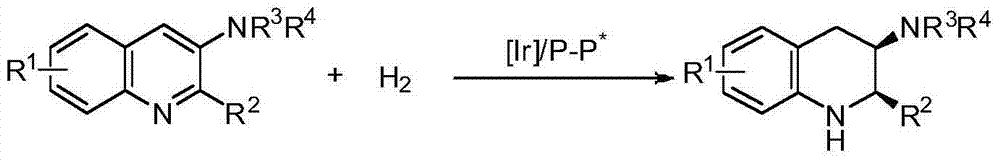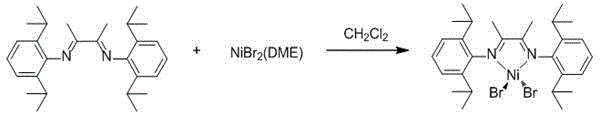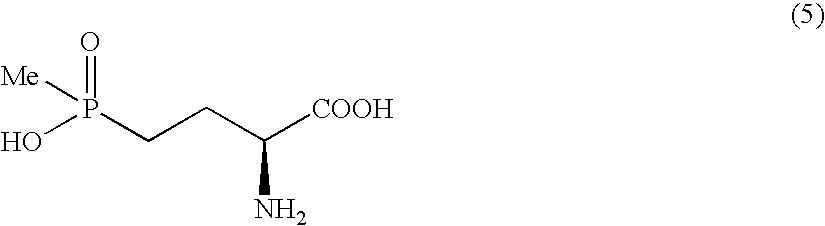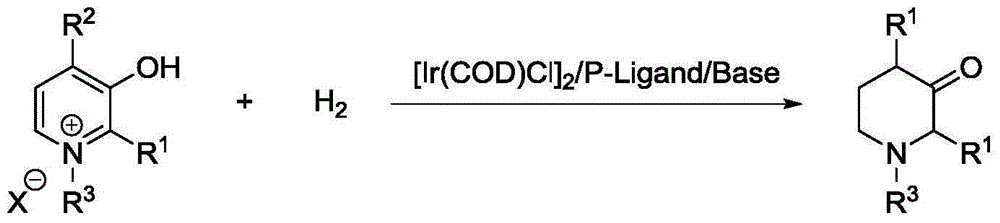Patents
Literature
178 results about "Cyclooctadiene" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
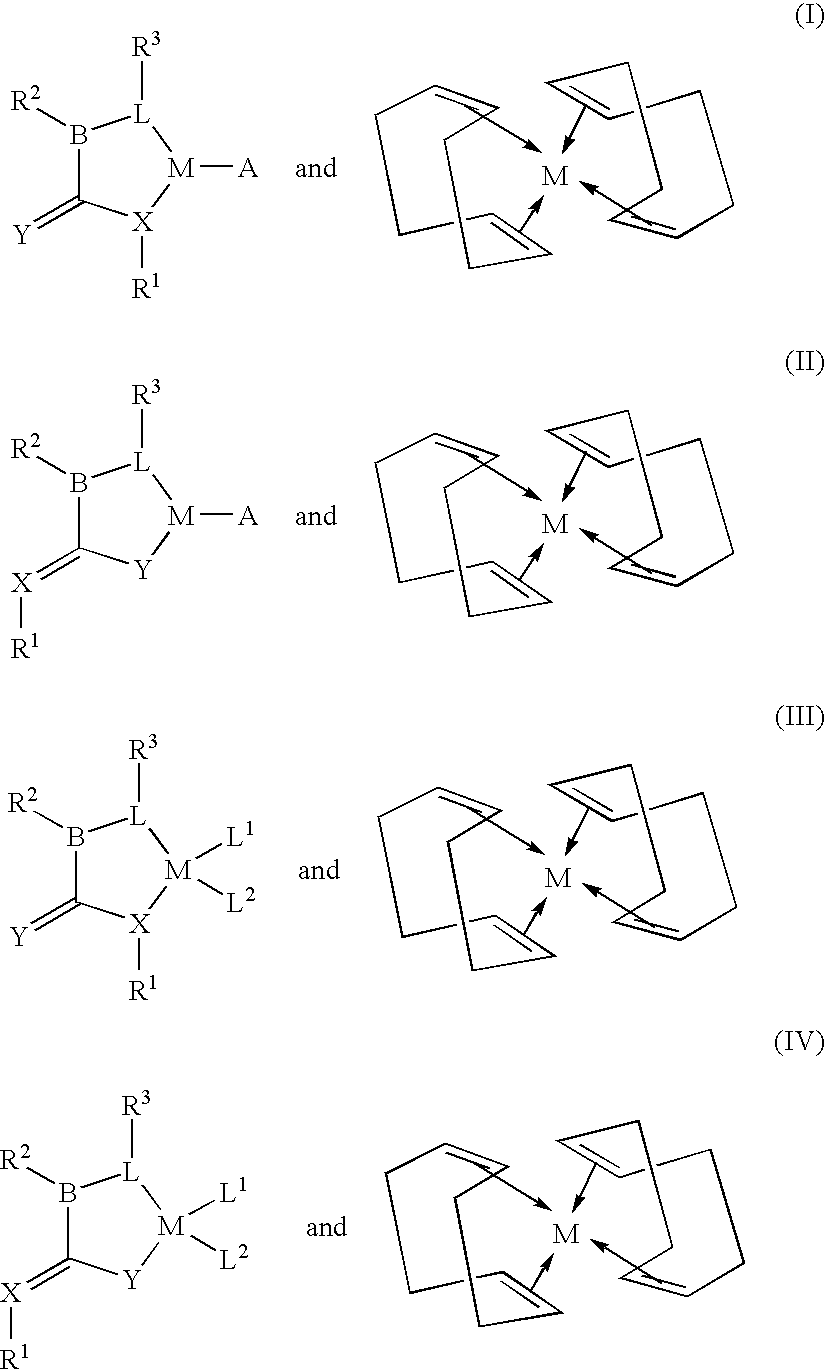
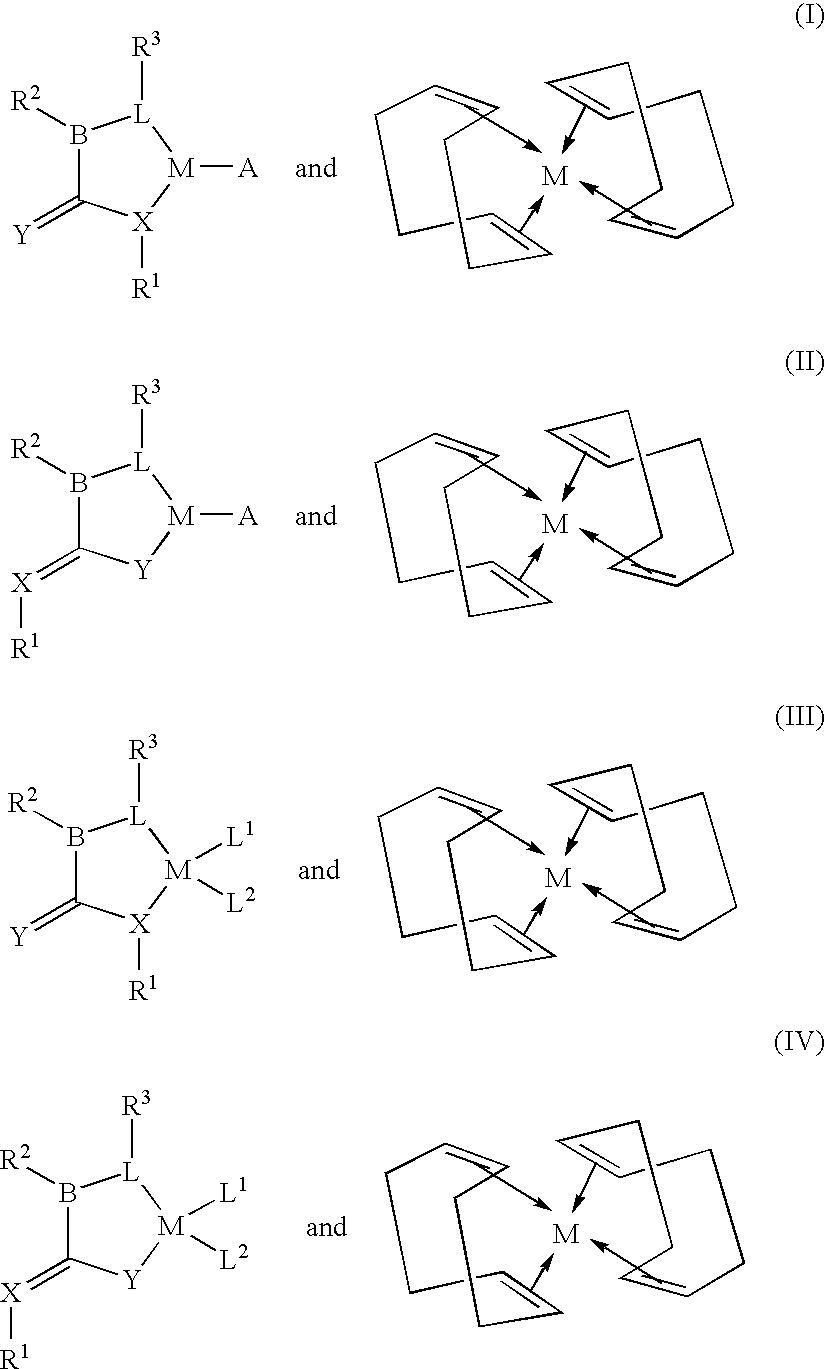
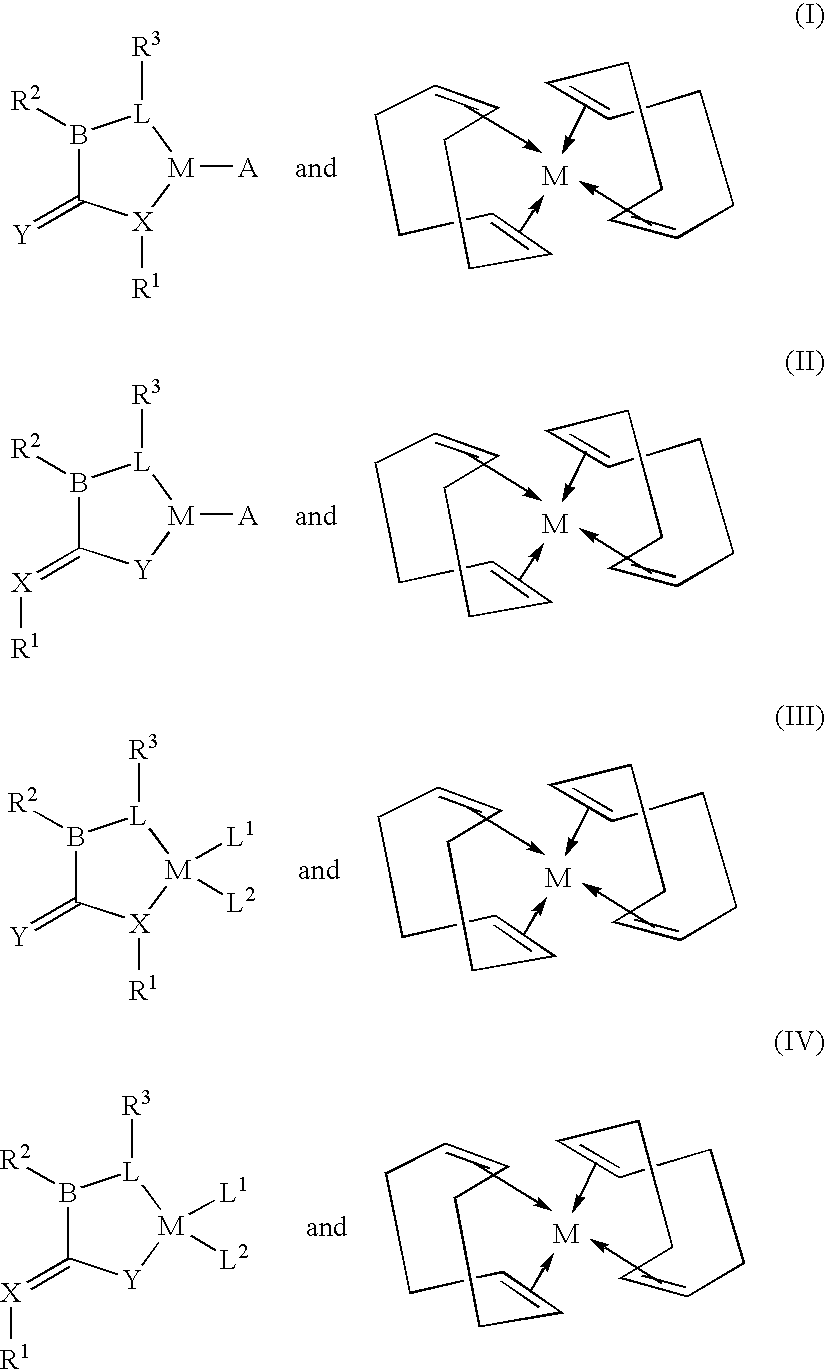
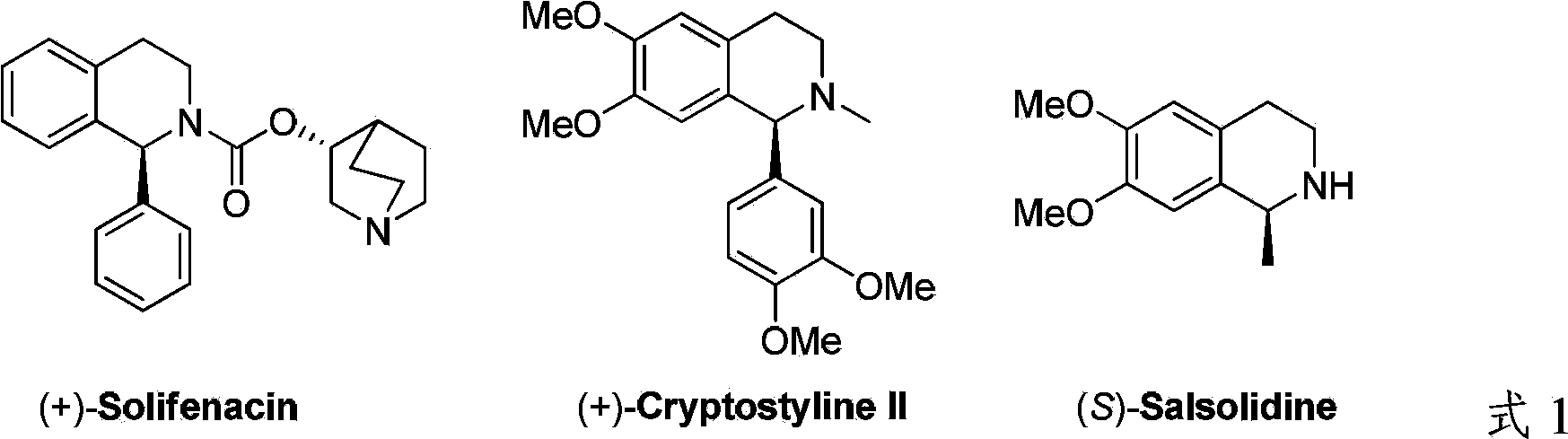
A cyclooctadiene (sometimes abbreviated COD) is any of several cyclic diene with the formula (CH₂)₄(C₂H₂)₂. Focusing only on cis derivatives, four isomers are possible: 1,2-, which is an allene, 1,3-, 1,4-, and 1,5-. Commonly encountered isomers are the conjugated isomer 1,3-cyclooctadiene and 1,5-cyclooctadiene, which is used as a ligand for transition metals. These dienes are colorless volatile liquids.
Class of volatile compounds for the deposition of thin films of metals and metal compounds
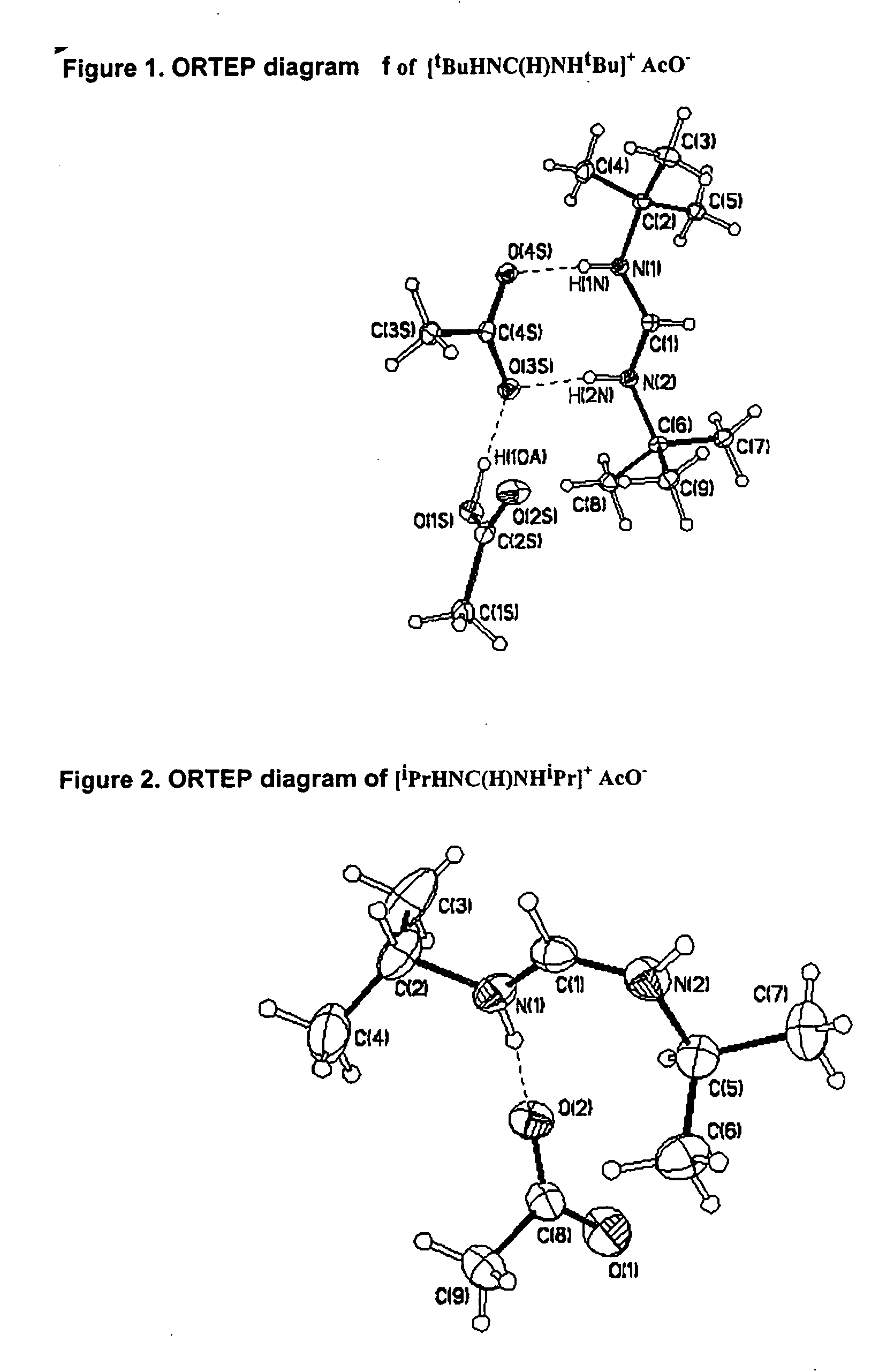
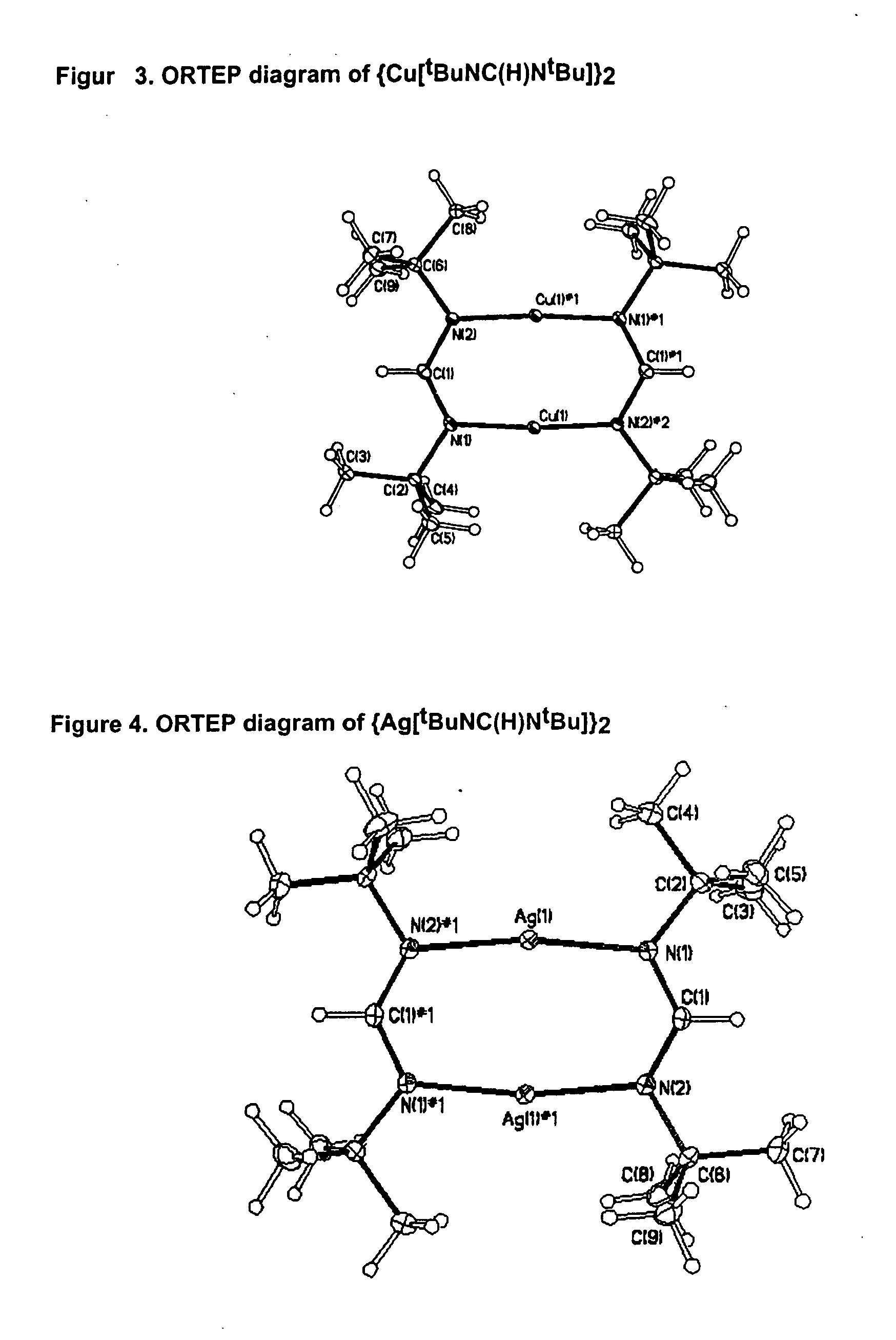
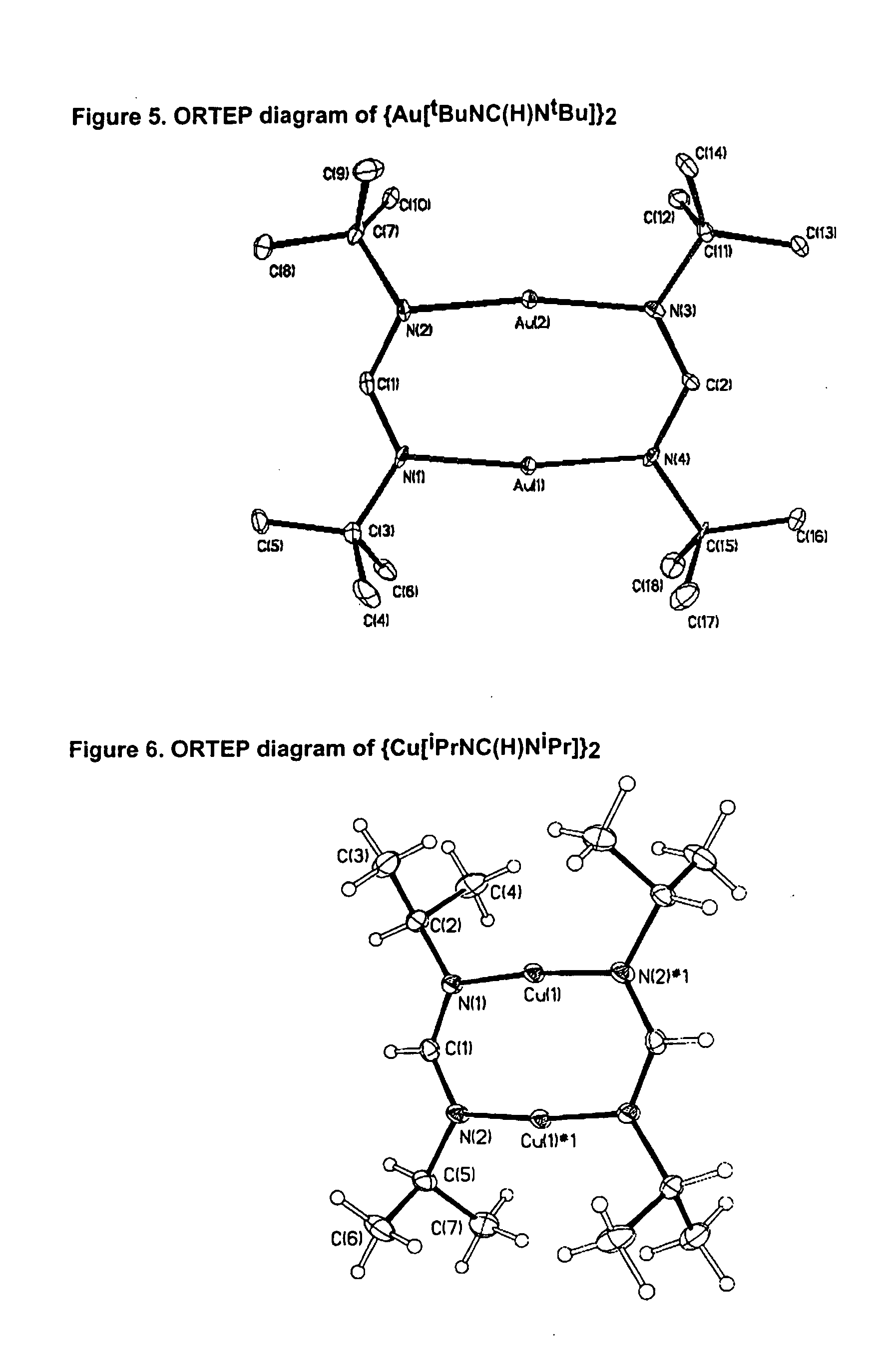
The invention provides an organometallic complex, containing oxygen free organic ligands, for the deposition of a metal, preferably copper, silver or gold, and preferably by way of chemical vapor deposition. The organometallic complex having the formula [(Do)nMLx]k where M is a metal preferably selected from the group consisting of Cu, Ag and Au; Do is selected from the group comprising ethers, phosphines, olefins, sulfides, pyridines, carbonyl, hydroxyl, cyclopentadiene, benzene derivatives, allyls, alkyls, amines, polyamines, aniline derivatives, cyclooctadiene and combinations thereof; n is an integer having a value from 0 to 4; k is an integer having a value from 1 to 4; x is an integer having a value from 1 to 4; and L is an amidinate ligand of the formula R1—NC(R2)N—R3 where R1, R2 and R3 are selected from the group consisting of alkyls, allyls, aryls, heteroaryls, hydrogen, non-metals and metalloids; and where R1, R2 and R3 are different or the same.
Owner:PRESIDENT & FELLOWS OF HARVARD COLLEGE
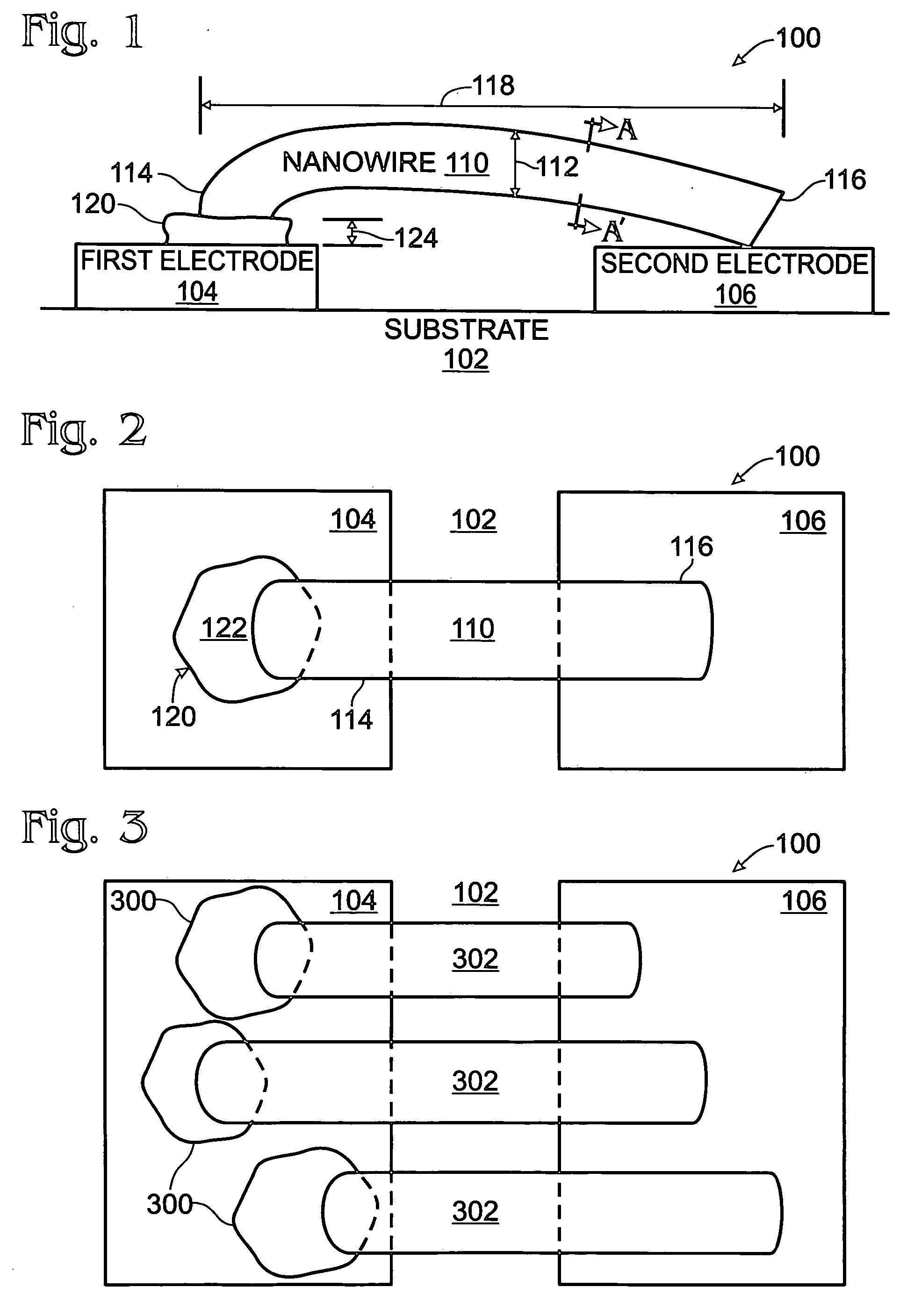
Iridium oxide nanowires and method for forming same
InactiveUS20060086314A1Good crystallinity and electrical propertyNanotechPolycrystalline material growthNanowireSingle crystal
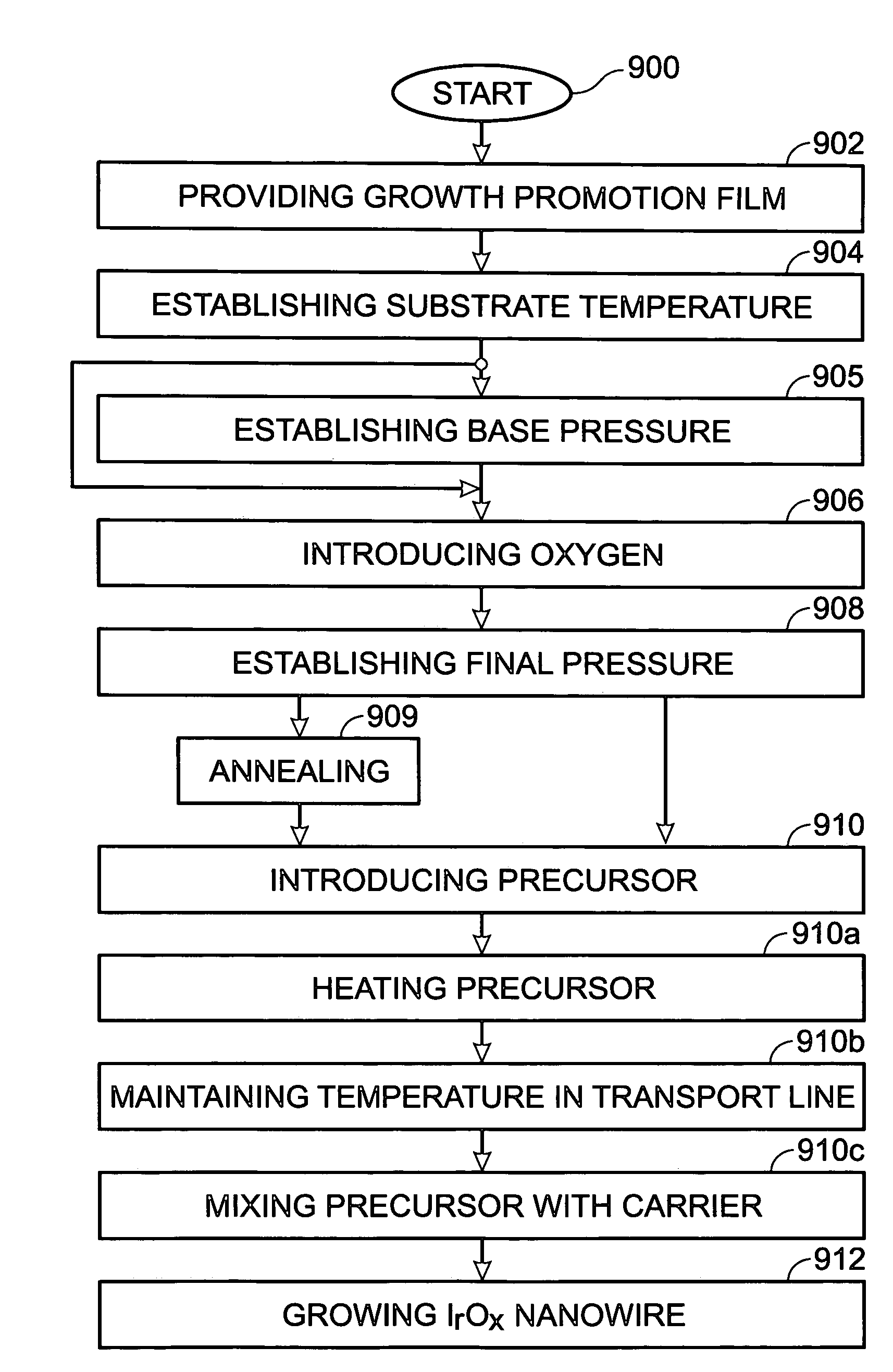
Iridium oxide (IrOx) nanowires and a method forming the nanowires are provided. The method comprises: providing a growth promotion film with non-continuous surfaces, having a thickness in the range of 0.5 to 5 nanometers (nm), and made from a material such as Ti, Co, Ni, Au, Ta, polycrystalline silicon (poly-Si), SiGe, Pt, Ir, TiN, or TaN; establishing a substrate temperature in the range of 200 to 600 degrees C.; introducing oxygen as a precursor reaction gas; introducing a (methylcyclopentadienyl)(1,5-cyclooctadiene)iridium(I) precursor; using a metalorganic chemical vapor deposition (MOCVD) process, growing IrOx nanowires from the growth promotion film surfaces. The IrOx nanowires have a diameter in the range of 100 to 1000 Å, a length in the range of 1000 Å to 2 microns, an aspect ratio (length to width) of greater than 50:1. Further, the nanowires include single-crystal nanowire cores covered with an amorphous layer having a thickness of less than 10 Å.
Owner:SHARP KK
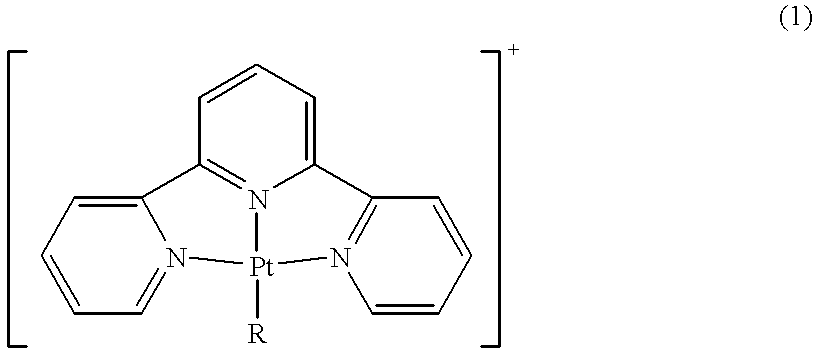
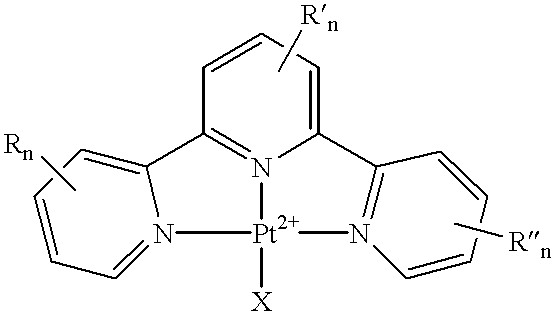
Terpyridine-platinum(II) complexes
InactiveUS20020013306A1Fast displacementImprove purification effectBiocideHeavy metal active ingredientsAntiparasiticChemical compound
A new class of 2,2':6',2''-terpyridine-platinum (II) and substituted 2,2':6',2''-terpyridine-platinum (II) complexes in which an N- or O- or halo nucleophile is the fourth ligand to platinum. The compounds are potent intercalators of DNA. Some have antitumour activity. Some have anti-parasitic activity. A new method of preparing the complexes involves reacting a Pt complex of 1,5-cyclooctadiene with a 2,2':6',2''-terpyridine.
Owner:ISIS INNOVATION LTD
Iridium oxide nanotubes and method for forming same
ActiveUS7098144B2Material nanotechnologyPolycrystalline material growthOxygenChemical vapor deposition
A method is provided for forming iridium oxide (IrOx) nanotubes. The method comprises: providing a substrate; introducing a (methylcyclopentadienyl)(1,5-cyclooctadiene)iridium(I) precursor; introducing oxygen as a precursor reaction gas; establishing a final pressure in the range of 1 to 50 Torr; establishing a substrate, or chamber temperature in the range of 200 to 500 degrees C.; and using a metalorganic chemical vapor deposition (MOCVD) process, growing IrOx hollow nanotubes from the substrate surface. Typically, the (methylcyclopentadienyl)(1,5-cyclooctadiene)iridium(I) precursor is initially heated in an ampule to a first temperature in the range of 60 to 90 degrees C., and the first temperature is maintained in the transport line introducing the precursor. The precursor may be mixed with an inert carrier gas such as Ar, or the oxygen precursor reaction gas may be used as the carrier.
Owner:SHARP KK
Metal catalyst for olefin polymerization and co-polymerization with functional monomers
InactiveUS20040171479A1Organic-compounds/hydrides/coordination-complexes catalystsCatalyst activation/preparationFunctional monomerPolyolefin
A catalyst is disclosed for the polymerization and co-polymerization of olefins with functionalized monomers. The catalyst is formed from a combination of two neutral metal complexes, L(Pr2)M(CH2Ph)(PMe3)[L=N-(2,6-diisopropylphenyl)-2-(2,6-diisopropylphenylimino)propanamide] and M(COD)2(COD=cyclooctadiene). The catalyst displays a unique mode of action and performs at ambient conditions producing high molecular weight polyolefins and co-polymers with functional groups. The polymerized olefins include ethylene, alpha-olefins and functionalized olefins.
Owner:RGT UNIV OF CALIFORNIA
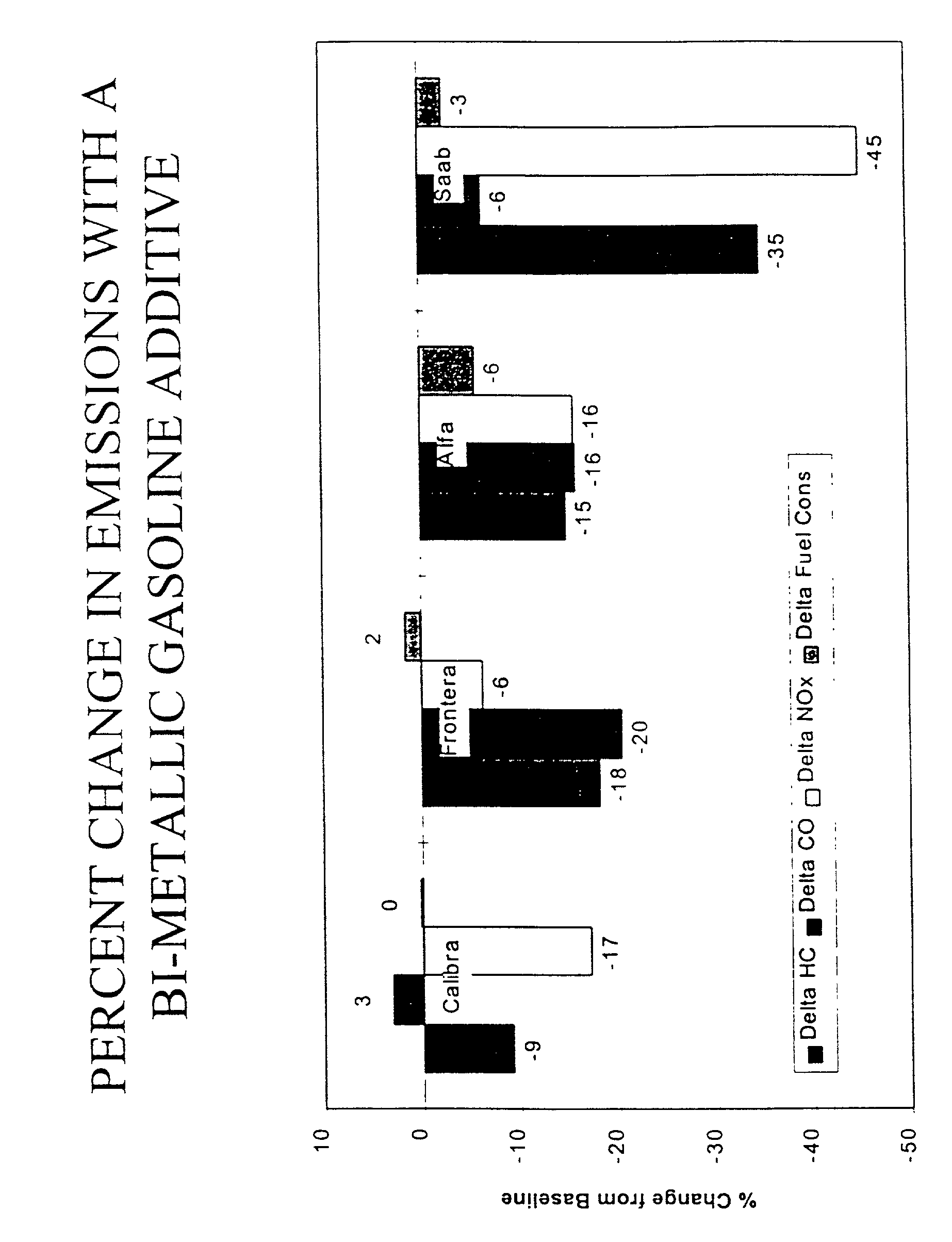
Method and composition for reducing emissions from a gasoline engine equipped with a three-way catalytic converter
InactiveUS20010001354A1Simple compositionSimple processLiquid carbonaceous fuelsFuel additivesGasolineTernary catalyst
Gasoline engines equipped with three-way catalysts emit less NOx, hydrocarbons and carbon monoxide when operated on fuels containing a bimetallic catalyst comprising rhodium acetylacetonate and a fuel-soluble platinum compound such as diphenyl cyclooctadiene platinum(II) or platinum acetyl acetonate. The total metals in the additive will be dosed at a concentration of less than about 2 ppm (milligrams of metal to liter of gasoline) based on the amount of gasoline burned in the engine. Preferred dosages will be from about 0.15 to about 1.5 ppm, with a ratio of platinum to rhodium of from about 3:1 to about 15:1.
Owner:PETER HOBLYN JEREMY D +2
Iridium oxide nanowires and method for forming same
InactiveUS7255745B2Good crystallinity and electrical propertyNanotechPolycrystalline material growthIridiumNanowire
Iridium oxide (IrOx) nanowires and a method forming the nanowires are provided. The method comprises: providing a growth promotion film with non-continuous surfaces, having a thickness in the range of 0.5 to 5 nanometers (nm), and made from a material such as Ti, Co, Ni, Au, Ta, polycrystalline silicon (poly-Si), SiGe, Pt, Ir, TiN, or TaN; establishing a substrate temperature in the range of 200 to 600 degrees C.; introducing oxygen as a precursor reaction gas; introducing a (methylcyclopentadienyl)(1,5-cyclooctadiene)iridium(I) precursor; using a metalorganic chemical vapor deposition (MOCVD) process, growing IrOx nanowires from the growth promotion film surfaces. The IrOx nanowires have a diameter in the range of 100 to 1000 Å, a length in the range of 1000 Å to 2 microns, an aspect ratio (length to width) of greater than 50:1. Further, the nanowires include single-crystal nanowire cores covered with an amorphous layer having a thickness of less than 10 Å.
Owner:SHARP KK
Preparation method for metal complex catalyst used for synthesis of acrylic acid
ActiveCN103785469AHigh yieldImprove solubilityOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsOrganic acidReaction temperature
The invention discloses a preparation method for a metal complex catalyst used for synthesis of acrylic acid. The method comprises the following steps: dissolving bis(1,5-cyclooctadiene)nickel [Ni(cod)2]; then adding ligand and organic acid, carrying out stirring at a temperature of -10 to 30 DEG C for 10 to 150 min, preferably, at a temperature of -5 to 20 DEG C for 20 to 120 min; and finally, carrying out filtering and drying so as to prepare the metal complex catalyst used for synthesis of acrylic acid. The catalyst prepared by using the method has the advantages of low reaction temperature, high yield of acrylic acid and the like when used in direct synthesis of acrylic acid from CO2 and ethene.
Owner:CHINA PETROLEUM & CHEM CORP +1
Method for synthesizing chiral cyclic amine through catalyzing asymmetric hydrogenation of quinolin-3-amine by iridium
ActiveCN104710406AEasy to separateHigh reactivityOrganic chemistryOrganic-compounds/hydrides/coordination-complexes catalystsDiphosphinesIridium chloride
A catalysis system used by a method for synthesizing chiral cyclic amine through catalyzing asymmetric hydrogenation of quinolin-3-amine by iridium is a chiral diphosphine complex of iridium. A reaction is carried out under the following conditions: the temperature is 25-70DEG C; a solvent is a toluene / tetrahydrofuran mixed solvent (V / V=3:1); the pressure is 2-14atm; a ratio of a substrate to a catalyst is 25:1; and the catalyst is a (1,5-cyclooctadiene)iridium chloride dimer and chiral diphosphine ligand complex. A corresponding chiral cyclic amine derivative is prepared from quinolin-3-amine, and the enantiomeric excess value can reach 94%. The method has the advantages of simple and practical operation, easily available raw material, high enantioselectivity, good yield, and green, atom-economic and environmentally-friendly reaction.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Ruthenium complex catalyzer for acetylene hydrochlorinate and preparation method and application thereof
ActiveCN108262072AImprove economyIncrease contentPreparation by halogen halide additionOrganic-compounds/hydrides/coordination-complexes catalystsChlorobenzeneRuthenium chloride
The invention discloses a ruthenium complex catalyzer for acetylene hydrochlorinate and a preparation method and application thereof. The catalyzer is characterized in that active carbon serves as catalyst support, and is loaded with ruthenium chloride and an organic ligand; the molar ratio of the ruthenium chloride and organic ligand is 1:1-6, and ruthenium accounts for 0.1-5.0% of the total weight of the catalyzer; the organic ligand is one or more of triphenylphosphine, pyridine, 2,2'-dipyridyl, acetylacetone, chlorodiphenylphosphine, chlorobenzene, cyclopentadiene, 4-methyl isopropyl benzene and 1,5-cyclooctadiene. The ruthenium complex catalyzer for the acetylene hydrochlorinate and the preparation method and application thereof have the advantages that the preparation process is simple, the catalytic activity and stability of the catalyzer are greatly improved, the catalyzer is more environmentally friendly, and the economy is high.
Owner:浙江天麟环境工程有限公司
Iridium complexes for electrocatalysis
ActiveUS20150021194A1Improve stabilityConvenient bindingPlatinum group organic compoundsFrom normal temperature solutionsIridiumPentamethylcyclopentadiene
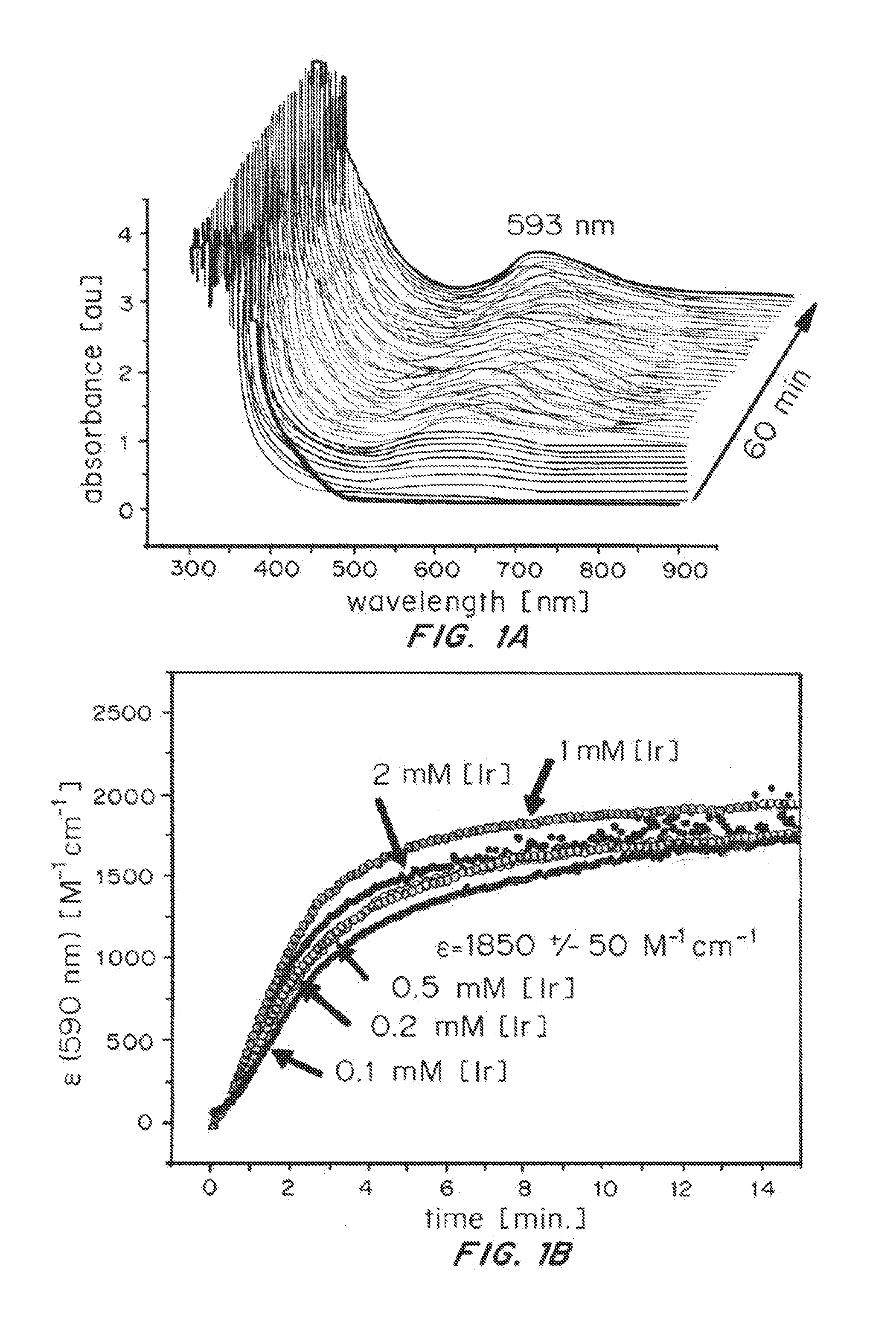
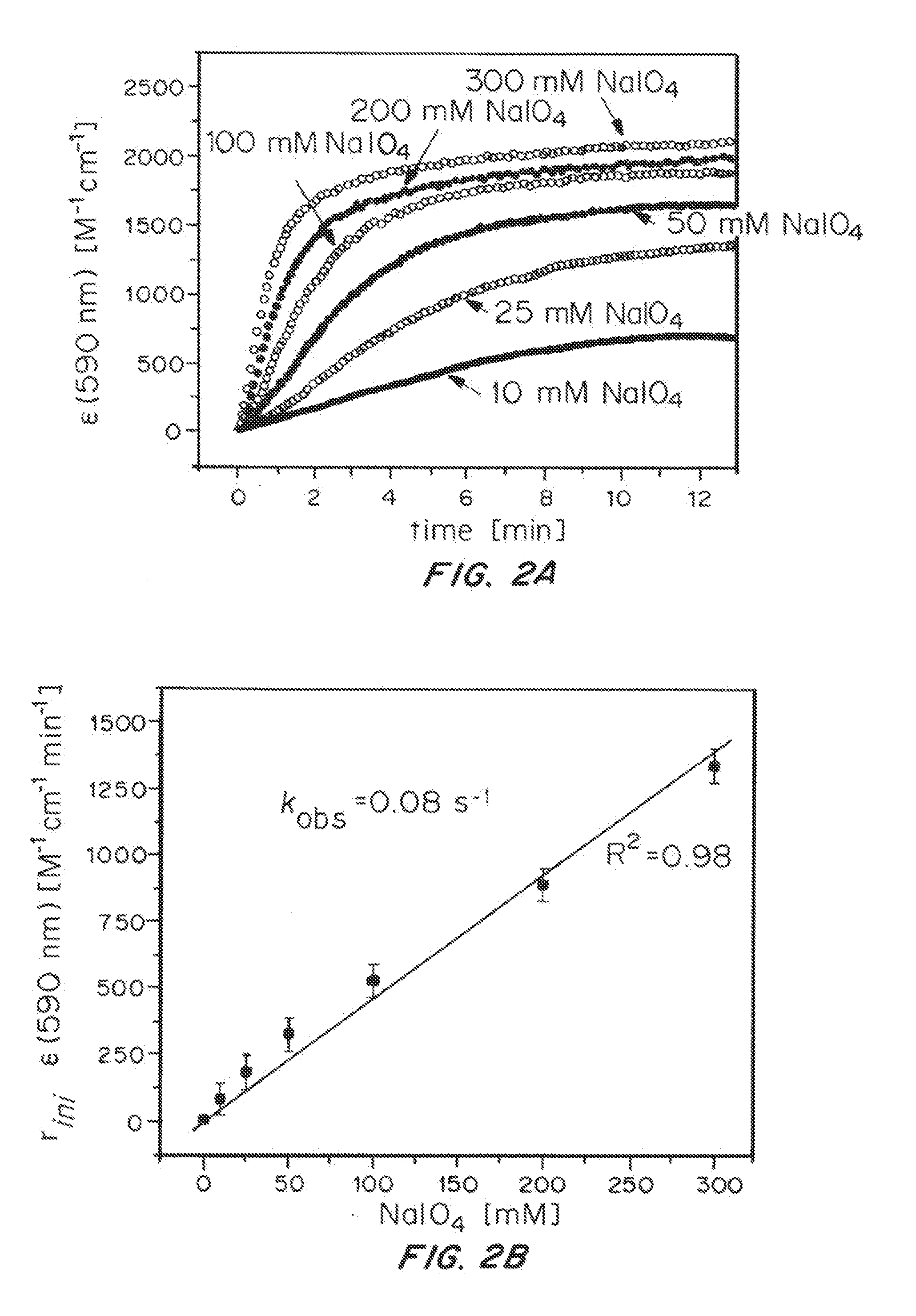
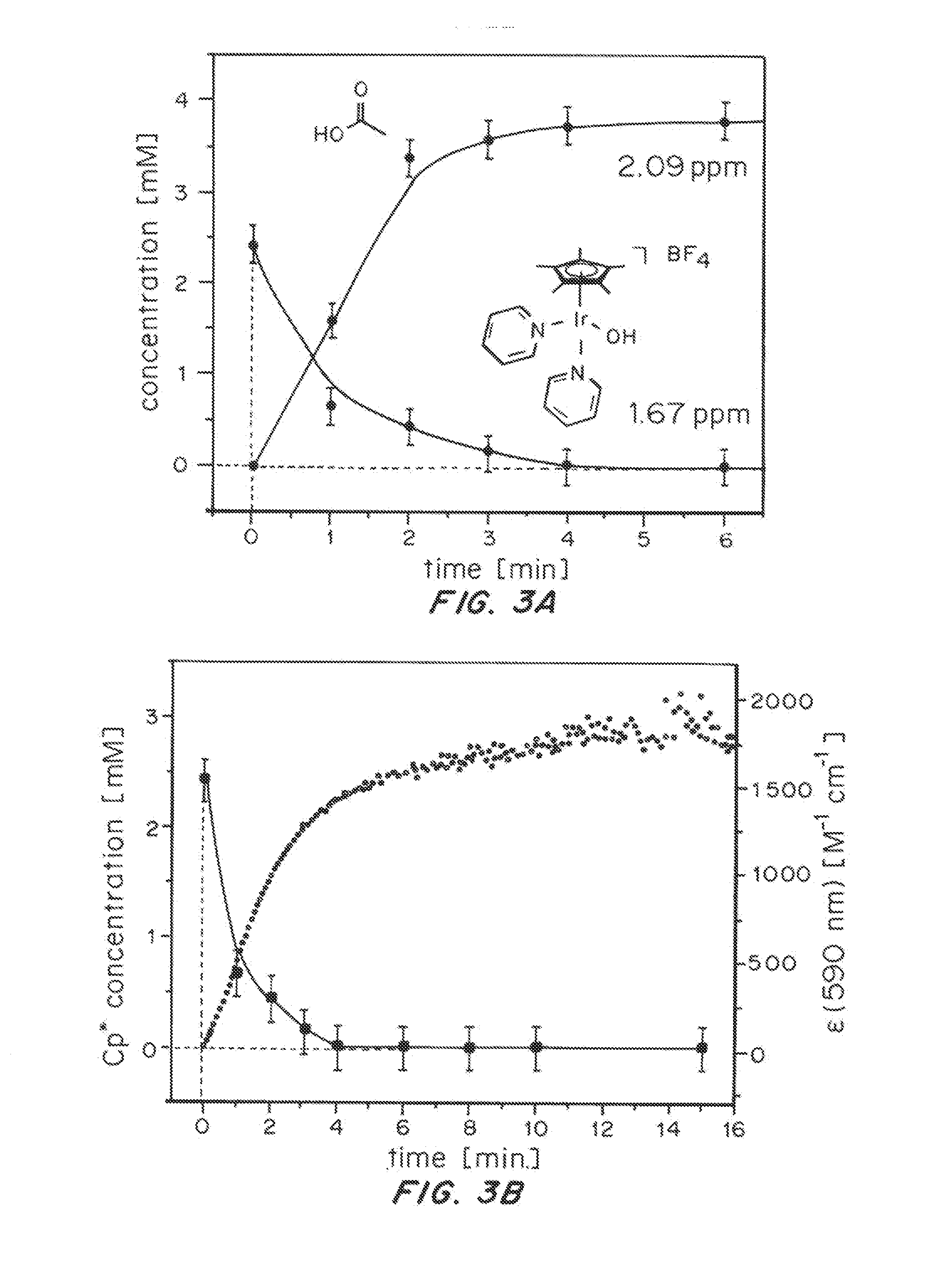
Solution-phase (e.g., homogeneous) or surface-immobilized (e.g., heterogeneous) electrode-driven oxidation catalysts based on iridium coordination compounds which self-assemble upon chemical or electrochemical oxidation of suitable precursors and methods of making and using thereof are. Iridium species such as {[Ir(LX)x(H2O)y(μ-O)]zm+}n wherein x, y, m are integers from 0-4, z and n from 1-4 and LX is an oxidation-resistant chelate ligand or ligands, such as such as 2(2-pyridyl)-2-propanolate, form upon oxidation of various molecular iridium complexes, for instance [Cp*Ir(LX)OH] or [(cod)Ir(LX)] (Cp*=pentamethylcyclopentadienyl, cod=cis-cis,1,5-cyclooctadiene) when exposed to oxidative conditions, such as sodium periodate (NaIO4) in aqueous solution at ambient conditions.
Owner:YALE UNIV
Method for synthesizing chiraltetrahydro naphthalenederivate through asymmetric hydrogenation on isoquinoline by means of iridium catalyst
The invention discloses a method for synthesizing a chiraltetrahydro naphthalenederivate through asymmetric hydrogenation on isoquinoline by means of an iridium catalyst. A catalysis system applied in the method is a chiral bi-phosphine complex of metal iridium. The reaction is carried out under the conditions that the temperature is 25-60 DEG C; the volume ratio of tetrahydrofuran to dichloromethane in a solvent, namely a mixed solvent of tetrahydrofuran and dichloromethane is 1:1; the pressure is 13-50 Mpa; the ratio of a substrate to a catalyst is 50:1; the catalyst is a coordination compound of a (1,5-cyclooctadiene) iridium chloridedipolymer and a chiral bi-phosphine ligand. The corresponding chiral 1-position or 3-position substituted tetrahydro naphthalenederivate through hydrogenation on isoquinoline, and the enantiomeric excess of the derivate can reach 96%. The method is simple and practical in operation, raw materials are easy to obtain, the enantioselectivity is high, the yield is high, the reaction has atom economy, and the environment is friendly.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Process for preparing cyclododecatriene
ActiveUS8168841B2High selectivityHigh yieldCosmetic preparationsHydrocarbon by isomerisationCyclododecatrieneDistillation
Preparation of cyclododecatriene in a continuous or discontinuous process by trimerization of butadiene in the presence of a catalyst system and a solvent, the crude cyclododecatriene obtained being able to be isolated by means of distillation. The cyclooctadiene formed as by-product can likewise be isolated from the crude product.
Owner:EVONIK OPERATIONS GMBH
Process for preparing cyclododecatriene
ActiveUS20070265184A1High selectivityHigh yieldCosmetic preparationsHydrocarbon by isomerisationDistillationCyclododecatriene
Preparation of cyclododecatriene in a continuous or discontinuous process by trimerization of butadiene in the presence of a catalyst system and a solvent, the crude cyclododecatriene obtained being able to be isolated by means of distillation. The cyclooctadiene formed as by-product can likewise be isolated from the crude product.
Owner:EVONIK OPERATIONS GMBH
Method for synthesizing derivatives of chiral tetrahydroquinoline by catalyzing asymmetric hydrosilylation with iridium
InactiveCN101845016AReduce usageHigh reactivityOrganic chemistryOrganic-compounds/hydrides/coordination-complexes catalystsIridiumQuinoline
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Class of volatile compounds for the deposition of thin films of metals and metal compounds
The invention provides an organometallic complex, containing oxygen free organic ligands, for the deposition of a metal, preferably copper, silver or gold, and preferably by way of chemical vapor deposition. The organometallic complex having the formula[(Do)nMLx]kwhere M is a metal preferably selected from the group consisting of Cu, Ag and Au;Do is selected from the group comprising ethers, phosphines, olefins, sulfides, pyridines, carbonyl, hydroxyl, cyclopentadiene, benzene derivatives, allyls, alkyls, amines, polyamines, aniline derivatives, cyclooctadiene and combinations thereof;n is an integer having a value from 0 to 4;k is an integer having a value from 1 to 4;x is an integer having a value from 1 to 4; andL is an amidinate ligand of the formulaR1—NC(R2)N—R3where R1, R2 and R3 are selected from the group consisting of alkyls, allyls, aryls, heteroaryls, hydrogen, non-metals and metalloids; and where R1, R2 and R3 are different or the same.
Owner:PRESIDENT & FELLOWS OF HARVARD COLLEGE
Alpha-diimine nickel metal organic compound and preparation method thereof
InactiveCN104892681AHigh yieldReaction raw materials are cheap and easy to obtainOrganic chemistry methodsNickel organic compoundsDiethyl etherOrganic compound
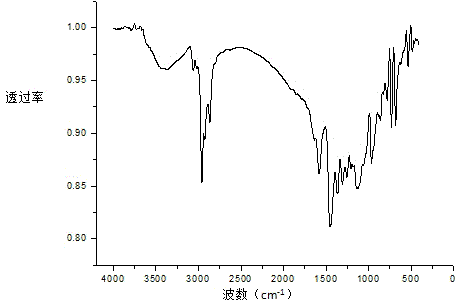
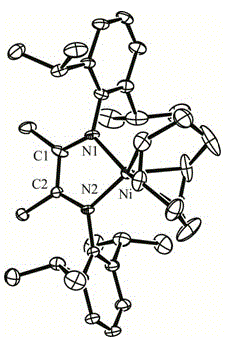
The invention relates to an alpha-diimine nickel metal organic compound and a preparation method thereof. The preparation method comprises the following steps: adding NiBr2 (DME) and alpha-diimine into a dichloromethane solution for reaction under the protection of an inert atmosphere, reducing an obtained product in a diethyl ether or n-hexane solution by using metallic sodium, and then reacting with 1,5-cyclooctadiene to obtain a mononuclear nickel metal organic compound of alpha-diimine. The preparation method provided by the invention is simple, is high in product yield and good in purity and can be used as an olefin polymerization catalyst.
Owner:NORTHWEST UNIV(CN)
Mercury-free catalyst with high catalytic activity for synthesizing vinyl chloride and preparation method thereof
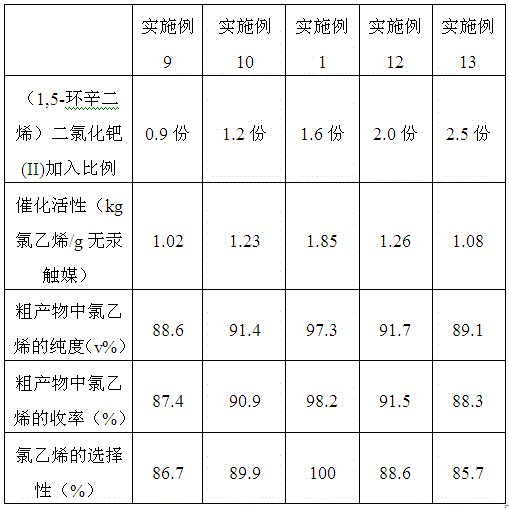
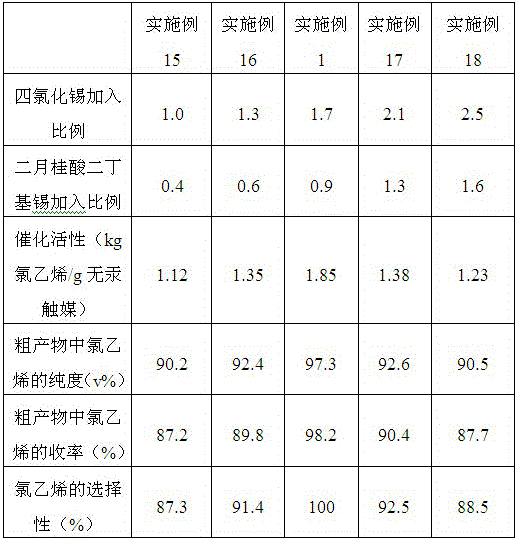
ActiveCN106215977AHigh catalytic activityThe catalytic effect did not change significantlyPreparation by halogen halide additionOrganic-compounds/hydrides/coordination-complexes catalystsMethylrhenium trioxideEthyl Chloride
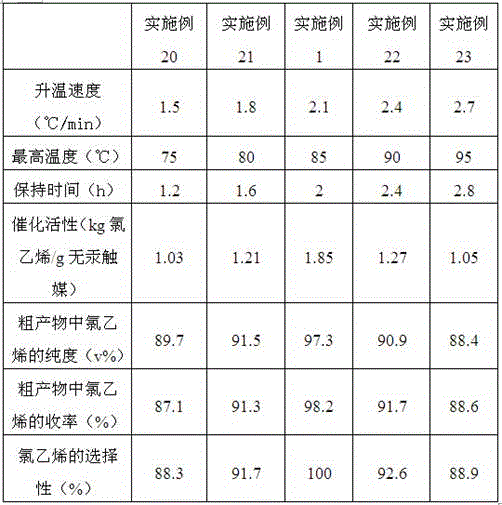
The invention provides a mercury-free catalyst with high catalytic activity for synthesizing vinyl chloride. The mercury-free catalyst is prepared from the raw materials of platinum, stannic chloride, cuprous chloride, methyltrioxorhenium, (1,5-cyclooctadiene)palladium dichloride(II), dibutyltin dilaurate and a carrier. The invention further provides a preparation method of the mercury-free catalyst. The mercury-free catalyst prepared by the method has high catalytic activity, and every gram of the mercury-free catalyst can be applied to catalysis to produce 1,80 to 1.85kg of vinyl chloride; in the prior art, every gram of low-mercury catalyst can be applied to catalysis to produce 1.25kg of vinyl chloride; the mercury-free catalyst prepared by the method is applied to a vinyl chloride synthetic reaction; as proved by chromatographic analysis, the purity of the vinyl chloride in an obtained crude product is 97.0 to 97.3 percent by volume, the yield of the vinyl chloride is 98.0 to 98.2 percent, and the selectivity of the vinyl chloride is 99.98 to 100 percent.
Owner:NINGXIA JINHAI CHUANGKE CHEM TECH
Pyrazolopyrimidines and their use for the treatment of CNS disorders
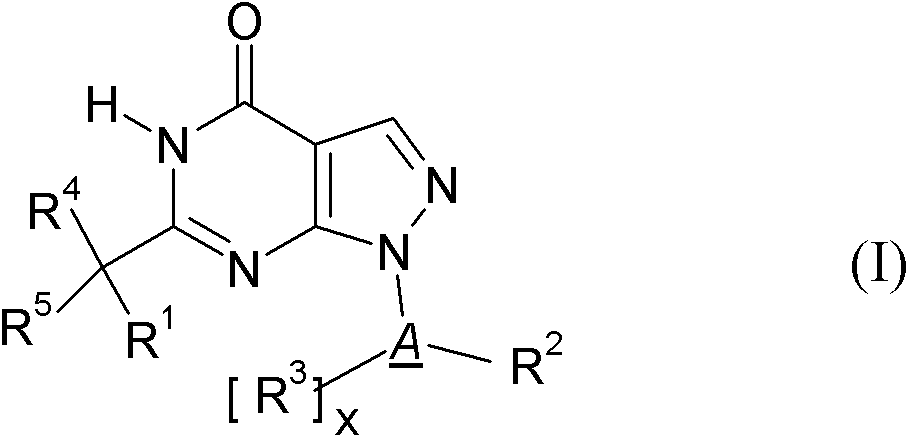
The invention relates to novel cycloalkyl- or cycloalkenyl-substituted pyrazolopyrimidinones of formula (I), wherein A is selected from the group A1 consisting of a C3-C8-cycloalkyl group or a C4-C8-cycloalkenyl group, whereby the members of C3-C8-cycloalkyl group being selected from the group of cyclopropyl, cyclobutyl, cyclopentyl, cyclohexyl, cycloheptanyl and cyclooctanyl; and the members of the C4-C8-cycloalkenyl group, being selected from cyclobutenyl, cyclopentenyl, cyclohexenyl, cycloheptenyl, cyclooctenyl, cyclopentadienyl, cyclohexadienyl, cycloheptadienyl, cyclooctadienyl, cycloheptatrienyl, cyclooctathenyl, cyclooctatetraenyl. The new compounds shall be used for the manufacture of medicaments, in particular medicaments for improving perception, concentration, learning and / or memory in patients in need thereof. Chemically, the compounds are characterised as pyrazolopyrimidinones with a cycloalkyl-moiety directly bound to the 1 position of the pyrazolopyrimidinone and a second substituent in the 6 position which is bound via an optionally substituted methylene-bridge. Further aspects of the present invention refer to a process for the manufacture of the compounds and their use for producing medicaments.
Owner:BOEHRINGER INGELHEIM INT GMBH
Adhesive monomer and method for improving adhesion strength between dental zirconia and resin
InactiveCN103601753AHigh bonding strengthGood biocompatibilityImpression capsDentistry preparationsPolymer sciencePhosphate
The invention discloses an adhesive monomer and a method for improving adhesion strength between dental zirconia and resin. The adhesive monomer is prepared from 1, 5-cyclooctadiene by performing epoxidation, ring-opening reaction, redox, exterification and the like, the main chain of the adhesive monomer is a n-octane carbon chain with one end of acrylic ester and the other end of phosphate ester, the forth position and the fifth position on the carbon chain are respectively connected with a R group which represents hydroxyl, sulfydryl or carboxyl, and the adhesive monomer has the structural formula shown in the description. The adhesive monomer helps to improve the adhesion strength between dental zirconia ceramic and resin, has the same improving effect with common 10-MDP-containing zirconia primer coating products in current market, has relatively good biological compatibility, is qualified in cytotoxicity tests, and has important application value during tooth repairing processing.
Owner:NANJING UNIV +1
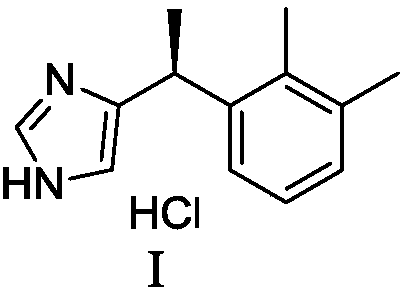
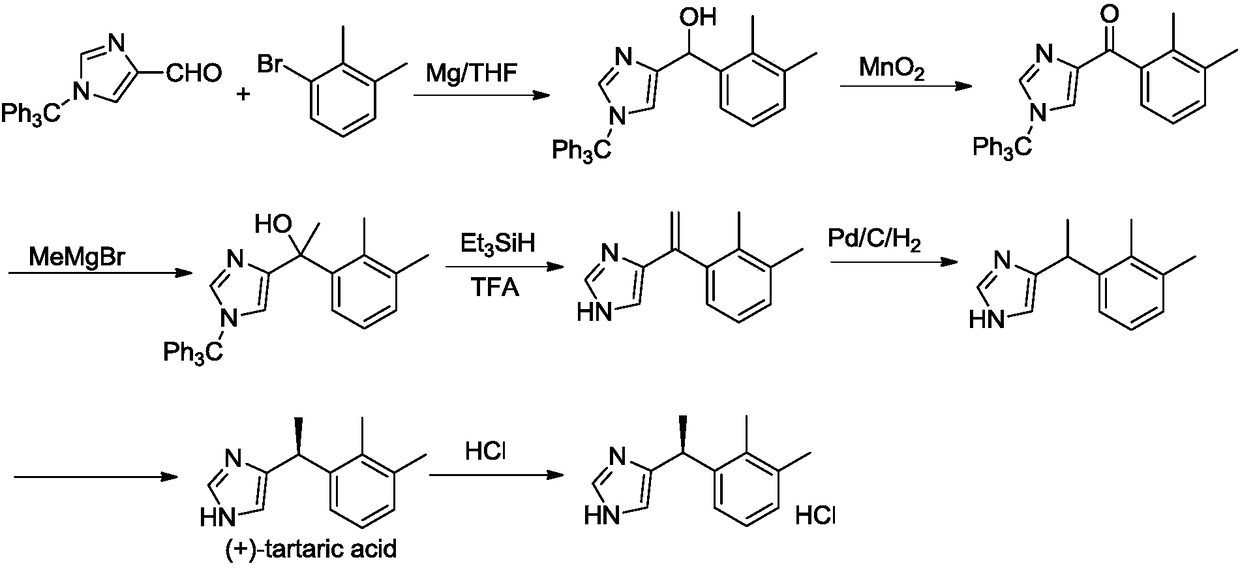
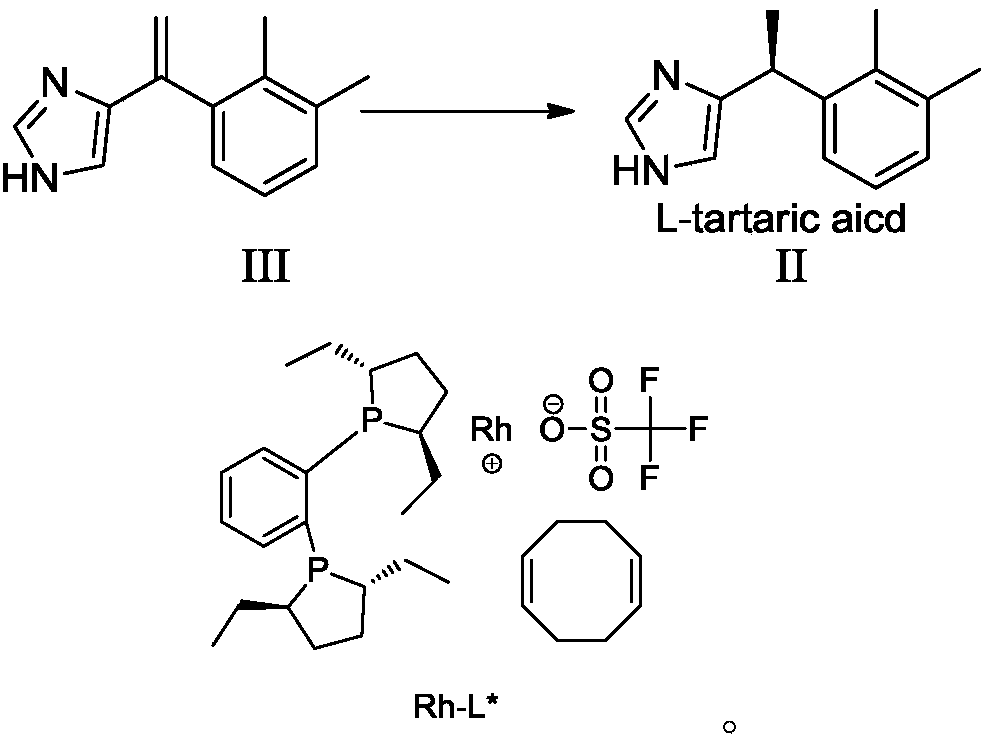
Preparation method of dexmedetomidine hydrochloride and its intermediate
ActiveCN108147999AShort route stepsHigh molar yieldSilicon organic compoundsPreparation by halogen halide additionBenzeneHydrogen
The invention discloses a preparation method of dexmedetomidine hydrochloride and its intermediate. A preparation method of dexmedetomidine L-tartrate comprises the steps of subjecting dexmedetomidineintermediate III and hydrogen to reduction reaction in an organic solvent in the presence of a chiral catalyst, and subjecting the reduced product and tartaric acid to neutralization reaction to obtain dexmedetomidine L-tartrate II, wherein the chiral catalyst is (+)-1,2-bis(2S-5S)-diethylphospholano-benzene(1,5-cyclooctadiene)rhodium trifluoromethanesulfonate. The preparation method herein has ashort step path, has no need for chiral splitting, and has high total molar yield; the product prepared herein has high purity, reaches the standard for bulk pharmaceutical chemicals and is suitablefor industrial production.
Owner:SHANGHAI BOCIMED PHARMA CO LTD
Additive for improving dynamic property of methanol gasoline
ActiveCN103695051AImprove combustion efficiencyImprove skillsLiquid carbonaceous fuelsFuel additivesBenzeneGasoline
The invention discloses an additive for improving dynamic property of methanol gasoline. The additive is prepared from the following raw materials by weight percent: 18.0-31.0% of 2,2-diisopropyl-1,3-dioxolane, 15.0-29.5% of aminoacetaldehyde diethyl acetal, 15.0-24.5% of 1,3-cyclooctadiene, 13.0-24.0% of 1-cyanoethyl-2-ethyl-4-methylimidazole, 10.0-20.5% of 5-methyl-2-heptylene-4-ketone and 9.0-19.5% of 1,4-bi(phenyl acetic diacyl) benzene in a manner of evenly stirring and mixing at normal temperature. According to the additive disclosed by the invention, the improvement effect on heat value power of the methanol gasoline is significant, and the dynamic properties such as acceleration are obviously superior to those of the same mark of methanol gasoline by adding 0.3-1.0% of methanol gasoline into the common methanol gasoline.
Owner:CRPC INNOVATION ENERGY
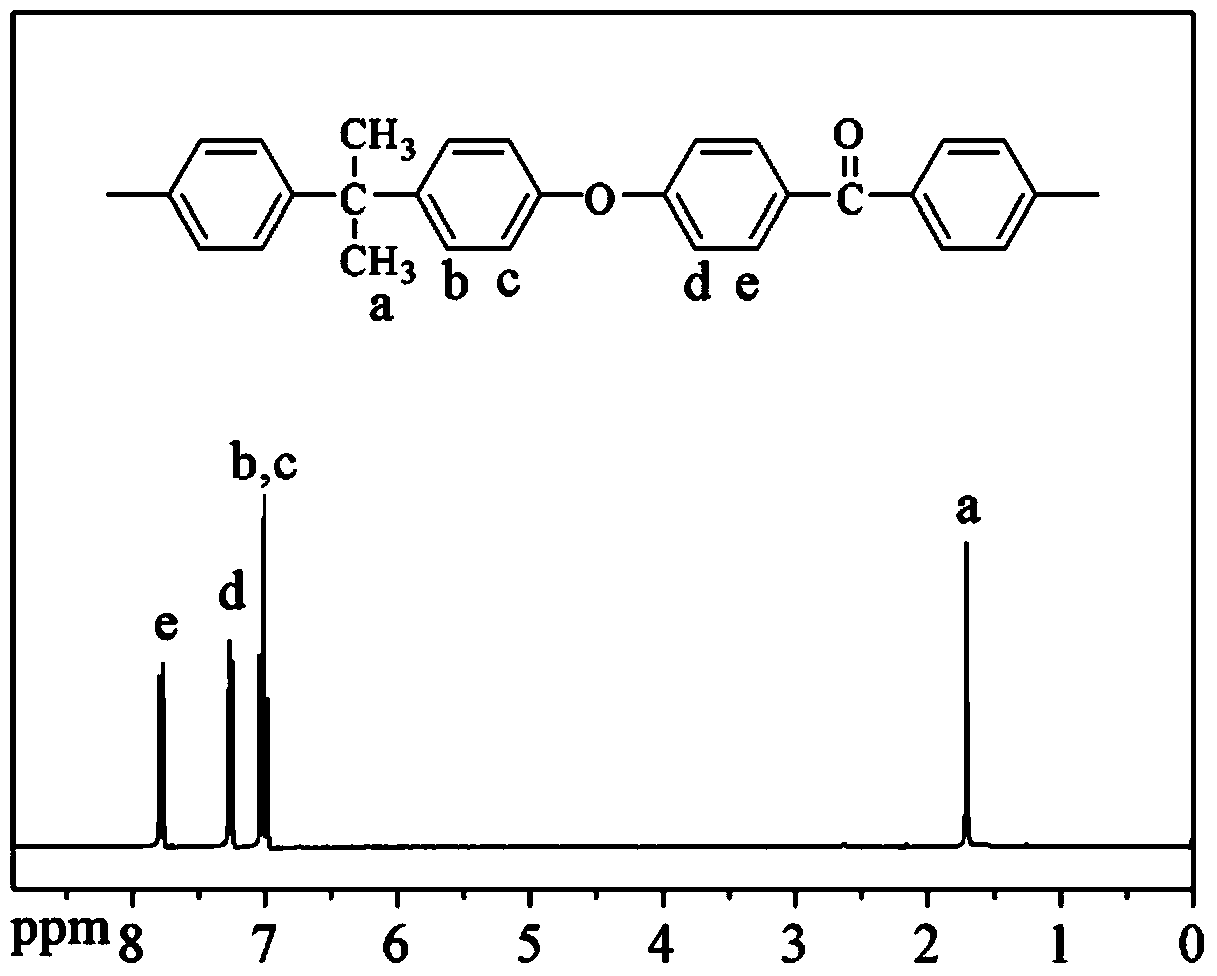
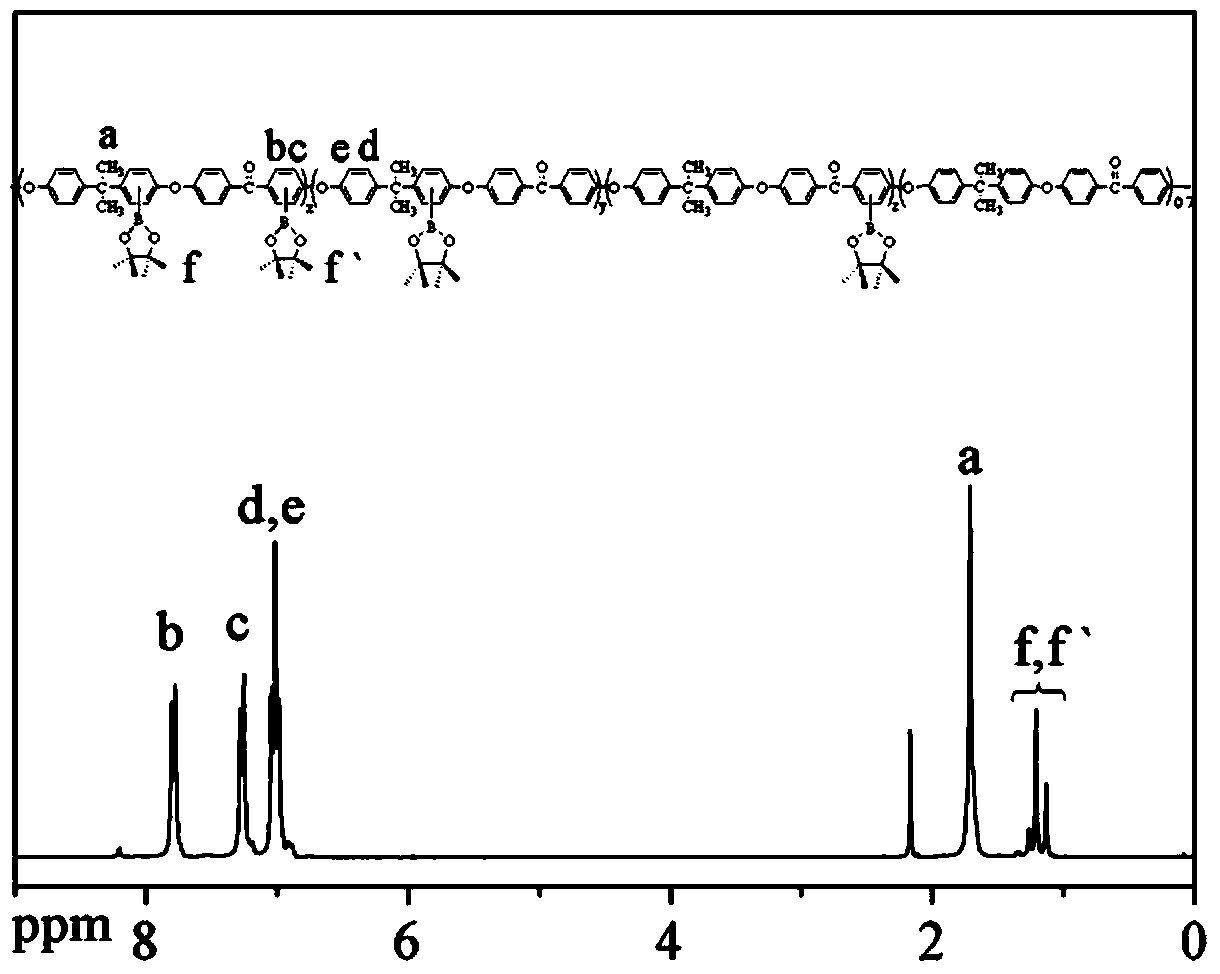
Polyaryletherketone containing boric acid ester, azo polyaryletherketone and preparation method thereof
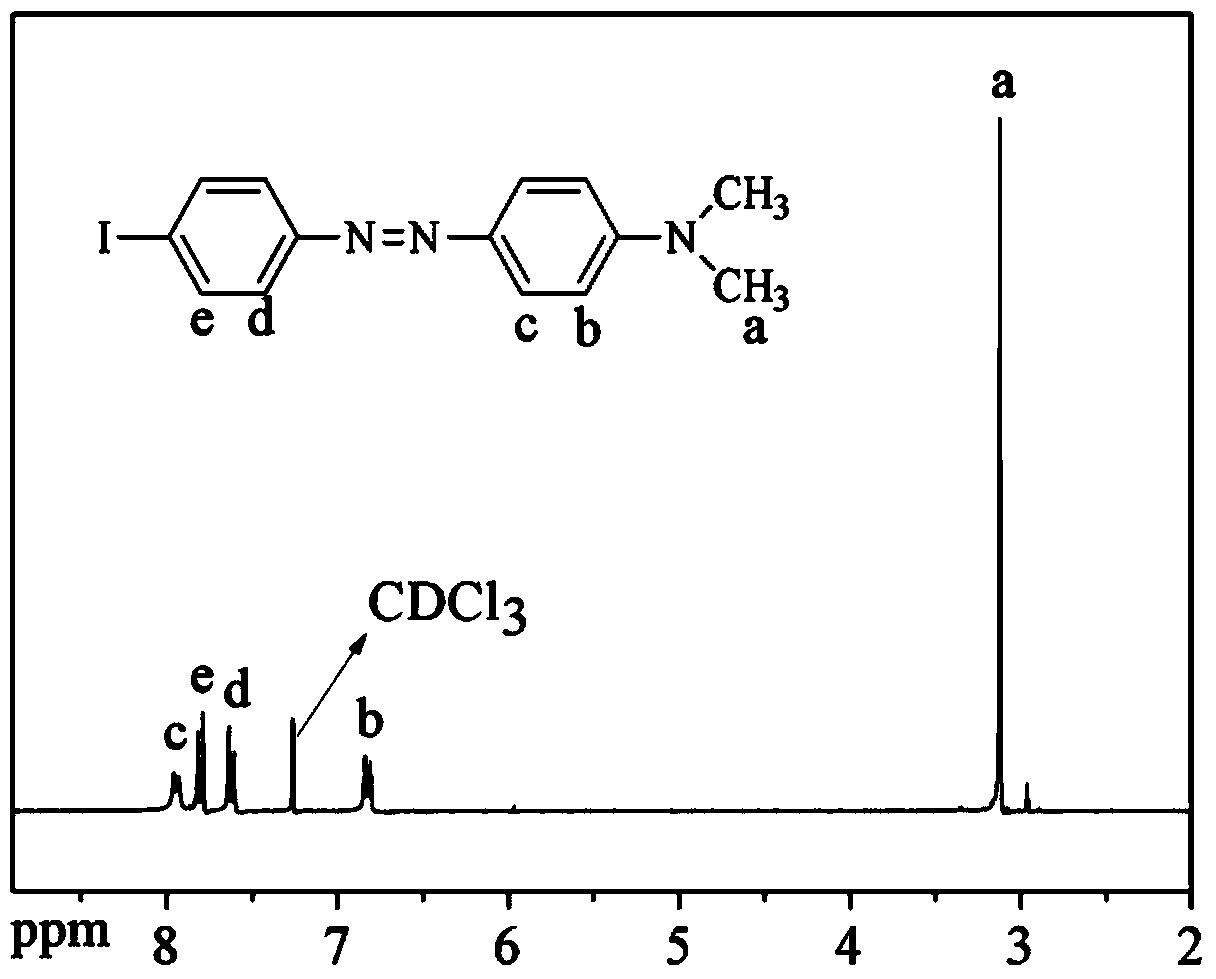
The invention discloses polyaryletherketone containing a boric acid ester, azo polyaryletherketone and a preparation method thereof, and belongs to the technical field of preparation of a high polymer material. The method comprises the following steps: firstly, synthetizing bisphenol A polyaryletherketone, and carrying out catalytic boriding reaction under a 1,5-cyclooctadiene chloride iridium dipolymer by using the polyaryletherketone and boron pinacol ester; secondly, synthetizing a 4-iodine-4'-(N,N-dimethyl amine) azobenzene monomer; finally, reacting with the 4-iodine-4'-(N,N-dimethyl amine) azobenzene monomer under catalysis of tetrakispalladium by using a polyaryletherketone material containing the boric acid ester, so as to prepare the azo polyaryletherketone. By adopting the method, an azo monomer can be successfully led to the polyaryletherketone, but the performance of the polyaryletherketone is not affected, biphenyl structure azo polyaryletherketone with stable performance also can be generated, and the storage stability of the azo material can be improved. Therefore, the method is expected to be applied in the aspect of preparation of a novel azo polymer material.
Owner:JILIN UNIV
Iridium oxide nanotubes and method for forming same
A method is provided for forming iridium oxide (IrOx) nanotubes. The method comprises: providing a substrate; introducing a (methylcyclopentadienyl)(1,5-cyclooctadiene)iridium(I) precursor; introducing oxygen as a precursor reaction gas; establishing a final pressure in the range of 1 to 50 Torr; establishing a substrate, or chamber temperature in the range of 200 to 500 degrees C.; and using a metalorganic chemical vapor deposition (MOCVD) process, growing IrOx hollow nanotubes from the substrate surface. Typically, the (methylcyclopentadienyl)(1,5-cyclooctadiene)iridium(I) precursor is initially heated in an ampule to a first temperature in the range of 60 to 90 degrees C., and the first temperature is maintained in the transport line introducing the precursor. The precursor may be mixed with an inert carrier gas such as Ar, or the oxygen precursor reaction gas may be used as the carrier.
Owner:SHARP KK
Method for producing L-2-amino-4-(hydroxymethylphosphinyl)-butanoic acid
InactiveUS7772426B2Efficient productionHigh enantiomeric excessOrganic compound preparationGroup 5/15 element organic compoundsHydrogen atomAsymmetric hydrogenation
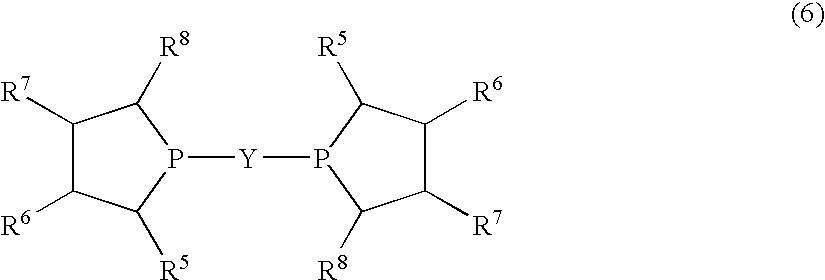
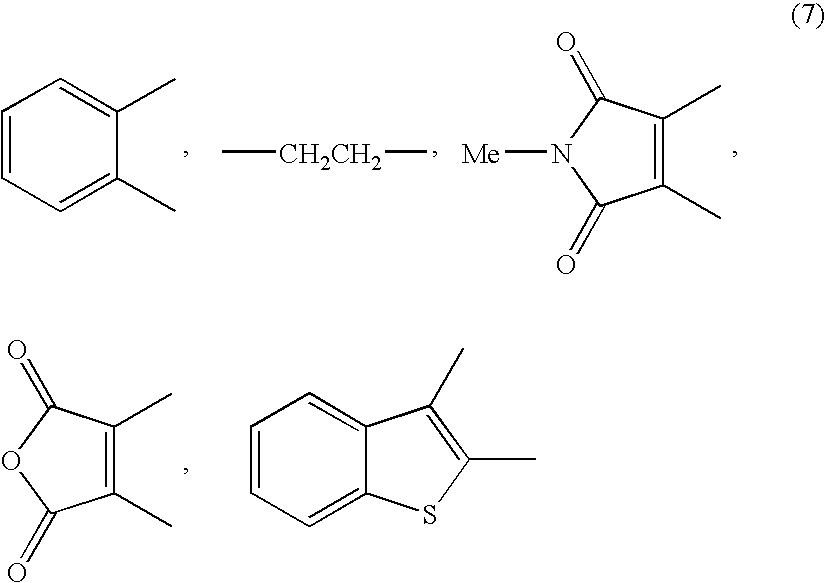
Disclosed is a method for efficiently and highly selectively producing L-2-amino-4-(hydroxymethylphosphinyl)-butanoic acid, which is useful as a herbicide, through a catalytic asymmetric synthesis reaction. Specifically disclosed is a method for producing L-2-amino-4-(hydroxymethylphosphinyl)-butanoic acid which is characterized in that a dehydroamino acid is subjected to an asymmetric hydrogenation by using a rhodium catalyst represented by the formula (2) below and having an optically active cyclic phosphine ligand, and then the resulting product is subjected to hydrolysis:[Rh(R4)(L)]X (2)[where R4 represents 1,5-cyclooctadien or norbornadien; L represents a substance represented by the following formula (6):(wherein R5 and R8 respectively represent a C1-4 alkyl group; R6 and R7 respectively represent hydrogen atom or hydroxyl group; and Y represents a group selected from groups represented by the following formula (7):(where Me represents methyl group)).].
Owner:MEIJI SEIKA KAISHA LTD
Method for preparing nitrogen, phosphor and fluorine codoping carbon-based mixed capacitor material
ActiveCN106992076AWith electric double layer capacitance performanceWith pseudocapacitive propertiesHybrid capacitor electrodesHybrid/EDL manufactureCapacitancePorous carbon
The invention relates to a method for preparing a nitrogen, phosphor and fluorine codoping carbon-based mixed capacitor material and belongs to a field of doping type porous carbon-based capacitor material technology. The preparation method is performed in an anhydrous and oxygen-free inert environment, a catalyst and 2, 2'-dipyridyl are added in N,N'-dimethylformamide, and 1,5-cyclooctadiene is added in the N,N'-dimethylformamide, and heating to dissolve is performed; nitrogen source and fluorine source are added in the solution, and after the heating reaction finished, reactants are taken out; concentrated hydrochloric acid is dropwise added in an obtained material, and a colloidal solution doped with flocculent precipitate is obtained; the material is filtered and cleaned to obtain a material containing N and F, the material containing N and F is transferred to a porcelain boat, and high temperature carbonization is performed on the material containing N and F at a temperature range of 500-1000 DEG C; and high temperature phosphorus doping is performed on the carbonized material containing N and F and a P source. According to the invention, the synthesis condition is mild, the experiment operation is simple, the specific capacitance of the material reaches up to 250Fg<-1>, and problems that the carbon-based supercapacitor has low energy density, and faradaic pseudocapacitance material has poor cycling stability are solved.
Owner:BEIJING UNIV OF CHEM TECH
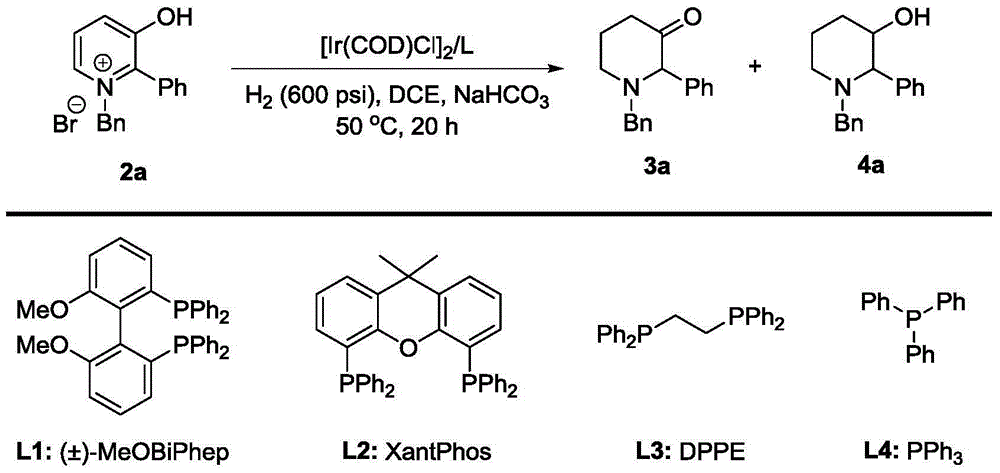
Method for synthesis of 3-piperidone derivatives through iridium catalytic hydrogenation
A method for synthesis of 3-piperidone derivatives through iridium catalytic hydrogenation is provided, wherein a used catalyst is a triphenylphosphine complex of iridium. A reaction can be carried out under the following conditions: the temperature is 40-60 DEG C; the solvent is 1,2-dichloroethane; the pressure is 20-50 atmospheric pressures; the ratio of a substrate to a catalyst is 100 to 1; and the catalyst is a complex of chloro(1,5-cyclooctadiene)iridium(I) dimer and triphenylphosphine. Hydrogenation of 3-hydroxypyridine benzyl bromide salt can obtain the 3-piperidone derivatives with excellent chemoselectivity, the highest yield can reach 97%, and the chemical selectivity of ketones and alcohols is greater than 20:1. The method has the advantages of simple and convenient operation, easily obtained raw materials, high chemoselectivity, and good yield, and provides an atom economic and environment-friendly route for synthesis of a series of 3-piperidone derivatives.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Preparation method of rhodium compounds
InactiveCN105585596AEasy to operateHigh purityRhodium organic compoundsOrganic-compounds/hydrides/coordination-complexes catalystsTetrafluoroborateDimmer
The invention discloses a preparation method of rhodium compounds. According to the method, bis(1.5-cyclooctadiene) rhodium(I) tetrafluoroborate compounds are synthesized in two steps, firstly, 1.5-cyclooctadiene rhodium chloride dimmers are synthesized with rhodium chloride trihydrate as raw materials; secondly, the bis(1.5-cyclooctadiene) rhodium(I) tetrafluoroborate compounds are synthesized with the 1.5-cyclooctadiene rhodium chloride dimmers as raw materials. The one-way total yield is 93% or above, operation is easy, industrial production can be achieved, and certain economic benefits are achieved.
Owner:JIANGXI HANS PRECIOUS METALS CO LTD
Method for co-production of urea and methanol by treating high-concentration ammonia nitrogen and 1, 5-cyclooctadiene (COD) sewage
ActiveCN102642876ANo secondary pollutionComprehensive utilization of circular economyUrea derivatives preparationHydrogen separationHigh concentrationExisting Treatment
The invention relates to a method for co-production of urea and methanol by treating high-concentration ammonia nitrogen and 1, 5-cyclooctadiene (COD) sewage. The existing treatment technique has the weaknesses of large investment expense, high operation cost and the like. The method comprises the steps of: firstly placing the high-concentration ammonia nitrogen, COD sewage, fine coal and coal slurry additive into a coal mill to be collectively ground so as to produce multi-element coal slurry with alkalescence concentration; and then mixing and atomizing the multi-element coal slurry with oxygen in a burner to be filled into a coal slurry gasification furnace and to be split, converted and oxidized to generate water gas; converting ammonia nitrogen and COD into carbon oxide (CO), carbon dioxide (CO2), hydrogen (H2) and nitrogen (N2), and washing, cooling and dedusting the water gas with washing water; and finally converting the water gas into H2 and CO2 through a total low temperature shift process, and separating the converted gas into H2, N2 and CO2 through a purification technique, and synthesizing H2 and N2 into ammonia and synthesizing ammonia and CO2 into urea. According tothe method, harmful substances are converted into useful chemical fertilizer raw material gas, the yield is increased without increasing the consumption, and no secondary pollution is caused.
Owner:ZHEJIANG JINJU CHEM
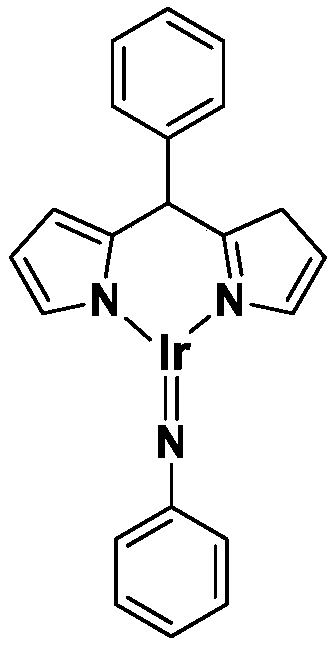
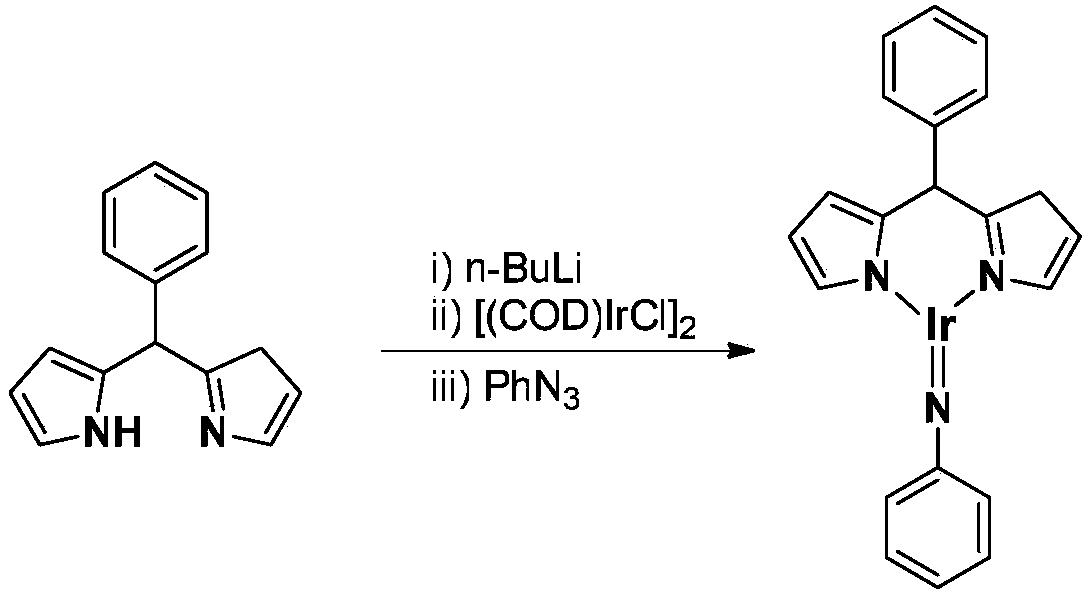
Trivalent iridium imine complex containing iridium nitrogen double bonds, preparation method and application of trivalent iridium imine complex
ActiveCN109293706AEasy to prepareHigh yieldIndium organic compoundsOrganic compound preparationMarkovnikov's ruleDouble bond
The invention belongs to the technical field of synthetic chemistry, and in particular relates to a trivalent iridium imine complex containing iridium nitrogen double bonds, a preparation method and an application of the trivalent iridium imine complex. A binuclear iridium compound cyclooctadiene iridium chloride dimer [ (COD) IrCl ]2 is taken as a raw material, and the binuclear iridium compoundiridium(I) cyclooctadiene chloride, dimer [ (COD) IrCl ]2 reacts with phenyl dipyrrole compounds under an alkaline condition to obtain a precursor containing a monovalent iridium complex, and the precursor is oxidized into the trivalent iridium imine compound containing the iridium nitrogen double bonds by using an azide oxidation method. The synthesis process is simple and green, and has excellent selectivity and higher yield. The trivalent iridium imine complex has the characteristics of stable physical and chemical properties, thermal stability and the like, and shows excellent activity andregioselectivity (anti-Markovnikov's rule) in hydroamination of olefins.
Owner:SHANGHAI INST OF TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com