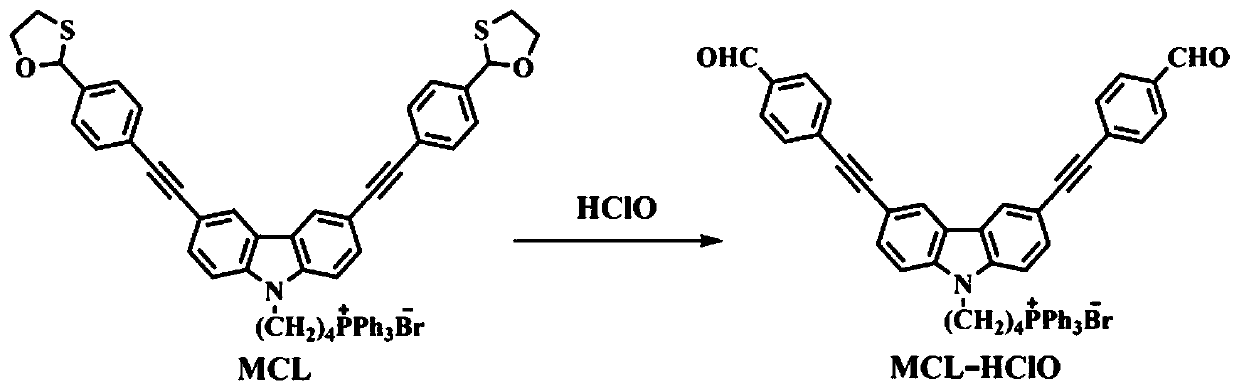
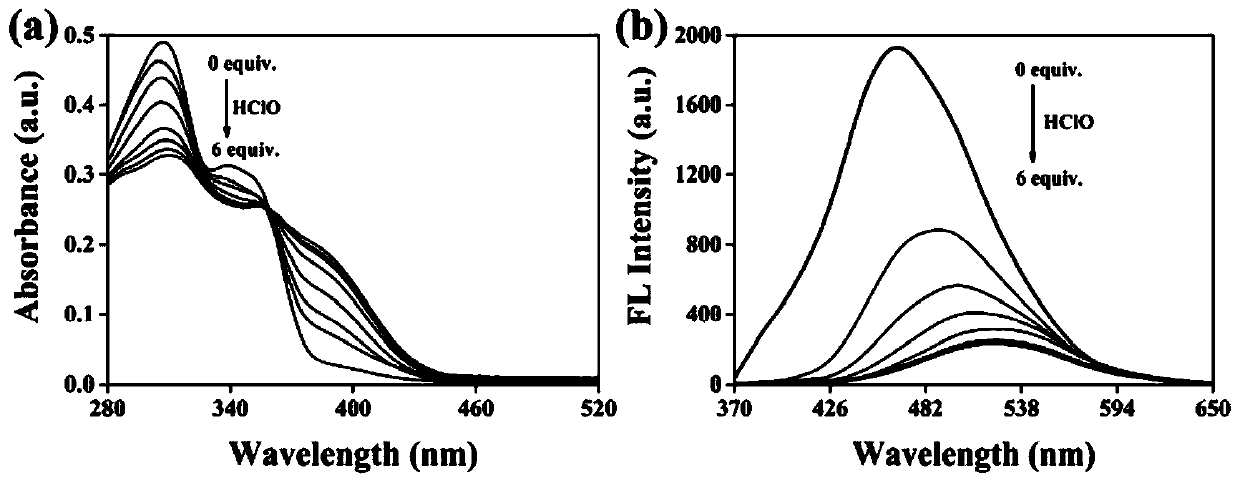
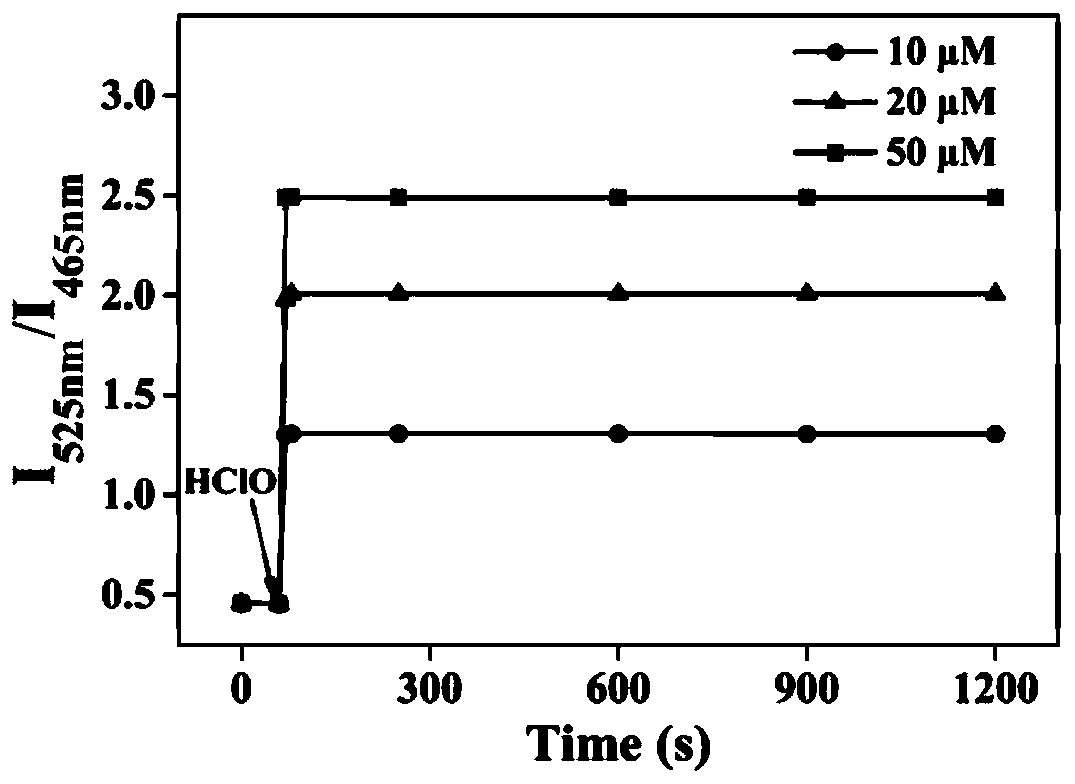
Mitochondria-targeted hypochlorous acid ratio type two-photon fluorescent probe as well as preparation method and application thereof
A two-photon fluorescence, mitochondrial technology, applied in fluorescence/phosphorescence, chemical instruments and methods, luminescent materials, etc., to achieve the effects of easy synthesis, good permeability, and simple structure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Embodiment 1: the synthesis of compound 1
[0035] Potassium hydroxide (2.0g, 35.8mmol), potassium iodide (0.4g, 2.39mmol) and 1,4-dibromobutane (7.73g, 35.8mmol) were added to acetone solution (200mL) and heated at 60°C for 1 Hour. 3,6-Diiodocarbazole (10 g, 23.9 mmol) was added slowly, and heating to reflux was continued for 12 hours. After cooling, it was spin-dried, and the crude product was obtained after washing with water. Purification by column chromatography (petroleum ether:dichloromethane=10:1 as eluent) gave Intermediate 1, 6.8g, with a yield of 51.4%. 1 H NMR (400MHz, CDCl 3 ,ppm)δ8.34(d,J=1.5Hz,2H),7.74(d,J=1.6Hz,1H),7.71(d,J=1.6Hz,1H),7.19(s,1H),7.17( s, 1H), 4.29(t, J=7.0Hz, 2H), 3.37(t, J=6.4Hz, 2H), 2.06–1.98(m, 2H), 1.86(m, 2H). 13 C NMR (100MHz, CDCl 3 , ppm) δ139.38, 134.69, 129.48, 124.08, 110.76, 81.93, 42.37, 32.82, 30.02, 27.44.
Embodiment 2
[0036] Embodiment 2: the synthesis of compound 2
[0037] Under the condition of nitrogen protection, compound 1 (2g, 3.6mmol), 4-ethynyl benzaldehyde (1.4g, 10.8mmol), bistriphenylphosphine palladium dichloride (0.0102g, 0.014mmol), iodide Cuprous (0.0054 g, 0.028 mmol) and triethylamine (7 mL) were dissolved in tetrahydrofuran (10 mL), and reacted at 30° C. for 12 hours. After cooling, it was spin-dried to obtain a crude product. Purification by column chromatography (petroleum ether:dichloromethane=4:1 as eluent) gave Intermediate 2, 1.3 g, with a yield of 64.5%. 1 HNMR (400MHz, CDCl 3 ,ppm)δ10.04(s,2H),8.33(s,2H),7.89(t,J=7.4Hz,4H),7.71(m,6H),7.42(d,J=8.5Hz,2H), 4.38(m, 2H), 3.42(t, J=6.3Hz, 1H), 3.19(t, J=6.6Hz, 1H), 2.15–2.01(m, 2H), 1.92(m, 2H). 13 C NMR (100MHz, CDCl 3 ,ppm)δ191.47,140.67,135.13,133.12,131.92,130.18,130.14,129.67,129.59,124.63,122.62,113.56,109.14,94.83,87.58,32.84,30.74,254.59,
Embodiment 3
[0038] Embodiment 3: the synthesis of compound 3
[0039] Under nitrogen protection, compound 2 (1 g, 1.8 mmol) was added to acetonitrile (10 mL), reacted at 80° C. for 1 hour, then added triphenylphosphine (2.8 g, 10.8 mmol), and continued heating for 36 hours. After cooling, it was spin-dried to obtain a crude product. Purification by column chromatography (dichloromethane:methanol=40:1 as eluent) gave intermediate 3, 0.83g, yield 56%. 1 HNMR (400MHz, DMSO-d 6 ,ppm)δ10.05(s,2H),8.53(s,2H),7.98(d,J=8.0Hz,4H),7.87(m,3H),7.79(d,J=8.0Hz,4H), 7.76–7.68(m,16H),4.51(t,J=6.9Hz,2H),3.62(t,J=15.1Hz,2H),2.00–1.93(m,2H),1.62(m,2H). 13 C NMR (100MHz, CDCl 3 ,ppm)δ191.43,140.73,135.13,135.08,135.05,133.60,133.50,132.15,132.05,131.88,130.50,130.37,130.20,130.08,129.67,128.57,128.45,124.24,122.31,118.10,117.24,113.34,110.05,94.88 ,87.59,42.55,29.19,22.66,20.15.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com