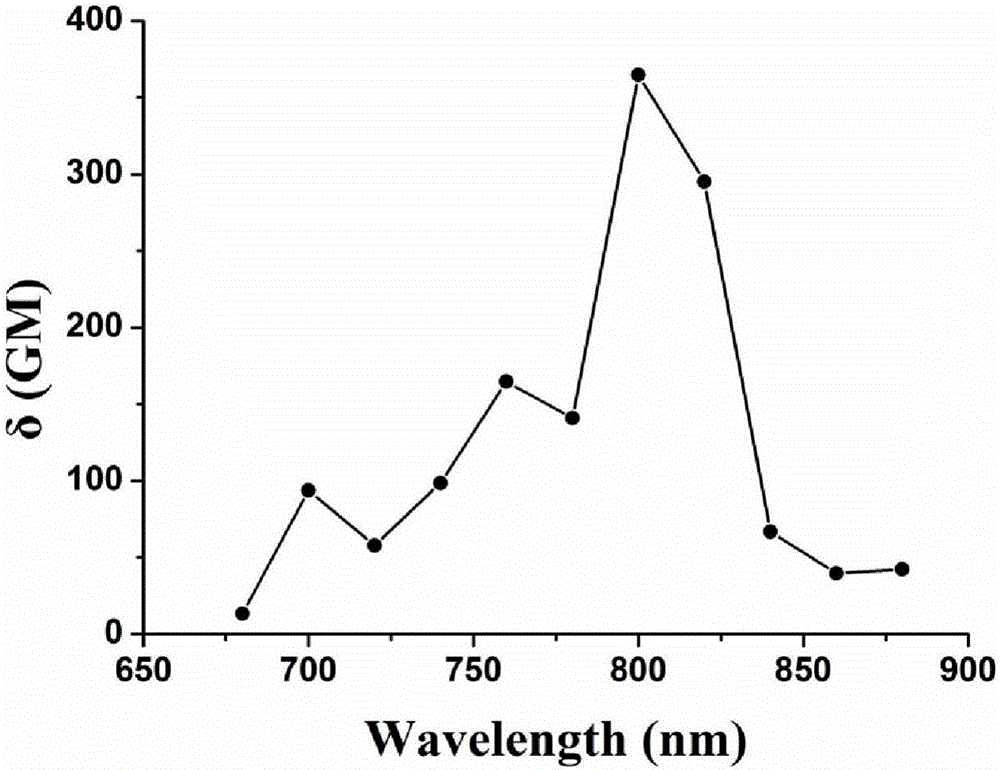
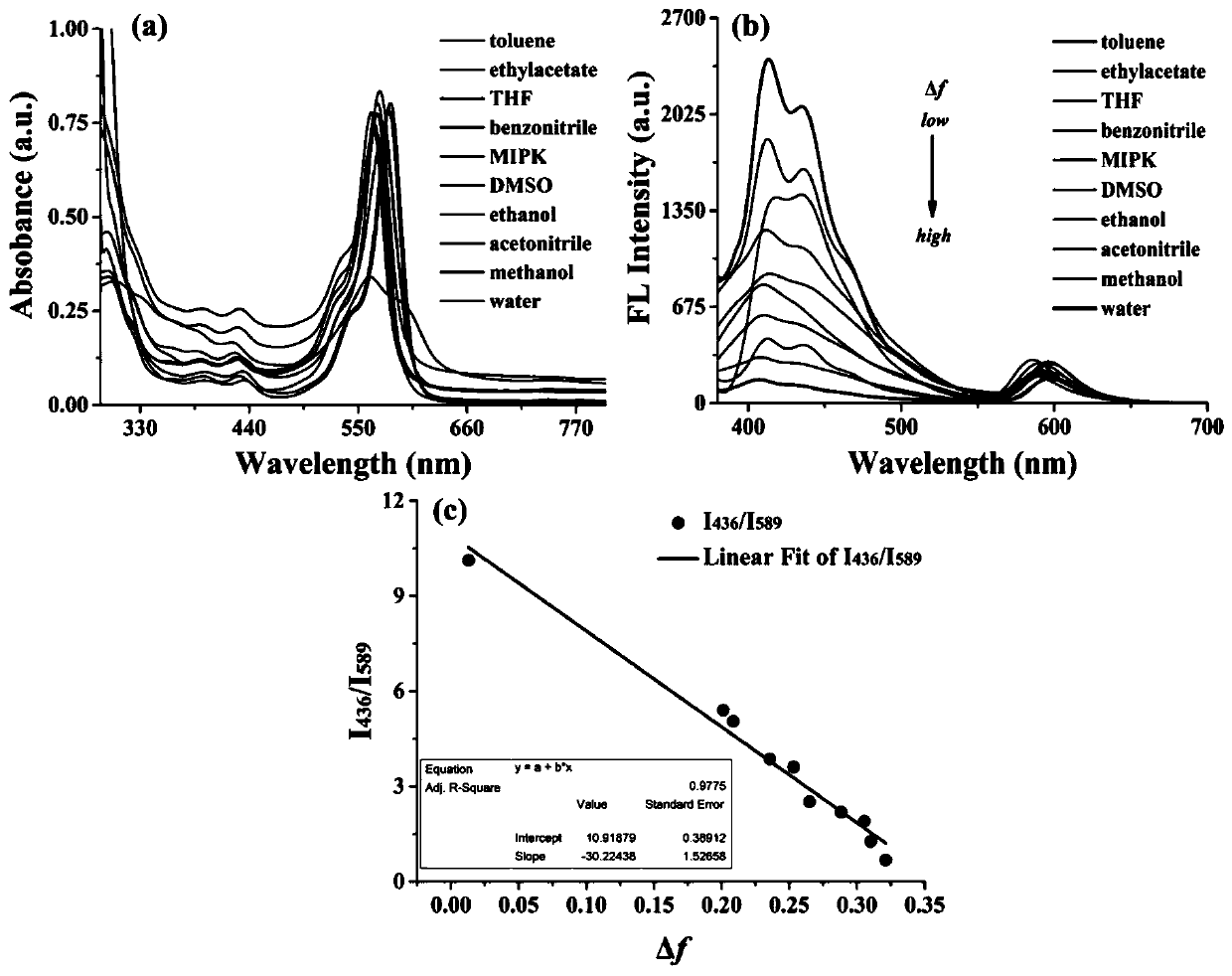
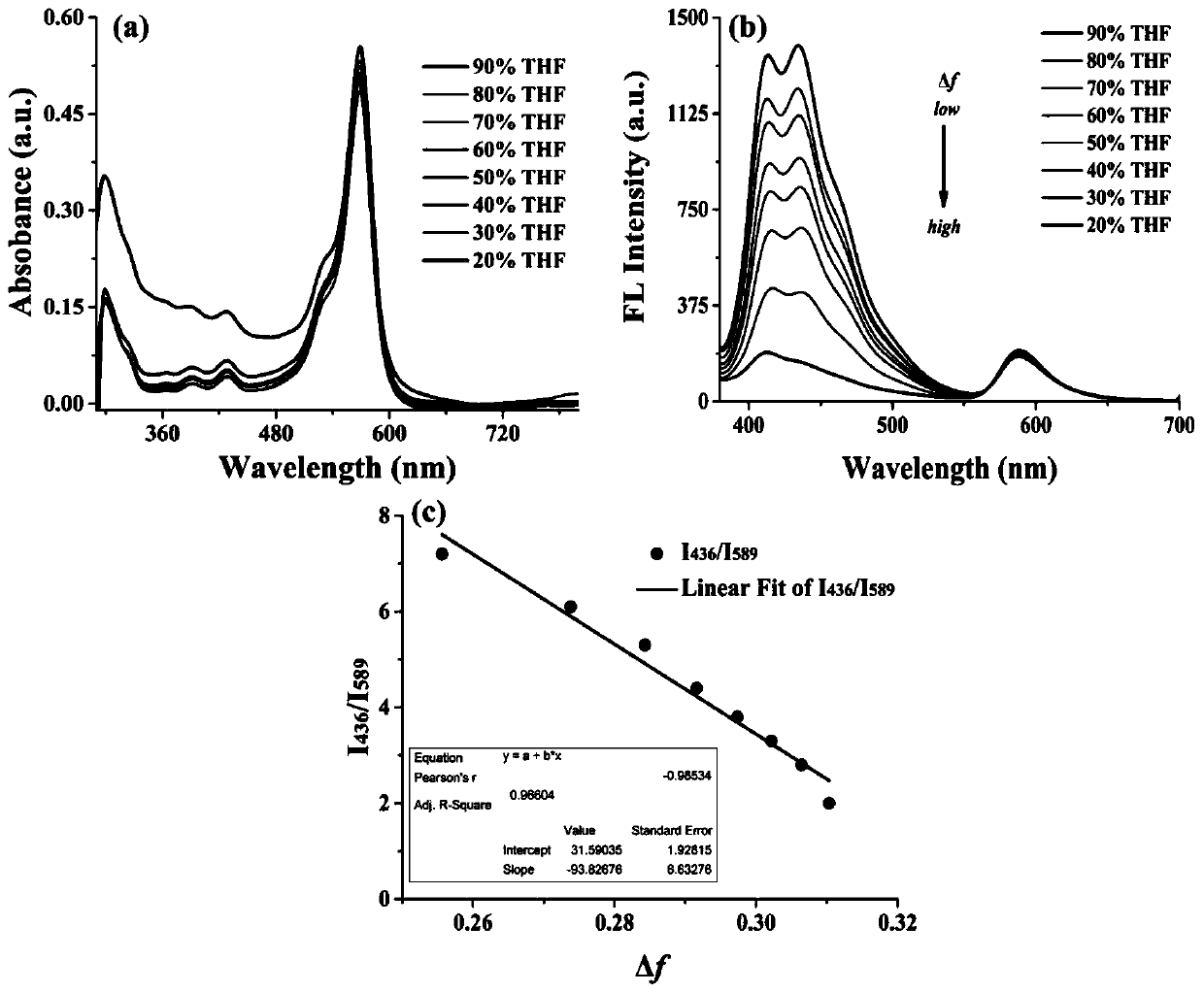
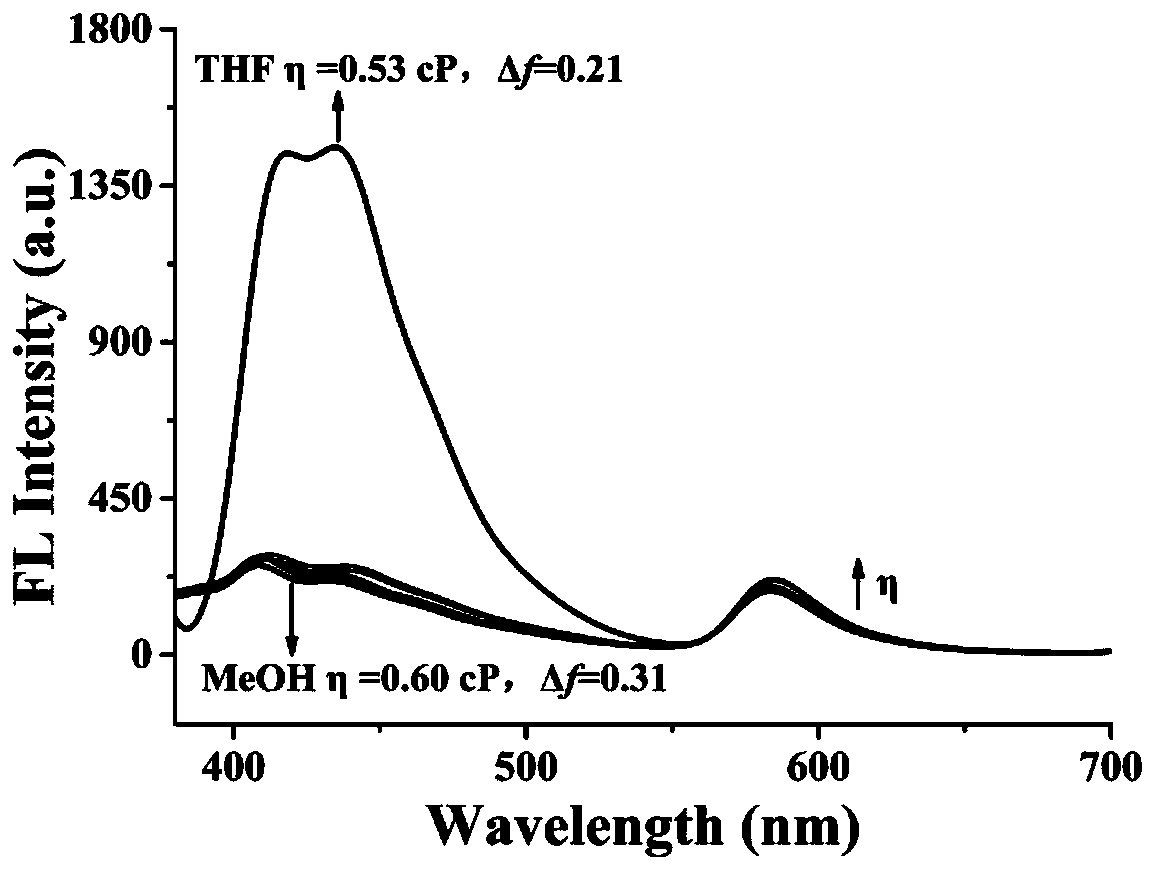
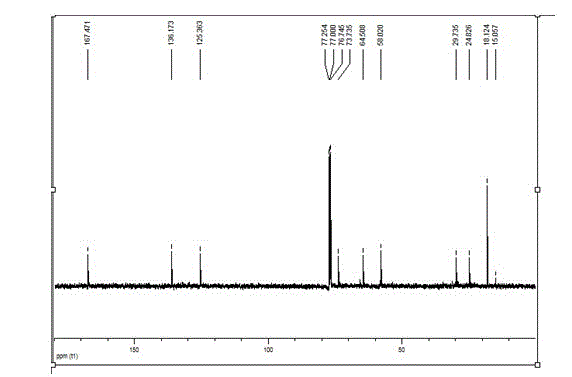
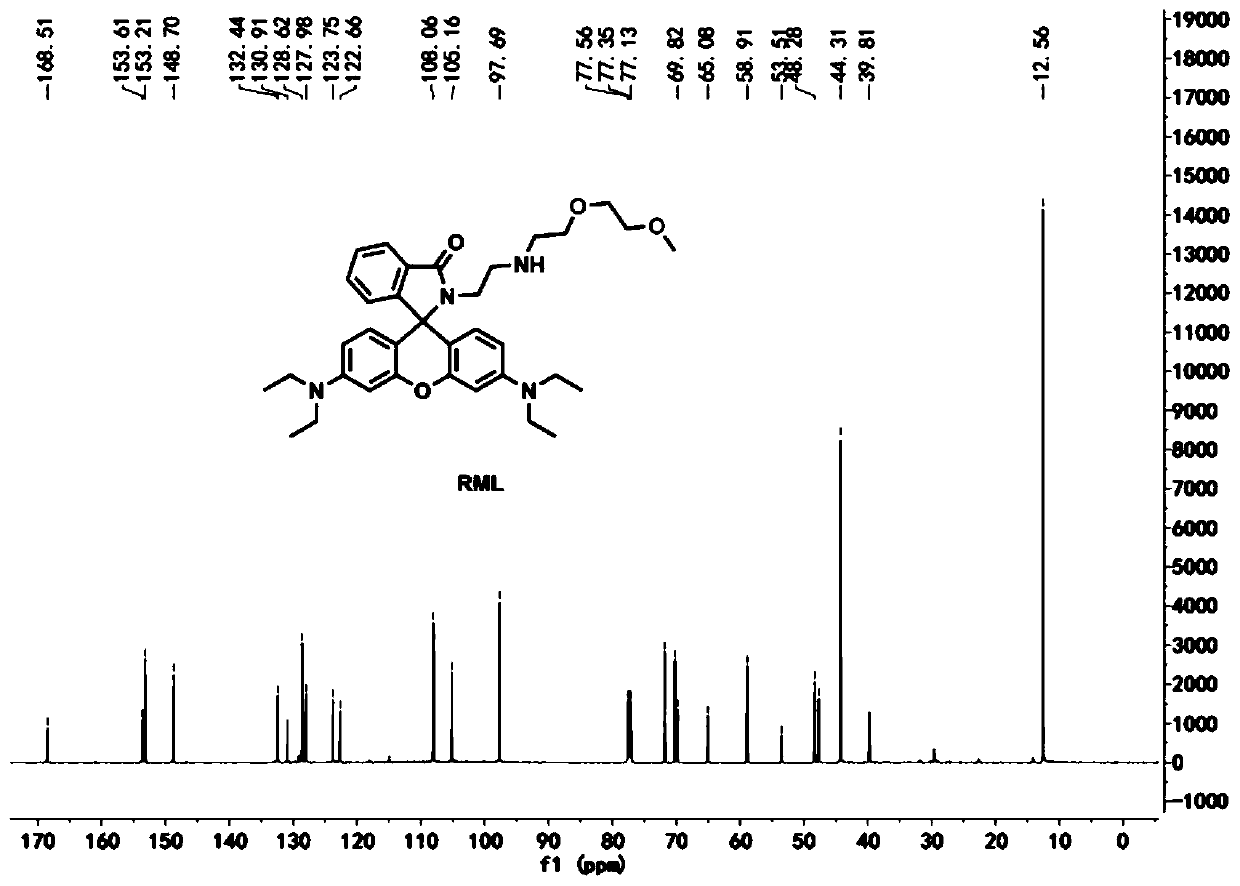
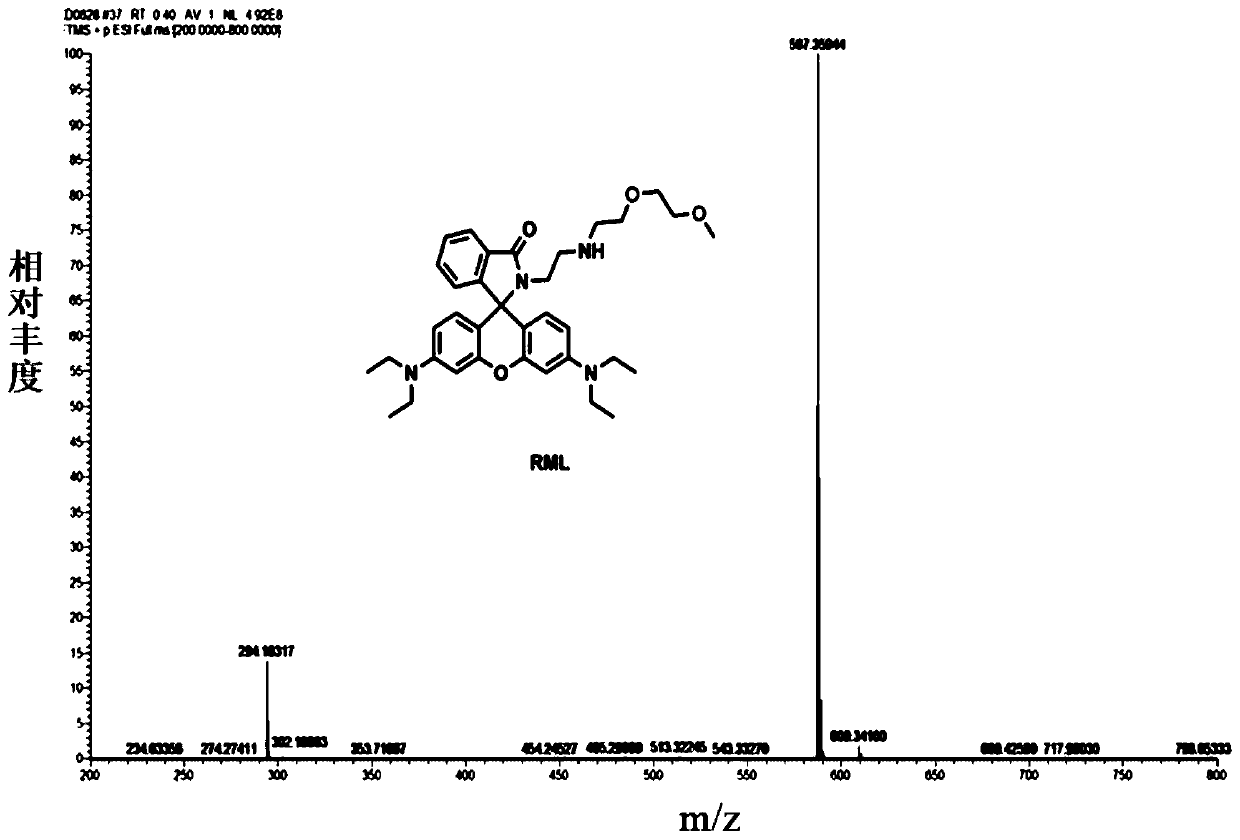
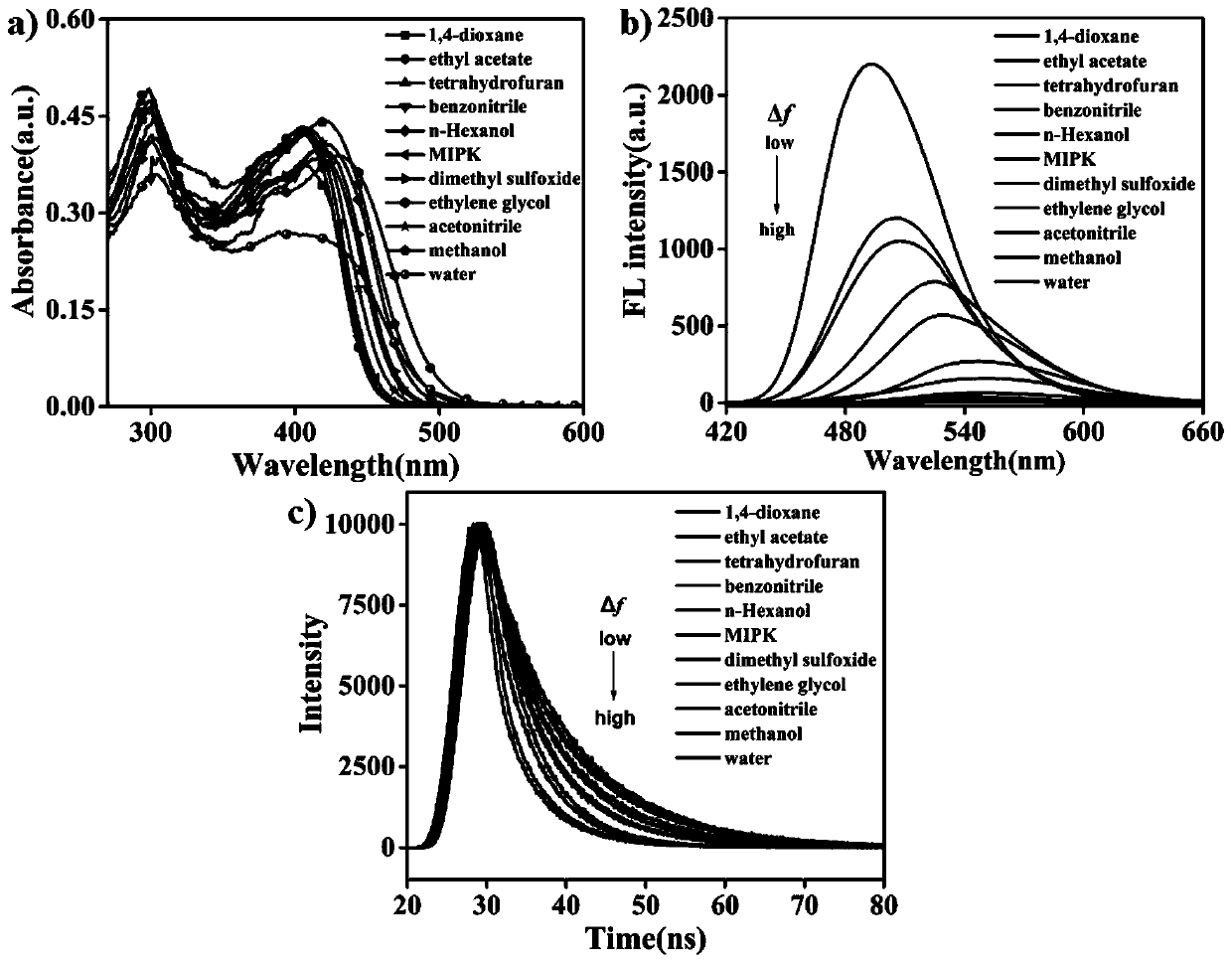
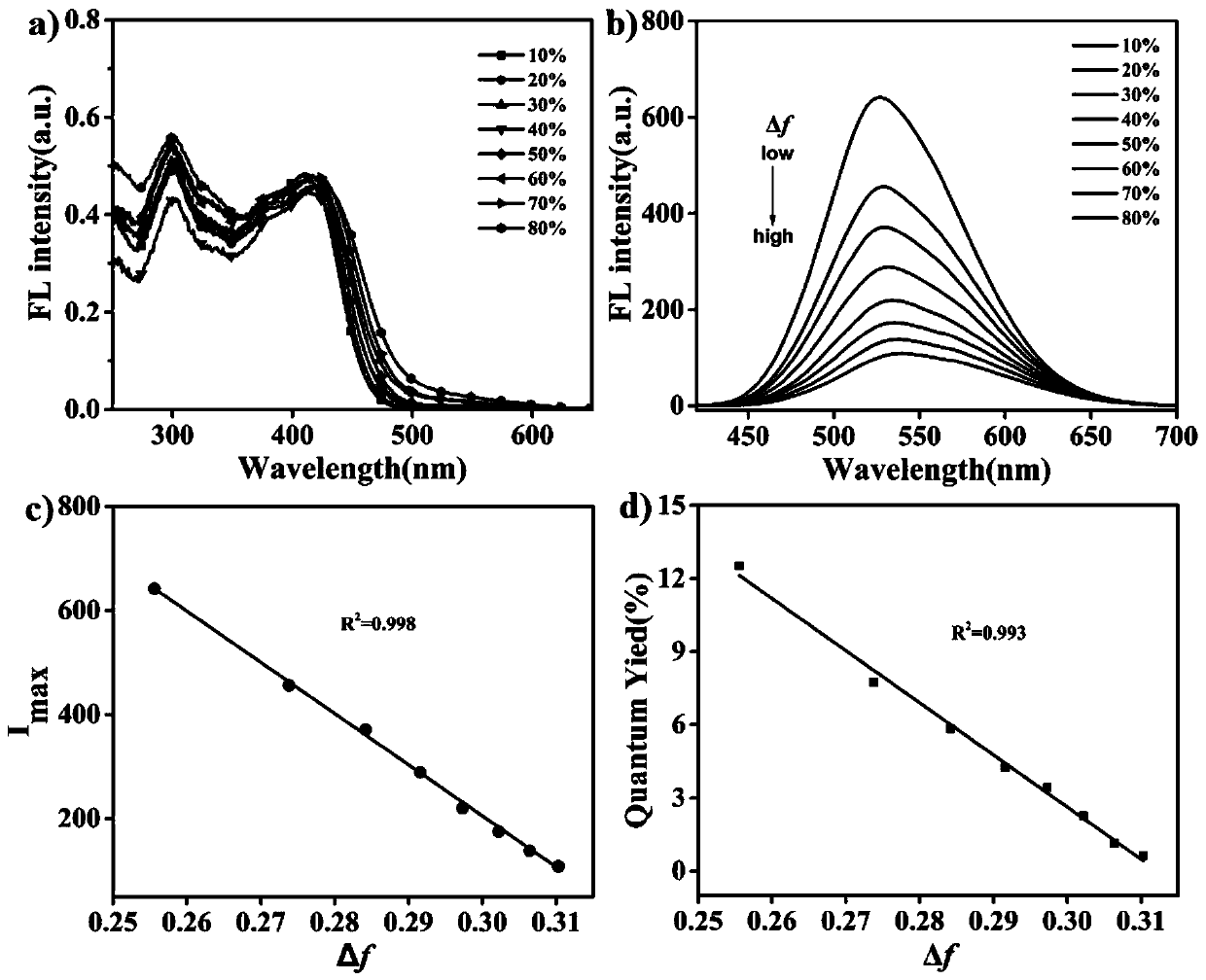
Patents
Literature
70 results about "Cytotoxicity testing" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Cytotoxicity tests are designed to determine the toxicity to cells of compounds either qualitatively or quantitatively. Qualitative cytotoxicity tests offered by Pacific BioLabs are the Direct Contact Test, the Indirect Contact Agar Diffusion Test, and the MEM Elution Test.
Two-photon fluorescent probe as well as preparation method and application thereof
InactiveCN103614135ALow fluorescence quantum yieldSimple structureOrganic chemistryFluorescence/phosphorescenceFluorescenceCytotoxicity testing
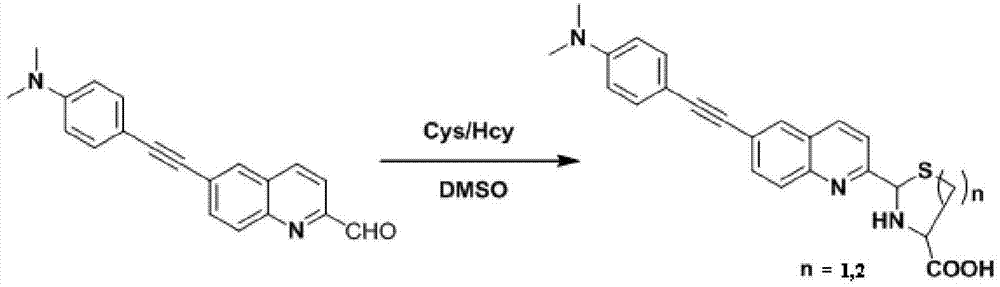
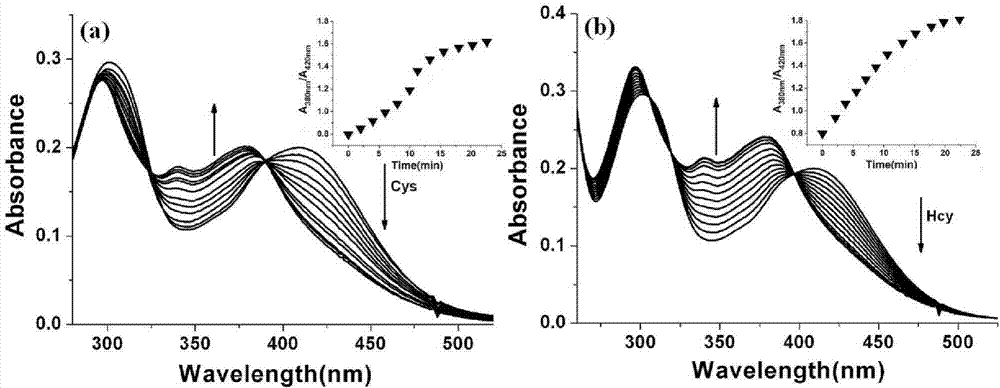
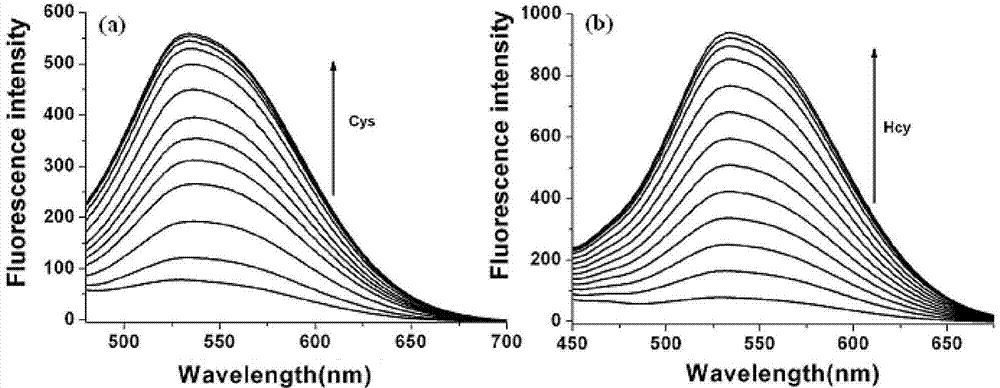
The invention discloses a two-photon fluorescent probe as well as a preparation method and an application thereof. The structure of the two-photon fluorescent probe is as shown in the specification. The two-photon fluorescent probe presents relatively high selectivity and high sensitivity in a coexisting system of aminothiopropionic acid or homocysteine and other amino acids. The cytotoxicity test shows that the two-photon fluorescent probe almost has no toxicity on cells. The two-photon fluorescent microscopic imaging experiment shows that the two-photon fluorescent probe is good in permeability on 293FT cells. The two-photon fluorescent probe is suitable for detecting the distribution of amino acid micromolecules in the cells.
Owner:ANHUI UNIVERSITY
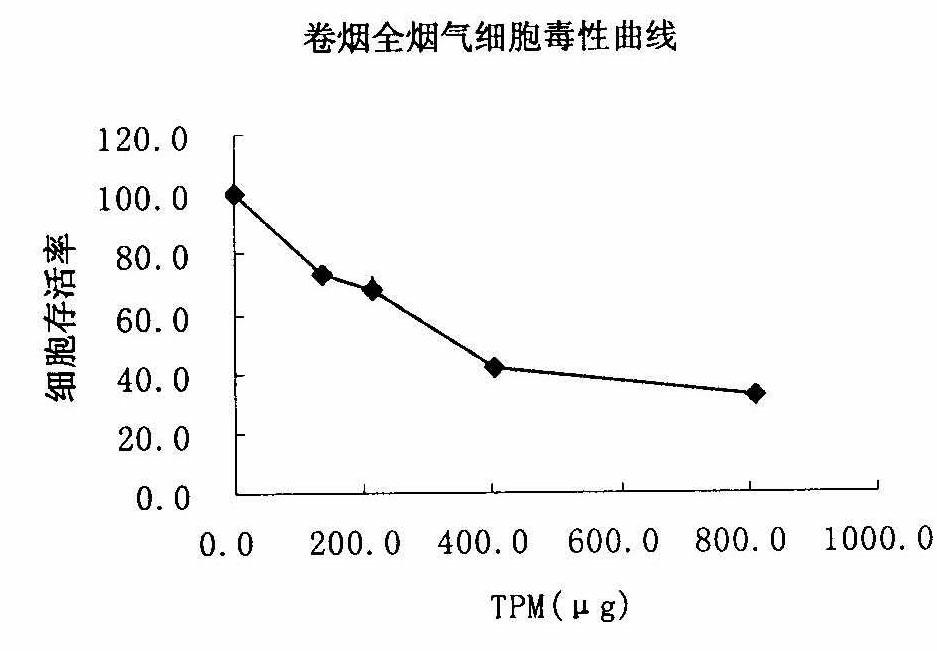
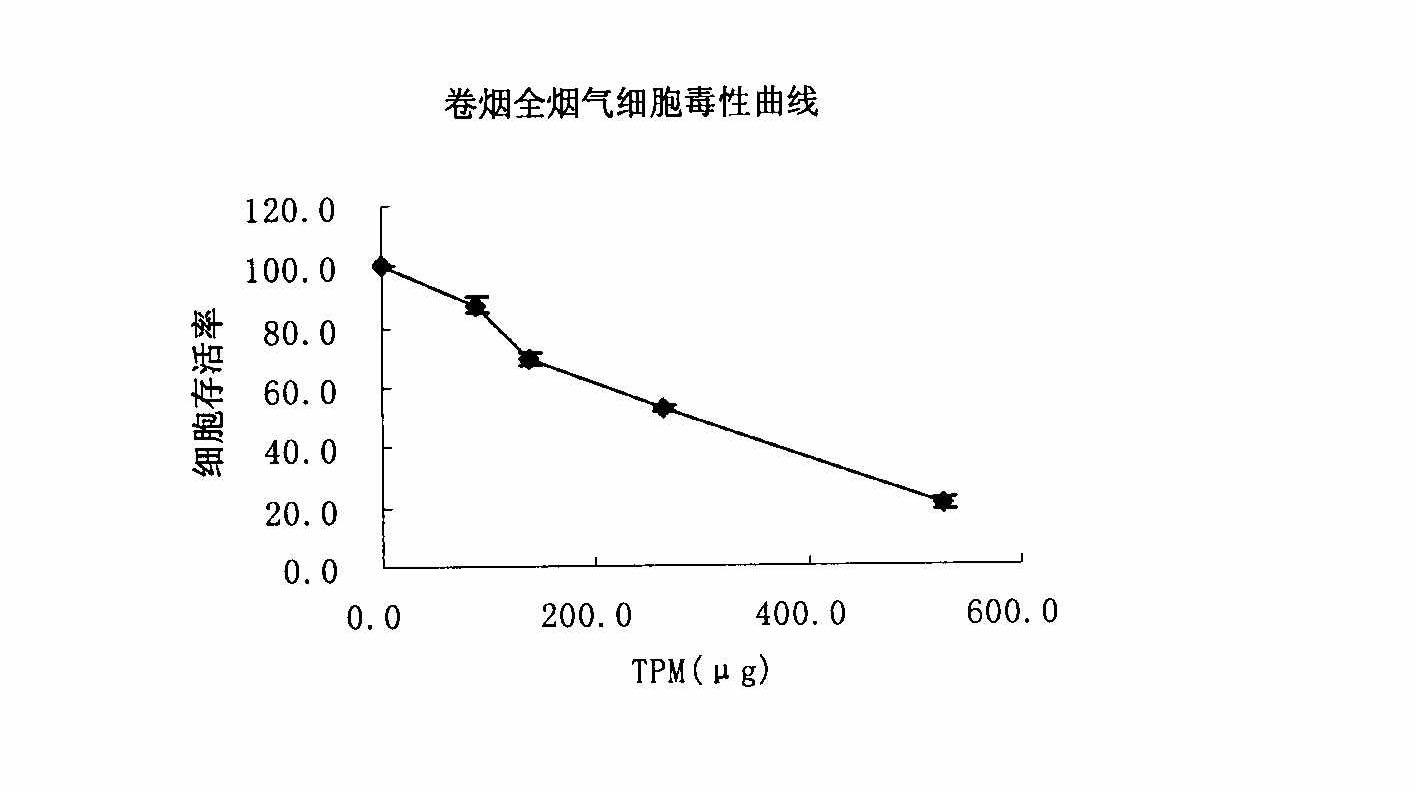
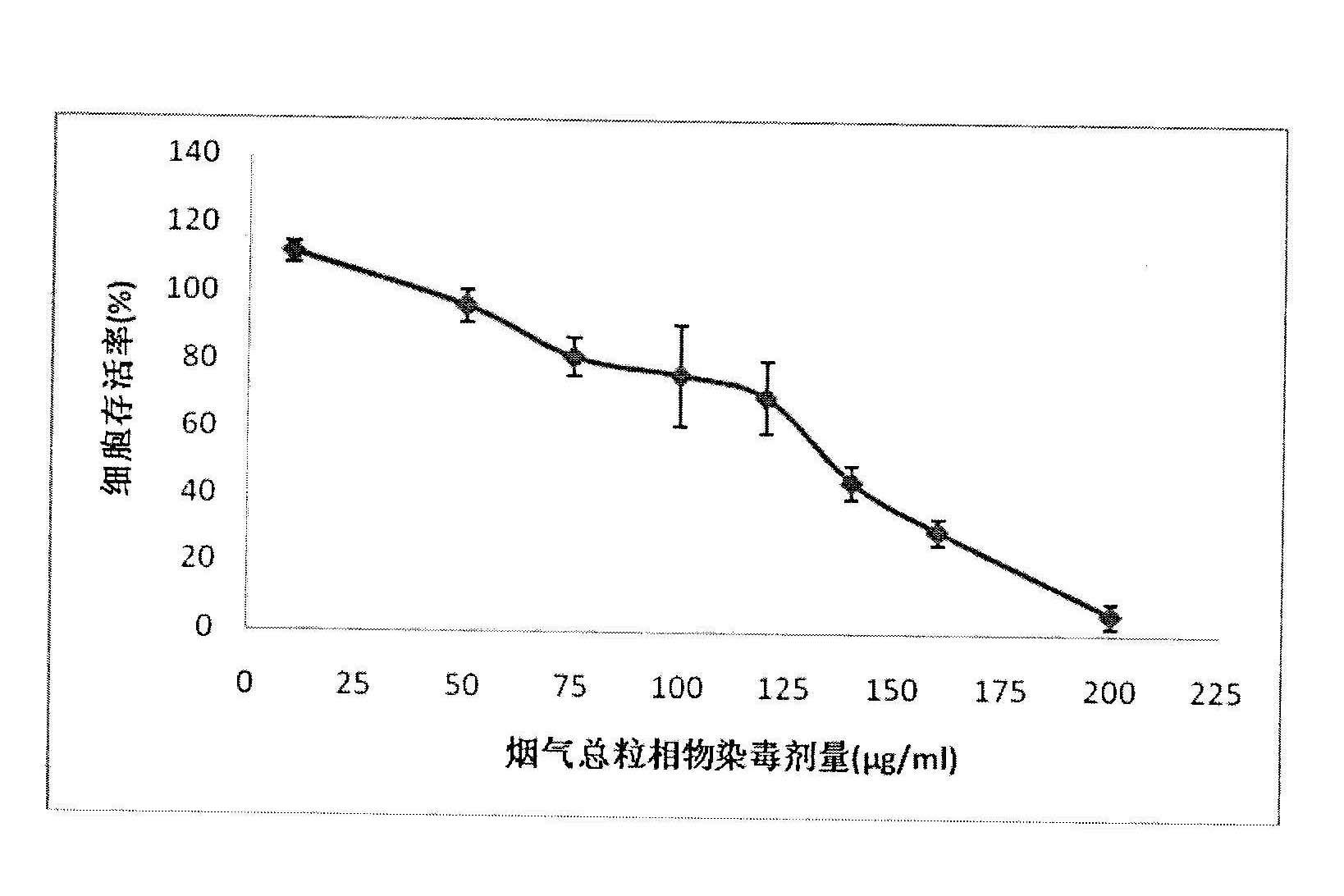
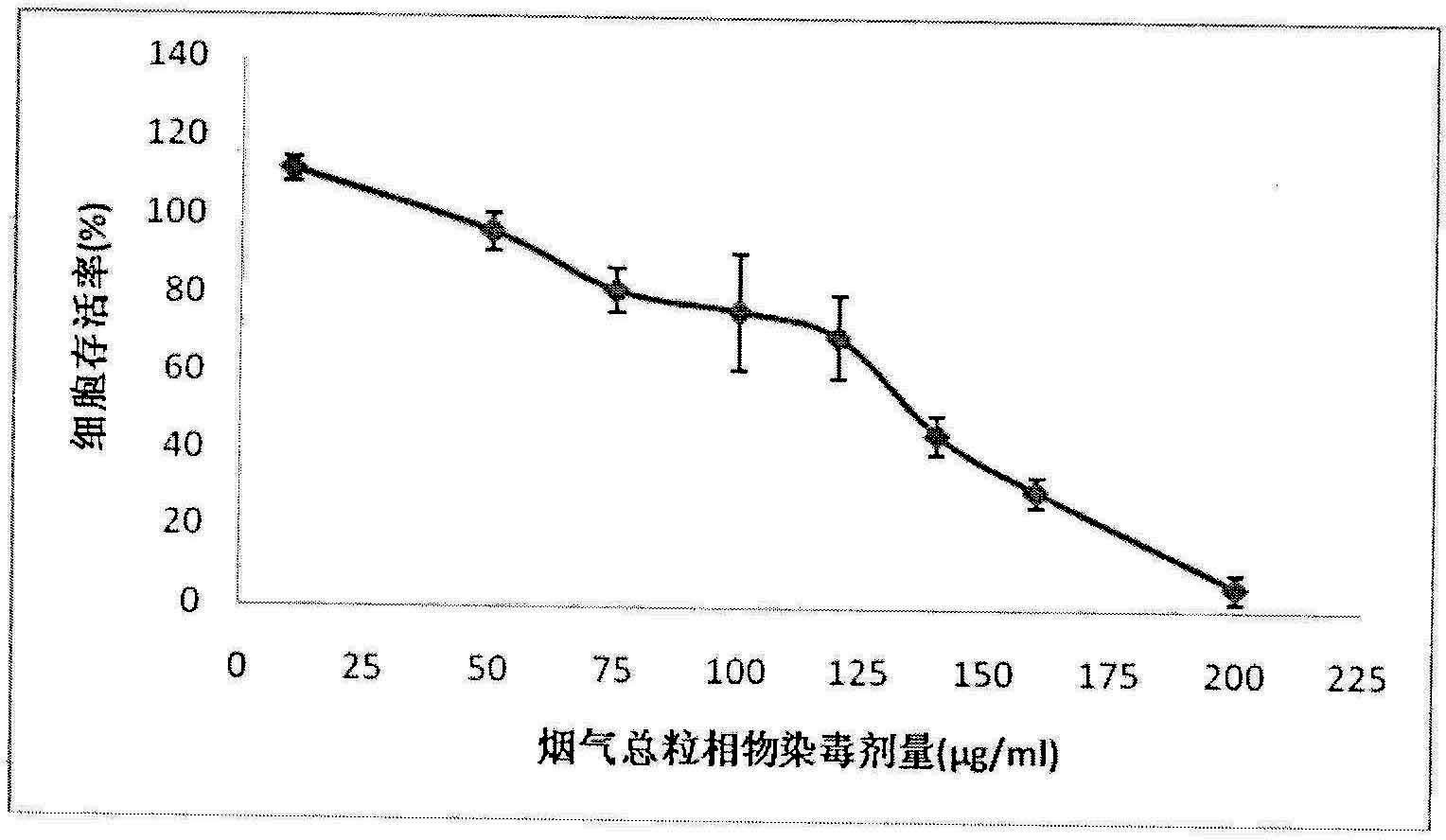
Method for testing cytotoxicity in full smoke contamination of cigarette
ActiveCN102140489ASolve the shortcomings of not being able to fully experience the full smoke of cigarettesReduce fixation stepsMicrobiological testing/measurementBiotechnologyPetri dish
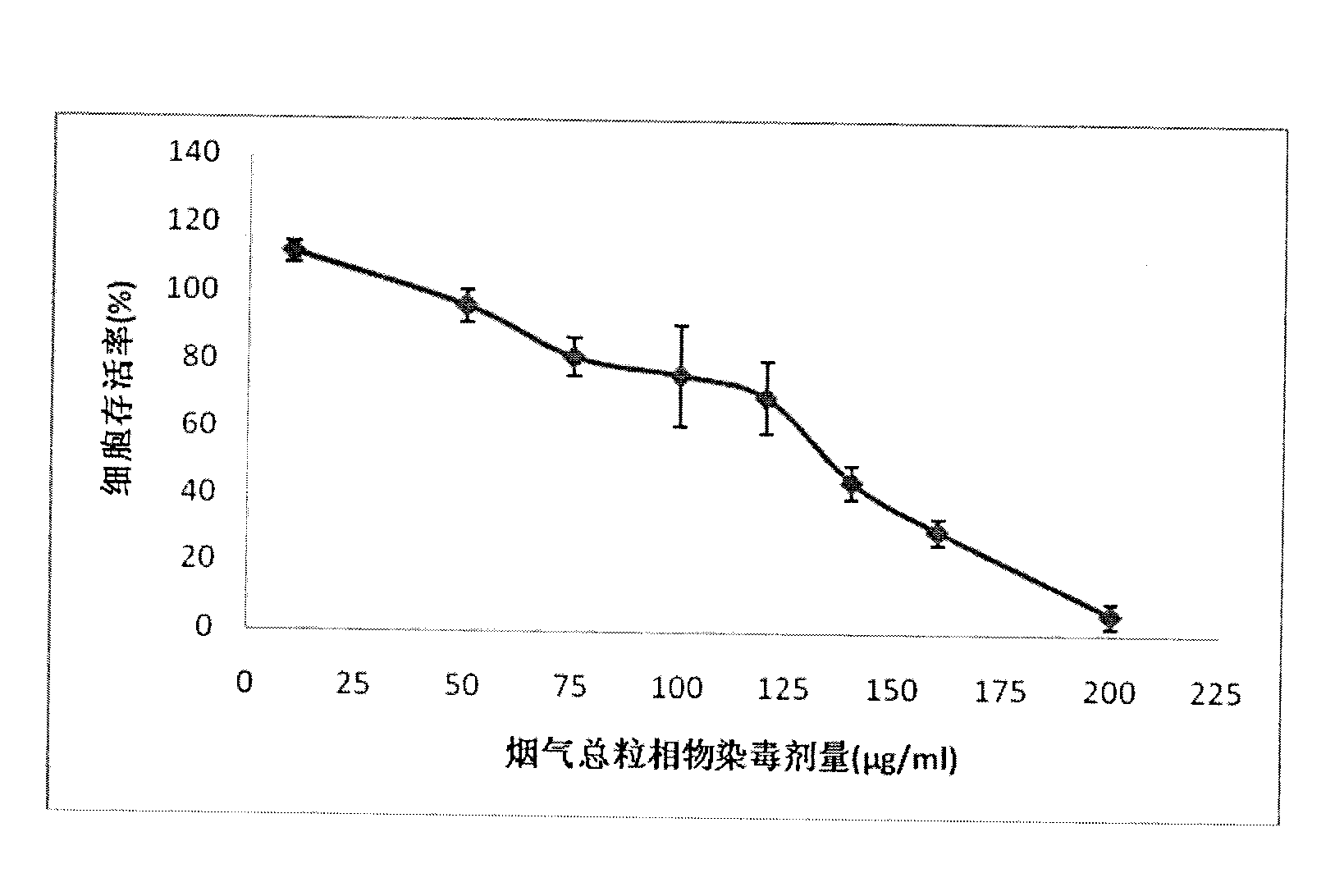
The invention relates to a method for testing cytotoxicity in full smoke contamination of cigarette. In the method, when full smoke exposure of the cigarette is carried out, an insertion type cell culture dish is used so that cultured cells are positioned at a gas-liquid interface between the smoke and the culture solution, and therefore, the purpose that the cells directly and fully contact withcigarette smoke is achieved, and the feel of the cells to the cigarette smoke can be comprehensively reflected. The method has high sensitivity, and the result can more truly reflect the cytotoxicityof the cigarette smoke. The insertion type cell culture dish is used so that a grain-phase part and a gas-phase part in the main-flow smoke of the cigarette directly and fully contact with the cultured cells, and when the full smoke exposure of the cigarette is carried out, the cells growing at microporous membrane attached to a wall are positioned at the gas-liquid interface between the cigarette smoke and the exposed culture solution, so that the problem that the cells can not comprehensively feel the full cigarette smoke in the previous experiments is solved; and meanwhile, in the method, the stationary step for formaldehyde stationary liquid is reduced so that the experimental process is simpler and more convenient.
Owner:ZHENGZHOU TOBACCO RES INST OF CNTC
Preparation process of strontium nano calcium phosphate containing biological active bone cement
InactiveCN1559888AHas the effect of slow-release strontium ionsPromote degradationBone implantPhosphorus compoundsBone cementCytotoxicity testing
The invention discloses a preparing technique of special pharmacological strontium sustained-release and no-toxicity strontium-containing nano calcium phosphate bioactive cement. It adopts the cement solid phase: the mixed powder of Ca4(PO4)2O, CaHPO4 and SrHPO4 distributed at a certain particle size and prepared in a certain mole ratio and the liquid phase: H3PO4 solution at 0.5-1 mol / l, where the solid-liquid ratio is 1.5-3.0. On physiological conditions, and the final condensate product is strontium-containing acalcerosis hydroxyl phosphorite with a microscopic daisy-petal or bent-stick shaped nano crystal structure. This cement not only has higher mechanical and operable properties, but also sustainedly releases strontium ions with special pharmacological functions, and the result of preliminary cell toxicity test shows it has no toxicity. It has wider application prospect than traditional calcium phosphate cement.
Owner:XI AN JIAOTONG UNIV
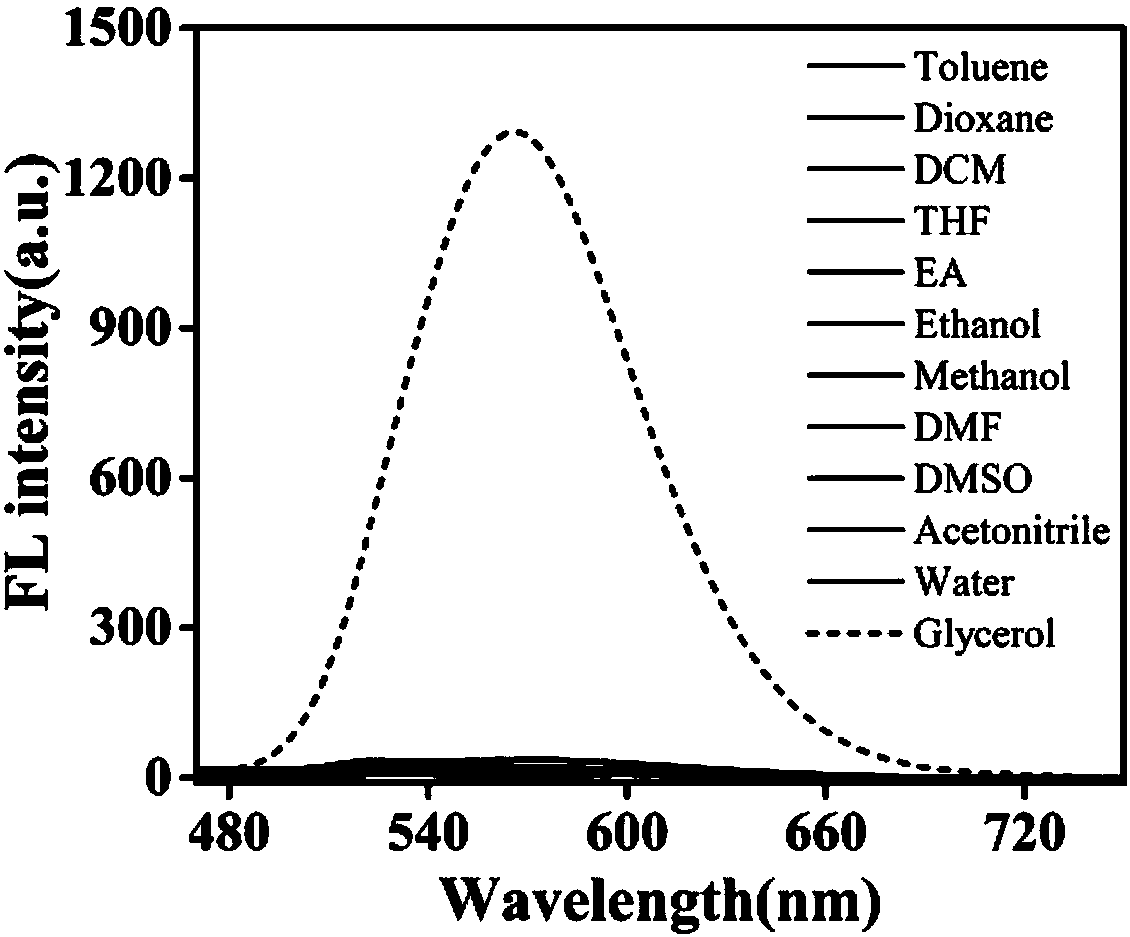
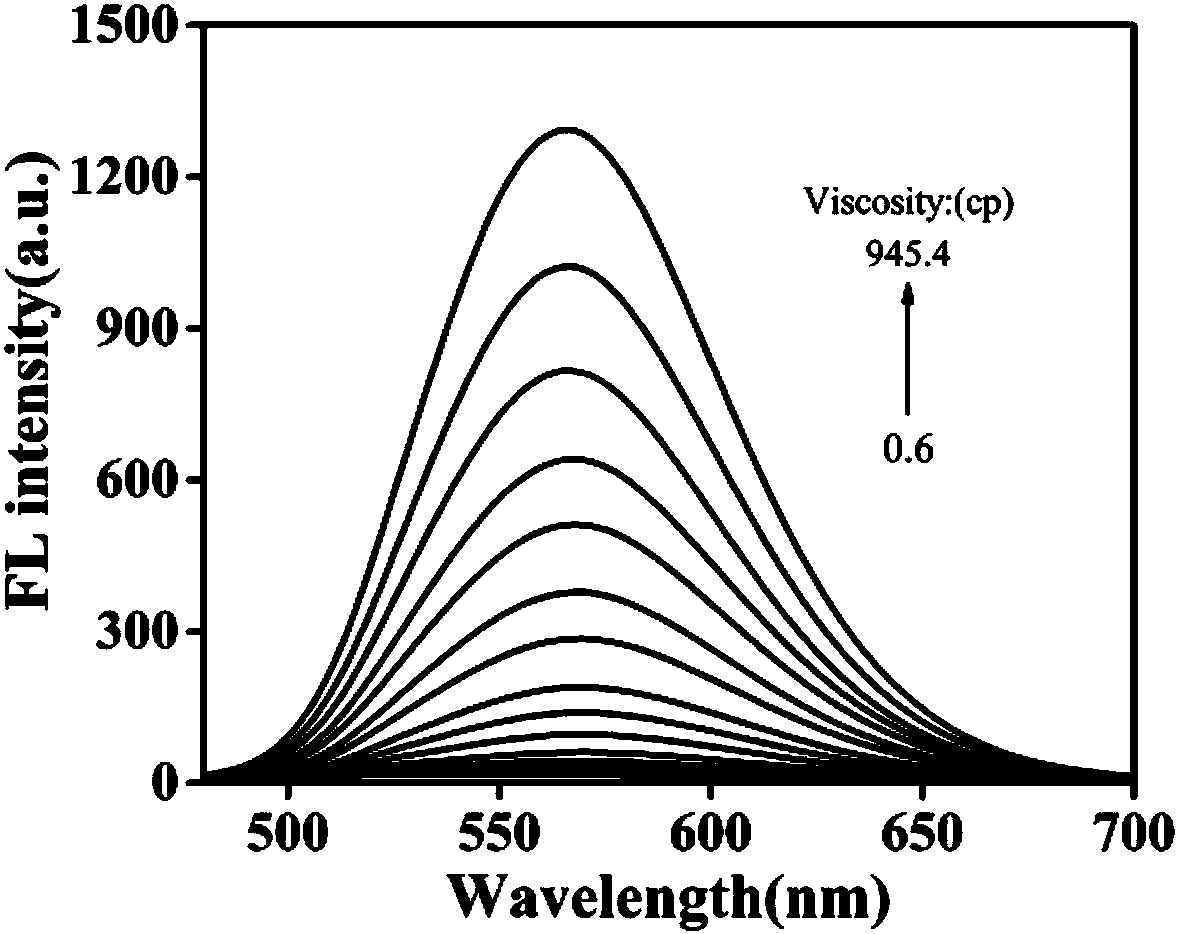
Carbazole-based two-photon viscosity fluorescence probe as well as preparation method and application thereof
ActiveCN108484590ASimple structureEasy to synthesizeOrganic chemistryFluorescence/phosphorescenceCarbazoleOrganic solvent
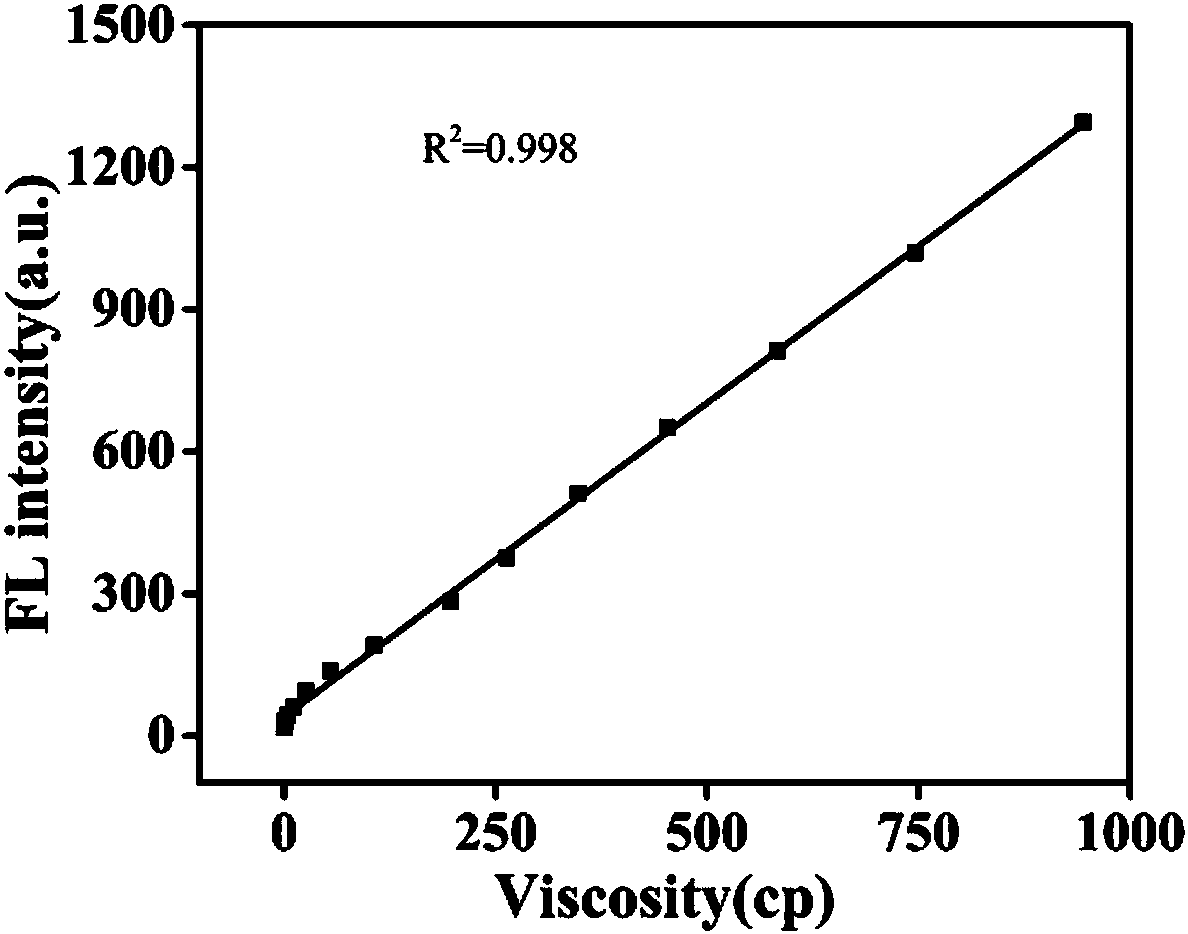
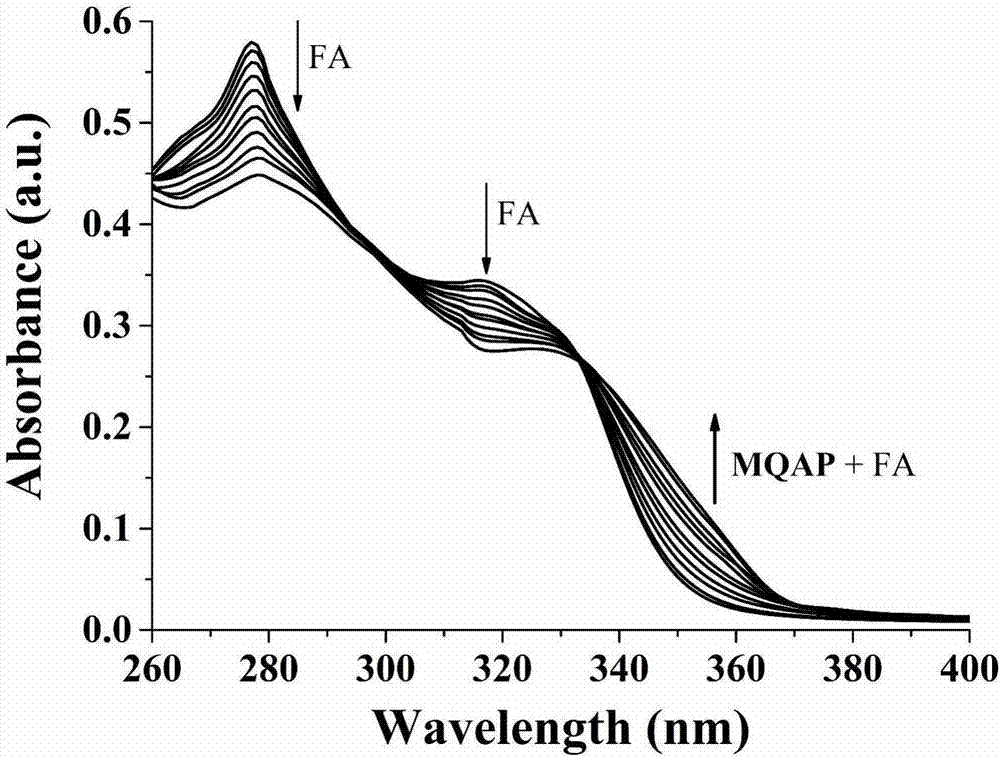
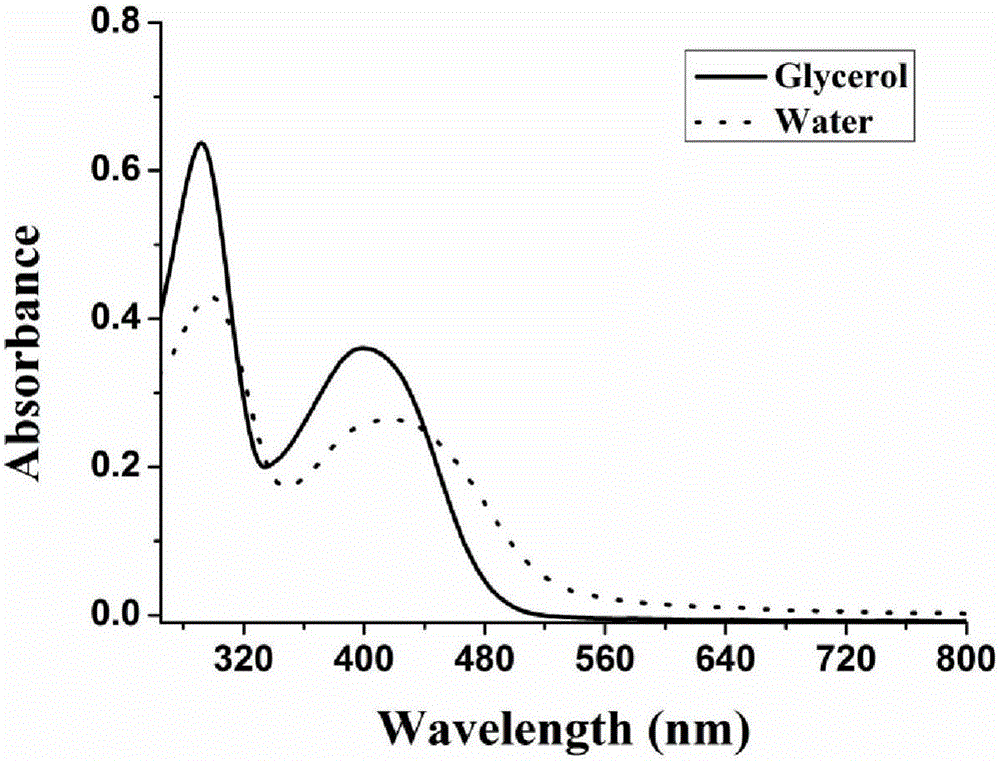
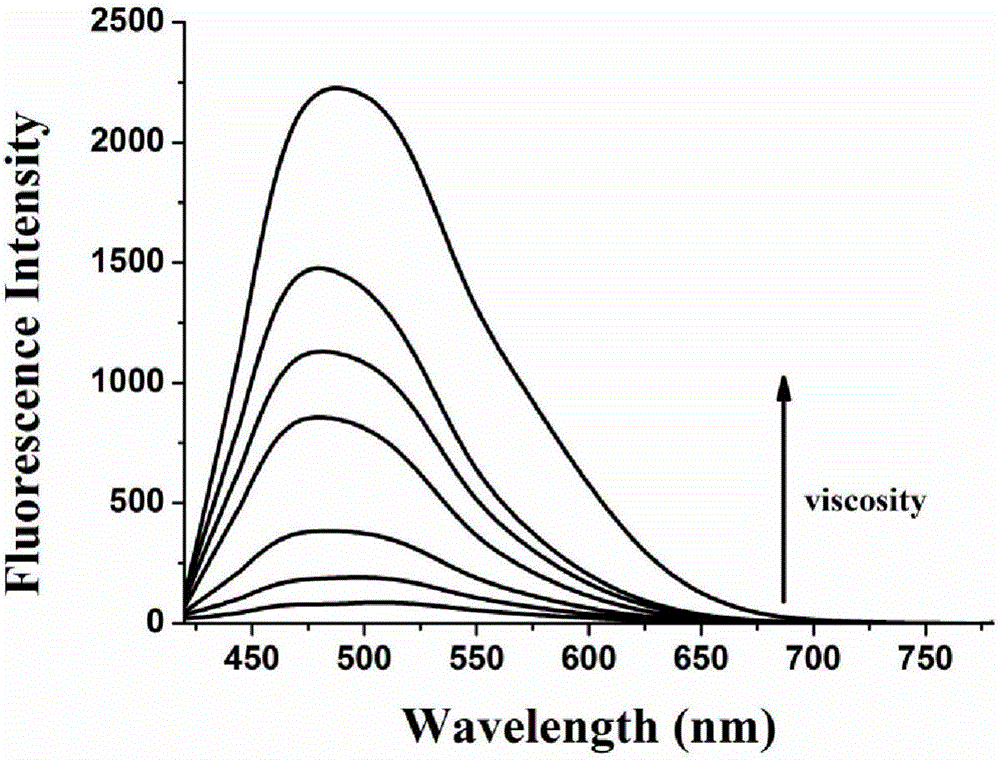
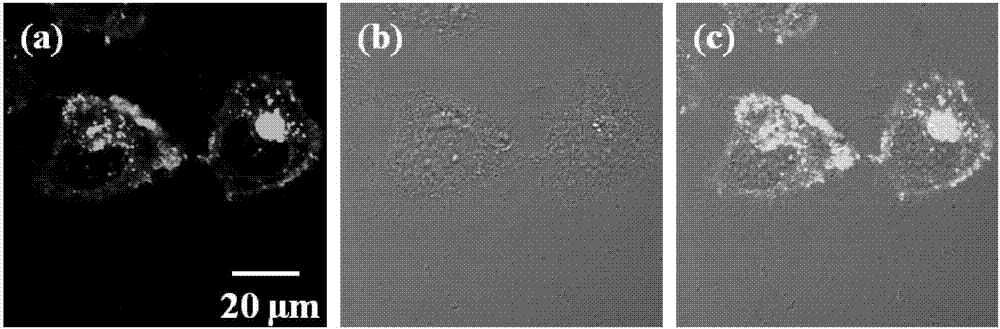
The invention discloses a carbazole-based two-photon viscosity fluorescence probe as well as a preparation method and application thereof. The two-photon viscosity fluorescence probe takes carbazole as a matrix, and the structural formula is as follows: as shown in the specification. The two-photon viscosity fluorescence probe aims at different organic solvent systems, and only in a viscosity solvent (glycerin), the fluorescence is obviously enhanced. Through the adjustment of the ratio of methanol to glycerin and changing of the system viscosity, tests show that the viscosity fluorescence probe has a good linear relation with viscosity response. Cytotoxicity tests indicate that the probe has hardly any toxic and side effect on cell, and two-photon confocal fluorescence microscopic imagingexperiments indicate that the probe has good permeability for cytomembrane and can be applied to the qualitative detection of viscosity change in mitochondria.
Owner:ANHUI UNIVERSITY
Epirubicin loaded graphene quantum dot drug carrying system and preparation method thereof
ActiveCN104984349ARealize the loadIncrease loading capacityOrganic active ingredientsPharmaceutical non-active ingredientsMedicineDrug carrier
The invention discloses an epirubicin loaded graphene quantum dot drug carrying system and a preparation method thereof. The preparation method includes the following steps: preparation of graphene quantum dots by a hydrothermal method; loading an anti-cancer drug with the graphene quantum dots; and performing a drug slow-release experiment, and performing a cytotoxicity test on the drug carrying system. The graphene quantum dots prepared by the hydrothermal method are used as a drug carrier for being loaded with epirubicin, greatly improve the drug loading rate, and show a quite good inhibitory effect on tumor HeLa cells. The preparation method of the drug carrying system is simple to operate, mild in reaction conditions and high in drug loading rate, and is expected to have a broad application prospect in the field of biological medicine.
Owner:南京美材科技有限公司
Ratio-type two-photon formaldehyde fluorescent probe, preparation method therefor and use of ratio-type two-photon formaldehyde fluorescent probe
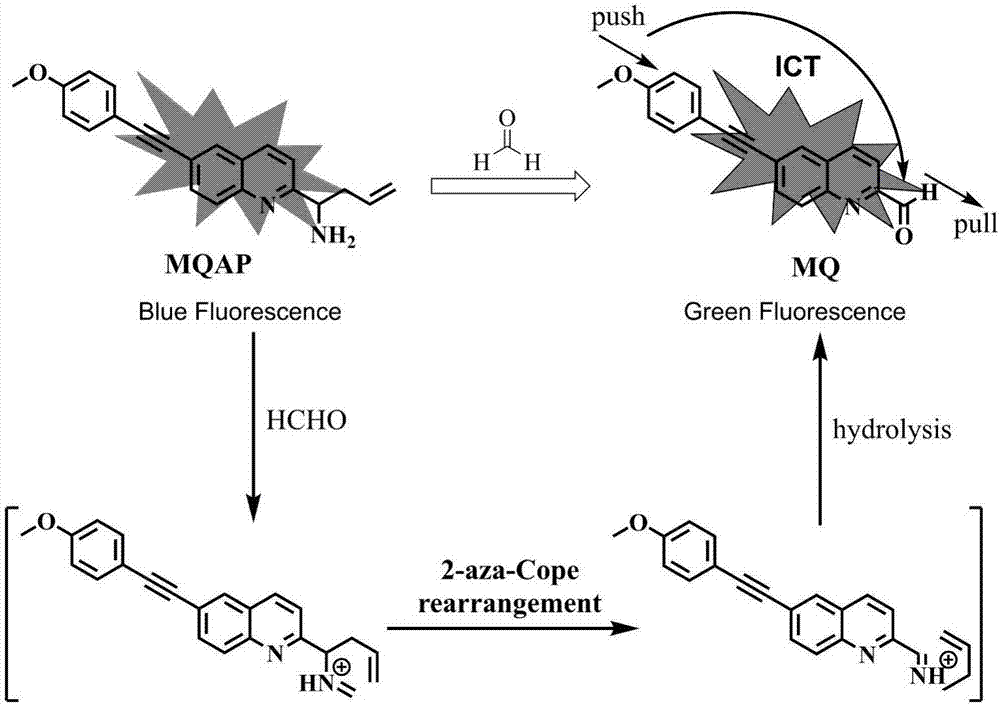
ActiveCN106946773ALow fluorescence quantum yieldSimple structureOrganic chemistryFluorescence/phosphorescenceMicro imagingCytotoxicity testing
The invention discloses a ratio-type two-photon formaldehyde fluorescent probe, a preparation method therefor and use of the ratio-type two-photon formaldehyde fluorescent probe. The ratio-type two-photon formaldehyde fluorescent probe has a structure as follows shown in the description. Molecules of the fluorescent probe disclosed by the invention show relatively high selectivity and sensitivity to formaldehyde in a coexisting system of molecules of the formaldehyde and other aldehydes, amino acids and the like. Proven by cytotoxicity tests, the molecules of the fluorescent probe disclosed by the invention are almost free of toxic action on cells; and shown by two-photon fluorescence microscopy imaging experiments, the molecules of the fluorescent probe disclosed by the invention have good cell permeability to MCF-7 cells, so that the fluorescent probe is applicable to the detection of presence of formaldehyde molecules in the cells.
Owner:ANHUI UNIVERSITY
Two-photon viscosity fluorescence probe as well as preparation method and application thereof
ActiveCN105801479ASimple structureEasy to synthesizeOrganic chemistryFluorescence/phosphorescenceSignal responseQuinoline
The invention discloses a two-photon viscosity fluorescence probe as well as a preparation method and application thereof. With quinoline as parent, the two-photon viscosity fluorescence probe has a structure shown in the specification. The two-photon viscosity fluorescence probe disclosed by the invention realizes specific fluorescence signal response to viscosity. Cell toxicity tests indicate that the two-photon viscosity fluorescence probe rarely causes toxic and side effects on cells; and two-photon confocal fluorescence microscopy imaging experiments show that the two-photon viscosity fluorescence probe has good cell permeability to HeLa cells and can be adapted to intracellular viscosity detection.
Owner:ANHUI UNIVERSITY
Two-channel two-photon fluorescent polar probe, preparation method and uses thereof
ActiveCN110194766AReal-time monitoring of apoptosisImprove permeabilityOrganic chemistryFluorescence/phosphorescenceElectrical polarityInterference factor
The invention discloses a two-channel two-photon fluorescent polar probe, a preparation method and uses thereof, wherein the structure of the two-channel two-photon fluorescent polar probe is definedin the specification. According to the present invention, the two-channel two-photon fluorescent polar probe has specific response to polarity in a system containing the probe and other interference factors; and the cytotoxicity test results show that the probe has almost no toxic side effects on cells, and the two-photon confocal fluorescence microscopy imaging experiment results show that the probe has good permeability to HeLa cells and can effectively locate the mitochondria in the cells (positioning coefficient of 0.95), such that the probe is suitable for the two-channel two-photon fluorescence imaging and quantitative detection of the polarity in cell mitochondrial.
Owner:ANHUI UNIVERSITY
Two-photon fluorescent probe for detecting polarity and viscosity through two channels as well as preparation method and application thereof
ActiveCN111253935ALow cytotoxicityImprove permeabilityOrganic chemistryFluorescence/phosphorescenceStructural formulaCytotoxicity testing
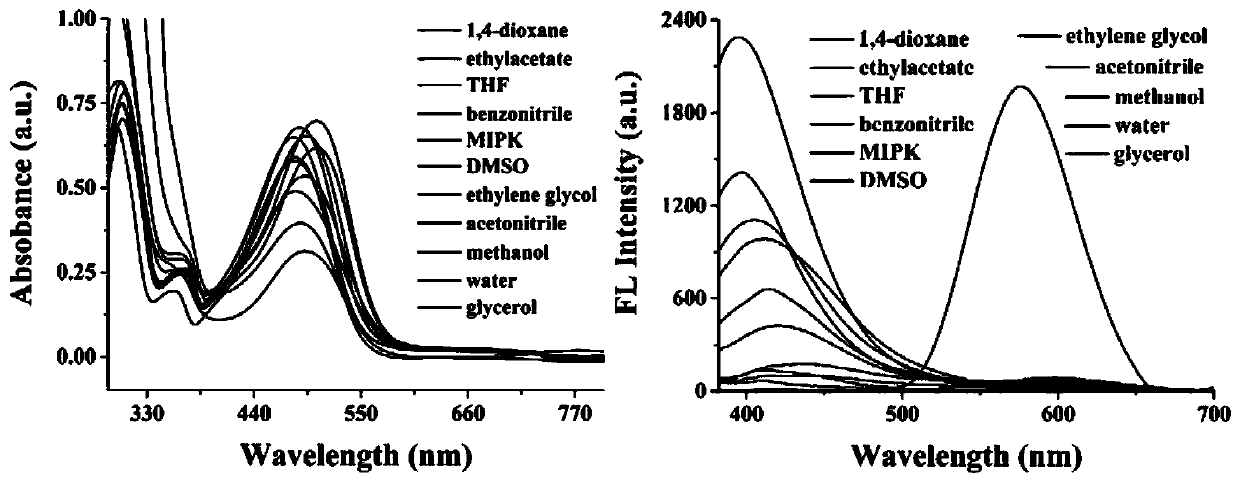
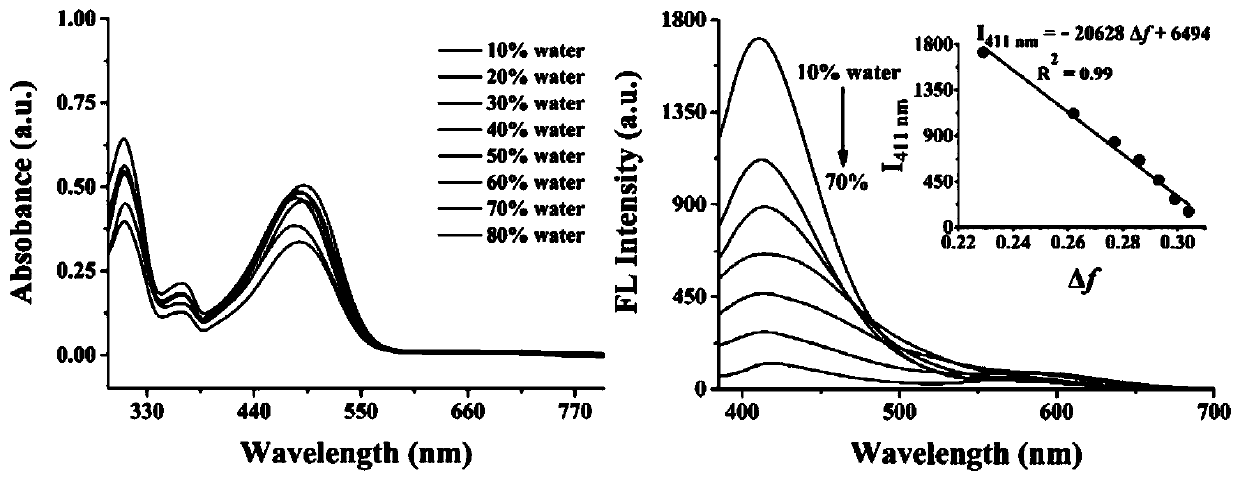
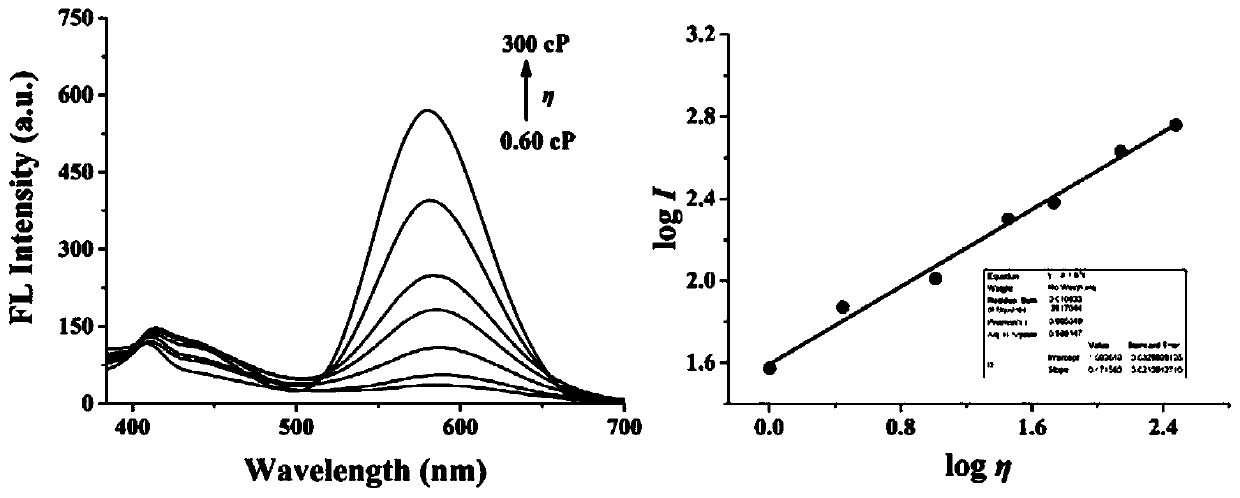
The invention discloses a two-photon fluorescent probe for detecting polarity and viscosity through two channels as well as a preparation method and application thereof. The two-photon fluorescent probe can monitor the real-time change of polarity and viscosity in cell mitochondria in two different channels at the same time, and the structural formula of the two-photon fluorescent probe is shown in the specification. The two-photon fluorescent probe molecule can perform fluorescence response on polarity (short wave with the wave length of 410 nm) and viscosity (long wave with the wave length of 580 nm) at the same time by utilizing two different fluorescence bands. Furthermore, a cytotoxicity test shows that the probe has almost no toxic or side effect on cells; a two-photon confocal fluorescence microimaging experiment shows that the probe has good permeability to HeLa cells, can effectively locate mitochondria (the locating coefficient is 0.97) in the cells, and is suitable for two-channel two-photon fluorescence imaging and detection of polarity and viscosity in the cell mitochondria.
Owner:ANHUI UNIVERSITY
Adhesive monomer and method for improving adhesion strength between dental zirconia and resin
InactiveCN103601753AHigh bonding strengthGood biocompatibilityImpression capsDentistry preparationsPolymer sciencePhosphate
The invention discloses an adhesive monomer and a method for improving adhesion strength between dental zirconia and resin. The adhesive monomer is prepared from 1, 5-cyclooctadiene by performing epoxidation, ring-opening reaction, redox, exterification and the like, the main chain of the adhesive monomer is a n-octane carbon chain with one end of acrylic ester and the other end of phosphate ester, the forth position and the fifth position on the carbon chain are respectively connected with a R group which represents hydroxyl, sulfydryl or carboxyl, and the adhesive monomer has the structural formula shown in the description. The adhesive monomer helps to improve the adhesion strength between dental zirconia ceramic and resin, has the same improving effect with common 10-MDP-containing zirconia primer coating products in current market, has relatively good biological compatibility, is qualified in cytotoxicity tests, and has important application value during tooth repairing processing.
Owner:NANJING UNIV +1
Strong signal and low toxicity composite nanometer material and preparation method thereof
InactiveCN103642491AHigh quantum yieldSolve the problem of water solubilityMaterial nanotechnologyInorganic non-active ingredientsCancer cellBiological imaging
The invention relates to a strong signal and low toxicity composite nanometer material and a preparation method thereof, and belongs to the technical field of material preparation, wherein Au-Cu2S quantum dots are adopted as an inner core, and ZnS is adopted as a shell to wrap the Au-Cu2S quantum dot inner core to prepare the Au-Cu2S / ZnS quantum dots. The preparation method comprises: preparing Au nanoparticles, preparing Au-Cu2S composite nanoparticles, and preparing Au-Cu2S / ZnS quantum dots. According to the present invention, the particle size of the obtained the composite nano-particles can be controlled in several nanometers to tens of nanometers, and the obtained the composite nano-particles can be used for cytotoxicity tests and biological labeling carriers or targeted drug delivery carriers, can be provided for observing fluorescence signals through a biological imaging system, can be combined with folic acid so as to provide biocompatibility, can further enter in vivo cells and be combined with the cells so as to be adopted as the biological probe marker to perform biological labeling, and can form the durg complex, wherein the durg complex can reside on the cancer cell surface.
Owner:CHANGCHUN UNIV OF SCI & TECH
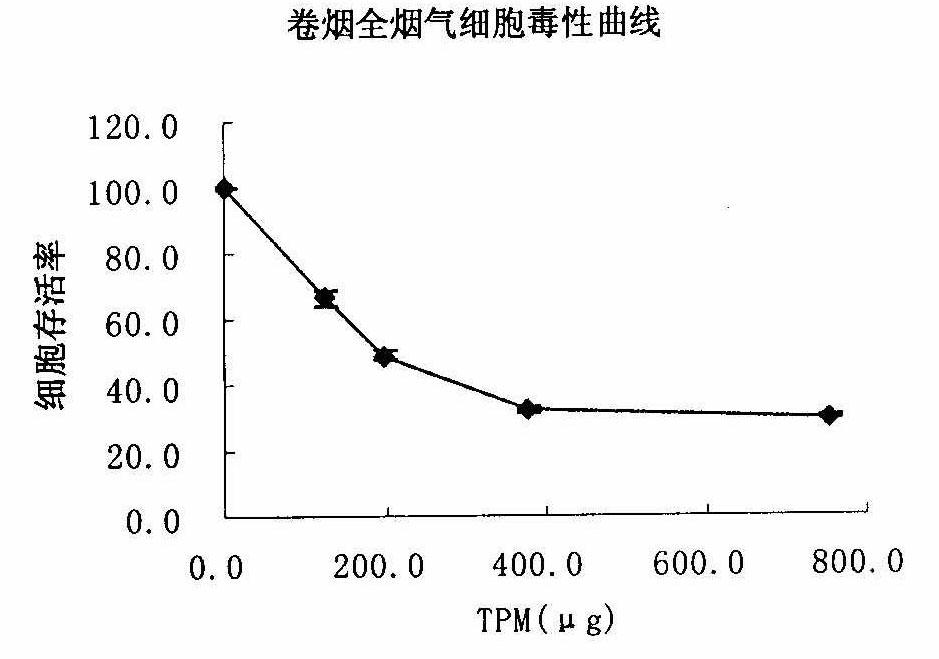
Method for testing in vitro cytotoxicity of cigarette smoke
ActiveCN102618619AWide linear rangeHigh sensitivityMicrobiological testing/measurementBiotechnologyStaining
The invention discloses a method for testing in vitro cytotoxicity of cigarette smoke. The method is characterized by comprising the following steps of: 1) preparing a laboratory reagent; 2) performing cell inoculation culture; 3) performing cigarette smoke contamination; 4) dyeing with water-soluble tetrazolium-1 (WST-1); and 5) obtaining a result and analyzing. Compared with the prior art, the method is characterized in that a test step for washing cells for multiple times is eliminated in the whole test process, so that the method is easy and convenient to operate; a step of replacing a nutrient solution is not required during dyeing with the WST-1, and a diluted nutrient solution can be directly added for reacting; formazan produced by the WST-1 is water-soluble, so that a subsequent dissolution step is eliminated; and absorbance detection can be finished in 2 hours after WST-1 dyeing, so that the test period is shortened, and the method is quick in comparison with the conventional testing method. The measuring method is quick and convenient to operate, and also has the advantages of high sensitivity and stable result; and the method can be applied to smoke cytotoxicity test of multiple cell lines.
Owner:ZHENGZHOU TOBACCO RES INST OF CNTC
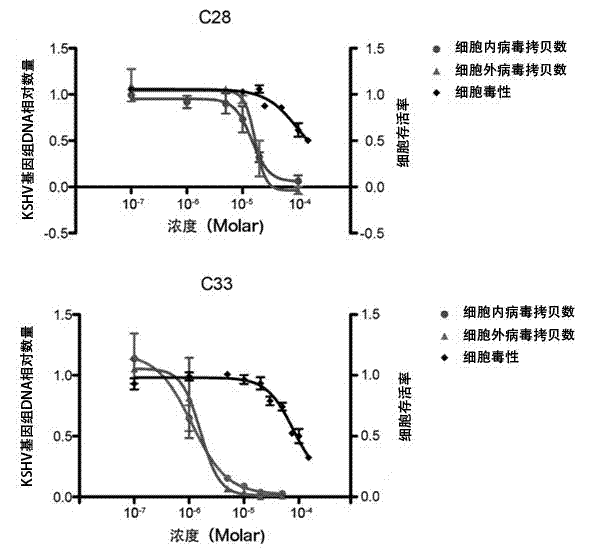
Application of benzo Alpha-pyrone compound as Gamma-type human herpes virus-resistant medicine
ActiveCN103070858AStrong inhibitory activityLow toxicityAntiviralsHeterocyclic compound active ingredientsDiseaseNovobiocin
The invention discloses an application of a benzo Alpha-pyrone compound as a Gamma-type human herpes virus-resistant medicine. According to the invention, the compound resistant to Gamma-type human herpes virus infection is higher in activity as compared with a novobiocin which is a marketed medicine, and is less in toxicity based on a cytoxicity test. The compound can be used for curing or preventing related diseases caused by KSHV infection.
Owner:SUN YAT SEN UNIV
Two-photon fluorescent probe as well as preparation method and application thereof
ActiveCN105859733ASimple structureSelectivityOrganic chemistryFluorescence/phosphorescenceMicro imagingCytotoxicity testing
The invention discloses a two-photon fluorescent probe as well as a preparation method and application thereof. The two-photon fluorescent probe takes coumarin and rhodamine as a matrix. The structure of the two-photon fluorescent probe is described in the specification. Molecules of the two-photon fluorescent probe show relatively high selectivity and sensitivity in a system in which palladium ions (II) and other positive ions coexist. A cytotoxicity test shows that the two-photon fluorescent probe disclosed by the invention has hardly any toxic effect on cells, and a two-photon fluorescence microscopy imaging experiment shows that the two-photon fluorescent probe has good HeLa cell permeability and is applicable to detection of distribution of palladium ions (II) in cells.
Owner:ANHUI UNIVERSITY
Two-photon fluorescent probe as well as preparation method and application thereof
ActiveCN105906619ASimple structureEasy to synthesizeOrganic chemistryFluorescence/phosphorescenceSide effectCarbazole
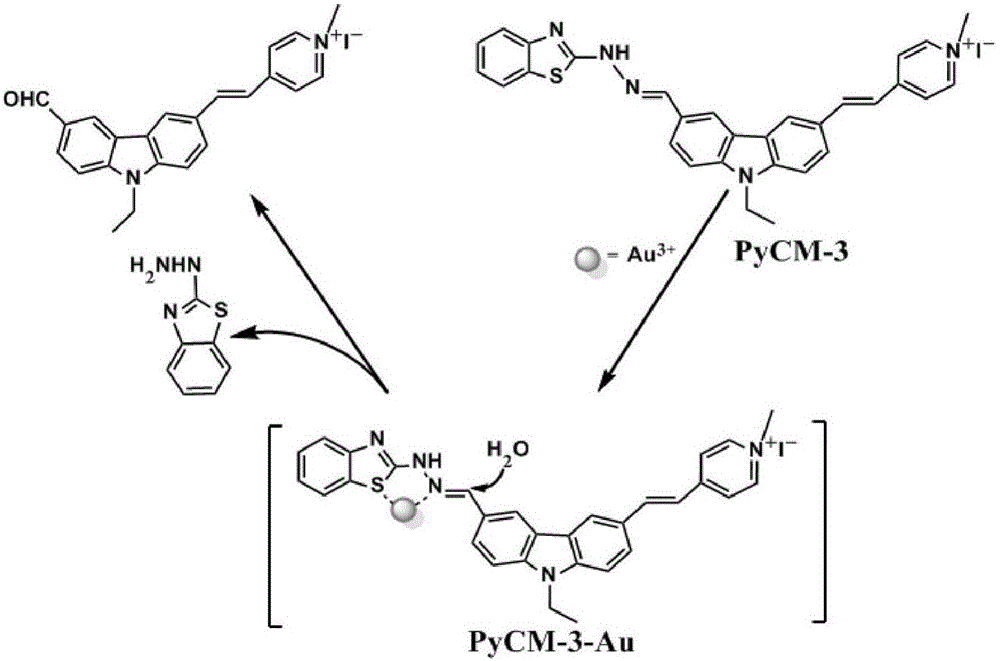
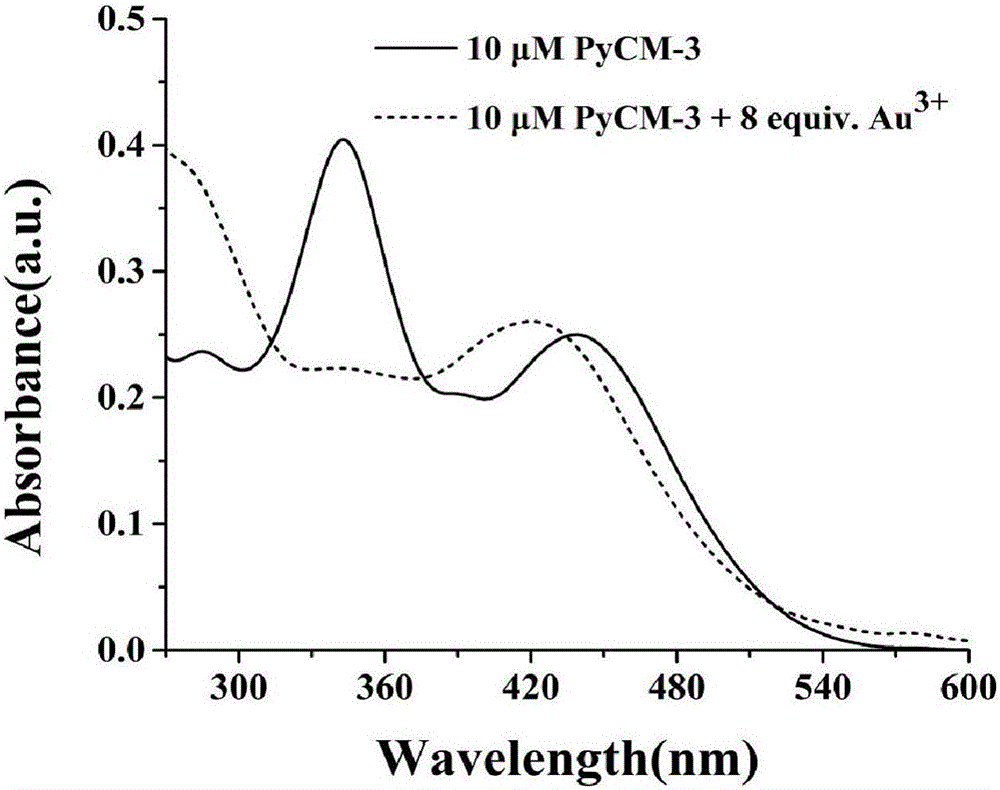
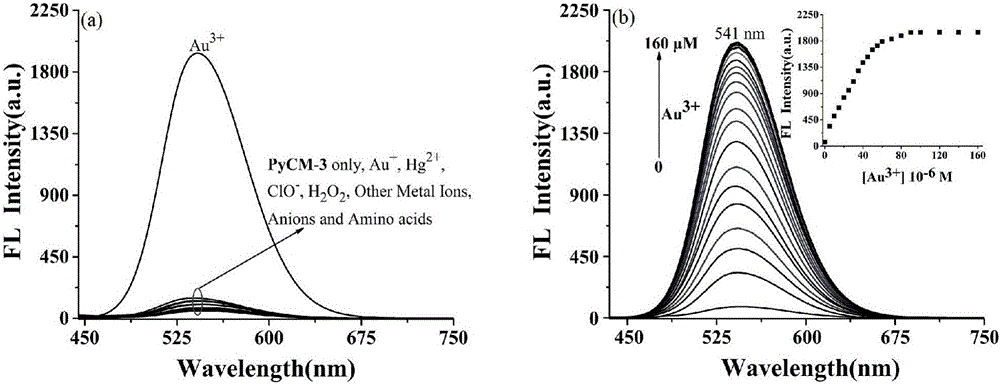
The invention discloses a two-photon fluorescent probe as well as a preparation method and application thereof. The two-photon fluorescent probe takes carbazole as matrix. The structure of the two-photon fluorescent probe is described in the specification. Molecules of the two-photon fluorescent probe show specificity response and high sensitivity in a system that trivalent gold ions (Au<3+>) and other interference reagents coexist. Cytotoxicity tests show that the probe almost has no toxic or side effect on cells, and two-photon confocal fluorescence microscopic imaging tests show that the probe has good HeLa cell permeability and is applicable to two-photon fluorescence imaging and qualitative detection on Au<3+> in a cell mitochondrion.
Owner:ANHUI UNIVERSITY
Lysosome targeted pH fluorescent probe for monitoring cell autophagy as well as preparation and application thereof
InactiveCN110951483AHas commercial application valueWith visual monitoringOrganic chemistryFluorescence/phosphorescenceFluoProbesLysosomal targeting
The invention relates to the technical field of pH fluorescent probes and particularly relates to a lysosome targeted pH fluorescent probe for monitoring cell autophagy as well as preparation and application of the lysosome targeted pH fluorescent probe. The preparation method comprises the following steps of dissolving 2-(2-aminoethyl)-3',6'-bis(diethylamino) spiro [isoindole-1,9'-xanthan]-3-ketone and 2-(2-methoxyethoxy)4-methyl benzene sulfonic acid ethyl ester in N, N-dimethylformamide, and carrying out heating reflux to obtain a crude product; and removing a solvent from the crude product, and separating through a silica gel column to obtain a pure product. Cytotoxicity tests show that the probe has almost no toxic or side effect on cells, a cell co-localization experiment determinesthat the probe can specifically target a cell lysosome, and a laser confocal microimaging experiment shows that the probe has good cell membrane permeability and can perform high-sensitivity detectionon pH change in the lysosome. The probe provided by the invention can monitor the autophagy process of the cells by detecting the change of pH in the lysosome.
Owner:SHANXI UNIV
Two-photon polar fluorescent probe based on carbazole and preparation method and application of two-photon polar fluorescent probe
ActiveCN110003173AExcellent two-photon absorption performanceGood light stabilityOrganic chemistryFluorescence/phosphorescenceQuantum yieldLysosome localization
The invention discloses a two-photon polar fluorescent probe based on carbazole and a preparation method and application of the two-photon polar fluorescent probe. The structure of the two-photon polar fluorescent probe based on the carbazole is shown in the description. The fluorescent quantum yield and the fluorescent lifetime of the two-photon polar fluorescent probe have a good linear relationship on polar response. Besides, fluorescent probe moleculars have an excellent two-photon absorption function. Through a cytotoxicity test, it is shown that the probe has little side and toxic effects on HeLa cells; through a two-photon confocal imaging experiment, it is shown that the probe has good lysosome localization capacity and cell permeability, is high in light stability and suitable forlong-time real-time two-photon fluorescence imaging and visualization research of changes in cell lysosome polarity.
Owner:ANHUI UNIVERSITY
Carbazolyl two-photon fluorescent probe and preparation method and application thereof
ActiveCN108484479ASimple structureEasy to synthesizeOrganic chemistryFluorescence/phosphorescenceCarbazoleCytolysosome
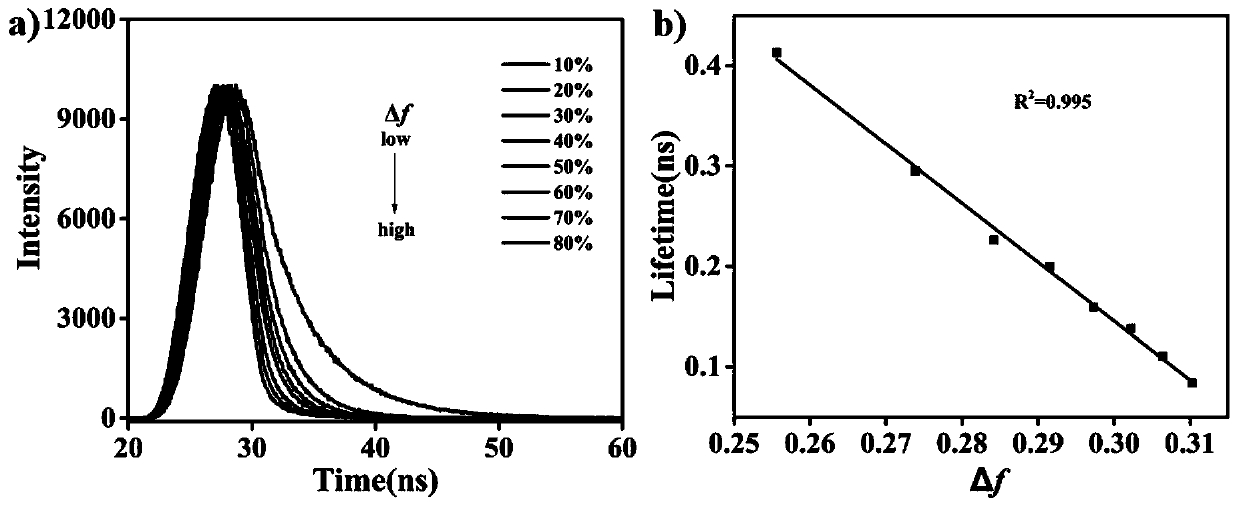
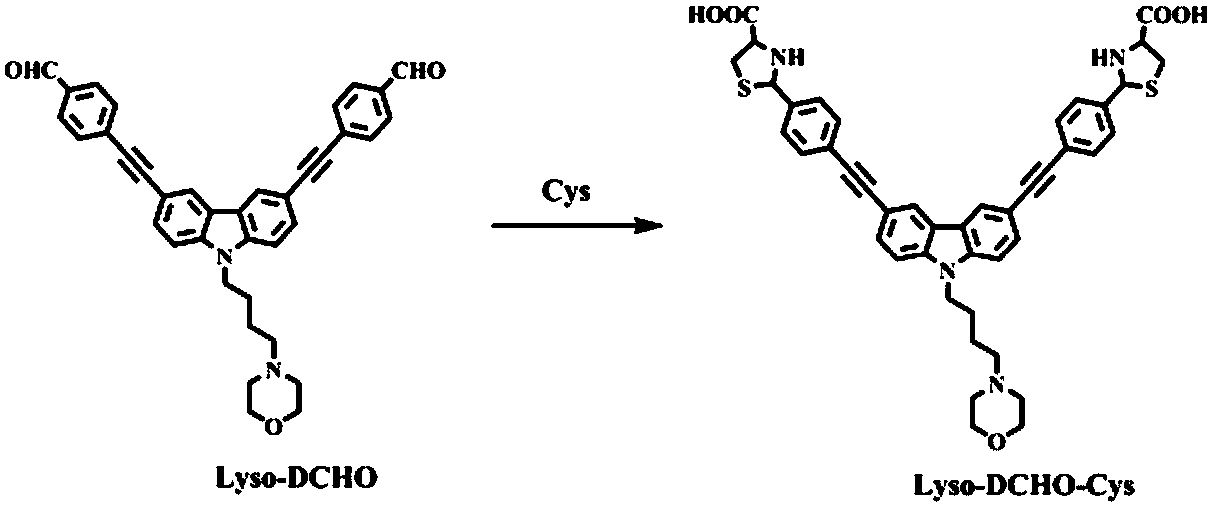
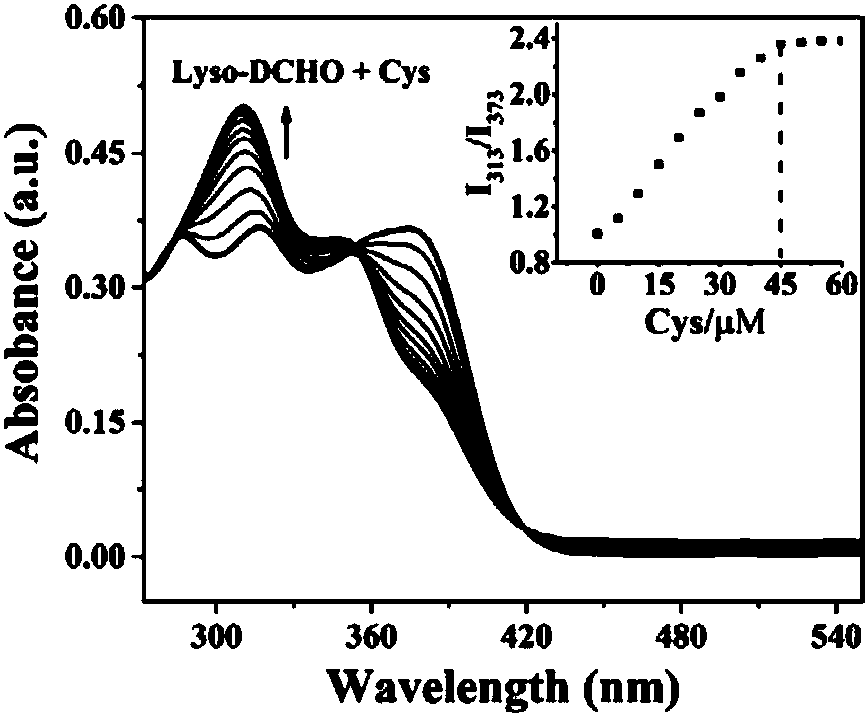
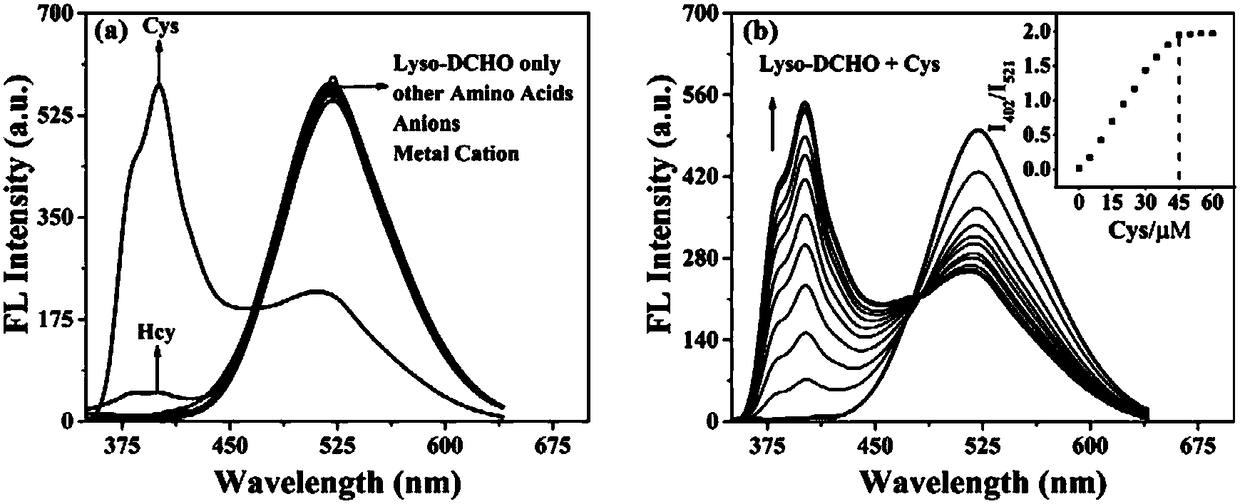
The invention discloses a carbazolyl two-photon fluorescent probe and a preparation method and application thereof. The carbazolyl two-photon fluorescent probe uses carbazole as a matrix and adopts astructural formula as follows: FORMULA. The two-photon fluorescent probe molecule shows specific response and high sensitivity in a system in which cysteine (Cys) coexists with other interfering reagents. Cytotoxicity tests show that the probe has almost no toxic and side effects on cells; in addition, the two-photon confocal fluorescent microscopic imaging experiments show that the fluorescent dye Lyso-DCHO has good permeability to an MCF-7 cell, can effectively position a lysosome in the cell (positioning coefficient is 0.90), and is suitable for visual detection of the Cys in the cytolysosome.
Owner:ANHUI UNIVERSITY
Biological ceramic and preparation method and application thereof
ActiveCN109293355AHigh solid contentReduce porosityImpression capsAdditive manufacturing apparatusPorosityApparent density
The invention provides a biological ceramic preparation method. The method includes: mixing aluminum hydroxide and phosphoric acid at 95-105 DEG C to obtain a phosphorus-containing adhesive; mixing ceramic powder with the phosphorus-containing adhesive, and performing ball milling to obtain ceramic slurry; printing with the ceramic slurry to obtain a ceramic green body in structural design by a 3Dprinting technique; dewatering the ceramic green body, and sintering to obtain biological ceramic. The biological ceramic prepared according to the method is free of evident surface cracks, high in compactness and uniform in texture, the linear shrinkage is 3.26-5.45%, the porosity is 5-8%, and the apparent density is 2.56-3.12g / cm<3>. According to cytotoxicity tests, cytotoxicity of the biological ceramic is level I and meets biomedical requirements.
Owner:LANZHOU INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Carbazole-based two-photon fluorescent probe as well as preparation method and application thereof
ActiveCN107286151ASimple structureEasy to synthesizeOrganic chemistryFluorescence/phosphorescenceSide effectCarbazole
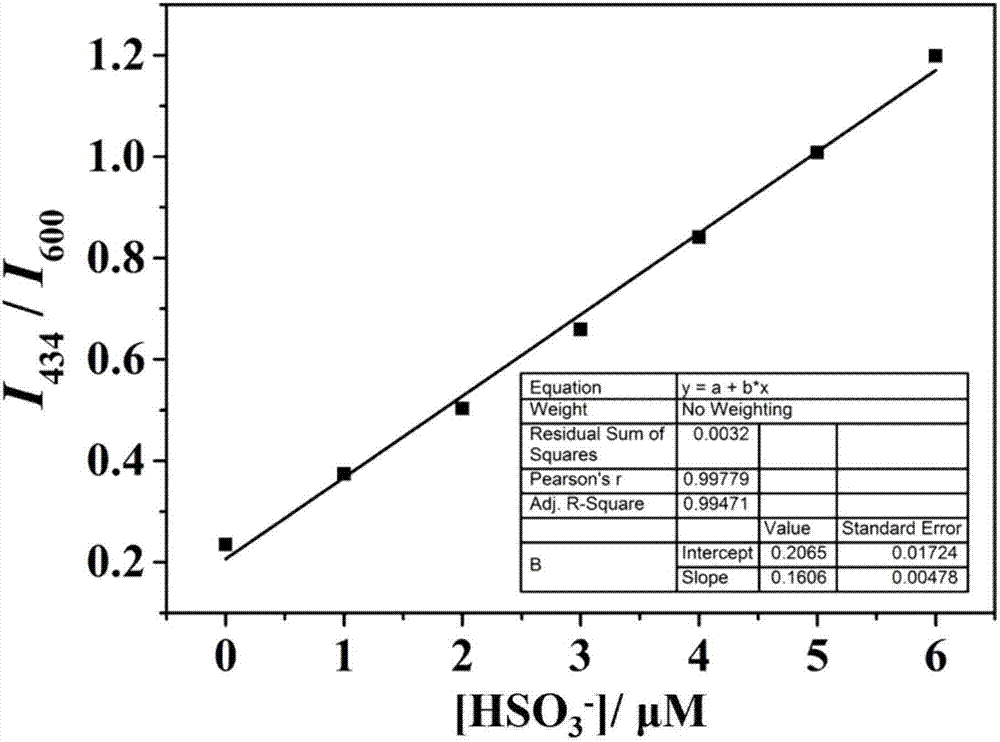
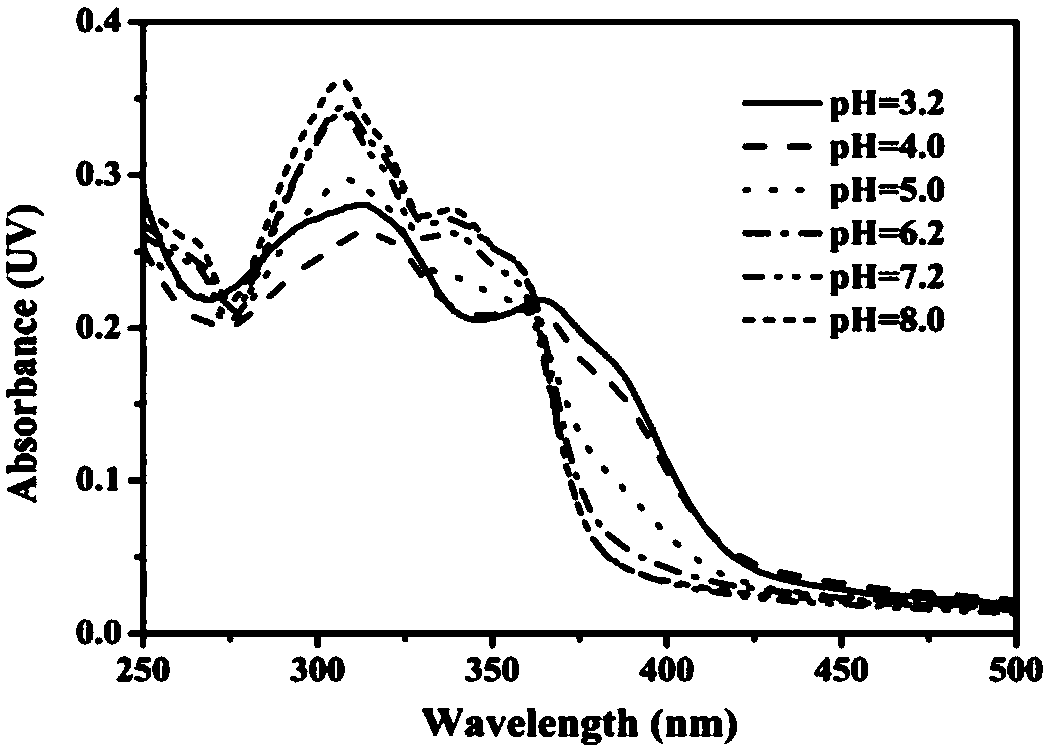
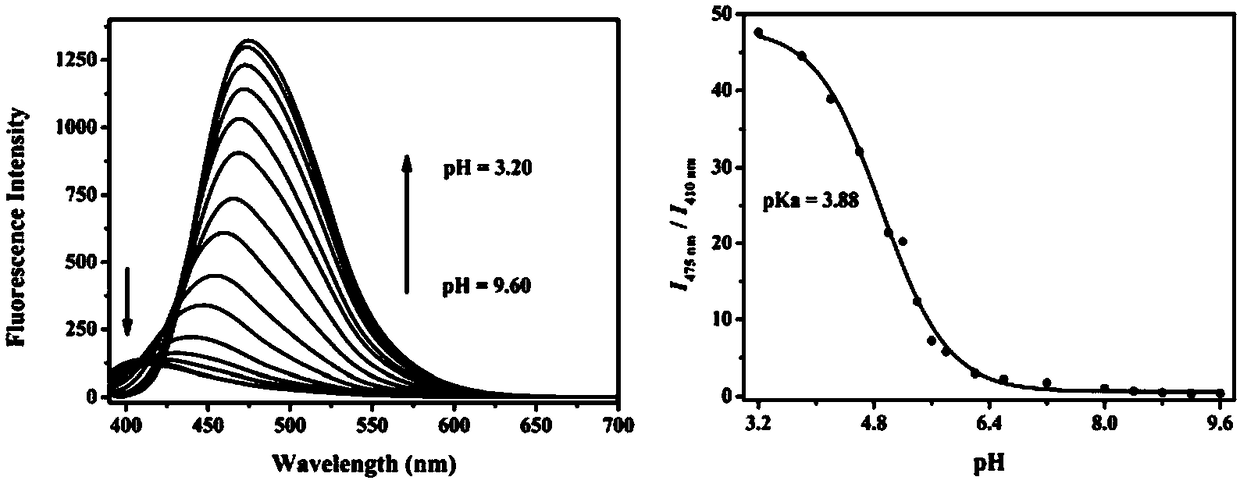
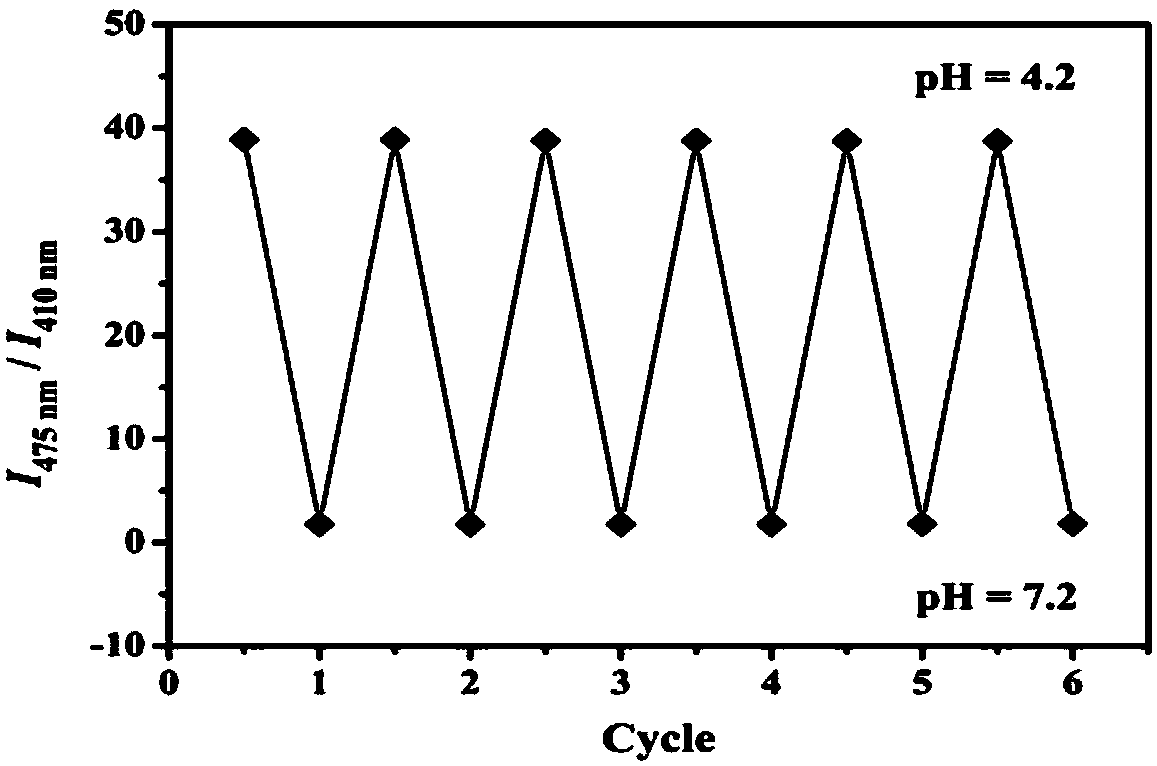
The invention discloses a carbazole-based two-photon fluorescent probe as well as a preparation method and application thereof. The carbazole-based two-photon fluorescent probe has the following structural formula, wherein the structural formula is as shown in the specification. The two-photon fluorescent probe disclosed by the invention shows specific response and high sensitivity in a system with coexistence of bisulfite (HSO3-) and other interference reagents. The cytotoxicity assay shows that the probe hardly has any toxic or side effect on cells. The two-photon confocal fluorescence microscopic imaging experiment shows that the probe has high permeability on MCF-7 cells. The probe is applicable to two-photon fluorescence imaging and qualitative detection of the HSO3- in cell mitochondria.
Owner:ANHUI UNIVERSITY
Two-photon pH (potential of hydrogen) ratio measurement fluorescence probe for monitoring cell autophagy, preparation method of fluorescence probe and application
ActiveCN108329301ASimple structureEasy to synthesizeOrganic chemistryFluorescence/phosphorescenceMicro imagingSide effect
Owner:ANHUI UNIVERSITY
Mitochondria-targeted hypochlorous acid ratio type two-photon fluorescent probe as well as preparation method and application thereof
ActiveCN110483573ASimple structureEasy to synthesizeGroup 5/15 element organic compoundsColor/spectral properties measurementsLinear relationshipStructural formula
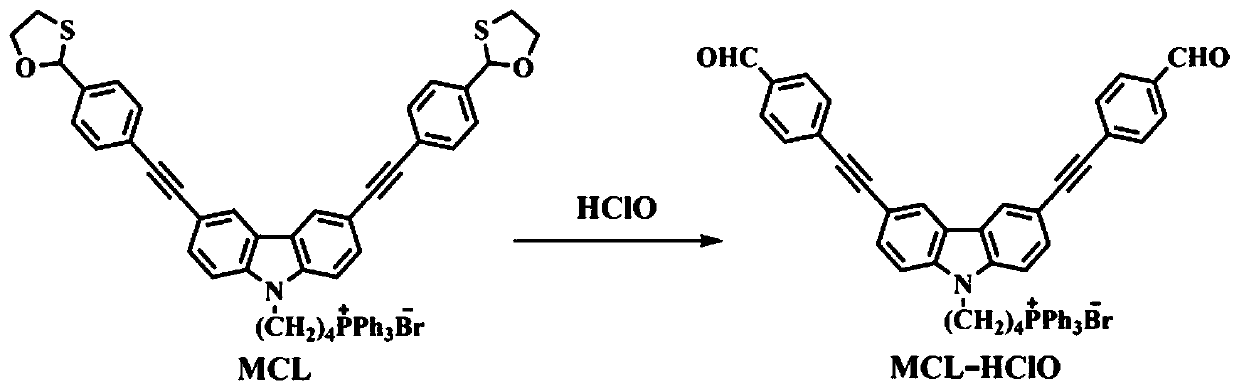
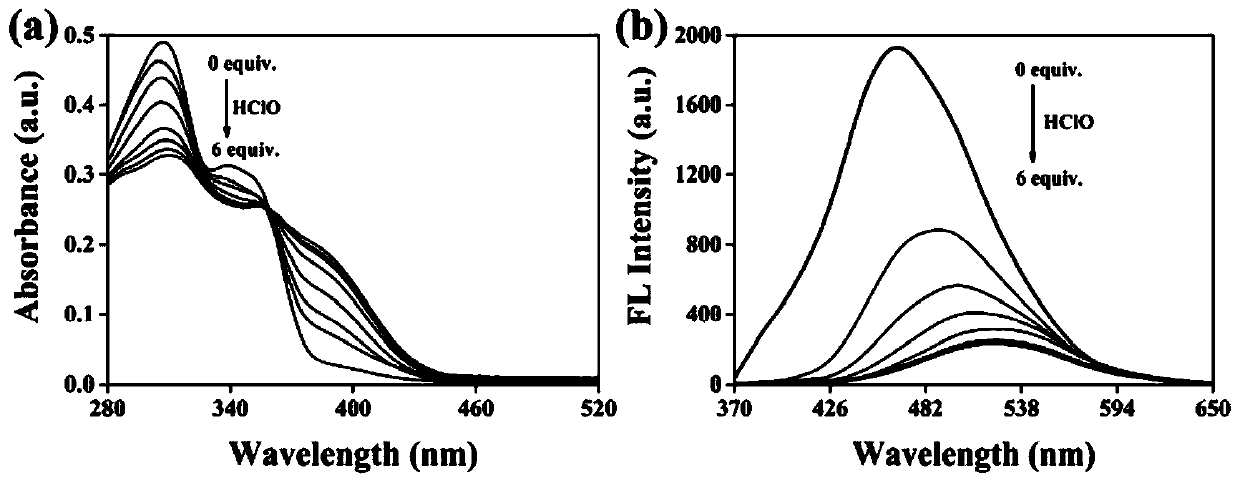
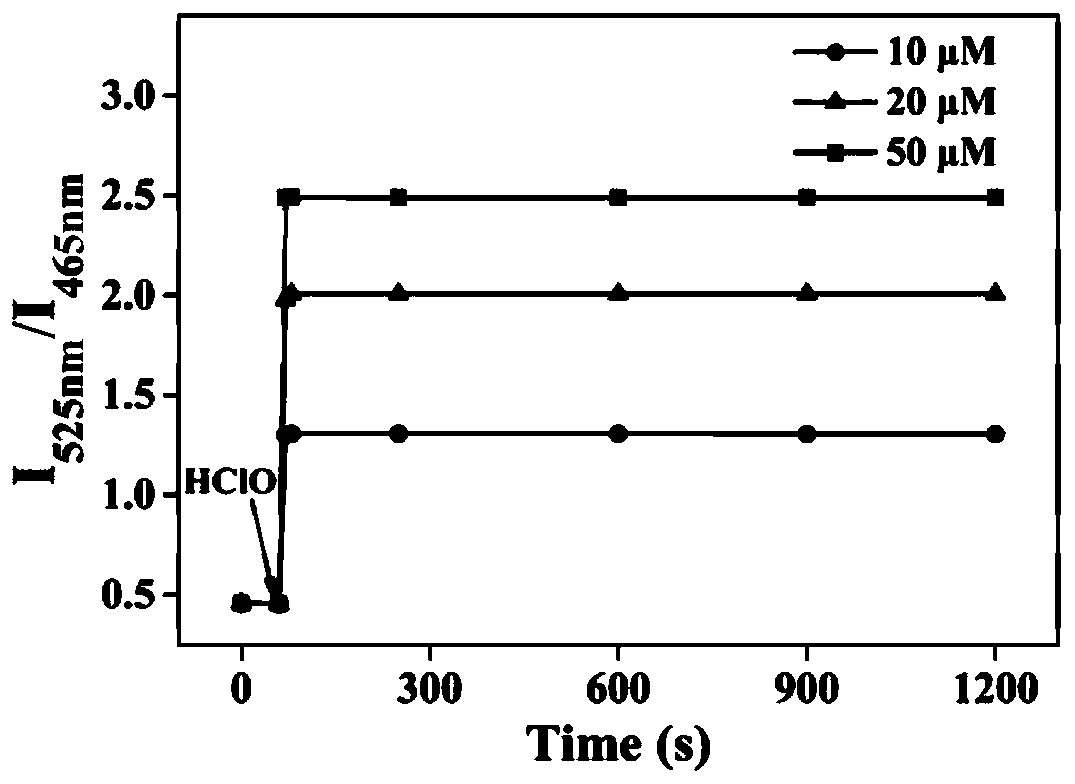
The invention discloses a mitochondria targeting hypochlorous acid ratio type two-photon fluorescent probe as well as a preparation method and application thereof. The mitochondria target hypochlorousacid ratio type two-photon fluorescence probe is prepared with carbazole as a parent and has the structural formula as the specification; when the fluorescent probe is applied to detection of HClO inan aqueous solution, the selectivity is high, the response speed is high, a good linear relationship exists between the fluorescence ratio (I525nm / I465nm) of the probe and the concentration of HClO,and the detection limit is as low as 35nM. In addition, the fluorescent probe molecule also has excellent two-photon absorption performance. Cytotoxicity tests show that the probe hardly has any toxicor side effect on cells. A two-photon confocal fluorescence microscopy imaging experiment shows that the probe has good mitochondria positioning capability and cell membrane permeability, and is suitable for two-photon fluorescence imaging and quantitative detection of HClO in cell mitochondria.
Owner:ANHUI UNIVERSITY
Method for testing in vitro cytotoxicity of cigarette smoke
ActiveCN102618619BWide linear rangeHigh sensitivityMicrobiological testing/measurementBiotechnologyStaining
The invention discloses a method for testing in vitro cytotoxicity of cigarette smoke. The method is characterized by comprising the following steps of: 1) preparing a laboratory reagent; 2) performing cell inoculation culture; 3) performing cigarette smoke contamination; 4) dyeing with water-soluble tetrazolium-1 (WST-1); and 5) obtaining a result and analyzing. Compared with the prior art, the method is characterized in that a test step for washing cells for multiple times is eliminated in the whole test process, so that the method is easy and convenient to operate; a step of replacing a nutrient solution is not required during dyeing with the WST-1, and a diluted nutrient solution can be directly added for reacting; formazan produced by the WST-1 is water-soluble, so that a subsequent dissolution step is eliminated; and absorbance detection can be finished in 2 hours after WST-1 dyeing, so that the test period is shortened, and the method is quick in comparison with the conventional testing method. The measuring method is quick and convenient to operate, and also has the advantages of high sensitivity and stable result; and the method can be applied to smoke cytotoxicity test of multiple cell lines.
Owner:ZHENGZHOU TOBACCO RES INST OF CNTC
Digital microarray organ chip and application thereof
PendingCN108641931AHigh simulationImprove throughputBioreactor/fermenter combinationsBiological substance pretreatmentsMicrosphereEngineering
The invention provides a digital microarray organ chip. The digital microarray organ chip comprises a top plate, a PET film and a bottom plate. An upper chamber is formed in the top plate, a lower chamber is formed in the bottom plate, and the upper chamber and the lower chamber are consistent in shape and are separated through the PET film; microarray holes used for microsphere culturing and digital detecting are arrayed in the lower chamber of the bottom plate; and an input pipeline and an output pipeline are connected to the top plate and communicate with the upper chamber. The digital microarray organ chip has the characteristics of being biomimetic, high in throughput and low in cost, and has good prospects in the aspects of clinical application of drug screening, disease modeling, cytotoxicity testing and the like.
Owner:ZHEJIANG UNIV
All-water polyurethane self-skinning foam composition
Provided is an all-water polyurethane self-skinning foam composition. The all-water polyurethane self-skinning foam composition is applied to armrests of treadmills, guard railings of highways, backrests of treatment facilities, and the like, and is composed of, by weight, 100 parts of combined polyether and 30-45 parts of isocyanate. The combined polyether mainly contains polypropylene oxide glycol with a low degree of unsaturation and with the hydroxyl value being 33-37 mgKOH / g, propylene oxide-ethylene oxide copolyether with the hydroxyl value being 21-27 mgKOH / g, a cross-linking agent, water, a catalyst, a surface active agent, a light stabilizer and nano-scale silicon carbide, wherein the cross-linking agent comprises at least two hydrogen atoms which can react with isocyanate, and the molecular weight of the cross-linking agent is 60-200. The all-water polyurethane self-skinning foam composition has excellent compression resistance and collision resistance, is wide in range of abrasion resistance, oil resistance and hardness and high in strength and elongation, and achieves shock absorption. The all-water polyurethane self-skinning foam composition can pass the standard ISO10993-5-2009 in-vitro cell toxicity test.
Owner:苏州库凌科高分子新材料有限公司
In-vitro cytotoxicity testing device for anesthesia breathing pipeline and working method of in-vitro cytotoxicity testing device
PendingCN111467627AEvaluation scienceAccurate evaluationRespiratorsBioreactor/fermenter combinationsBiocompatibility TestingBreathing system
The invention discloses an in-vitro cytotoxicity testing device for an anesthesia breathing pipeline and a working method of the in-vitro cytotoxicity testing device, and belongs to the technical field of medical instrument inspection. The testing device comprises a gas power control device, an in-vitro artificial lung breathing system and gas condensate water collecting equipment, wherein the gaspower control device comprises a cylinder for simulating breathing movement; the in-vitro artificial lung breathing system comprises a gas storage container; a gas bag is arranged in the gas storagecontainer; an airtight water seal device is arranged below the gas storage container; the in-vitro artificial lung breathing system is positioned in a heat insulation box body; and the gas condensatewater collecting equipment comprises a condensing pipe and a condensate liquid collecting bottle. According to the technical scheme of the embodiment of the invention, the cytotoxicity index of the gas pipeline is tested by simulating clinical actual use condition, the collected gas condensate liquid can be used for other biocompatibility testing requirements, and the biocompatibility testing problem of gas pipeline type medical instruments for anesthesia breathing is fundamentally solved.
Owner:山东省医疗器械产品质量检验中心
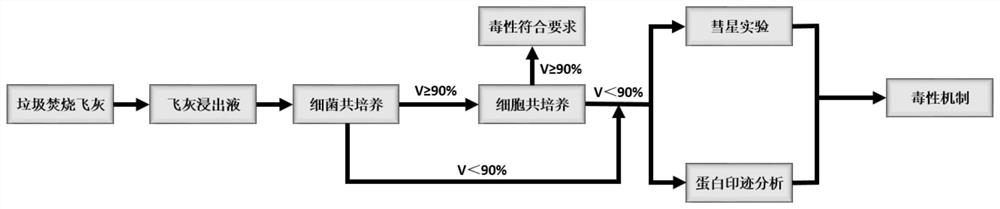
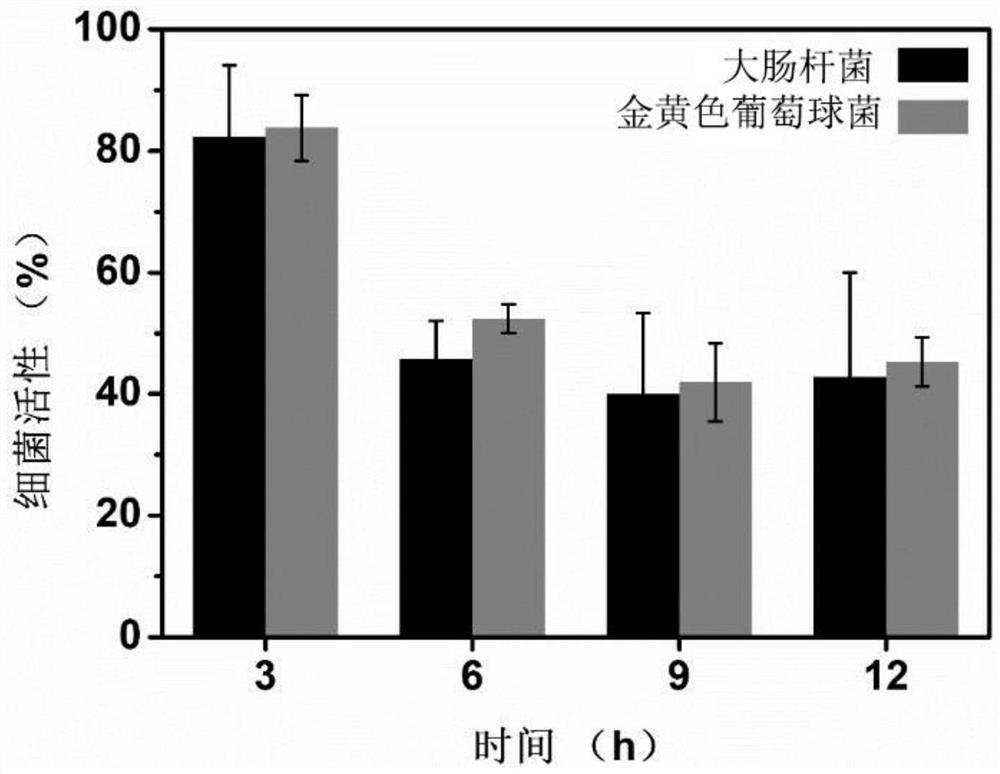
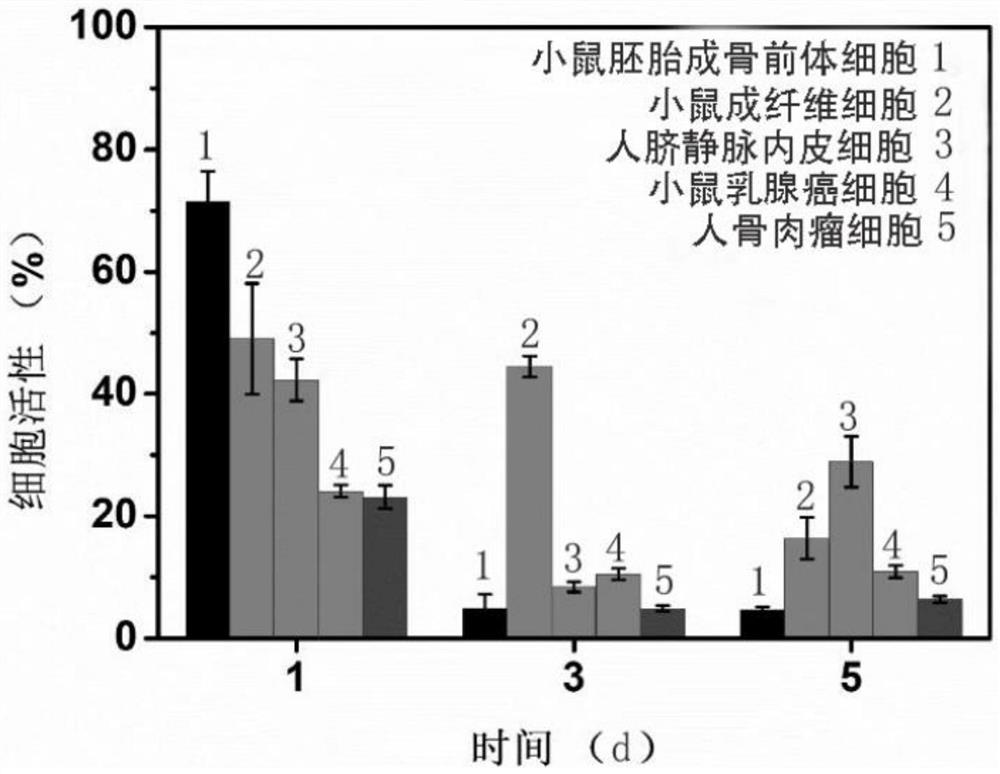
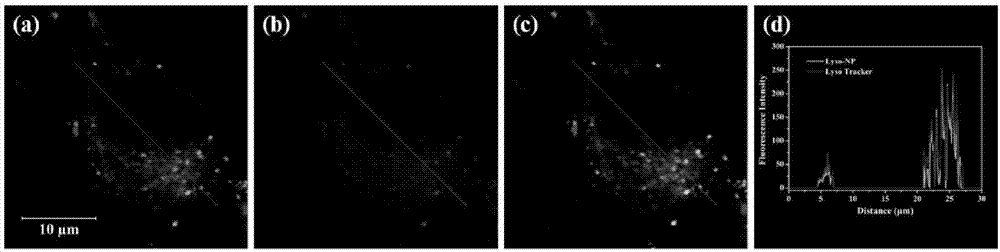
Method for evaluating leaching toxicity of municipal waste incineration fly ash
ActiveCN112029813AToxicity Testing and Evaluation ScienceHarm reductionMicrobiological testing/measurementColor/spectral properties measurementsWestern blotCulture bacteria
The invention discloses a method for evaluating leaching toxicity of municipal waste incineration fly ash. The method comprises the following steps: S1, preparing a waste incineration fly ash sample;S2, preparing a leaching agent; S3, adding the sample prepared in the step S1 into the leaching agent prepared in the step S2 to prepare a leaching solution; S4, preparing a bacterial culture solution; S5, culturing the bacteria and evaluating the activity of the bacteria; S6, if the bacterial activity in the step S5 is qualified after being detected, further preparing a cell culture solution, andif the bacterial activity in the step S5 is unqualified after being detected, performing comet assay and western blot detection; S7, culturing cells; S8, taking the cells cultured in the step S7 formulti-cytotoxicity test, and evaluating the cell activity; S9, if the cell activity in the step S8 is detected to be qualified, ending the test, and if the cell activity is detected to be unqualified,performing comet assay; and S10, performing western blot analysis by the cells obtained in the step S7. According to the method, the toxic reaction of bacteria and biological cells to the leaching solution is directly utilized, and the method can be used for scientifically evaluating the toxicity detection of the waste incineration fly ash.
Owner:SICHUAN UNIV
Lysosome targeted two-photonviscosityfluorescence probe and preparation method and application thereof
ActiveCN106892870ALow fluorescence quantum yieldSimple structureOrganic chemistryFluorescence/phosphorescenceSide effectLysosomal targeting
The invention discloses a lysosome targeted two-photon viscosityfluorescence probe and a preparation method and application thereof. The viscosityfluorescence probe uses naphthalimide as a parent, and the structure of the probe is shown in the description. The viscosityfluorescence probe can respond to fluorescence signals having specificity to viscosity. A cytotoxicity test indicates that the lysosome targeted two-photon viscosityfluorescence probe has almost no toxic and side effect on cells, a confocal fluorescence microimaging experiment indicates that the probe has good cell permeability to MCF-7 cells, a lysosome co-positioning experiment indicates that the probe has the good positioning effect on cell lysosome, and the probe can be suitable for detection of the viscosity in the cell lysosome.
Owner:ANHUI UNIVERSITY
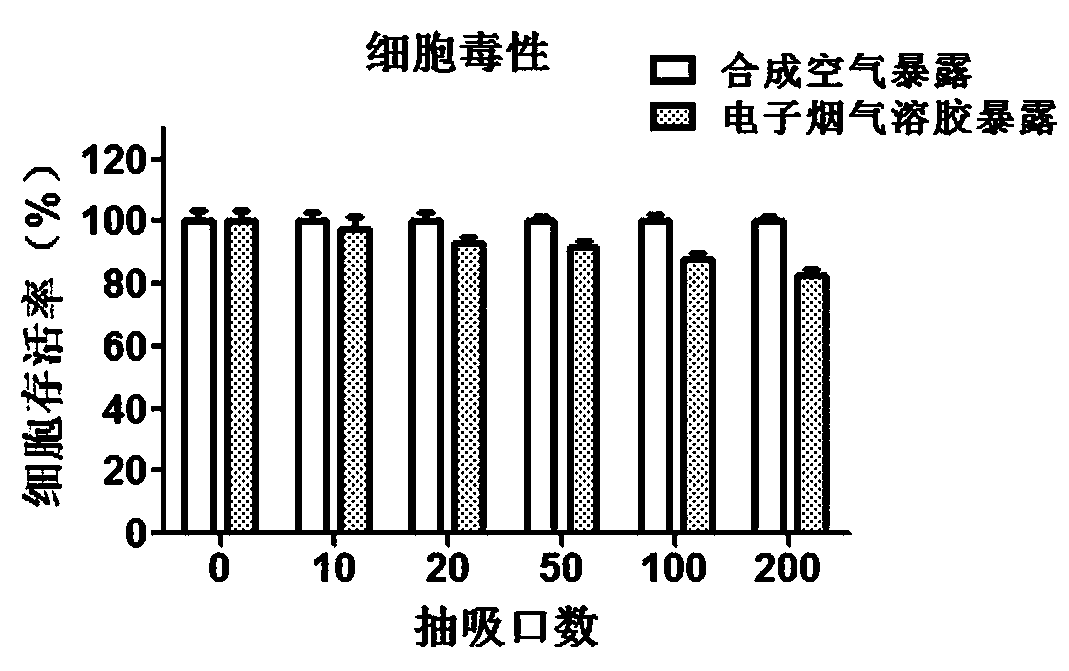
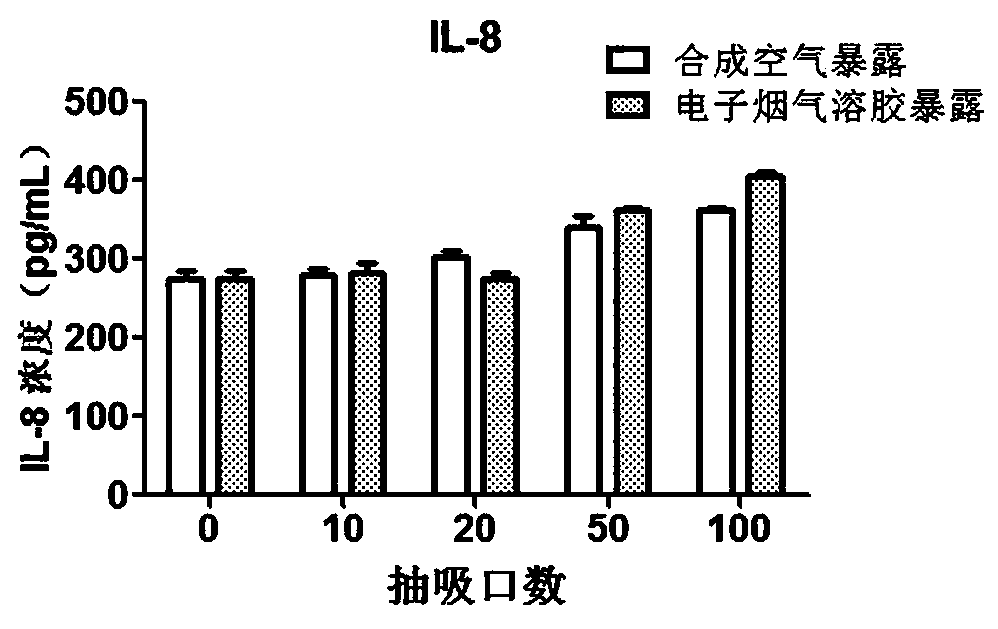
Direct exposure in-vitro toxicity test method for electronic cigarette aerosol
PendingCN109988809AReflect biological effectsEvaluate damage effectsMicrobiological testing/measurementDiagnosticsInflammatory factorsElectronic cigarette
The invention provides a direct exposure in-vitro toxicity test method for electronic cigarette aerosol. The method is characterized in that testing of various cytotoxic terminal points and detectionof release levels of various inflammatory factors can be carried out, and the in-vitro toxicity effect of the electronic cigarette aerosol is reflected more comprehensively. The method specifically comprises the following steps of generation of the electronic cigarette aerosol, direct exposure of cells, testing of cytoxicity and detection of the inflammatory factors. According to the method, an in-vitro toxicity determination method of the electronic cigarette aerosol is established, and an in-vitro cell direct exposure experiment of the electronic cigarette aerosol, testing of the cytotoxic terminal points and the detection of the release levels of the inflammatory factors are integrated. After one-time exposure, the testing of the cytotoxic terminal points can be carried out on the cells, the detection of the inflammatory factors can be carried out on a cell supernatant, the evaluated toxic terminal points are numerous and efficient, the flux is high, and the in-vitro toxicity effectof the electronic cigarette aerosol can be represented comprehensively from multiple aspects.
Owner:ZHENGZHOU TOBACCO RES INST OF CNTC
Preparation method for TIA yeast peptide fermentation product filtrate and function application thereof in skin-whitening and freckle-removing
InactiveCN108066172AAvoid harmAvoid stabilityCosmetic preparationsToilet preparationsWhitening AgentsPreparing skin
The invention discloses a preparation method for TIA yeast peptide fermentation product filtrate and a function application thereof in skin-whitening and freckle-removing. The preparation method comprises the following steps: S1, carefully selecting rice, washing for 1-2 times by using deionized water, and soaking for 6-12 hours in the room temperature; S2, crushing the soaked rice by using a crushing machine, adding distilled water and stirring until the homogenate, saccharifying for 2-12 hours, and cooling to 25-30 DEG C; S3, using pseudomonas saccharomycetes screened out by the amplification, enabling the bacteria density to be 0.5-2.5 OD, for the future fermentation adding and using; and S4, adding the saccharified rice homogenate to a culture medium. The preparation method is capableof performing the saccharomycetes fermentation by using the food material rice and vegetable edible fat and oil. The obtained wall-broken filtrate is safe completely without any external stimulation,and a cytotoxicity test shows the zero. The raw material obtained by using the preparation method can be used for preparing skin-whitening freckle-removing drugs or cosmetics, the cytotoxicity is zero, the raw material is safe and effective without the stimulation, good in stability, and is capable of avoiding the defects that a chemical whitening agent and a plant extractant can injure and stimulate a human body while used for whitening the skin, and the stability is bad.
Owner:重庆雅素生物科技有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com