Pyrazolopyrimidines and their use for the treatment of CNS disorders
A kind of alkyl, the technology selected from, is applied in the field of preparing these compounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
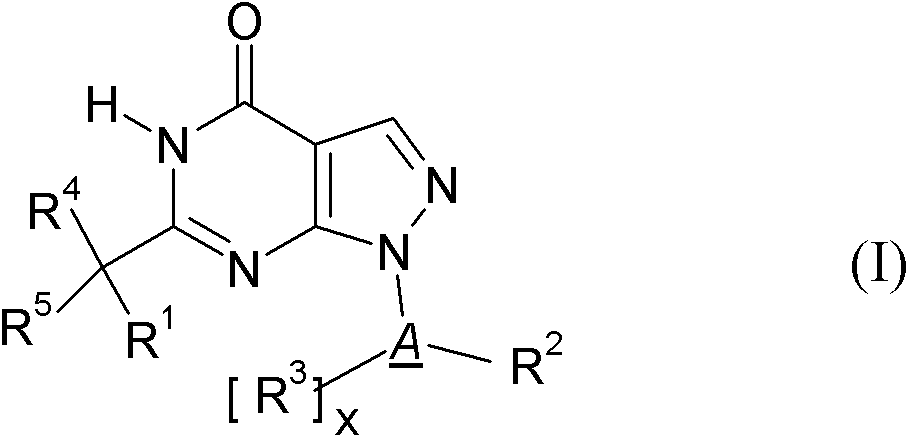
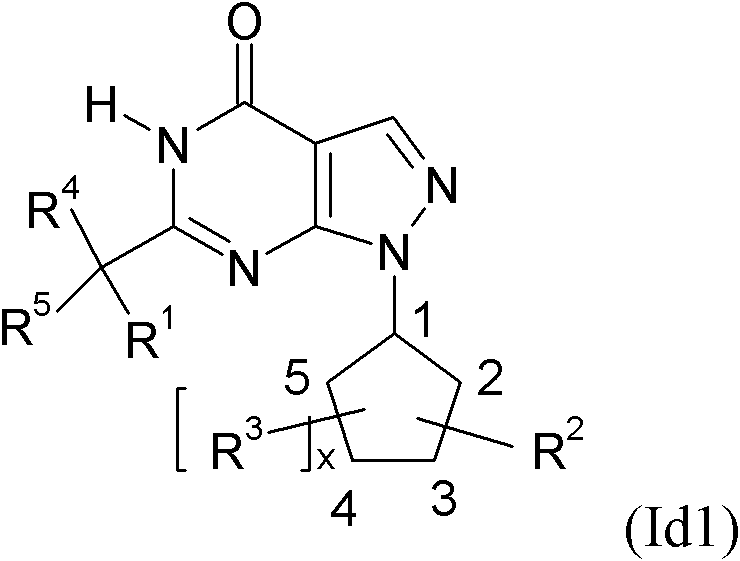
[0031] R 1.1 R 1 is a substituent selected from the following:
[0032] C 1-8 Alkyl-, C 2-8 Alkenyl-, C 2-8 Alkynyl-, R 10 -S-C 1-3 Alkyl-, R 10 -O-C 1-3 Alkyl-, C 3-7 Cycloalkyl-, C 3-7 Cycloalkyl-C 1-6 Alkyl-, C 3-7 Cycloalkyl-C 2-6 Alkenyl-, C 3-7 Cycloalkyl-C 2-6 Alkynyl-, C 3-8 Heterocycloalkyl-, C 3-8 Heterocycloalkyl-C 1-6 Alkyl-, C 3-8 Heterocycloalkyl-C 2-6 Alkenyl-, C 3-8 Heterocycloalkyl-C 2-6 Alkynyl-, aryl, aryl-C 1-6 Alkyl-, aryl-C 2-6 alkenyl-, aryl-C 2-6 Alkynyl-, Heteroaryl, Heteroaryl-C 1-6 Alkyl-, Heteroaryl-C 2-6 alkenyl- and heteroaryl-C 2-6 Alkynyl-, wherein the above-mentioned groups are optionally independently selected from each other by one or more groups selected from the group R 1.1.S1 The substituent is substituted, the group R 1.1.S1 From fluorine, chlorine, bromine, iodine, oxo (oxo) (wherein the oxo group is preferably only the substituent of cycloalkyl or heterocycloalkyl), HO-, NC-, O 2 N-, F 3 C-, HF 2 C-, FH 2...
Embodiment
[0359] pharmaceutical composition
[0360] The following examples present pharmaceutical preparations which illustrate the invention but do not limit the scope of the invention:
[0361] The term "active substance" means one or more compounds of the invention (including salts thereof).
Embodiment A
[0363] Tablets containing 100 mg of active substance
[0364] composition:
[0365] One tablet contains:
[0366] Active substance 100.0mg
[0367] Lactose 80.0mg
[0368] Corn starch 34.0mg
[0369] Polyvinylpyrrolidone 4.0mg
[0370] Magnesium stearate 2.0mg
[0371] 220.0 mg
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com