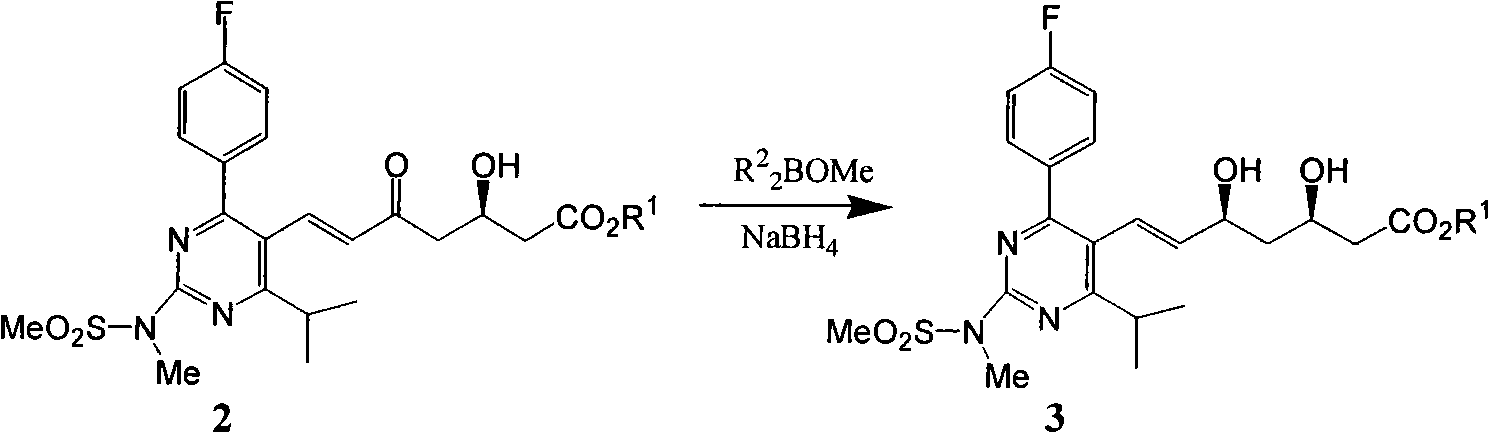Preparation method of 3, 5-dihydroxy heptyl-6-gadoleic acid derivative
A dimethyl propyl and benzyl technology, applied in the field of preparation of rosuvastatin calcium, can solve the problems of low total yield, high cost, low purity of rosuvastatin calcium, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
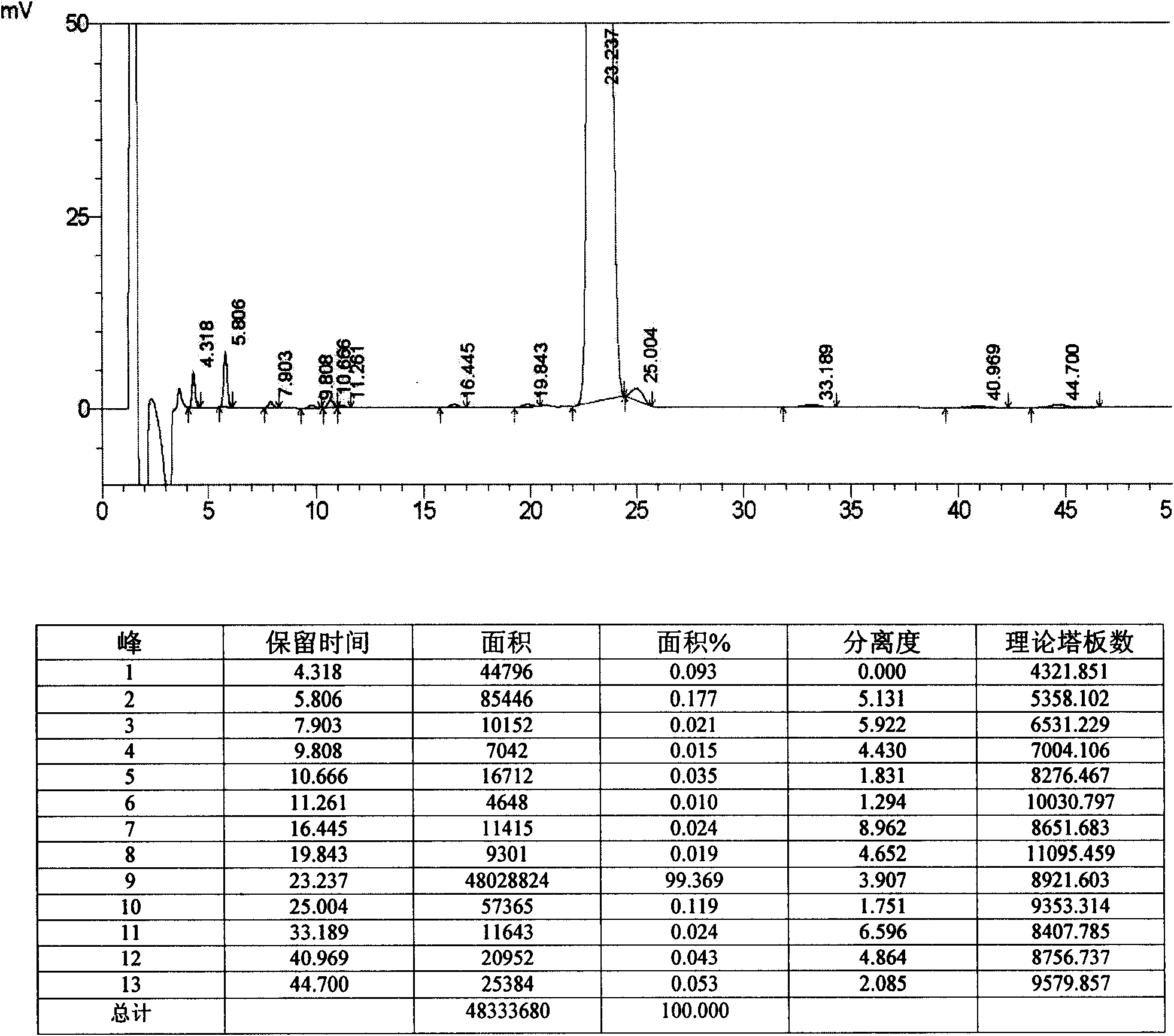
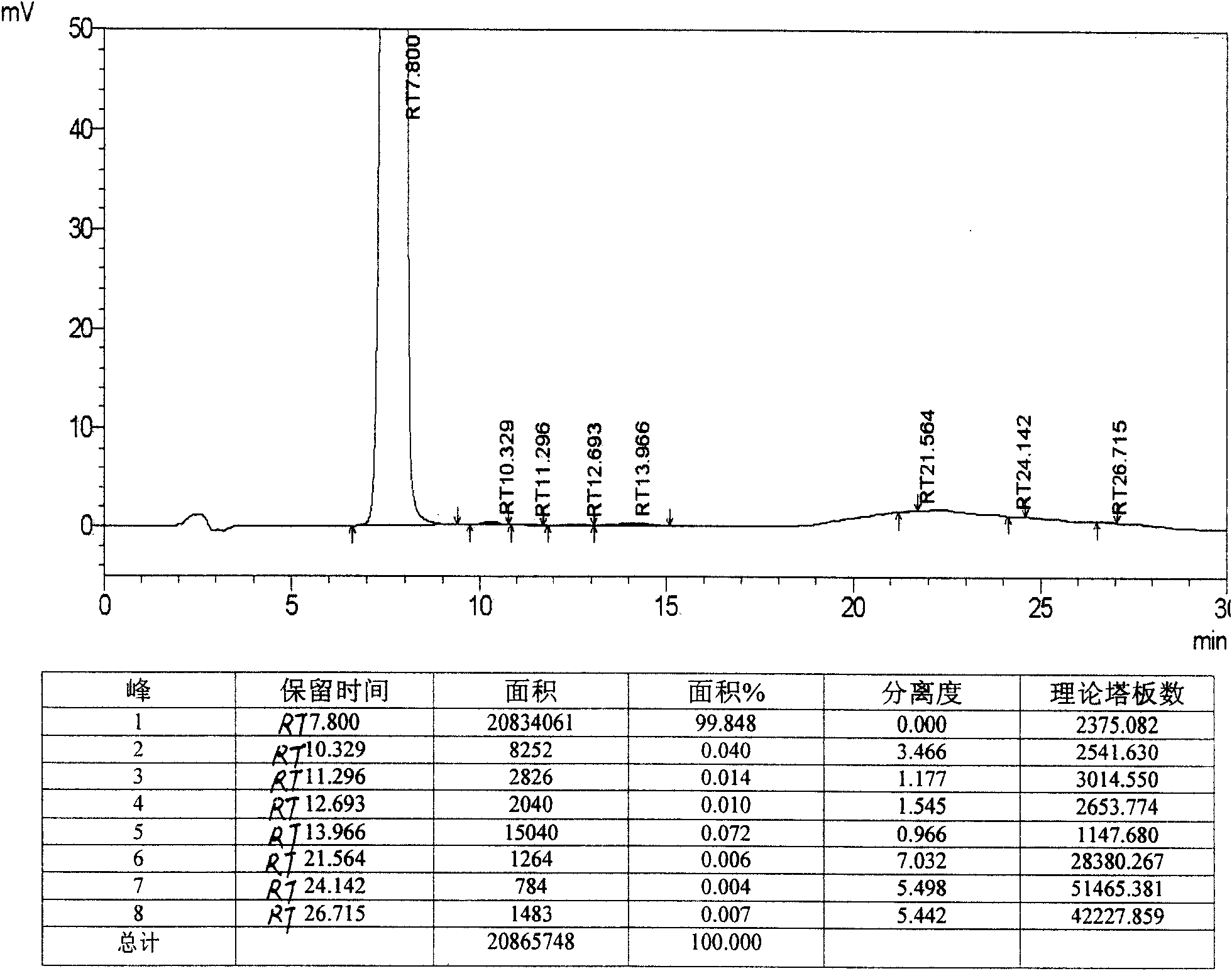
[0098] 1. Restore
[0099]
[0100]Add 300 mL of anhydrous THF and 85 mL of anhydrous methanol into a 500 mL dry reaction flask, add 6.3 g of TP-13, replace the reaction system with nitrogen, and stir until TP-13 is completely dissolved. Cool down to -80~-85°C with liquid nitrogen, add 12.8 mL of 1M diethylmethoxyborane tetrahydrofuran solution dropwise, control the rate of addition to keep the temperature between -80~-85°C, and drop it in about 30 minutes. Maintain the temperature at -80 to -85°C and continue stirring for 55 to 60 minutes. Then add 0.7g of sodium borohydride evenly in batches, and after about 80 to 90 minutes, maintain the temperature at -80 to -85°C. This process takes about 5.5 to 6 hours, and the reaction ends. Raise the temperature to 20-30°C within 2 hours, continue the heat preservation reaction for 2-3 hours, add 1.2g of glacial acetic acid, and distill under reduced pressure at 45-55°C to remove the methanol-tetrahydrofuran mixed organic solvent. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com