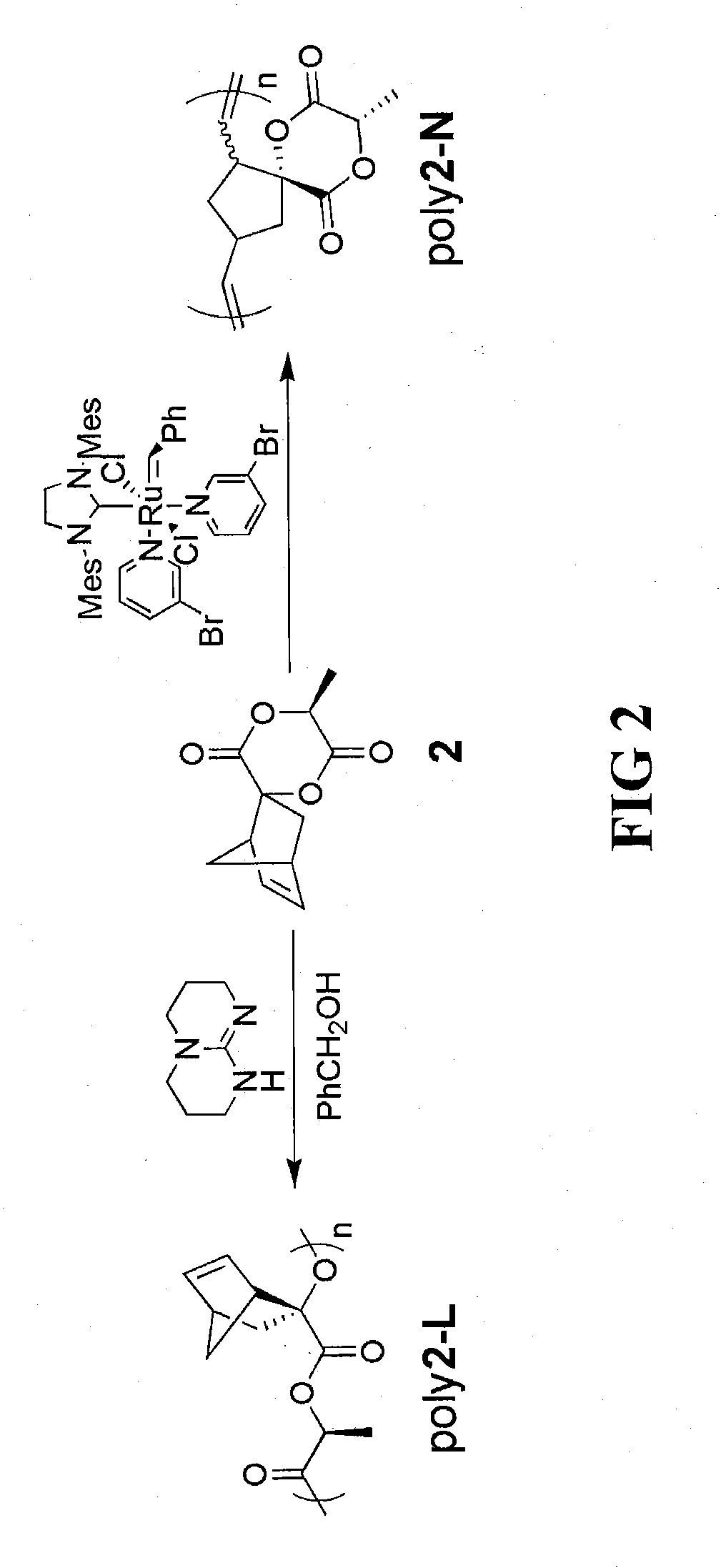
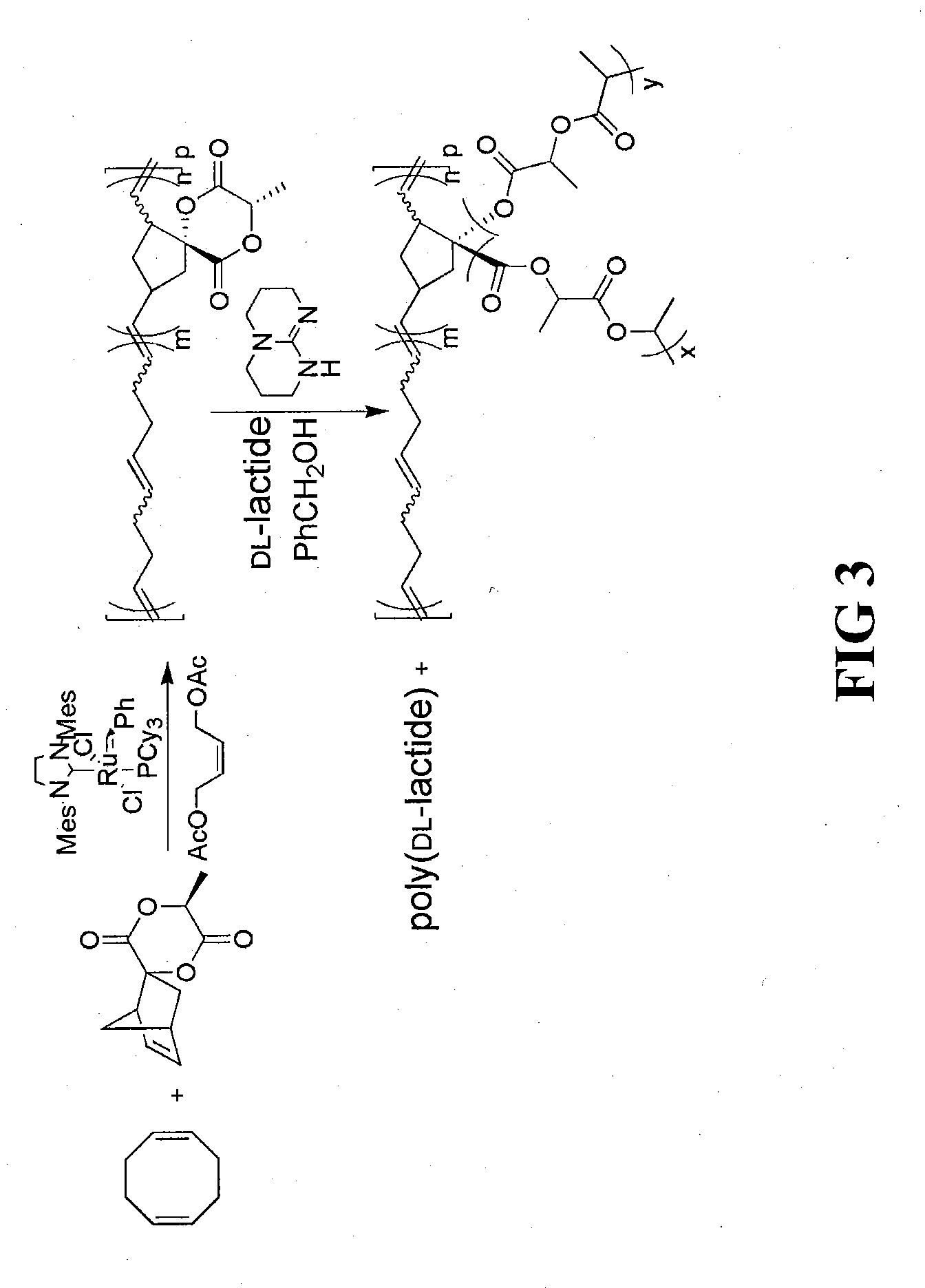
The invention described herein provides a novel lactide monomer derivative and process for preparing the lactide monomer derivative. The monomer derivative of the invention is bifunctional in nature, and can be employed a variety of efficient synthesis processes to prepare various polymers. Further, the bifunctional monomer derivative can be used to prepare various intermediate-stage compounds and polymers, which in turn can be used to synthesize other compounds, polymers, copolymers and composites. The lactide monomer derivative has a bifunctional norbornene spiro lactide structure, spiro[6-methyl-1,4-dioxane-2,5-dione-3,2′-bicyclo[2.2.1]hept[5]ene], and structure as follows:and stereoisomers thereof. The lactide monomer derivative is bifunctional in that either 1) the norbornene ring, 2) lactide ring, or 3) both, can be opened and used in polymer synthesis for the backbone or the reactive branch for other polymeric syntheses.
Owner:RGT UNIV OF MINNESOTA
Azeotrope compositions comprising tridecafluoro-3-heptene and hydrogen fluoride and uses thereof
ActiveUS20070100176A1Preparation by hydrogen halide split-offHalogenated hydrocarbon separation/purificationHydrogen fluoridePhysical chemistry
Disclosed herein are azeotrope and near-azeotrope compositions comprising 1,1,1,2,2,4,5,5,6,6,7,7,7-tridecafluoro-3-heptene (HFC-162-13mczy, CF3CF2CH═CFCF2CF2CF3) and 1,1,1,2,2,3,5,5,6,6,7,7,7-tridecafluoro-3-heptene (HFC-162-13mcyz, CF3CF2CF═CHCF2CF2CF3) and hydrogen fluoride (HF) and to azeotrope and near-azeotrope compositions comprising 1,1,1,2,2,3,4,5,5,6,6,7,7,7-tetradecafluoroheptane and hydrogen fluoride (HF). These compositions are useful in processes to produce and purify HFC-162-13mcyz, HFC-162-13mczy and HFC-63-14mee.
Owner:THE CHEMOURS CO FC LLC
Olefin polymerization catalysts and olefin polymer production methods using said olefin polymerization catalysts
InactiveUS6525150B1Organic-compounds/hydrides/coordination-complexes catalystsCatalyst activation/preparation1-OcteneOctene
Olefins are homopolymerized or copolymerized in the presence of a catalyst containing a reaction product obtained from vanadium oxytrichloride and 2,2'-thiobis(6-t-butyl-4-methylphenyl), an organic aluminum compound, such as trimethylaluminum, and an ionized ionic compound, such as N,N-dimethylanilinium tetrakis(pentafluorophenyl)borate, and the like. By using this catalyst to polymerize ethylene with an alpha-olefin, such as propylene, 1-heptene, 1-octene, and the like, or a non-conjugated diene, such as 5-ethylidene-2-norbomene, and the like, a polymer having a high degree of copolymerization is obtained.
Owner:JSR CORPORATIOON
Olefin compositions
A composition comprising: a) at least 76 mol % C10 monoolefins, the C10 monoolefins comprising i) at least 3 mol % 2-butyl-1-hexene, ii) at least 8 mol % 3-propyl-1-heptene, iii) at least 6 mol % 4-ethyl-1-octene, and iv) at least 20 mol % 5-methyl-1-nonene; and b) at least 1 mol % C14 monoolefins. A composition comprising at least 95 mol % C10 monoolefins, the C10 monoolefins comprising i) at least 3 mol % 2-butyl-1-hexene, ii) at least 10 mol % 3-propyl-1-heptene, iii) at least 7 mol % 4-ethyl-1-octene, and iv) at least 24 mol % 5-methyl-1-nonene. Processes to prepare a composition comprising at least 76 mol % C10 monoolefins and at least 1 mol % C14 monoolefins, or a composition comprising at least 95 mol % C10 monoolefins, where the C10 monoolefins comprise i) at least 3 mol % 2-butyl-1-hexene, ii) at least 10 mol % 3-propyl-1-heptene, iii) at least 7 mol % 4-ethyl-1-octene, and iv) at least 24 mol % 5-methyl-1-nonene.
Owner:CHEVRON PHILLIPS CHEMICAL CO LP
Controlled odor mimic permeation system
The primary aspect of the Controlled Odor Mimic Permeation System (COMPS) is that it provides a field deployable instant and reproducible source of known amounts of target odors. This technology consists of a permeable polymer container (chosen to suit target odor and release rate required), stored inside a non-permeable package. The design allows for the pre-equilibration of the target odors such that the outer surface of the inner package can saturate with odor during storage. Removal of the inner item then provides an instant and reproducible source of known target vapor flux. We have successfully demonstrated this technology by placing the target odor chemicals within permeable membranes such as low density polyethylene which are then sealed within a non-permeable membranes such as metallized polyester. This design has multiple advantages including preventing cross contamination when storing multiple odor targets (5-10 targets are commonly employed) as well as being light-weight disposable, low unit cost potential, no external power / operating unit / machinery / hardware, simple to use and providing a known reproducible concentration of the target odors to the detector in the field. The applications of these COMPS include the whole range of biological (e.g. detector dog) and electronic (e.g. field sensors) detectors with examples such as explosives (e.g. 2-ethyl-1-hexanol simulating plasticized explosives), drugs (e.g. 3,4-methylenedioxybenzaldehyde simulating ecstasy), human remains (including dimethyl disulfide and pentanoic acid) and live human scent (including 5-heptene-2-one and nonanol).
Owner:FLORIDA INTERNATIONAL UNIVERSITY
Organic compound having dibenzo heptene structure and application thereof
InactiveCN106467468AImprove applicabilityHigh color purityOrganic chemistrySolid-state devicesQuantum efficiencySimple Organic Compounds
The invention discloses an organic compound having a dibenzo heptene structure and an application thereof, and a structure of the compound is shown as a general formula (1). When the compound as a luminescent layer doping material of an organic light emitting diode (OLED) luminescent device for usage, current efficiency, power efficiency and external quantum efficiency of the device are greatly improved; purity of the device is obviously improved, and the device life can be obviously increased. The compound has good application effect in the OLED luminescent device, and has good industrial prospect.
Owner:VALIANT CO LTD
Mixed decyl mercaptans compositions and use thereof as mining chemical collectors
Disclosed herein is a process for the recovery of a metal from an ore using a collector composition. The process includes contacting the ore with the collector composition. The collector composition can include sulfur-containing compounds comprising (i) mercaptans comprising branched C10 mercaptans compounds selected from the group consisting of 5-methyl-1-mercapto-nonane, 3-propyl-1-mercapto-heptane, 4-ethyl-1-mercapto-octane, 2-butyl-1-mercapto-hexane, 5-methyl-2-mercapto-nonane, 3-propyl-2-mercapto-heptane, 4-ethyl-2-mercapto-octane, 5-methyl-5-mercapto-nonane, and combinations thereof; and (ii) sulfides comprising branched C20 sulfides represented by the structure R1—S—R2, wherein R1 and R2 are each independently a functional group derived from an olefin, wherein the olefin comprises 5-methyl-1-nonene, 3-propyl-1-heptene, 4-ethyl-1-octene, 2-butyl-1-hexene, or combinations thereof.
Owner:CHEVRON PHILLIPS CHEMICAL CO LP
Hyphantria cunea Drury attractant composition, its application and attracting core
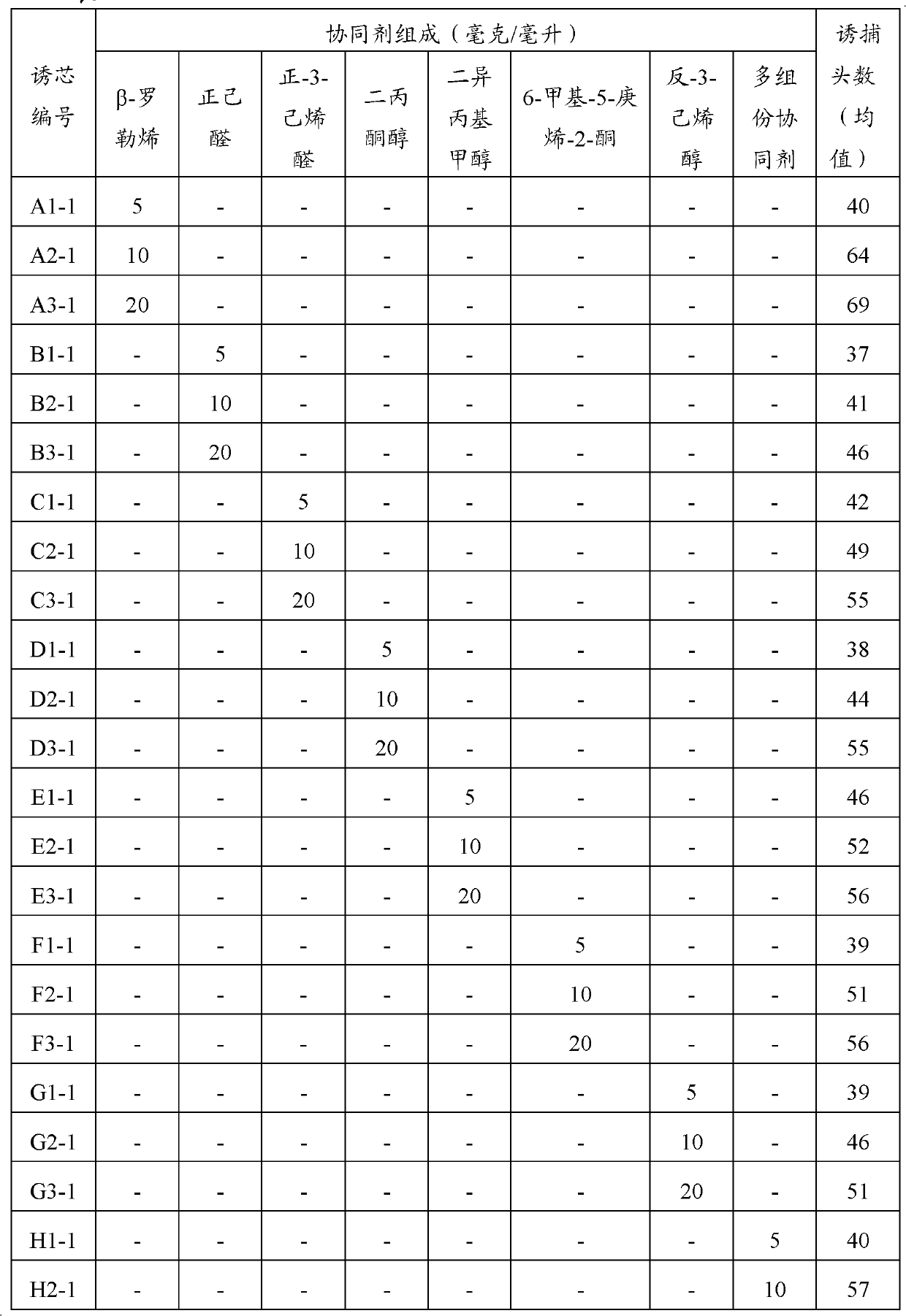
InactiveCN103315002AFlexible usageStrong autonomyBiocidePest attractantsDiacetone alcoholCis-3-Hexenal
The invention provides a Hyphantria cunea Drury attractant composition, its application and attracting core. The Hyphantria cunea Drury attractant composition includes Hyphantria cunea Drury sex pheromone and its synergistic agent. The synergistic agent contains one or more of the following plant source smell components: beta-ocimene, hexanal, cis-3-hexenal, diacetone alcohol, diisopropylcarbinol, 6-methyl-5-heptene-2-one, trans-3-hexenol, o-xylene, hemimellitene, trans-2-hexenal, and limonene. The attractant composition provided in the invention can be prepared into an attracting core so as to be used cooperatively with the existing Hyphantria cunea Drury sex pheromone. The invention provides the attracting core containing the Hyphantria cunea Drury attractant composition to prevent, control and detect Hyphantria cunea Drury, so that the defects of high cost and difficult popularization of imported and domestic Hyphantria cunea Drury sex pheromone attracting cores in the prior art are solved. The attracting core provided in the invention has the advantages of low cost, flexible use, and higher trapping efficiency.
Owner:INST OF ZOOLOGY CHINESE ACAD OF SCI
Preparation method of 3, 5-dihydroxy heptyl-6-gadoleic acid derivative
ActiveCN101591301AAddress impurity controlSolving Mass Manufacturing ProblemsOrganic chemistryCardiovascular disorderHydrolysisMedicinal chemistry
The invention improves a preparation method of 3, 5-dihydroxy heptyl-6-gadoleic acid derivative. Asymmetric reduction is performed on intermediate 3-hydroxy-5-oxo-6-heptenoic acid ester derivative, alkali hydrolysis is performed on the obtained 3, 5-dihydroxy heptyl-6-heptenoic acid ester derivative crude product which is treated by alkali hydrolysis to obtain 3, 5-dihydroxy heptyl-6-gadoleic acid sodium salt derivative which is extracted and purified to obtain 3, 5-dihydroxy heptyl-6-gadoleic acid sodium salt derivative solution, then 3, 5-dihydroxy heptyl-6-gadoleic acid sodium salt derivative is converted into 3, 5-dihydroxy heptyl-6-gadoleic acid derivative, and 3, 5-dihydroxy heptyl-6-gadoleic acid sodium salt derivative is again converted into 3, 5-dihydroxy heptyl-6-gadoleic acid ester derivative with high yield, and purification methods such as recrystallization are performed to obtain purified 3, 5-dihydroxy heptyl-6-gadoleic acid ester derivative. The purified 3, 5-dihydroxy heptyl-6-gadoleic acid ester derivative is then hydrolyzed into 3, 5-dihydroxy heptyl-6-gadoleic acid sodium salt derivative which is finally converted into 3, 5-dihydroxy heptyl-6-gadoleic acid calcium salt derivative. 3, 5-dihydroxy heptyl-6-gadoleic acid derivative with high quality is obtained.
Owner:CHANGZHOU PHARMA FACTORY
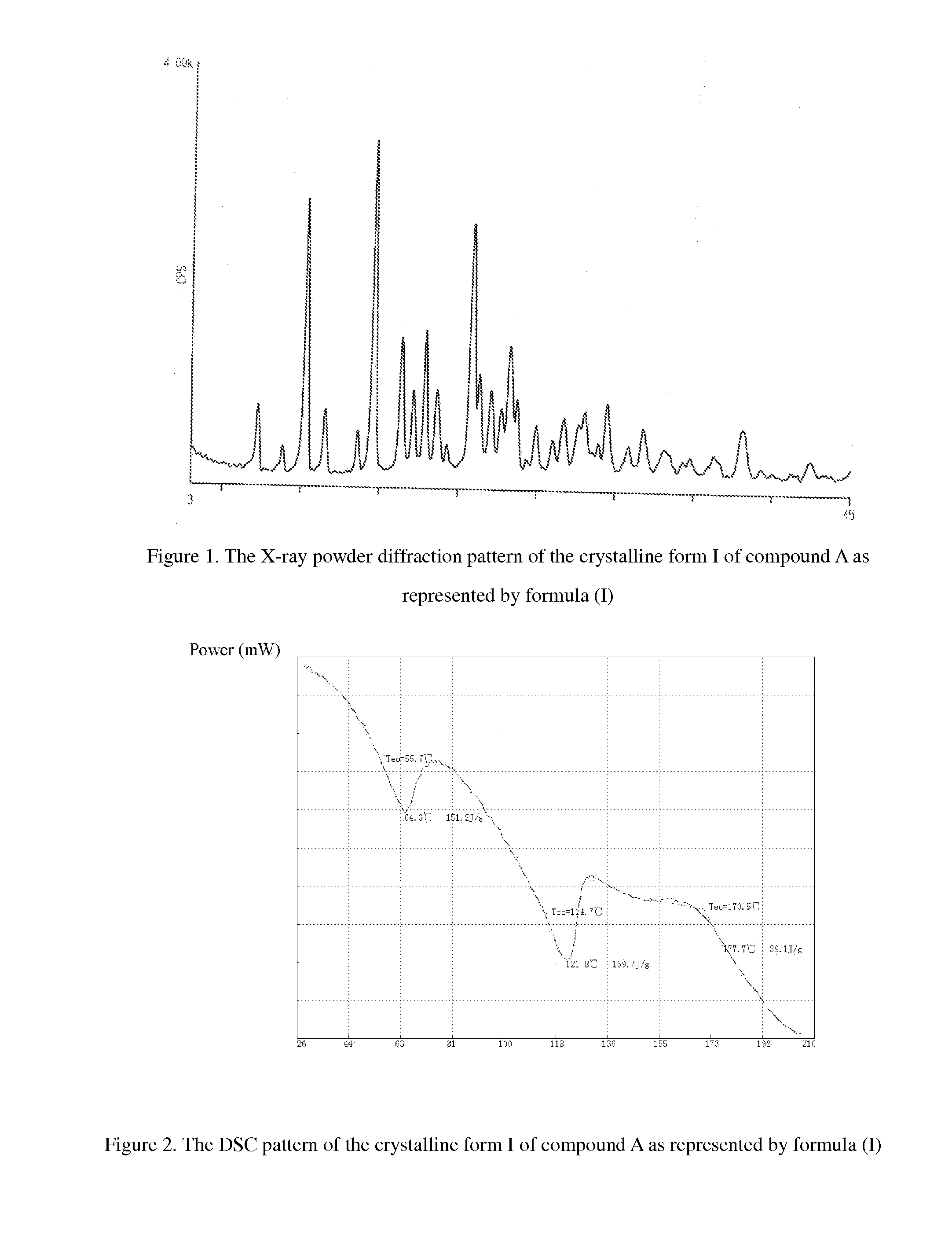
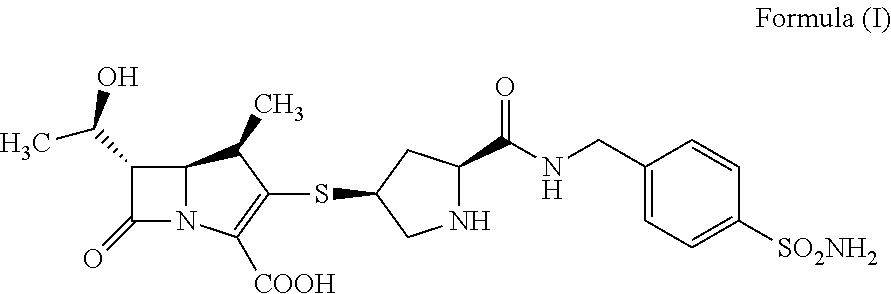
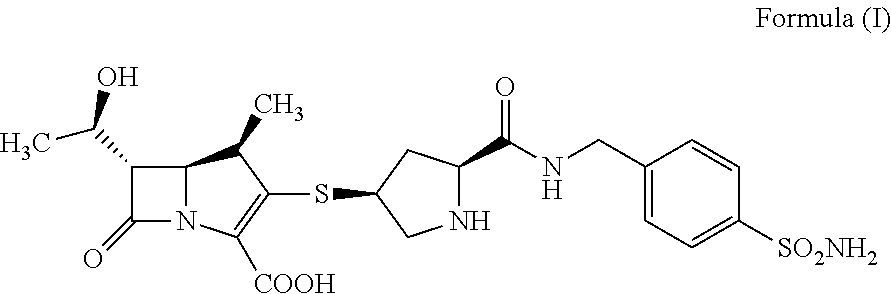
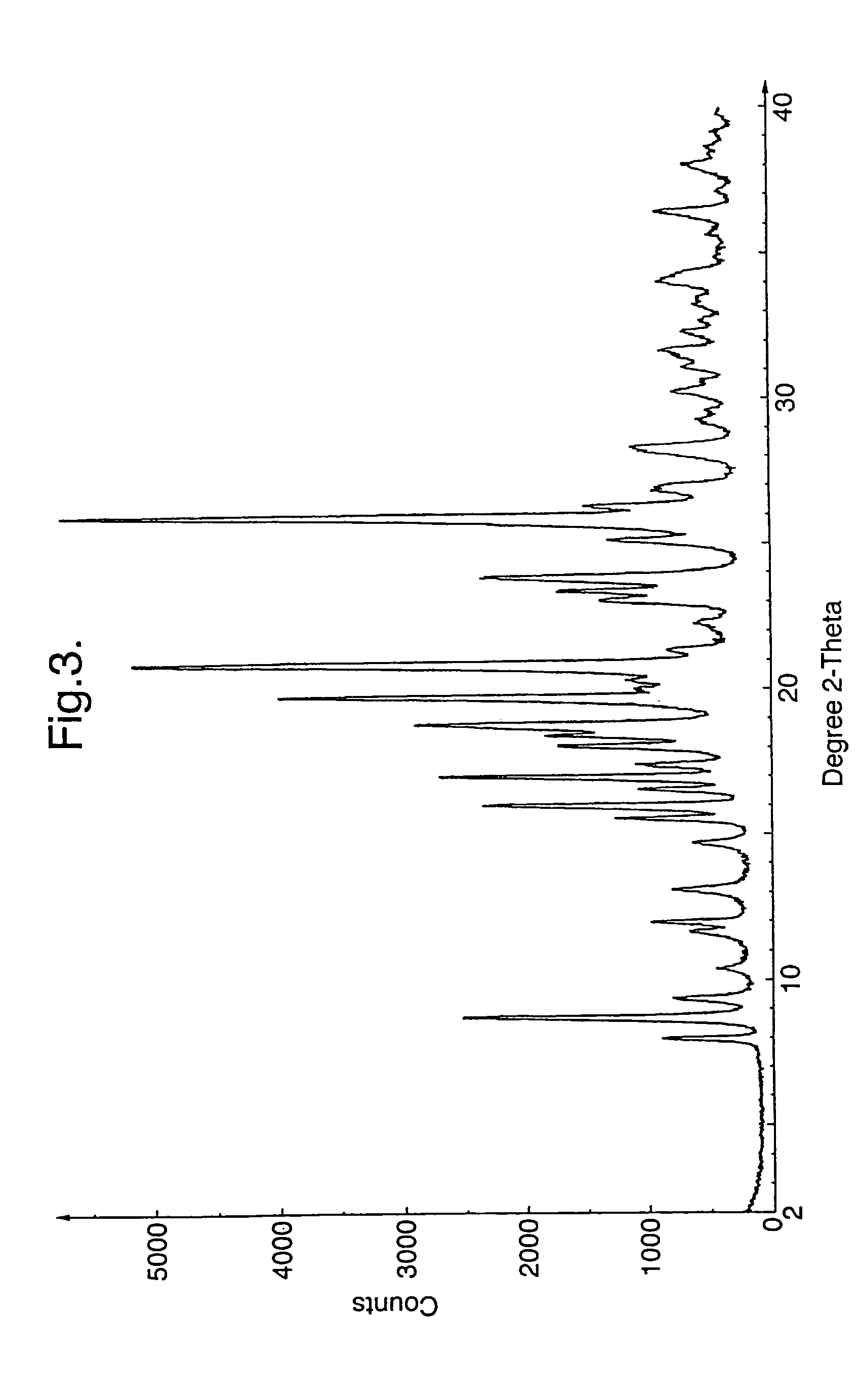
Crystalline of carbapenem derivative or its hydrate, preparation methods and uses thereof
InactiveUS20130079322A1Superior stabilityImprove stabilityAntibacterial agentsOrganic active ingredientsThio-Organic solvent
The present invention relates to a crystalline form of carbapenems derivative (4R,5S,6S)-6-((R)-1-hydroxyethyl)-4-methyl-7-oxo-3-(((3S,5S)-5-((4-sulfamoylbenzyl)carbamoyl)pyrrolidin-3-yl)thio)-1-azabicyclo[3.2.0]hept-2-ene-2-carboxylic acid_as represented by formula (I) or hydrate thereof and the preparation methods thereof, wherein said method comprise: dissolving the compound as represented by formula (I) by an aqueous solution of N,N′-dimethylformamide (DMF), dimethyl sulfoxide (DMSO), and then adding a poor solvent dropwise to this solution, filtering and drying to obtain a crystal. Another method comprises: formulating the compound as represented by formula (I) as an aqueous suspension; after adjusting pH until complete dissolution, adding a mixed solvent of organic solvent / water with a certain volume ratio; adjusting pH to 5.4-7.0, cooling to low temperature, filtering and drying to obtain a crystal. The invention also relates to the use of the crystalline form of compound A or hydrate thereof in the preparation of a medicament for treating and / or preventing infectious diseases. The invention further relates to a pharmaceutical composition comprising the crystalline form of compound A or hydrate thereof and one or more pharmaceutical carriers and / or diluents.
Owner:SHANDONG XUANZHU PHARMA TECH CO LTD
Azeotropic and azeotrope-like compositions of methyl perfluoroheptene ethers and ethanol and uses thereof
Owner:EI DU PONT DE NEMOURS & CO
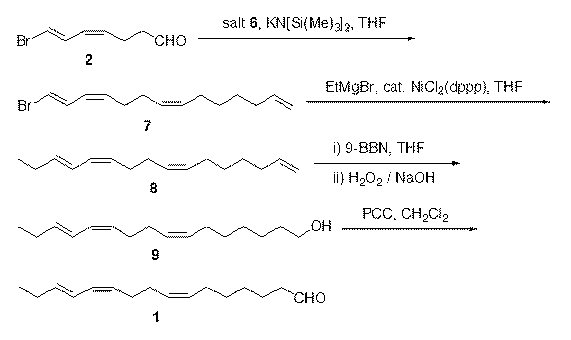
Synthesis method of (7Z,11Z,13E)-hexadecatrienal
InactiveCN102795977ALow costLower synthesis costCarbonyl compound preparation by oxidationPhenyl groupToxicology
The present invention relates to a synthesis method of (7Z,11Z,13E)-hexadecatrienal as main constituent of citrus leaf-miner sex pheromone. 7-Bromo-(4Z,6E)-heptadienal and 4-bromo-1-butenol are used as start raw materials, the 4-bromo-1-butenol is coupled with allylmagnesium bromide to obtain 6-hepten-1-ol and then the 6-hepten-1-ol is brominated and salified with triphenylphosphine to obtain 1-bromo-(1E,3Z,7Z,13)-tetradecatetraene, and then the 1-bromo-(1E,3Z,7Z,13)-tetradecatetraen is subjected to Kumada coupling to obtain (1,7Z,11Z,13E)-hexadecatetraene, and then the (1,7Z,11Z,13E)-hexadecatetraene is subjected to hydroboration-oxidation reaction to obtain (7Z,11Z,13E)-hexadecatrienol, and finally the (7Z,11Z,13E)-hexadecatrienol is oxidized into (7Z,11Z,13E)-hexadecatrienal by pyridinium chlorochromate. The synthesis method of (7Z,11Z,13E)-hexadecatrienal has advantages of cheap and easily obtained raw materials, simple synthesis path, mild reaction conditions, high yield, low cost, convenient and safe operation.
Owner:昆明博鸿科技有限公司
Fermentation beef ham identification method
InactiveCN101393178AComponent separationMaterial analysis by electric/magnetic meansBenzaldehydeKetone
The invention discloses a method for discriminating the truth of fermented beef ham through detecting the compositions of a flavor substance by a gas chromatography-smelling method / a gas chromatography-mass spectrometry method. The method comprises the following steps: when a sample of a flavor substance of fermented beef ham to be detected is prepared through dynamic headspace purging and trapping, the suitable purging time of nitrogen gas is 30 minutes; when the flavor substance is detected by utilizing the gas chromatography-mass spectrometry method, the fermented beef ham contains fifteen flavor substance compositions: namely, 2-propylene-1-mercaptan, dimethyl benzene methanol, 3-methyl butyraldehyde, benzaldehyde, methyl-5-heptene-2-ketones, acetophenone, alpha-pinene, beta-pinene, styrene, dipentene, biphenyl, naphthalene, 2-methyl naphthalene, phenol, eucalyptus essential oil and anethole; and when the compositions of the smelling flavor substance are detected by the chromatography-smelling method, the fermented beef ham contains trimethyl butyraldehyde, benzaldehyde, dimethyl disulfide, naphthalene, alpha-pinene and benzothiazole. The method can be widely applied to quality detection and discrimination; and the process design, improvement and management of the fermented beef ham.
Owner:INST OF ANIMAL SCI OF CHINESE ACAD OF AGRI SCI
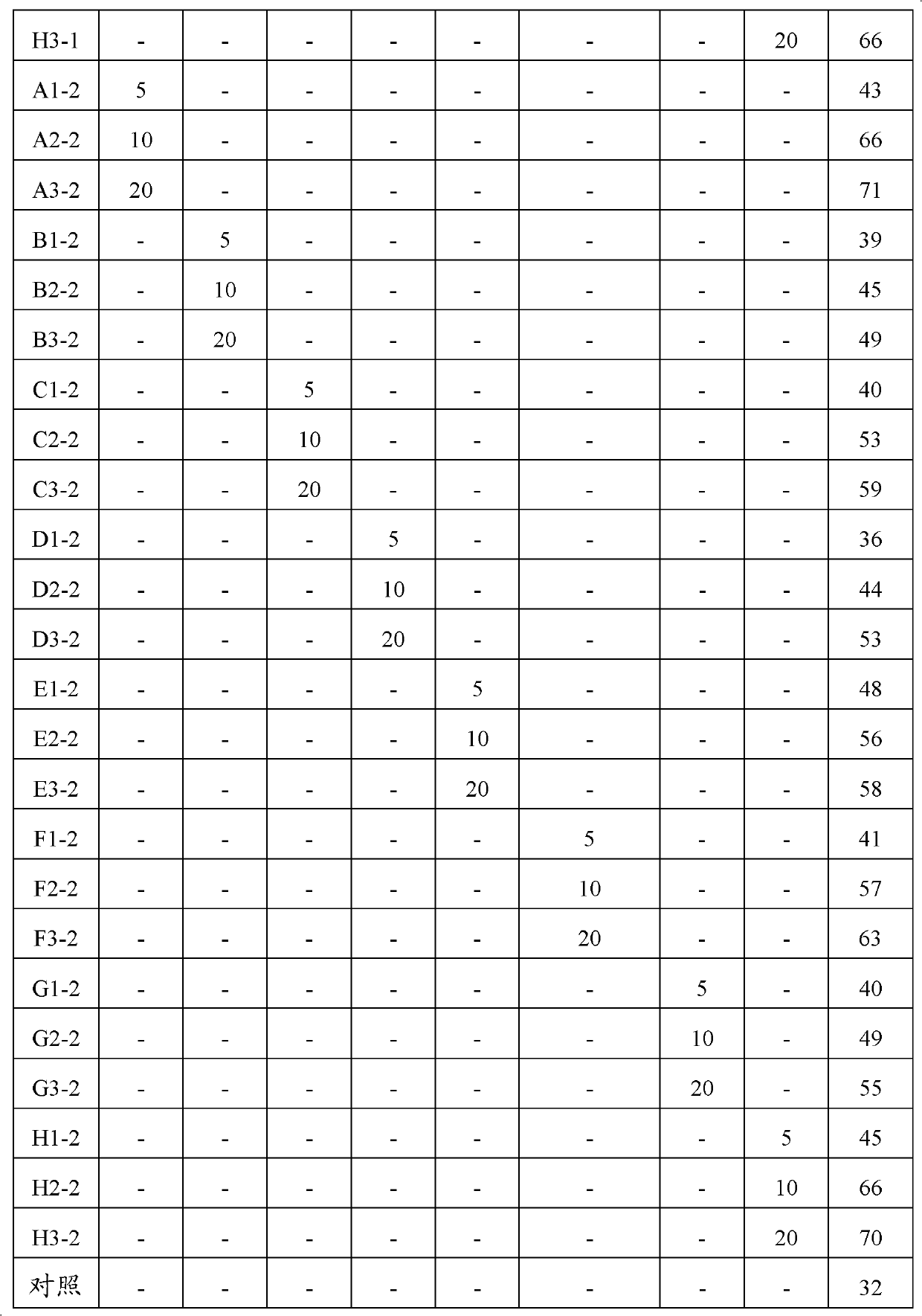
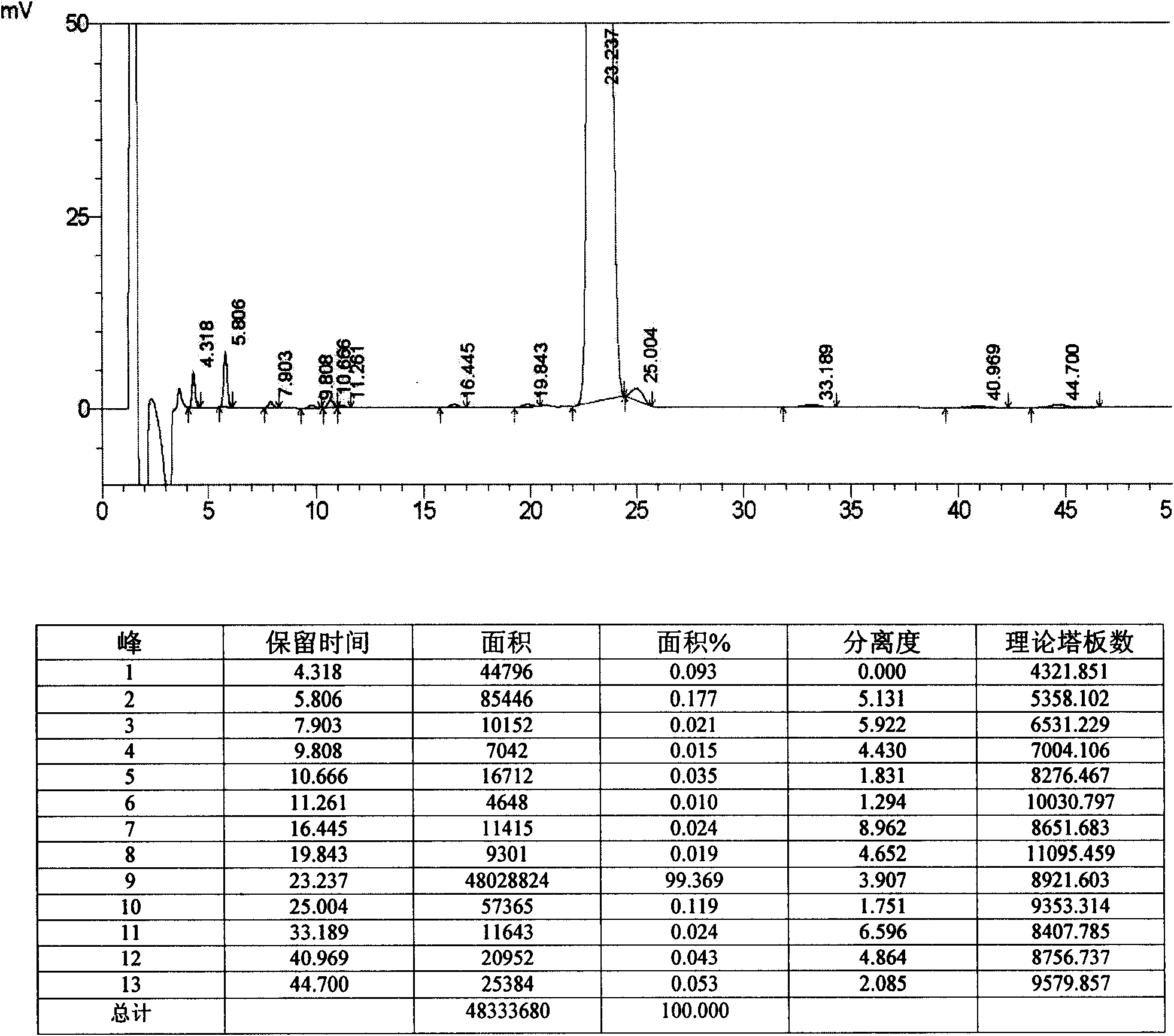
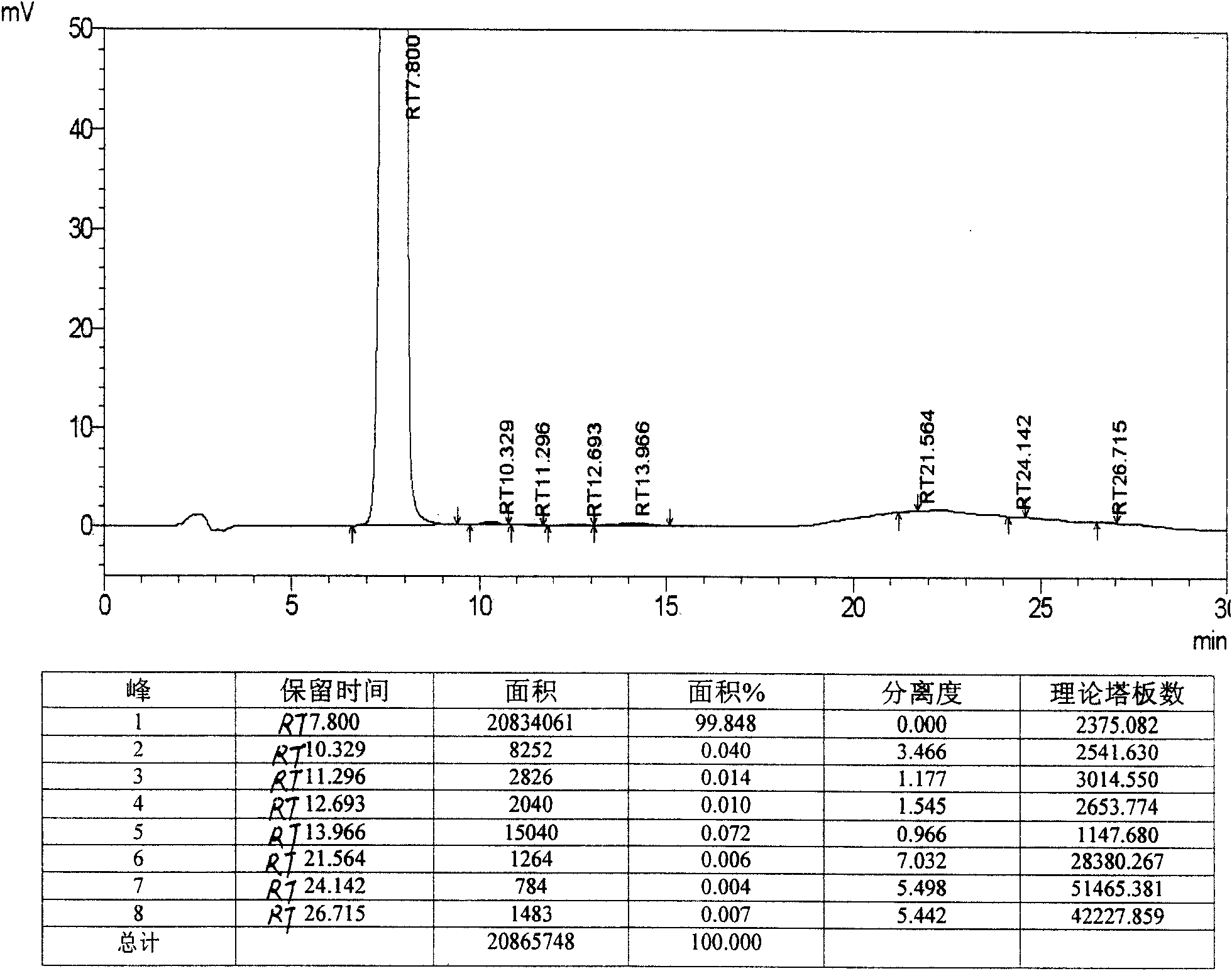
Crystalline salts of 7-′4-(4-fluorophenyl) -6-isopropyl-2-′methyl (methylsulfonyl) amino!pyrimidin-5-yl!- (3R, 5S) -3, 5-dihydroxyhept-6-enoic acid
The invention relates to crystalline salts of the compound (E)-7-[4-(4-fluorophenyl)isopropyl-2-[methyl(methylsulfonyl)amino]pyrimidin-5-yl]-(3R,5S)-3,5-dihydroxyhept-6-enoic acid of formula (I), as well as processes for their manufacture, pharmaceutical compositions containing them, and their uses
Owner:ASTRAZENECA AB +1
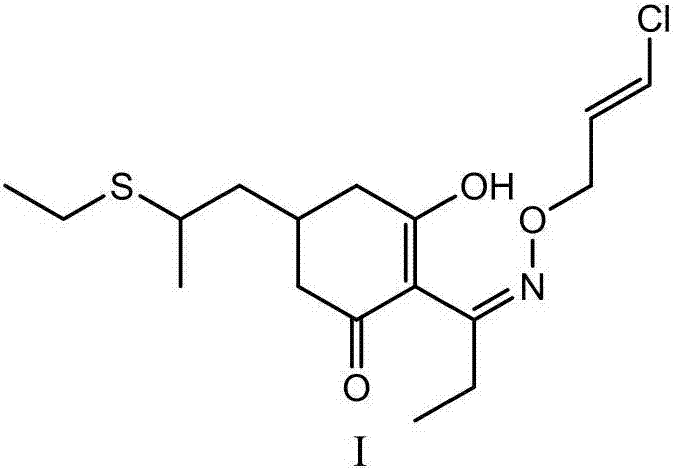
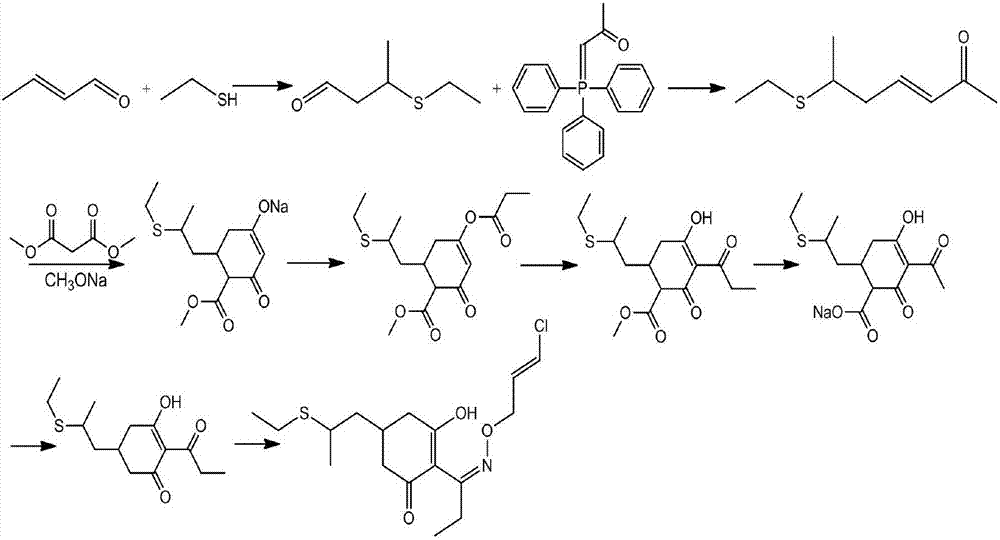
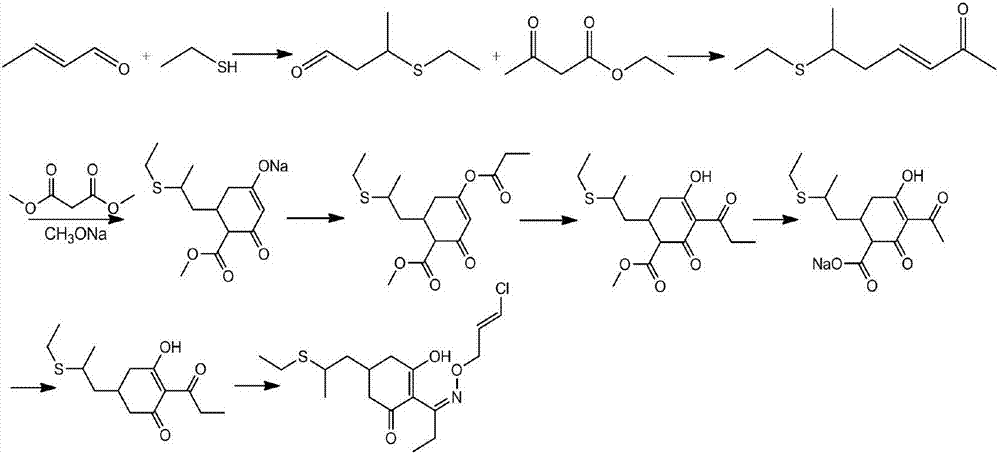
Method for synthesizing clethodim
InactiveCN107162945AHigh yieldImprove product qualitySulfide preparationPropionyl chlorideEthanethiol
The present invention proposes a method for synthesizing clethodim, comprising 3,5-heptadiene-2-one (formula II) and ethanethiol for nucleophilic addition to prepare clethodim intermediate 6-ethylthio ‑3‑heptene‑2‑ketone (formula III); formula III reacts with dimethyl malonate and propionyl chloride to prepare (±)‑3‑[propionyloxy]‑5‑[ 2-(Ethylthio)propyl]-6-[methoxyformyl]cyclohex-2-enone (Formula V); Formula V is then rearranged, hydrolyzed and decarboxylated to obtain (±)-2- [Propionyl]-3-[hydroxyl]-5-[2-(ethylthio) propyl]cyclohex-2-enone (formula VIII); formula VIII reacts with chloroallyloxyamine to obtain alkene Clethodim (formula I), the method for preparing clethodim by using intermediate 3,5-heptadiene-2-one (formula II) in the present invention is proposed for the first time, and the clethodim (formula I) prepared by this method is generally The yield is high, the product quality is good, and the method has low production cost and little environmental pollution, and is suitable for large-scale production.
Owner:江苏威格瑞斯化工有限公司
Mixed decyl mercaptans compositions and use thereof as mining chemical collectors
Owner:CHEVRON PHILLIPS CHEMICAL CO LP
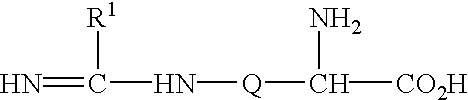
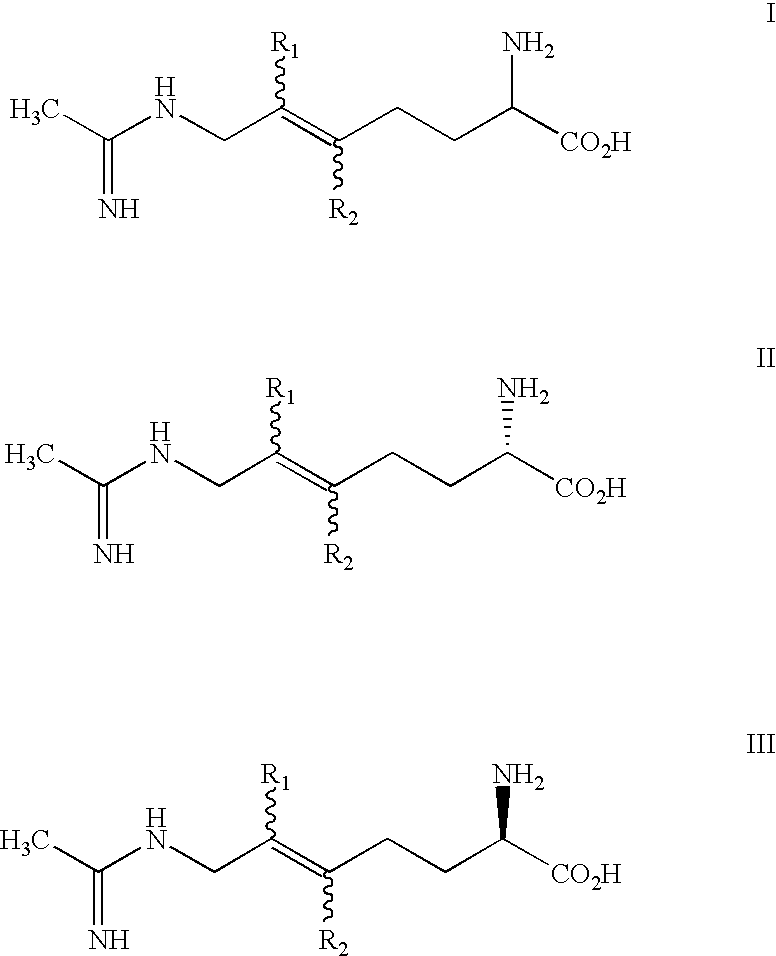
2-amino-5, 6 heptenoic acid derivatives useful as nitric oxide synthase inhibitors
InactiveUS20020143061A1BiocideOrganic active ingredientsAcid derivativeNitric Oxide Synthase Inhibitors
Owner:PHARMACIA CORP
Oxygen bridge bicyclo-[2.2.1]-heptene compound containing different functional side chain structures, as well as preparation and application thereof
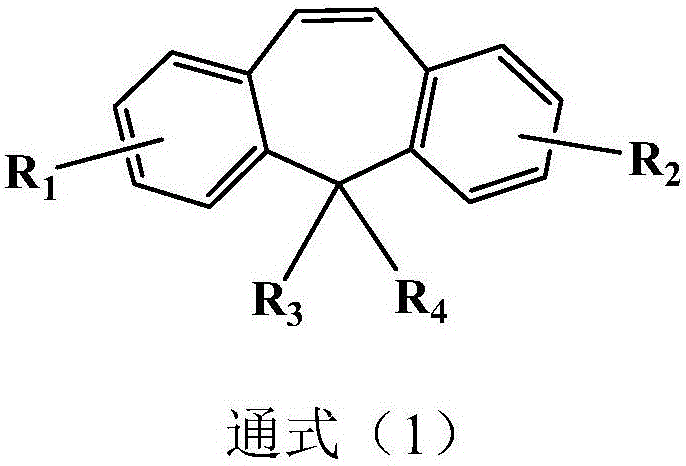
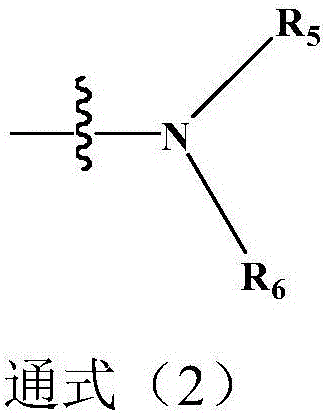
ActiveCN109942595AStrong inhibitory activityGood proteolytic activityOrganic active ingredientsOrganic chemistryFuranSide chain
The invention discloses an oxygen bridge bicyclo-[2.2.1]-heptene compound containing different functional side chain structures, as well as preparation and application thereof. The preparation comprises the following steps that furan derivatives which contain different functional side chain structures (2,3-glycol butyl, N,N-diethoxy ethylamino, 2-pyrrolidinyl ethyl, 2-piperidine ethyl and alkyl acid) and are synthesized from 3,4-bis(4-methyoxy-phenyl)furan reacts with vinyl sulfonamide derivatives at 90 DEG C for 12 hours in one step without solvent or catalyst to prepare the oxygen bridge bicyclo-[2.2.1]-heptene compound containing basic side chains and other functional side chain groups. In-vitro experiments indicate that, compared with an existing anti-breast cancer drug tamoxifen, thenovel sulfamide type oxygen bridge bicyclo-[2.2.1]-heptene compound has stronger inhibiting activity on MCF-7 cells, and has excellent protein degrading activity and equivalent degrading performance as that of existing drug fulvestrant.
Owner:WUHAN UNIV
Method for synthesizing delta-dodecalactone
The present invention discloses a method for synthesizing delta-dodecalactone, which comprises the following steps: (1) condensating and dehydrating: under the present of a phase-transfer catalyst under an alkaline condition, causing aldol condensation between cyclopentanone and heptanal, and dehydrating for generating 2-heptene cyclopentanone under the function of an acid catalyst; (2) hydrogenating: causing the 2-heptene cyclopentanone to hydrogenate under the presence of ion exchange resin for obtaining 2- heptyl cyclopentanone; (3) oxidizing: causing a Baeyer-Villiger oxidation reaction between the 2- heptyl cyclopentanone and hydrogen peroxide for obtaining a crude product; and (4) refining: obtaining a pure product from the crude product through molecular distillation. The method for synthesizing delta-dodecalactone has the following advantages: easy method application, simple operation, higher yield, and easy available raw material. The yield of the target product delta-dodecalactone is improved, and the purity is greatly improved. The product purity is larger than 99.0%, and the yield is above 80%. The catalyst used in the method has the characteristics such as capability of being used repeatedly.
Owner:ANHUI UNIV OF SCI & TECH
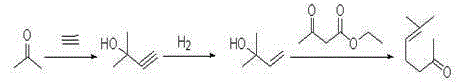
Method for preparing methyl heptenone by using 3-methylcrotonaldehyde
ActiveCN105218339AHigh molar yieldHigh selectivityOrganic compound preparationCarbonyl compound preparationPtru catalystHydrogenation reaction
The invention provides a method for preparing methyl heptenone by using 3-methylcrotonaldehyde, which comprises the steps of condensation and selective reduction, wherein the step of condensation comprises the sub-steps of adding acetone and a catalyst, and dropwise adding 3-methylcrotonaldehyde; and the step of selective reduction comprises the sub-steps of adding materials and carrying out hydrogenation reaction; and the sub-step of adding materials, namely adding 1 part of methyl diheptenone, 0-10 parts of a solvent, and 0.001-0.05 part of transition metal Pd / Rh catalyst precursor compounds and diphosphine ligand compounds into a reaction kettle. According to the invention, through two-step reaction, the mole yield of the methyl diheptenone to 3-methylcrotonaldehyde is high, and the methyl heptenone product content reaches 98.0-98.7%; in the step of selective reduction, the conversion rate of methyl diheptenone is greater than or equal to 99.0%, and the selectivity reaches 90.8-94%; and in the step of condensation, the conversion rate of 3-methylcrotonaldehyde is 99.0%, and the mole yield of the methyl diheptenone to 3-methylcrotonaldehyde reaches 89.8-92.7%.
Owner:SHANDONG NHU PHARMA +1
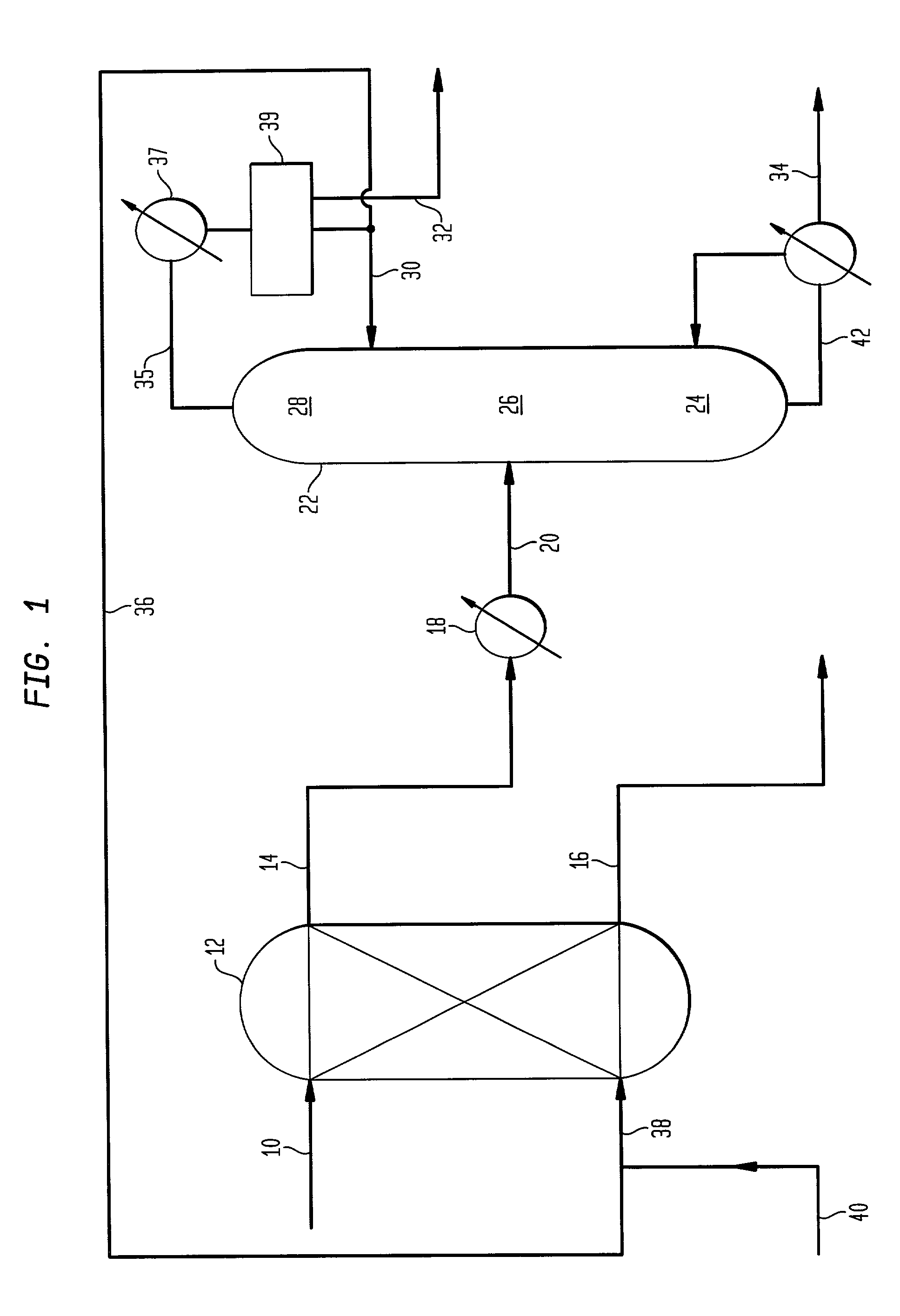
Acrylic acid recovery utilizing ethyl acrylate and selected co-solvents
InactiveUS20030146081A1Organic compound preparationSolvent extractionPolymer scienceMethyl palmoxirate
A method of recovering acrylic acid from a mixture comprising acrylic acid, water and acetic acid is disclosed, which includes: (a) extracting acrylic acid from the mixture with a solvent mixture comprising ethyl acrylate as the preponderant component thereof and an organic co-solvent selected from the group consisting of toluene, heptane, 1-heptene, methylcyclohexane, cycloheptane, cycloheptadiene, cycloheptatriene, 2,4-dimethyl-1,3 pentadiene, methylcyclohexene and methylenecyclohexene to form an extracted composition; and (b) azeotropically distilling the extracted composition to recover acrylic acid. Also disclosed is an alternate method of recovering acrylic acid which includes: (a) providing a feed stream containing acrylic acid, water, acetic acid, ethyl acrylate and an organic co-solvent selected from the group consisting of toluene, heptane, 1-heptene, methylcyclohexane, cycloheptane, cycloheptadiene, cycloheptatriene, 2,4-dimethyl-1,3 pentadiene, methylcyclohexene and methylenecyclohexene to a distillation column, wherein the weight ratio of ethyl acrylate to the organic co-solvent is from about 80:20 to about 95:5; and (b) azeotropically distilling said feed stream to provide an acrylic acid residue stream.
Owner:DOW GLOBAL TECH LLC
Load type polymerization catalyzed system, preparation method and application
This invention relates to a catalyst system for bicyclo [2.2.1] hept-2-ene and its derivatives, its preparation method and its application. The catalyst system comprises: carrier, major catalyst and auxiliary catalyst. The carrier is MgCl2.xROH (x = 1-3, ROH is C3-C8 alcohol) or silica gel. The auxiliary catalyst is methylaluminoxane. The major catalyst is nickel complex with ligands containing nitrogen and oxygen. The catalyst system is prepared by supporting nickel complex with spherical MgCl2 carrier or silica gel carrier. This invention has such advantages as simple process, abundant raw materials and high catalytic activity.
Owner:PETROCHINA CO LTD
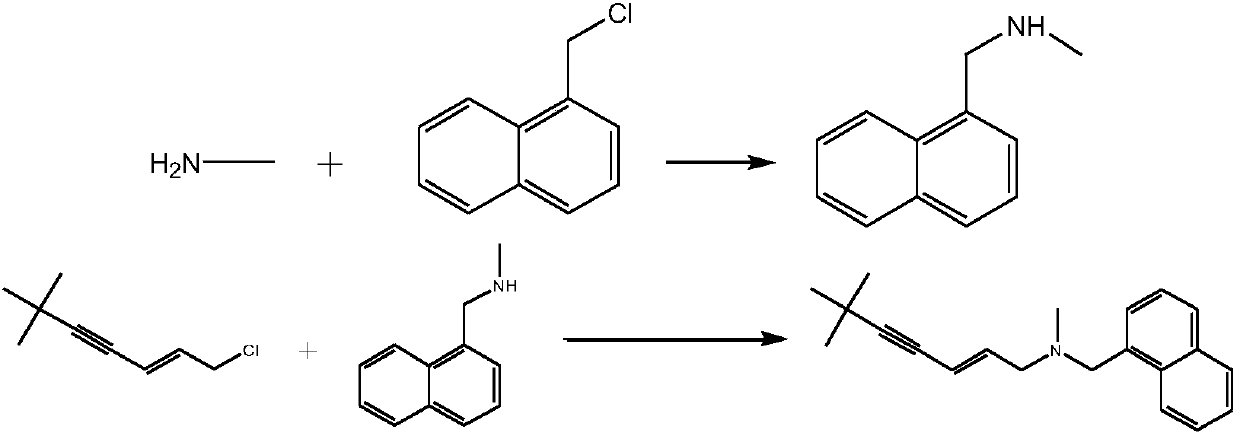
Synthetic method of terbinafine
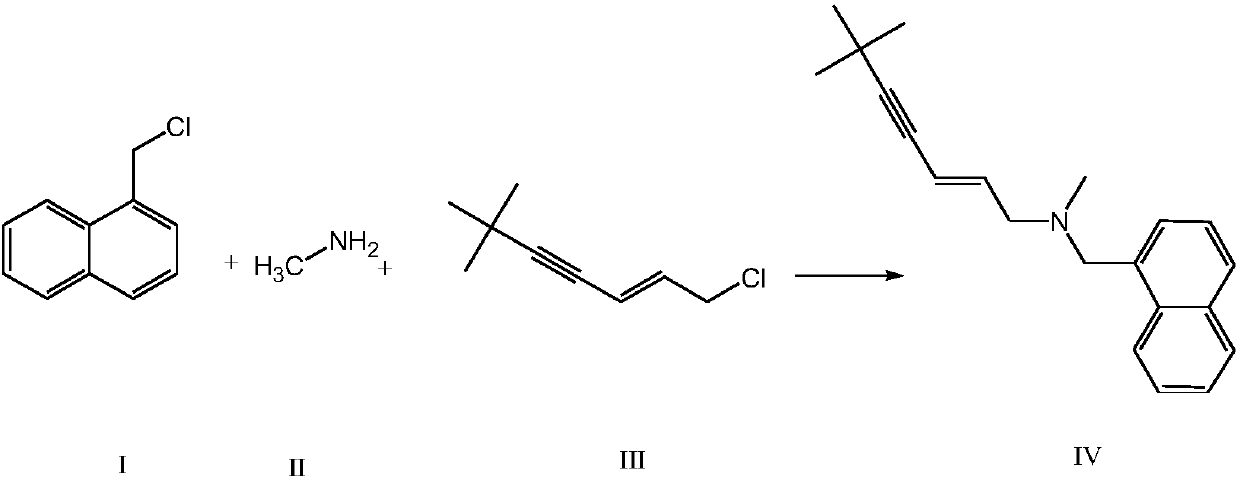
ActiveCN108017544AHigh yieldOvercome yieldAmino preparation by functional substitutionOrganic layerAlkyne
The invention provides a synthetic method of terbinafine. Monomethylamine, 1-chloromethyl naphthalene and 1-chloro-6,6-dimethyl-2-heptene-4-alkyne are subjected to a one-step reaction to generate theterbinafine. Particularly, the monomethylamine is slowly added into a solvent, and then an acid-binding agent is added; the 1-chloromethyl naphthalene and the 1-chloro-6,6-dimethyl-2-heptene-4-alkyneare simultaneously slowly dropwise added, and a dropwise adding speed is controlled so as to enable both the 1-chloromethyl naphthalene and the 1-chloro-6,6-dimethyl-2-heptene-4-alkyne to be simultaneously dropwise added up; a temperature control reaction is performed for 2 to 3 hours at a temperature of 10 to 20 DEG C; chloroform is added to extract reaction liquid, vacuum concentration is carried out on an organic layer to a dry state, and ethyl acetate is added into residues to carry out crystallization to obtain the terbinafine. The synthetic method is high in yield, simple in step and convenient to operate, generates few byproducts, and is beneficial to industrial production.
Owner:SHANDONG BOYUAN PHARM CO LTD
Melt blown fiber
ActiveCN102639769AImprove mechanical propertiesImprove the blocking effectMonocomponent synthetic polymer artificial filamentMelt spinning methodsFiber1-Octene
Melt-blown fiber having an average diameter of not more than 5.0 [mu]m, said fiber comprises at least 85 wt% of a propylene copolymer, wherein DEG said melt blown fiber and / or said propylene copolymer has / have a melt flow rate MFR2 (230 DEG C) measured according to ISO 1133 of at least 200 g / 10min, DEG said propylene copolymer has a comonomer content of 0.5 to 5.5 wt%, the comonmers are ethylene and / or at least one C4 to C20 a-olefin selected from the group consisting of 1-butene, 1-pentene, 1-hexene, 1-heptene, and 1-octene, DEG the propylene copolymer has 13C-spectroscopy, and DEG said melt blown fiber and / or said propylene copolymer fulfill(s) the equation (1).; wherein Tm [DEG C] is the melting temperature [given in DEG C] of melt blown fiber said and / or of said propylene copolymer measured according to ISO 11357-3, C2 [wt%] is the amount [given in weight percentage] of comonomers within said melt blown fiber and / or within said propylene copolymer determined with Fourier transform infrared spectroscopy (FTIR).
Owner:BOREALIS AG
Cycloolefin addition copolymer and optical transparent material
The present invention provides a cyclic olefin addition copolymer composed of endo-tricyclo[4.3.0.1 2,5 ]dec-3,7-diene, endo-tricyclo[4.3.0.1 2,5 ] Cyclic olefin compounds with branched chain substituents such as dec-3-ene and other cyclic olefin compounds such as bicyclo[2.2.1]hept-2-ene, and cyclic olefin compounds with hydrolyzable silyl groups when necessary A cyclic olefin addition copolymer obtained by copolymerization or hydrogenation of a olefin compound after copolymerization; and a crosslinking composition in which a specific crosslinking agent is added to the copolymer, and the crosslinked product formed by crosslinking the composition contains The optical material of its copolymer, composition or cross-linked product, and the production method of the copolymer by addition polymerization using a specific nickel catalyst.
Owner:JSR CORPORATIOON
Popular searches
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
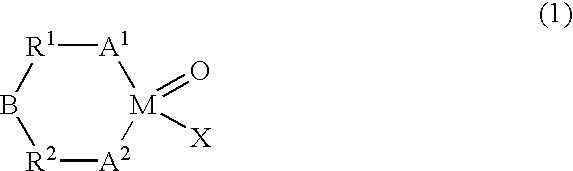

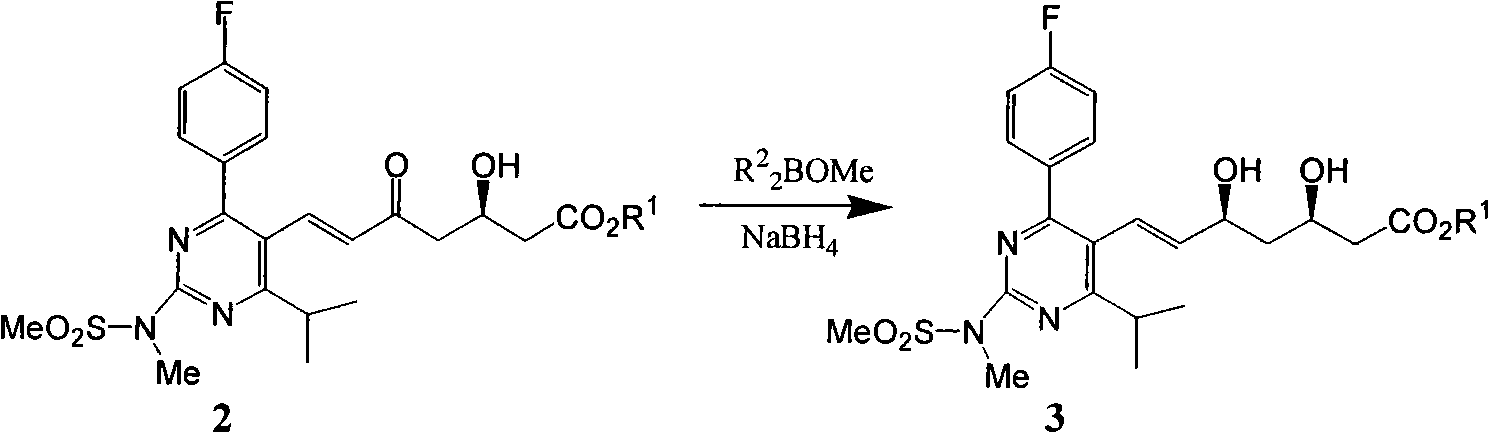
![Oxygen bridge bicyclo-[2.2.1]-heptene compound containing different functional side chain structures, as well as preparation and application thereof Oxygen bridge bicyclo-[2.2.1]-heptene compound containing different functional side chain structures, as well as preparation and application thereof](https://images-eureka.patsnap.com/patent_img/c14bd2dc-6736-46a2-84dc-751d28dab027/HDA0001960300290000011.png)
![Oxygen bridge bicyclo-[2.2.1]-heptene compound containing different functional side chain structures, as well as preparation and application thereof Oxygen bridge bicyclo-[2.2.1]-heptene compound containing different functional side chain structures, as well as preparation and application thereof](https://images-eureka.patsnap.com/patent_img/c14bd2dc-6736-46a2-84dc-751d28dab027/FDA0001960300270000011.png)
![Oxygen bridge bicyclo-[2.2.1]-heptene compound containing different functional side chain structures, as well as preparation and application thereof Oxygen bridge bicyclo-[2.2.1]-heptene compound containing different functional side chain structures, as well as preparation and application thereof](https://images-eureka.patsnap.com/patent_img/c14bd2dc-6736-46a2-84dc-751d28dab027/FDA0001960300270000031.png)