Cycloolefin addition copolymer and optical transparent material
A cyclic olefin and copolymer technology, applied in the field of cyclic olefin addition copolymers, can solve the problems of unknown cyclic olefins and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0327] Hereinafter, the present invention will be specifically described with examples, but the present invention is not limited by the examples.
[0328] In addition, "parts" and "%" are based on weight unless otherwise specified.
[0329] In addition, molecular weight, thermal stability test, hue change, total light transmittance, glass transition temperature, linear expansion coefficient, adhesion-adhesion, toluene swelling degree, tensile strength, elongation, solution viscosity, solubility test, etc. , Determination according to the following method.
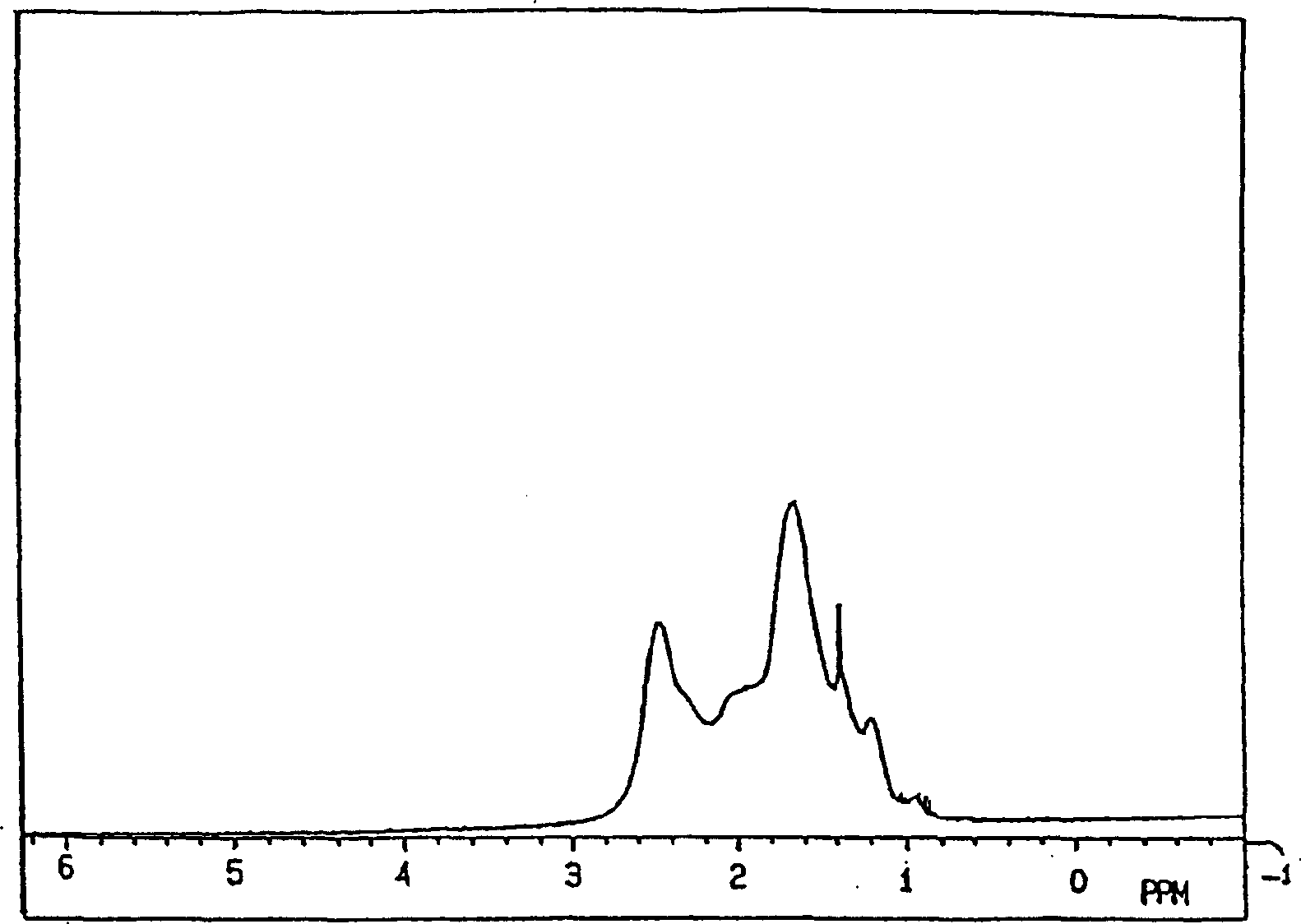
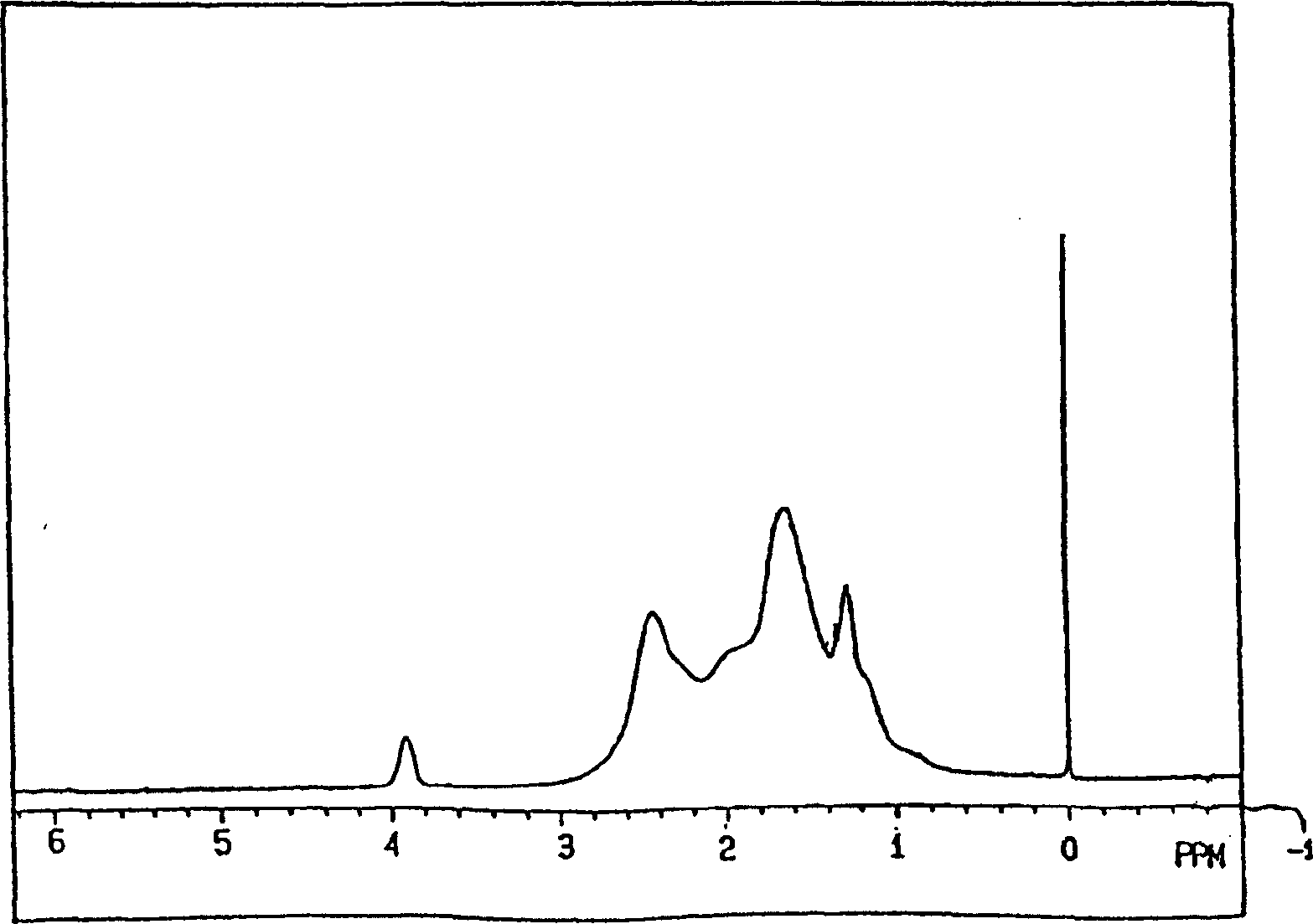
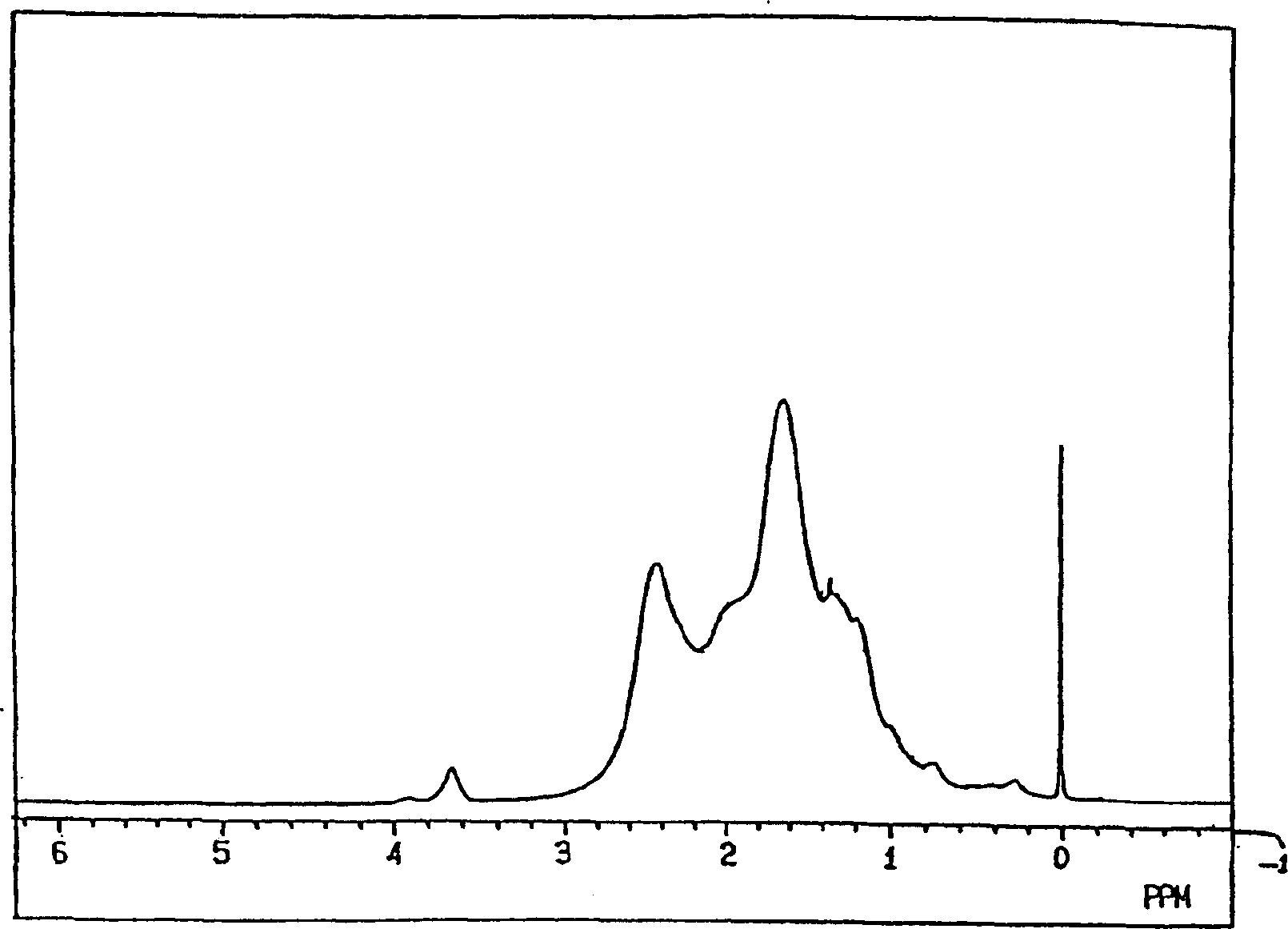
[0330] (1) 1 H-NMR:
[0331] in benzene-d 6 In a mixed solvent with O-dichlorobenzene (volume ratio 60 / 40), it can be measured at a resonance frequency of 270MHz under heating if necessary.
[0332] (2) Weight average molecular weight, number average molecular weight:
[0333] Using a 150C gel permeation chromatography (GPC) device manufactured by Water Co., Ltd. and an H-type column manufactured by Tosoh Corporation, ...
Synthetic example
[0362] endo-tricyclic [4.3.0.1 2,5 ] Deca-3,7-diene, which is obtained by refining commercially available products through vacuum distillation. Its endo / exo ratio was determined to be above 99 / 1 by gas chromatography.
[0363] endo-tricyclic [4.3.0.1 2,5 ] Dec-3-ene, synthesized with reference to the US Patent No. 4,139,569 specification, and the method described in Macromol.Chem.Vol.95, 179 (1966), and using a distillation apparatus with 40 theoretical stages to carry out vacuum distillation Refined, and use a refined product with a purity of more than 99%, endo / exo=90 / 10, or endo / exo=96 / 4.
[0364] exo-tricyclic [4.3.0.1 2,5 ]Deca-3,7-diene is synthesized by referring to J.Am.Chem.Soc., 69, 2553 (1947) and Synthesis 105 (1975), and the purity obtained by distillation under reduced pressure is over 99%. Endo / exo = Refined product of 4 / 96.
[0365] exo-tricyclic [4.3.0.1 2,5 ]Deca-3-ene is synthesized by referring to methods described in J.Am.Chem.Soc., 69, 2553 (1947), ...
Embodiment 1
[0369] Bicyclo[2.2.1]hept-2-ene 47g (500mmol), endo-tricyclo[4.3.0.1 2,5 ] 66 g (500 mmol) of dec-3,7-diene, 0.42 g (5 mmol) of 1-hexene, 480 g of toluene as a solvent, and 85 g of cyclohexane were added to a 2-liter stainless steel reactor under a nitrogen atmosphere.
[0370] The hexane solution of nickel octoate and hexafluorotitanic acid were reacted at -10°C with a molar ratio of 1:1, and the by-products bis(hexafluorotitanate) nickel, [Ni(SbF 6 ) 2 ] The precipitate was removed by filtration and diluted with toluene. The nickel atom of the obtained hexafluoroantimonic acid modified product of nickel octoate was 0.40 mmol, and 1.2 mmol of boron trifluoride ethyl vinyl ester, 8.0 mmol of methylaluminoxane, 0.4 mmol of 1,5-cyclooctadiene, Subsequently, 8.0 mmol of methyltriethoxysilane, 1,5-cyclooctadiene, methylaluminoxane, and hexafluorotitanic acid modified product of boron trifluoride ethylvinyl octanoate nickel were added in order, Start to aggregate. The polymeriz...
PUM
| Property | Measurement | Unit |
|---|---|---|
| glass transition temperature | aaaaa | aaaaa |
| glass transition temperature | aaaaa | aaaaa |
| thermal degradation temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com