Adhesive monomer and method for improving adhesion strength between dental zirconia and resin
A kind of cohesive, monomeric technology, applied in the direction of dentistry, dental preparations, dental prosthesis, etc., can solve the problem of high price of functional monomers, and achieve the effect of improving bonding strength and good biocompatibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] The synthetic steps of adhesive monomer H are as follows:
[0053]
[0054] Step (a):
[0055] A chloroform solution containing 6.91 g (0.04 mol) of m-chloroperoxybenzoic acid was added to a chloroform solution containing 6.14 g (0.05 mol) of 1,5-cyclooctadiene A, and stirred at room temperature for 18 hours. After the reaction, wash with aqueous sodium bisulfite, then with aqueous sodium bicarbonate, and finally with distilled water until neutral. The solvent was removed from the organic layer to obtain an oily liquid, and the oily liquid product B was obtained by column chromatography.
[0056] Step (b):
[0057] Dissolve 5g (0.04mol) of product B in a mixture of 100mL 1,4-dioxane and 75mL of water, add 11.4g (0.05mol) of periodic acid, and stir at room temperature for two hours. After the reaction was finished, it was washed with water, and then extracted with dichloromethane, and the solvent was removed from the organic layer to obtain an oily product C.
[0...
Embodiment 2
[0069] The synthesis steps of adhesive monomer H1 are as follows:
[0070]
[0071] Step (a) to step (e): Same as Example 1.
[0072] Step (f):
[0073] The product F (2.0g, 0.007mol) and S 8 O (7.6 g, 0.028 mol) dissolved in 10 mL of CS 2 In the dark, stir and reflux for two hours. After the reaction, the solvent was removed by rotary evaporation, and the yellow oily product G1 was obtained by separation by column chromatography.
[0074] Step (g):
[0075] Dissolve the product G1 (0.005mol) in 10 mL of ether, add LiAlH to the ether mixture in batches under ice-cooling 4 (0.020mol), stirred for two hours, and then sequentially added ammonium chloride aqueous solution, ether extraction. The organic phase was evaporated to remove the solvent, and the yellow oily product H1 was obtained by separation by column chromatography.
Embodiment 3
[0077] The synthetic steps of binding monomer H2 are as follows:
[0078]
[0079] Step (a) to step (e): Same as Example 1.
[0080] Step (f):
[0081] Under the protection of nitrogen, add activated zinc powder (0.52g, 0.008mol), product F (2.0g, 0.007mol) and 20mL of anhydrous ether to the three-necked flask with reflux stirring successively, and then add dropwise to the reaction system 10mL dissolved in Cl 3 CCOCl (1.5g, 0.008mol) and POCl 3 (1.22g, 0.008mol) in anhydrous ether solution, refluxed and stirred for two hours, and filtered to remove the solid to obtain the ether solution of product G2.
[0082] Step (g):
[0083] Synthesis of H2: Add NaHCO to the ether solution of product G2 3 aqueous solution, stirred, and extracted with ether. The organic phase was evaporated to remove the solvent, and the yellow oily product H2 was obtained by separation by column chromatography.
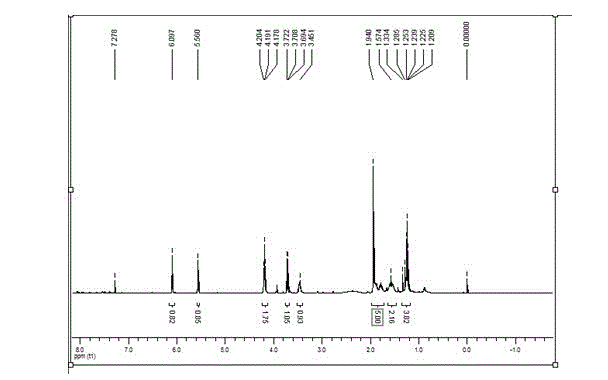
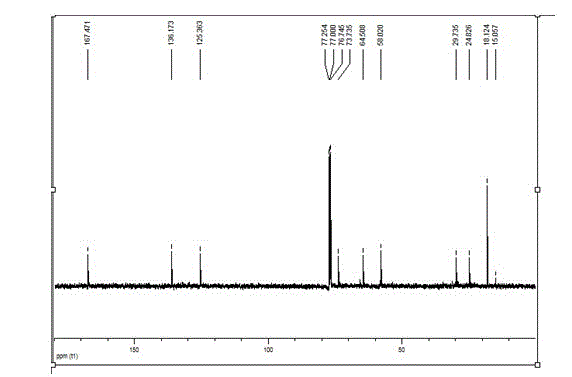
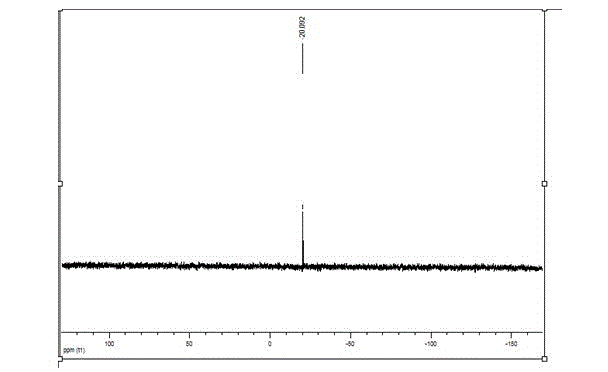
[0084] Taking the adhesive monomer H as an example, the structure and performance ar...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com