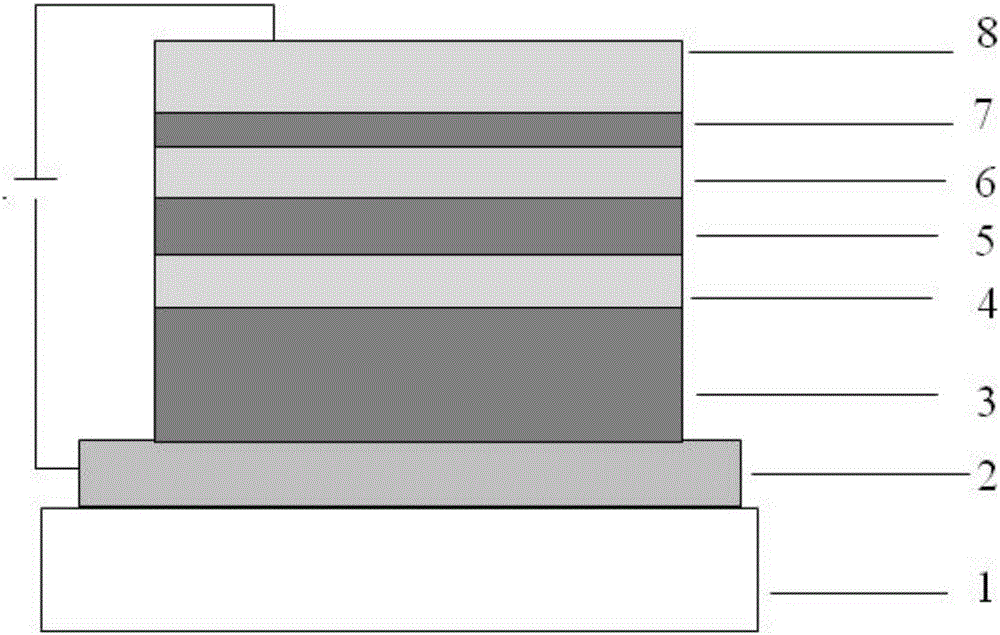
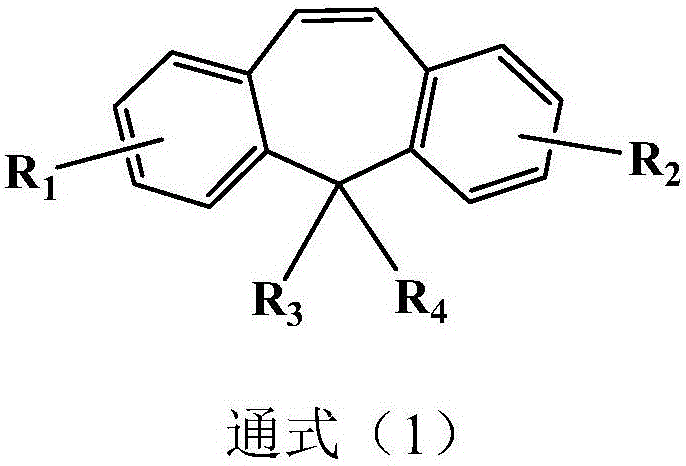
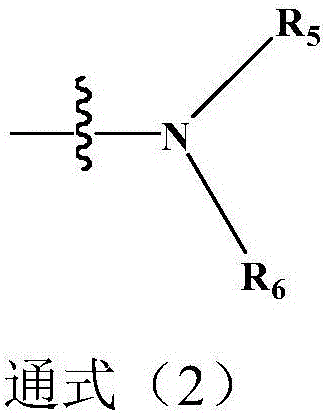
Organic compound having dibenzo heptene structure and application thereof
An organic compound, benzoheptene technology, applied in the field of organic fluorescent compounds, can solve problems such as backwardness and insufficient fluorescent materials, and achieve the effects of increasing device life, improving color purity, and improving thermal stability and color purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Example 1 (Compound 01)
[0046]
[0047] The concrete synthetic route of this compound is provided now:
[0048]
[0049] In a 250ml four-neck flask, under nitrogen protection, add 5.02 grams (0.01M) of intermediate A, 4.62 grams (0.025M) of bis(3,4-dimethylphenyl)amine, 1.15 grams (0.012M) of tert-butanol Sodium, 0.1 gram of palladium acetate, 0.05 gram of CXA (n-butyl bis(1-adamantyl) phosphine), 200ml of toluene, heated to reflux for 16 hours, the reaction was complete; naturally cooled, filtered, and the filtrate was rotary evaporated, passed through a silica gel column, and used Toluene:ethanol=2:1 (volume ratio) mixed solvent was beaten, and 5.81 g of white solid was obtained after recrystallization, the purity (HPLC) was 98.6%, and the yield was 73.5%.
[0050] Elemental analysis structure (molecular formula C 59 h 54 N 2 ): theoretical value C, 89.58; H, 6.88; N, 3.54; test value: C, 89.42; H, 6.74; N, 3.84.
Embodiment 2
[0051] Example 2 (Compound 05)
[0052]
[0053] The concrete synthetic route of this compound is provided now:
[0054]
[0055] In a 250ml four-neck flask, under nitrogen protection, add 5.0 grams (0.01M) of intermediate A, 7.94 grams (0.025M) of intermediate a, 1.15 grams (0.012M) of sodium tert-butoxide, 0.1 grams of palladium acetate, and 0.05 grams of CXA (n-butyl bis (1-adamantyl) phosphine), 200ml toluene, heated to reflux for 22 hours, the reaction was complete; natural cooling, filtration, filtrate rotary evaporation, silica gel column, with toluene:ethanol=2:1 (volume ratio ) mixed solvent for beating, and after recrystallization, 7.25 grams of white solid were obtained, the purity (HPLC) was 98.85%, and the yield was 68.4%.
[0056] Elemental analysis structure (molecular formula C 75 h 66 N 2 S 2 ): theoretical value C, 85.02; H, 6.28; N, 2.64; S, 6.05; test value: C, 84.85; H, 6.35; N, 2.70; O, 6.10.
Embodiment 3
[0057] Example 3 (Compound 06)
[0058]
[0059] The concrete synthetic route of this compound is provided now:
[0060]
[0061] In a 250ml four-neck flask, under nitrogen protection, add 5.0 grams (0.01M) of intermediate A, 8.18 grams (0.025M) of intermediate b, 1.15 grams (0.012M) of sodium tert-butoxide, 0.1 grams of palladium acetate, and 0.05 grams of CXA (N-butyl bis (1-adamantyl) phosphine), 200ml toluene, refluxed 24 hours, reaction is complete; Natural cooling, filtration, filtrate rotary steaming, pass through silica gel column, with toluene: ethanol=2:1 (volume ratio ) mixed solvent for beating, and after recrystallization, 6.98 grams of white solid were obtained, the purity (HPLC) was 98.35%, and the yield was 70.1%.
[0062] Elemental analysis structure (molecular formula C 75 h 66 N 2 ): theoretical value C, 90.50; H, 6.68; N, 2.81; test value: C, 90.28; H, 6.75; N, 2.97.
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com