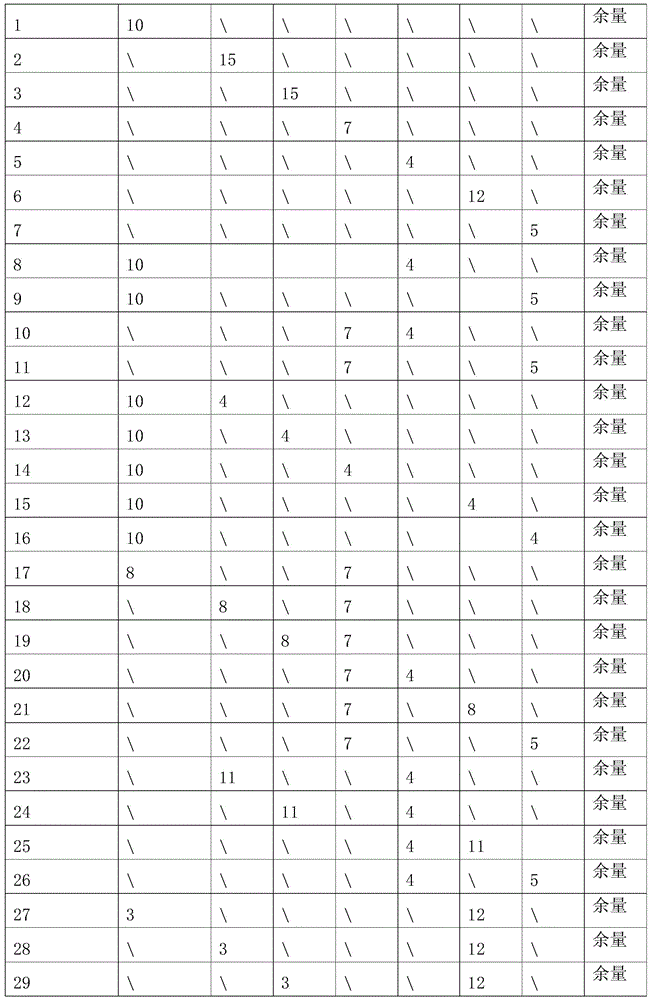
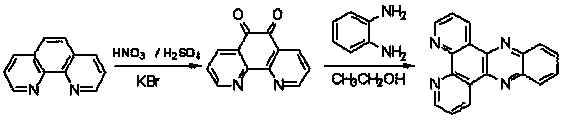
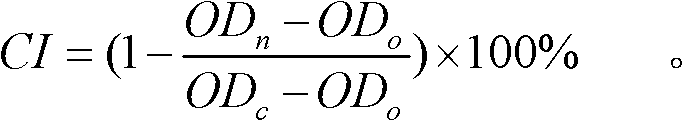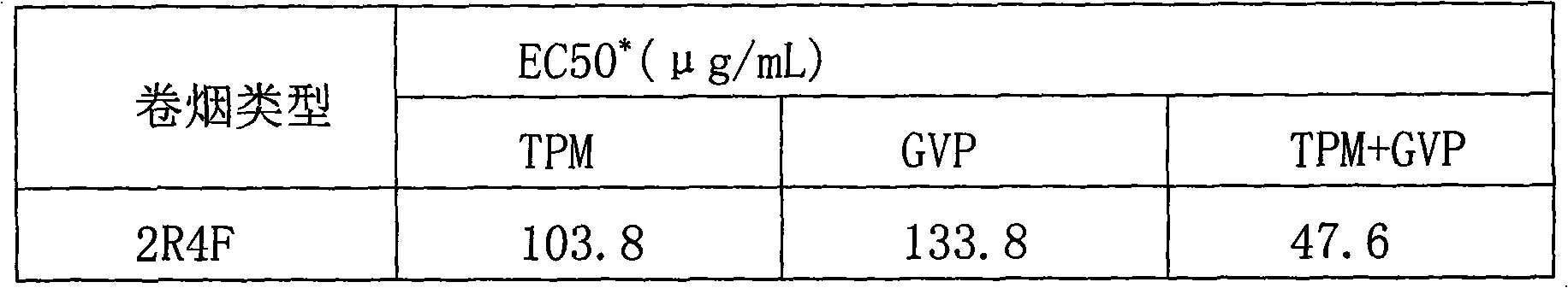Patents
Literature
173 results about "Cytotoxicity test" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Cytotoxicity tests are in-vitro assays used to assess the possibility of a test article to cause the death of cells in culture or to prevent their multiplication. Tests for in vitro cytotoxicity specifies procedures for testing devices by direct or indirect contact, extracts of devices, and filter diffusion.
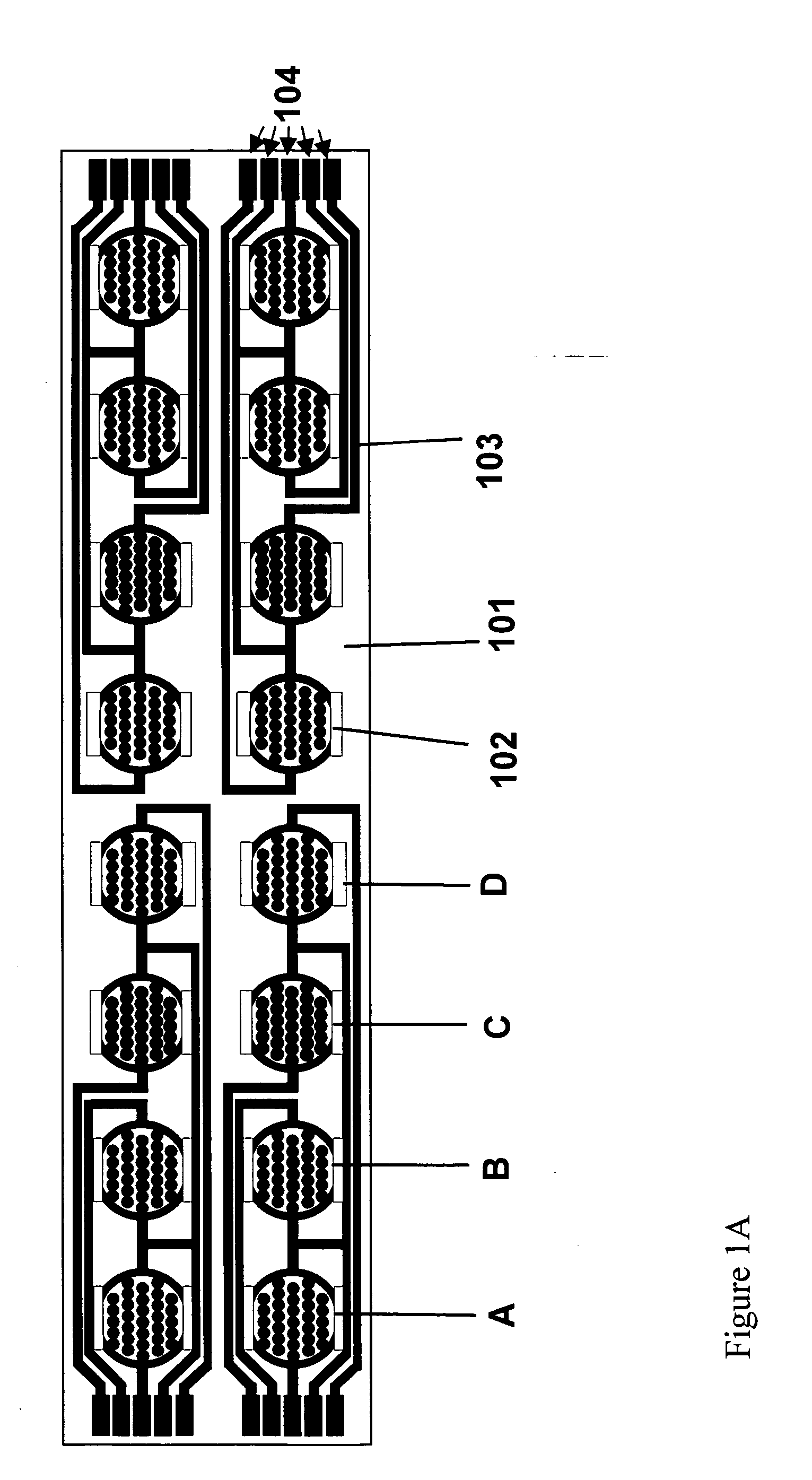
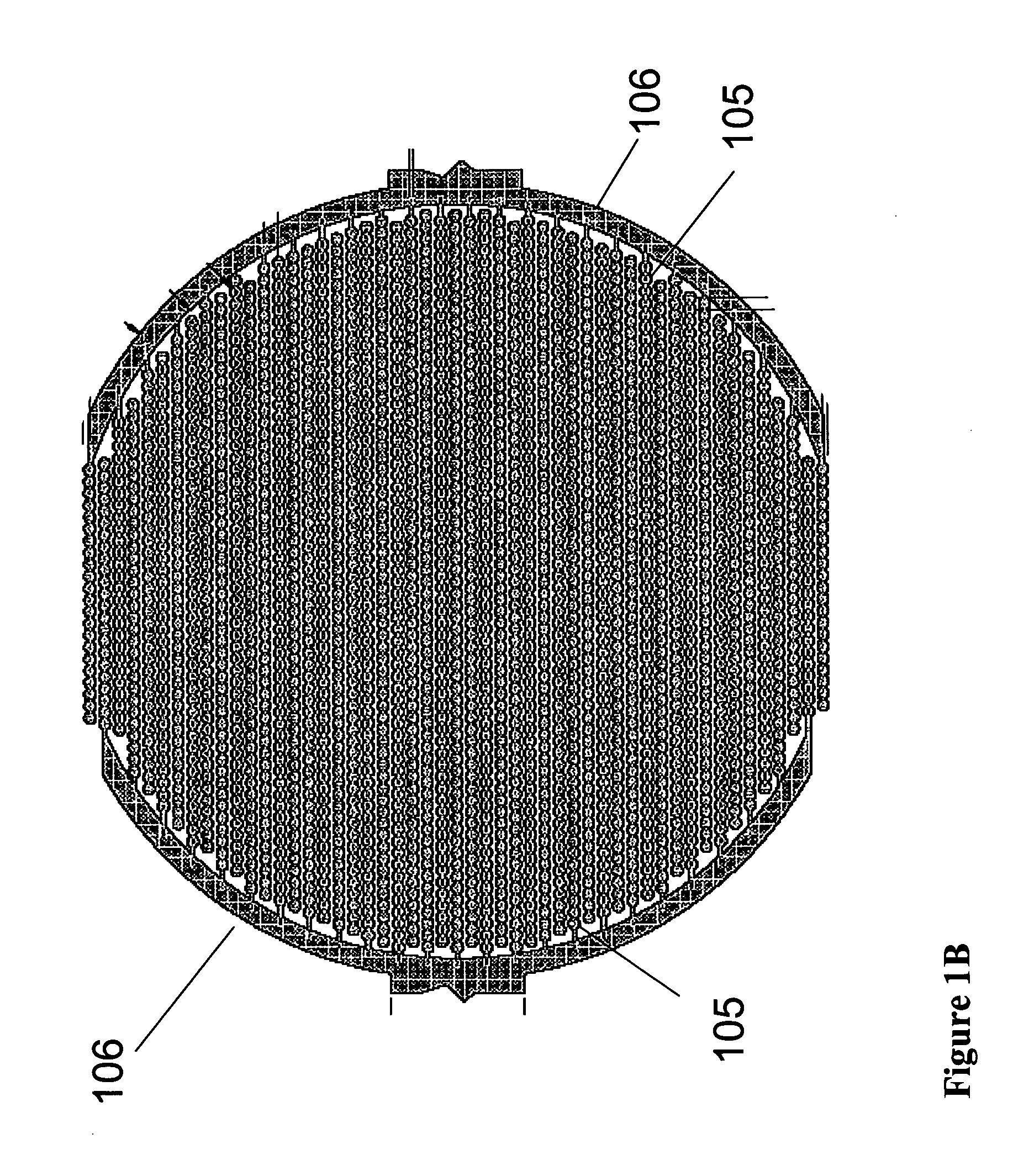
Real time electronic cell sensing systems and applications for cell-based assays
InactiveUS7192752B2Reducing cell-impedance response of cellImmobilised enzymesBioreactor/fermenter combinationsAntigenCytotoxicity
The present invention includes devices, systems, and methods for assaying cells using cell-substrate impedance monitoring. In one aspect, the invention provides cell-substrate impedance monitoring devices that comprise electrode arrays on a nonconducting substrate, in which each of the arrays has an approximately uniform electrode resistance across the entire array. In another aspect, the invention provides cell-substrate monitoring systems comprising one or more cell-substrate monitoring devices comprising multiple wells each having an electrode array, an impedance analyzer, a device station that connects arrays of individual wells to the impedance analyzer, and software for controlling the device station and impedance analyzer. In another aspect, the invention provides cellular assays that use impedance monitoring to detect changes in cell behavior or state. In some preferred aspects, the assays are designed to investigate the affects of compounds on cells, such as cytotoxicity assays. In other preferred aspects, the assays are designed to investigate the compounds that effect IgE-mediated responses of cells to antigens.
Owner:AGILENT TECH INC
Real time electronic cell sensing systems and applications for cell-based assays
InactiveUS20050153425A1Reducing cell-impedance response of cellBioreactor/fermenter combinationsCompound screeningAntigenEngineering
The present invention includes devices, systems, and methods for assaying cells using cell-substrate impedance monitoring. In one aspect, the invention provides cell-substrate impedance monitoring devices that comprise electrode arrays on a nonconducting substrate, in which each of the arrays has an approximately uniform electrode resistance across the entire array. In another aspect, the invention provides cell-substrate monitoring systems comprising one or more cell-substrate monitoring devices comprising multiple wells each having an electrode array, an impedance analyzer, a device station that connects arrays of individual wells to the impedance analyzer, and software for controlling the device station and impedance analyzer. In another aspect, the invention provides cellular assays that use impedance monitoring to detect changes in cell behavior or state. In some preferred aspects, the assays are designed to investigate the affects of compounds on cells, such as cytotoxicity assays. In other preferred aspects, the assays are designed to investigate the compounds that effect IgE-mediated responses of cells to antigens.
Owner:AGILENT TECH INC
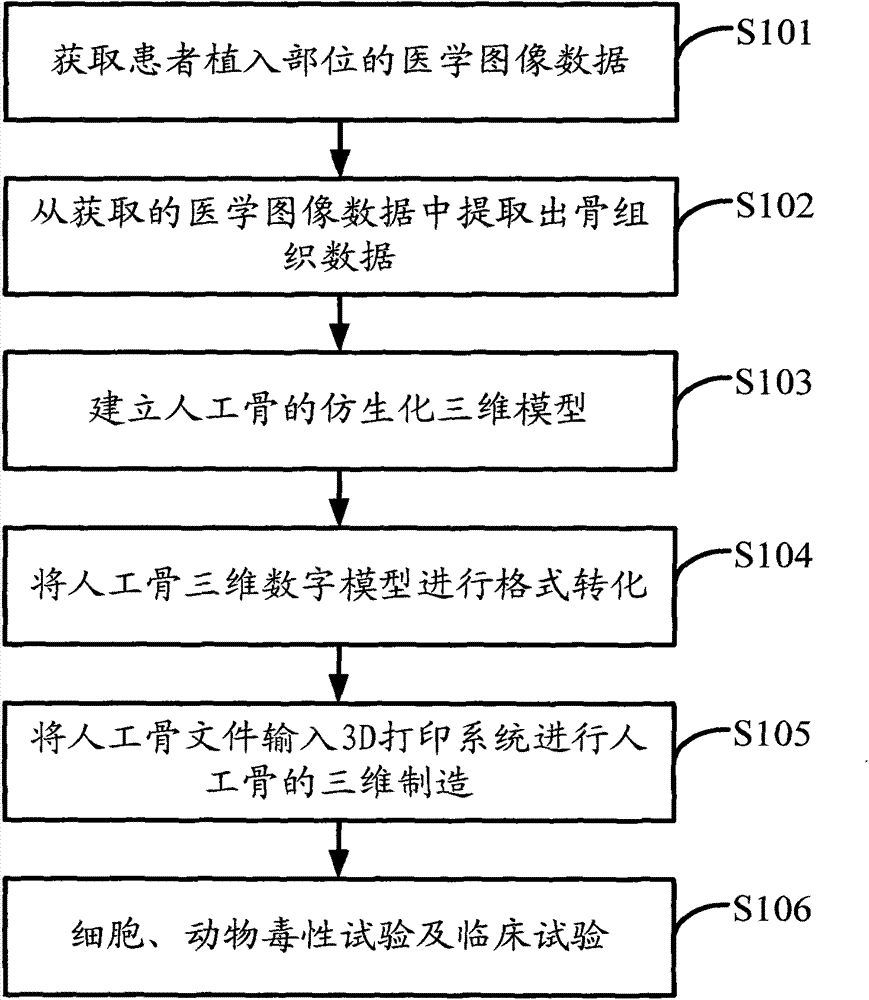
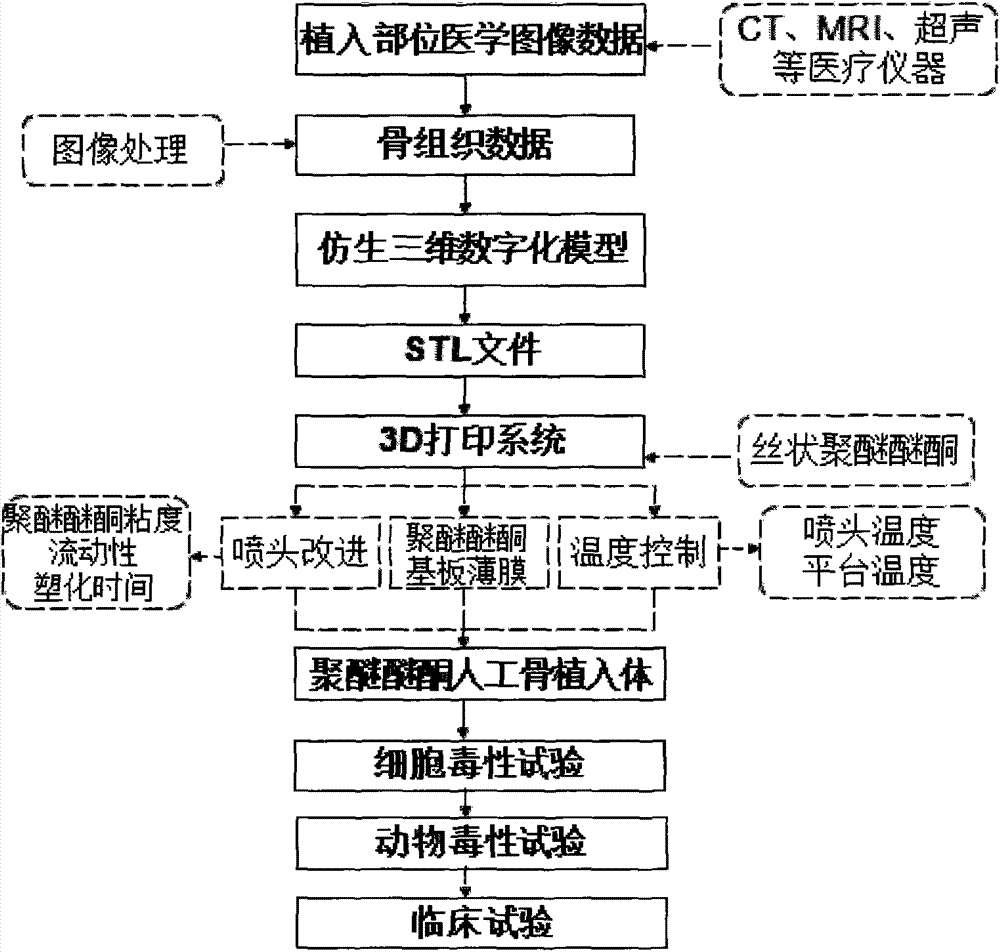
Polyether-ether-ketone biomimetic artificial bone 3D printing manufacturing method
ActiveCN103707507ASave timeSave costSpecial data processing applications3D modellingBiocompatibility TestingArtificial bone
The invention discloses a polyether-ether-ketone biomimetic artificial bone 3D printing manufacturing method, wherein the artificial bone can replace metal and has an excellent biocompatibility. The method comprises the following steps: first, collecting the bone tissue image data of the part, which is about to be implanted with an artificial bone, of a patient by using a medical instrument; secondly, establishing a three-dimensional digital model of the artificial bone on the basis of the collected data; thirdly, carrying out a format conversion on the three-dimensional digital model of artificial bone, inputting the converted file into a 3D printing system to manufacture the artificial bone; and finally carrying out cell toxicity tests, animal tests, and clinical tests. The invention utilizes a self-made polyether-ether-ketone 3D printing system to manufacture artificial bones, thus the time and cost for manufacturing moulds are saved, the manufacture period is shortened; at the same time, the shape of parts can be adjusted at any time according to the setting of the forming software; so that an crystalline polymer polyether-ether-ketone artificial bone, which has excellent biocompatibility, can be implanted into the human body, and has the advantages of high melting point, large viscosity, and bad fluidity, can be manufactured through the 3D printing method.
Owner:JILIN UNIV
Natural polymer-based nano-fibrous membrane prepared by freeze-drying method
The invention relates a biodegradable and absorbable natural polymer-based nano-fibrous membrane prepared by a freeze-drying method, and the application thereof. The natural polymer-based nano-fibrous membrane is prepared through the following steps of: dissolving natural polymer powder into a corresponding solvent so as to prepare an extremely-dilute solution with the concentration of 0.001-0.1wt %; after the natural polymer powder is completely dissolved in the solvent, transferring the obtained natural polymer solution into a liquid nitrogen refrigerating device, so that the natural polymer solution is rapidly frozen in a liquid nitrogen environment; then, carrying out freeze-drying treatment on the obtained product in a freeze drier for 12-48 hours to obtain natural polymer-based nano fibers; and carrying out cross-linking on the obtained natural polymer-based nano fibers by a corresponding cross-linking agent to obtain a natural polymer-based nano-fibrous membrane, and then carrying out MTT (methyl thiazolyl tetrazolium) cytotoxicity test and cell vaccination experiments on the natural polymer-based nano-fibrous membrane, with the obtained results showing that the obtained fibrous membrane has no toxicity but has excellent cell adhesion and proliferation properties. The natural polymer-based nano-fibrous membrane disclosed by the invention is simple in the operation process, easy to control and low in cost; and by using the nano-fibrous membrane disclosed by the invention, ultra-fine natural polymer-based nano fibers can be prepared continuously on a large scale.
Owner:BEIJING UNIV OF CHEM TECH
Two-photon fluorescent probe as well as preparation method and application thereof
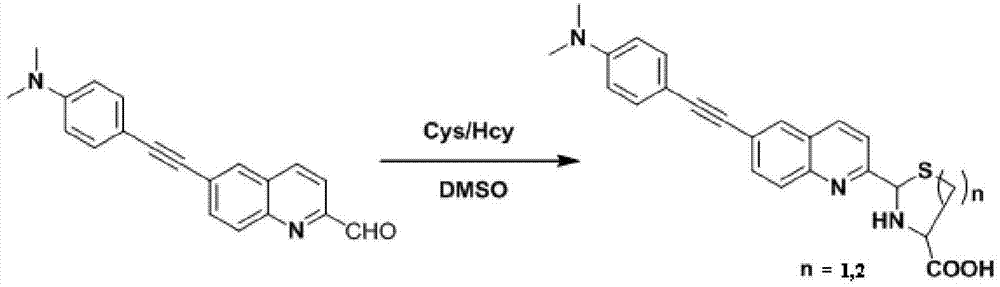
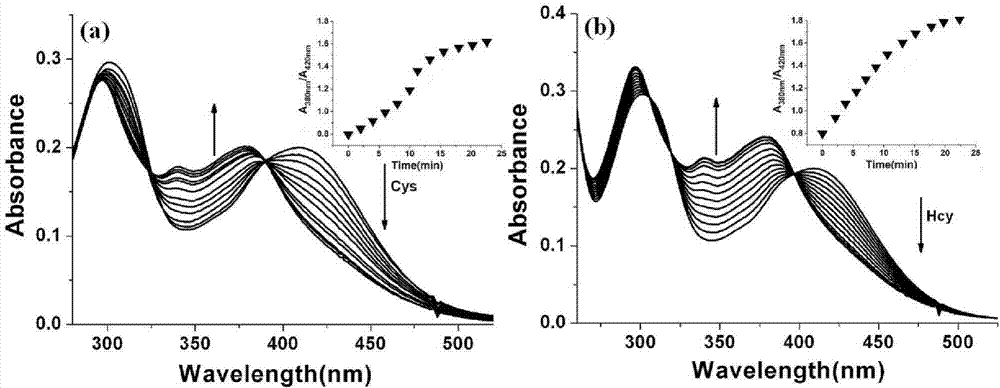
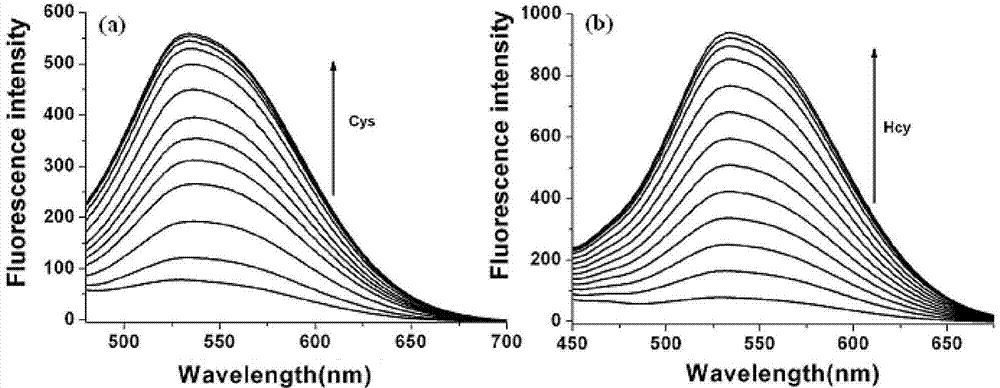
InactiveCN103614135ALow fluorescence quantum yieldSimple structureOrganic chemistryFluorescence/phosphorescenceFluorescenceCytotoxicity testing
The invention discloses a two-photon fluorescent probe as well as a preparation method and an application thereof. The structure of the two-photon fluorescent probe is as shown in the specification. The two-photon fluorescent probe presents relatively high selectivity and high sensitivity in a coexisting system of aminothiopropionic acid or homocysteine and other amino acids. The cytotoxicity test shows that the two-photon fluorescent probe almost has no toxicity on cells. The two-photon fluorescent microscopic imaging experiment shows that the two-photon fluorescent probe is good in permeability on 293FT cells. The two-photon fluorescent probe is suitable for detecting the distribution of amino acid micromolecules in the cells.
Owner:ANHUI UNIVERSITY
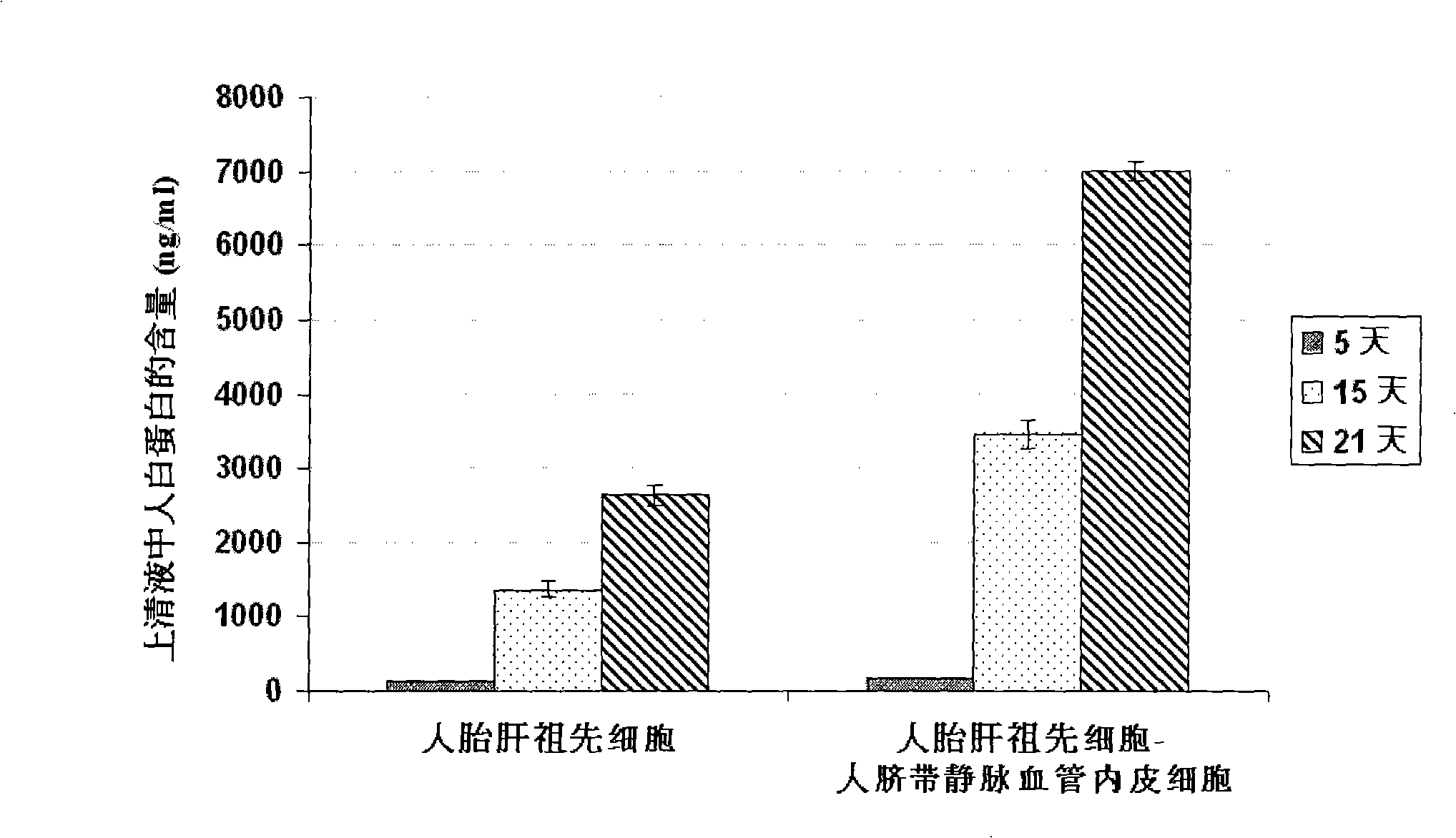
In vitro culture-amplified human liver progenitor cell and preparation thereof
InactiveCN101275121AAccelerate self-renewalConvenient sourceArtificially induced pluripotent cellsNon-embryonic pluripotent stem cellsCell-Extracellular MatrixECM Protein
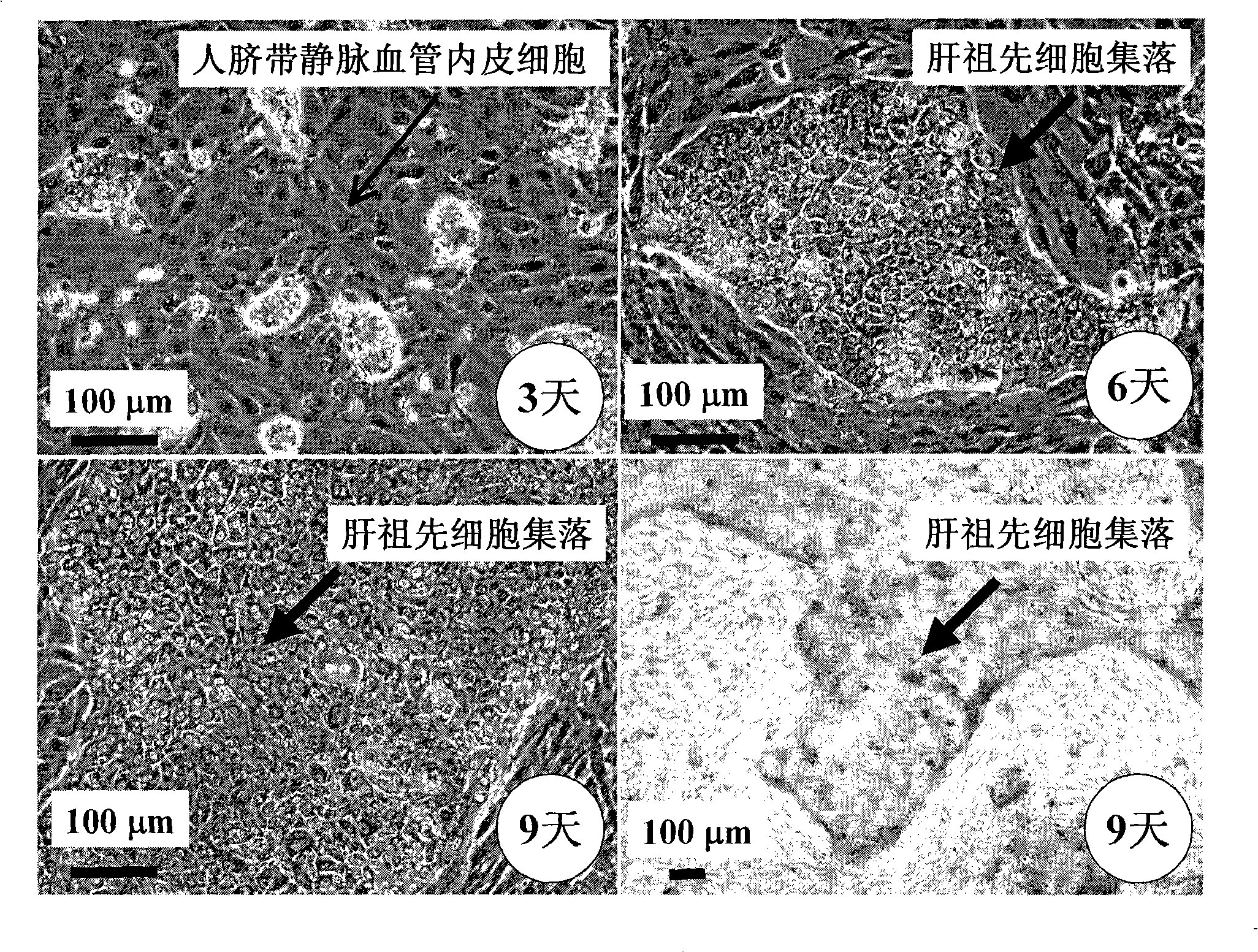
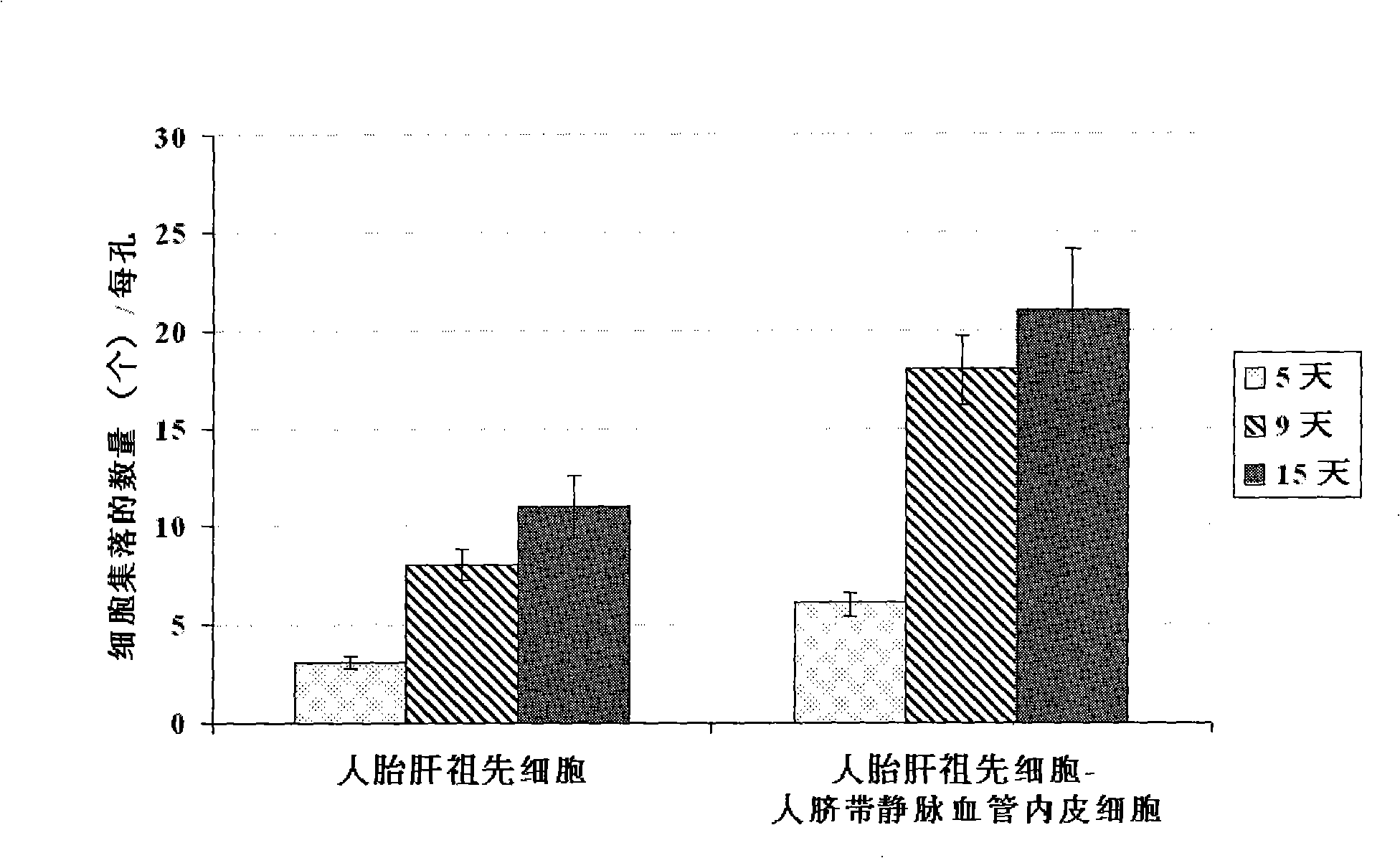
The present invention provides a preparing method of a human hepatic progenitor cell which is amplified and cultivated in vitro, including: a. separating the human hepatic progenitor cell; b. co-cultivating a feeder cell and the human hepatic progenitor cell separated from the step a by a medium having no serum on an extracellular matrix containing human fibrin sealant or other analogues, obtaining a human hepatic progenitor cell colony by amplification. The human hepatic progenitor cell colony is easy to separate from the surface of human fibrin sealant by the simple gelatinolytic band process, purified or / and subcultured by further single cell preparation technology process such as enzymatic degradation etc. The human hepatic progenitor cell prepared by the method is hepatic progenitor cell treatment, including a cell transplantation and a bioartificial liver support system, providing excellent human hepatocyte source for cytotoxicity test platform in the drug screening, virus infection and drug screening platform etc.
Owner:芦银雪
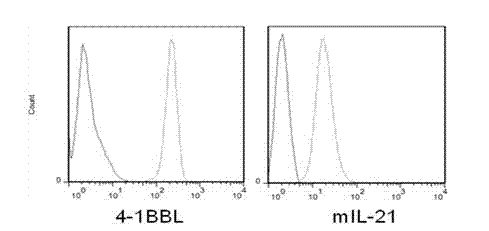
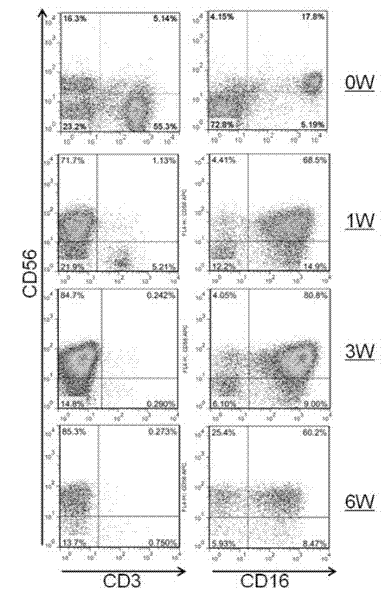
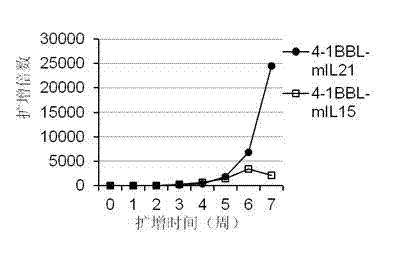
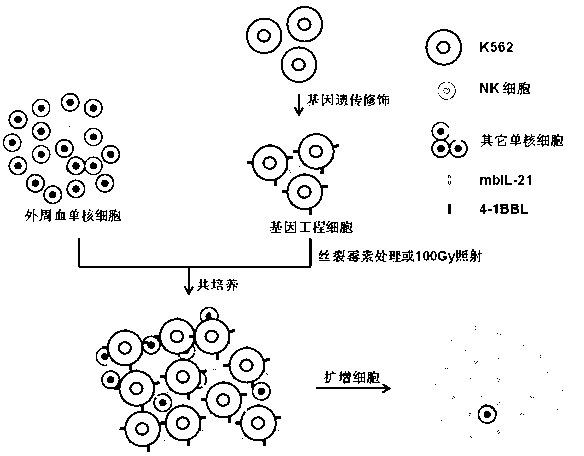
Artificial antigen presenting cell and application thereof in NK (natural killer) cell amplification
InactiveCN102559600AHigh purityStrong cytotoxicityBlood/immune system cellsRecombinant DNA-technologyNatural Killer Cell Inhibitory Receptors4-1BB ligand
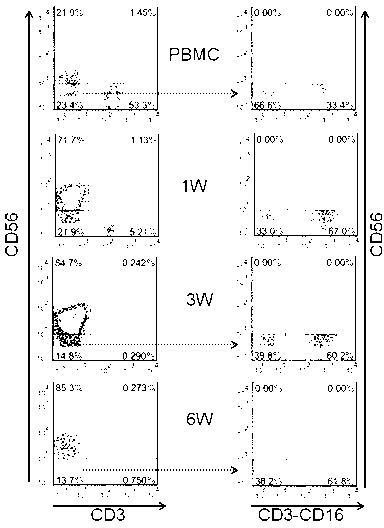
The invention provides an in vitro amplification method for efficient and highly cytotoxic natural killer (NK) cells. Novel artificial antigen presenting cells 4-1BBL-mIL-21-aAPC, such as 4-1BBL-mIL-21-K562 cells and the like, are constructed through stably expressing 4-1BB ligands (4-1BBL) and membrane immobilized interleukin 21 (mIL-21) on the surfaces of cell membranes, and by using the novel artificial antigen presenting cells as feeder cells for amplification, the NK cells are directly amplified from peripheral blood lymphocytes. Flow cytometry, cytotoxicity test and the like suggest that the amplified cells NK have high purity and strong cytotoxicity and have obvious killing effect on tumor cells.
Owner:SHANGHAI JIAOTONG UNIV SCHOOL OF MEDICINE
3-methoxylflavonoid compound, preparation method and application thereof
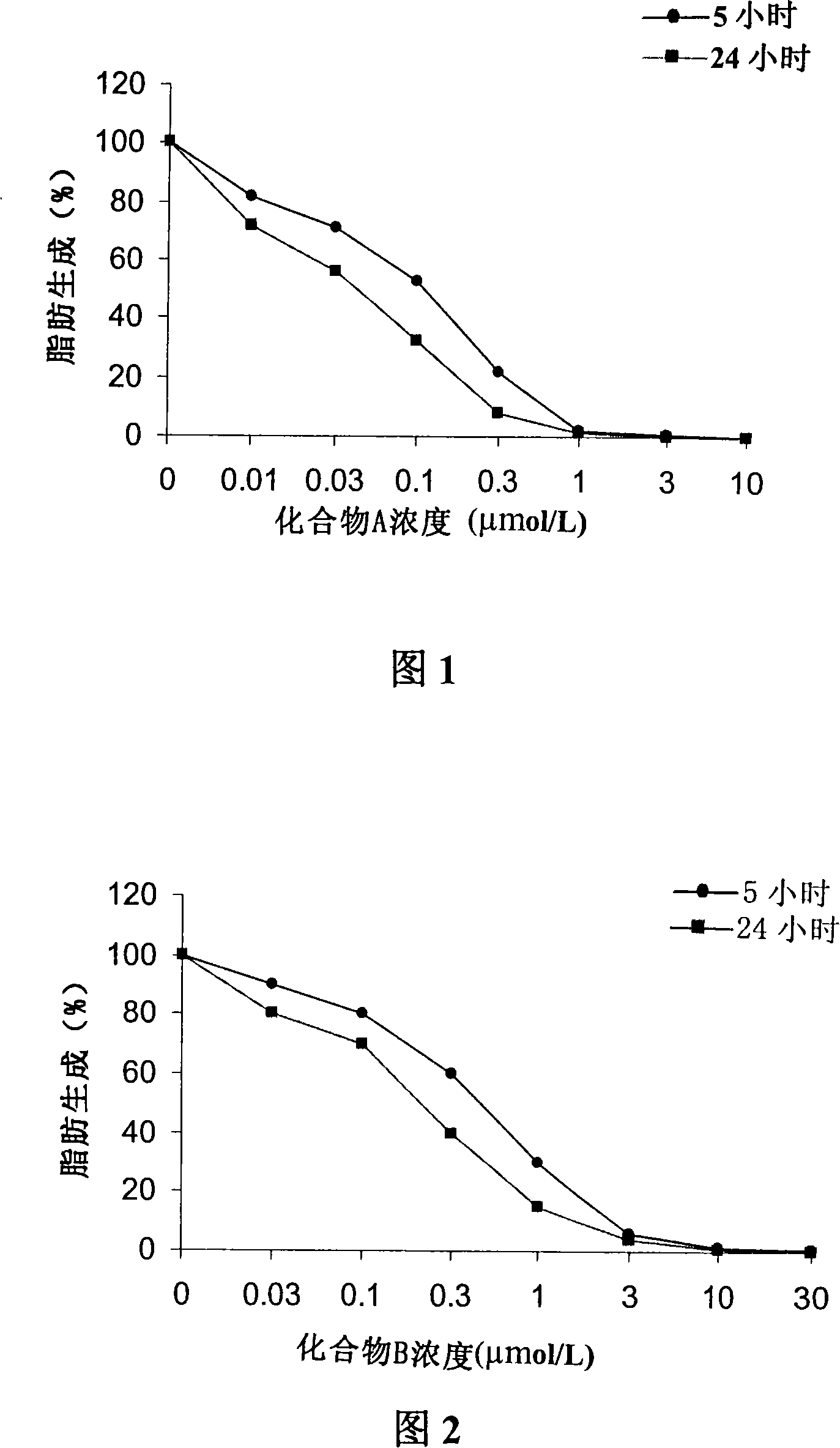
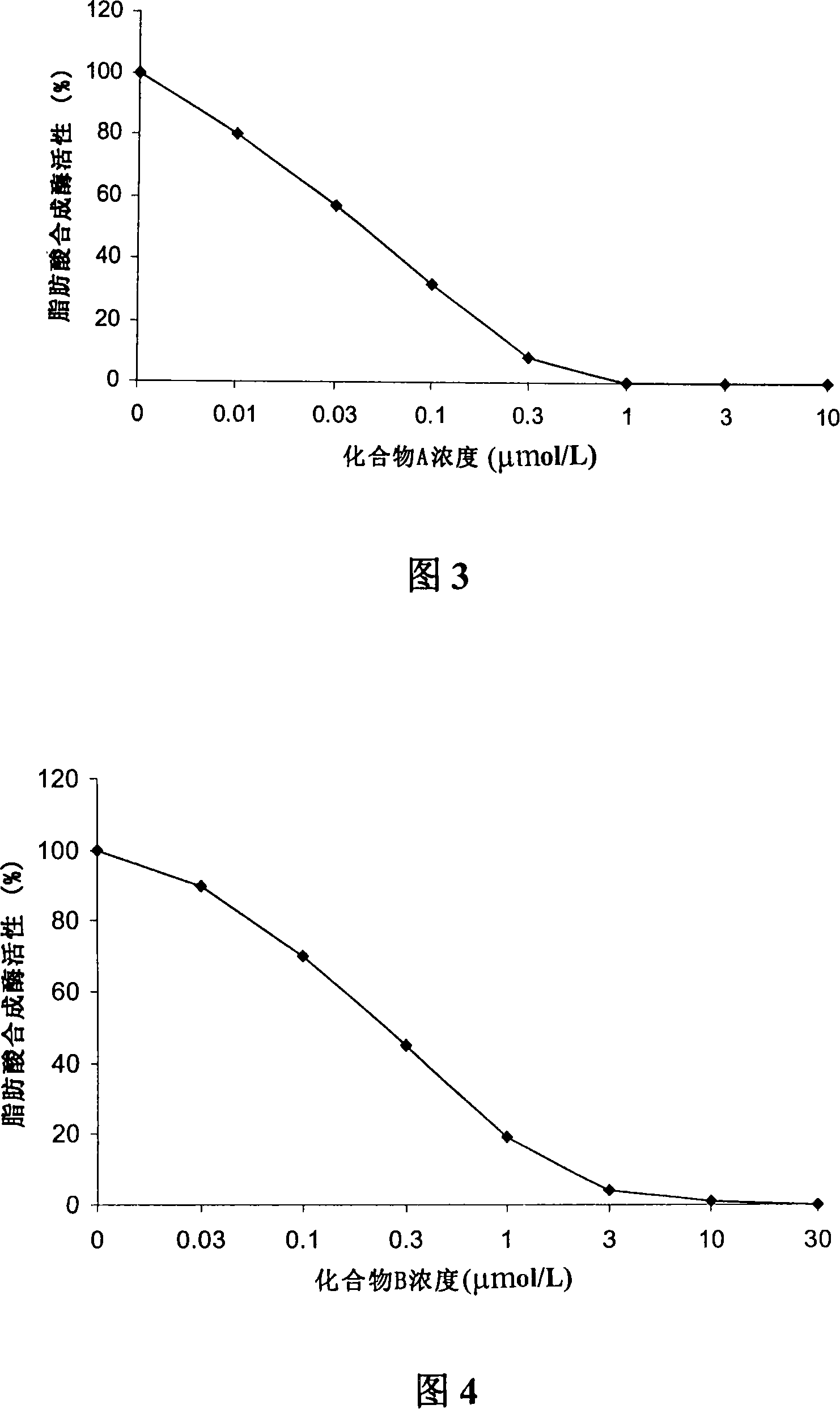
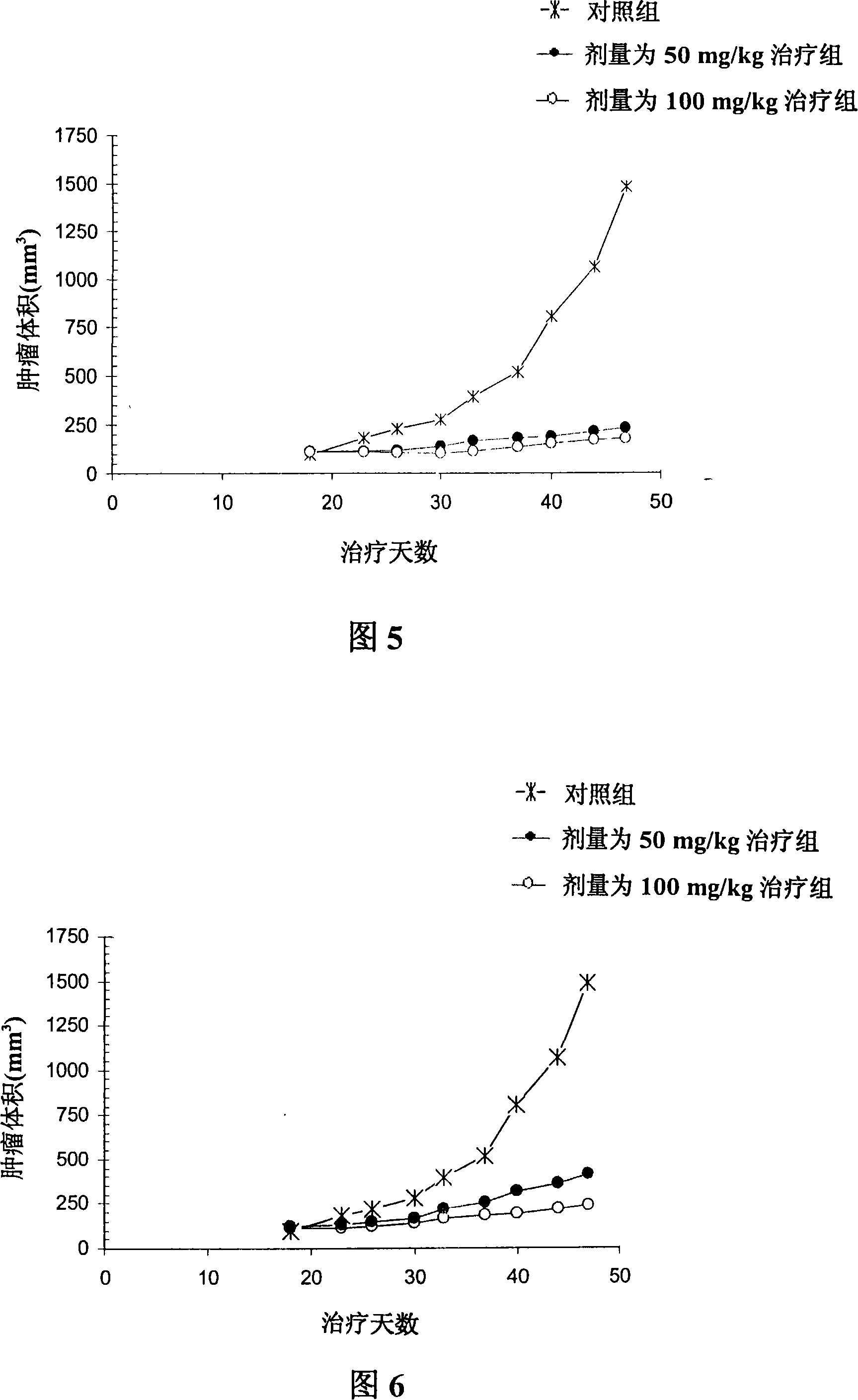
A 3-methoxy-flavone compound comprises 5,7-dihydroxy-8-(3,3-dimethyl diallyl)-3,3', 4'-trimethoxy flavone and 5, 7-2 dihydroxy-8- (3,3-dimethyl allyl)-3,4'-dimethoxy flavonoe. harmacological test results prove that: the 3-methoxy-flavone compound is an effective fatty acid synthase inhibitor, which shows the broad-spectrum anti-tumor effect in the cytotoxicity tests of various tumor cell lines and has strong tumor growth inhibiting effect on human prostate cancer cell LnCAP, human breast cancer cell ZR-75-1, human lung cancer cell NCI-H23 or human colon cancer cell HCT-116 when applied to human tumor transplant nude mice model tests. In addition, the 3-methoxy-flavone compound reveals no toxicity in mice acute toxicity tests, which then can be used as anti-tumor drug and is a new broad-spectrum anti-tumor drug with great development prospects.
Owner:殷正丰 +2
Full-degradable magnesium alloy and preparation method thereof
InactiveCN105671391AGood biocompatibilityControllable degradation rateSurgeryCardiovascular stentBiocompatibility Testing
The invention discloses a novel full-degradable magnesium alloy cardiovascular stent material. A full-degradable magnesium alloy comprises magnesium and alloy elements, wherein the weight ratio of the magnesium is not smaller than 85%, and the alloy elements include a combination of one or more of gadolinium, erbium, thulium, yttrium, neodymium, holmium and zinc. Mechanical properties of the full-degradable magnesium alloy can meet requirements of cardiovascular biological stents, in-vitro immersion corrosion tests and electrochemical corrosion tests prove that the in-vitro corrosion resistance of the full-degradable magnesium alloy is good, in-vitro cytotoxicity tests of the full-degradable magnesium alloy indicate the good biocompatibility, the degradation velocity is controllable, and the biocompatibility is good.
Owner:周倩 +1
Gene engineering cell and application thereof in NK (Nature Killer) cell proliferation
InactiveCN102911918AStrong cytotoxicityHigh purityBlood/immune system cellsForeign genetic material cellsHigh cellCancer cell
The invention discloses a gene engineering cell and application thereof in NK (Nature Killer) cell proliferation, and provides an in vitro proliferation method of an NK cell with high efficiency and high cytotoxicity. A 4-1BB ligand (4-1BBL) and a membrane fixed interleukin 21 (mbIl-21) are stably expressed on the surface of a cell membrane by adopting a genetic engineering technology for constructing 4-1BBL-mbIl-21-gene engineering cells (4-1BBL-mbIl-21-Gene Engineering Cells, 4-1BBL-mbIl-21-GEC), such as 4-1BBL-mbIl-21-K562 cells, to be used as feeder cells for proliferation, and the NK cells are directly proliferated from peripheral blood monouclear cells, thus the operation is simpler and easier. Studies such as cell counting, flow cytometry and cytotoxicity tests indicate that high cell proliferation times, high NK cell purity, strong cytotoxicity and remarkable cancer cell killer effect are achieved.
Owner:SHANGHAI JIAOTONG UNIV SCHOOL OF MEDICINE
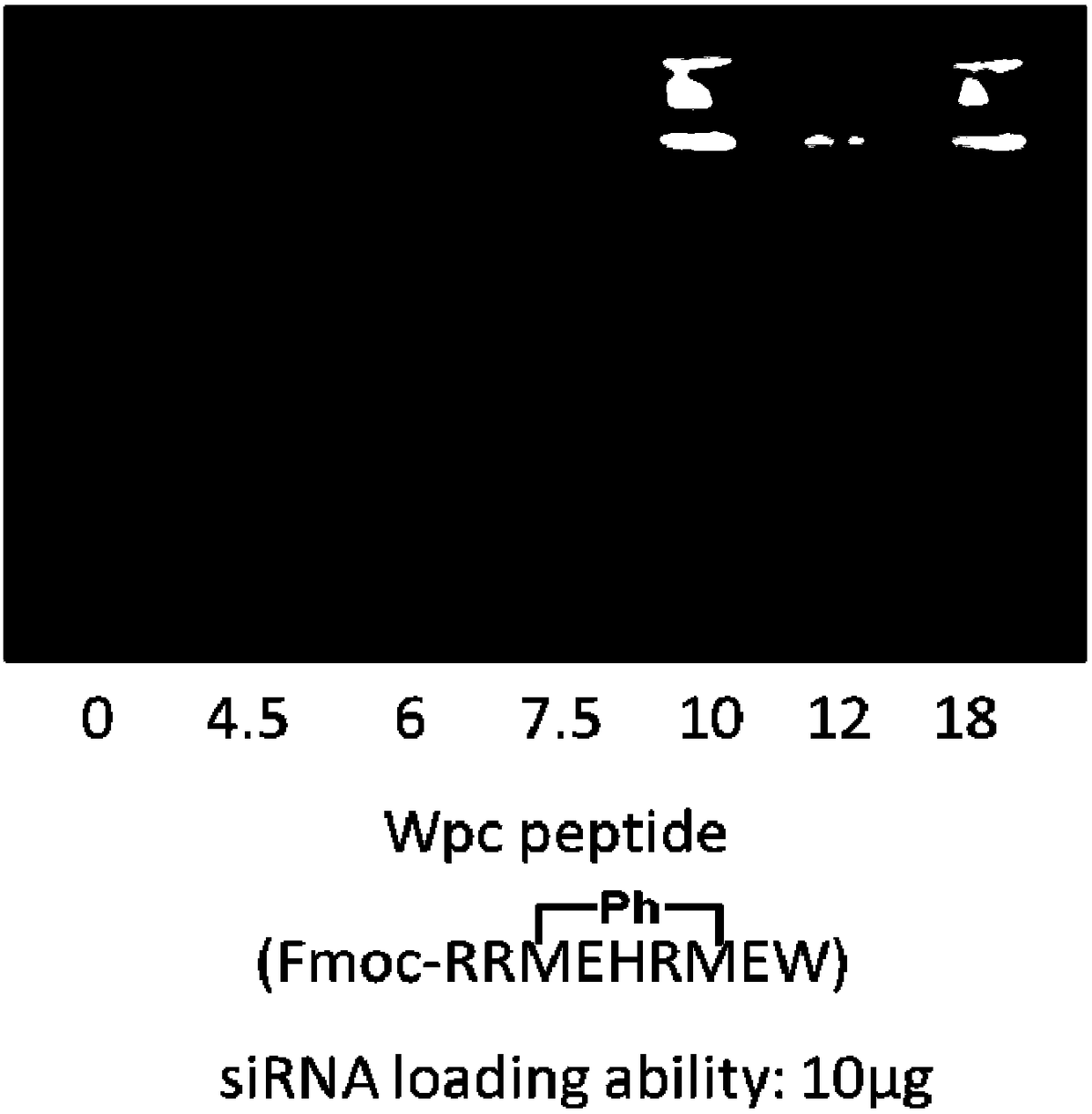
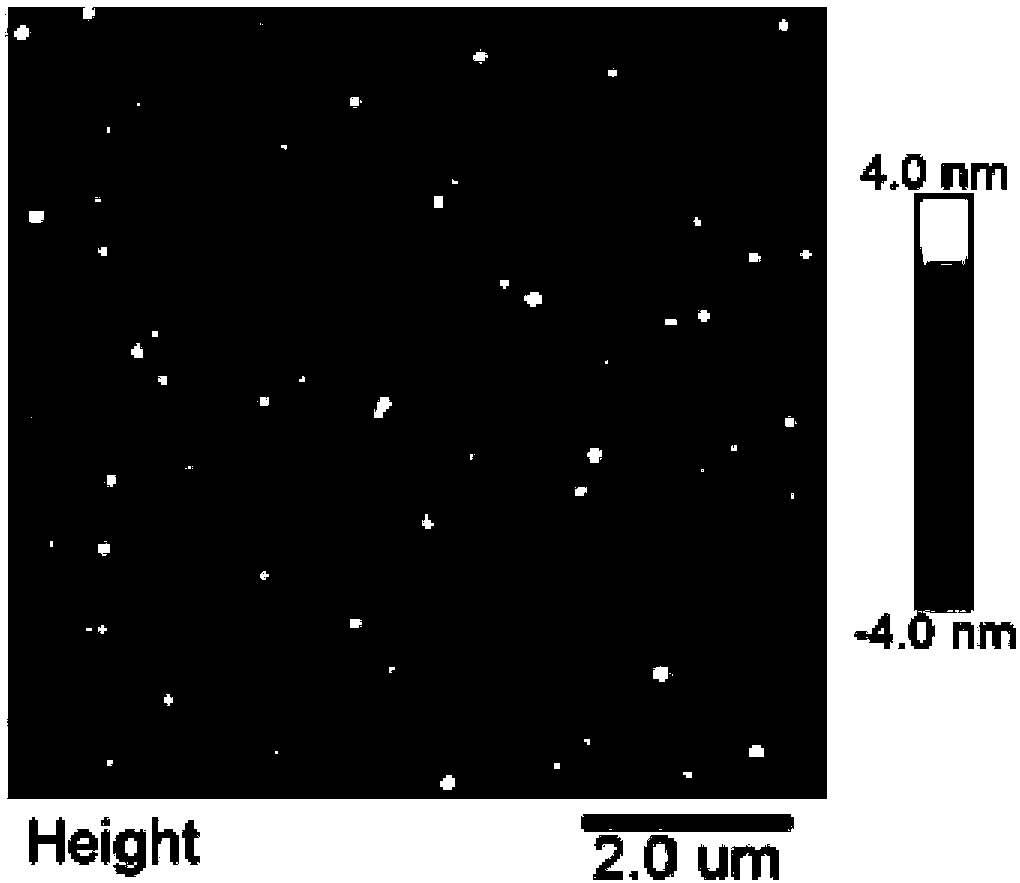
Polypeptide, polypeptide-siRNA (ribonucleic acid) induction coassembly and application thereof
ActiveCN108250267AGrowth inhibitionIncrease electropositivityOrganic active ingredientsPeptidesHemolysisMedicine
The invention provides polypeptide. The structural formula of the polypeptide is as shown below (the structural formula is shown in the description). The invention further provides a polypeptide-siRNAinduction coassembly which is composed of polypeptide and siRNA. The invention further provides application of the polypeptide-siRNA induction coassembly to cell carrying. The invention further provides application of the polypeptide-siRNA induction coassembly to preparation of drugs for treating cervical cancer. After polypeptide and target siRNA are mixed and hatched for 5 minutes at the room temperature, stable and uniform nano particles can be formed. A cytotoxicity test proves that the coassembly nano particles have high biocompatibility, and the cytotoxicity and hemolysis toxicity of the coassembly nano particles are both low. A cell proliferation resistant test further verifies that the polypeptide-siRNA nano particles can effectively block a cervical cancer cell line HeLa in the G2 stage, so that growth of the cervical cancer line HeLa is hindered.
Owner:PEKING UNIV SHENZHEN GRADUATE SCHOOL
Epirubicin loaded graphene quantum dot drug carrying system and preparation method thereof
ActiveCN104984349ARealize the loadIncrease loading capacityOrganic active ingredientsPharmaceutical non-active ingredientsMedicineDrug carrier
The invention discloses an epirubicin loaded graphene quantum dot drug carrying system and a preparation method thereof. The preparation method includes the following steps: preparation of graphene quantum dots by a hydrothermal method; loading an anti-cancer drug with the graphene quantum dots; and performing a drug slow-release experiment, and performing a cytotoxicity test on the drug carrying system. The graphene quantum dots prepared by the hydrothermal method are used as a drug carrier for being loaded with epirubicin, greatly improve the drug loading rate, and show a quite good inhibitory effect on tumor HeLa cells. The preparation method of the drug carrying system is simple to operate, mild in reaction conditions and high in drug loading rate, and is expected to have a broad application prospect in the field of biological medicine.
Owner:南京美材科技有限公司
Ratio-type two-photon formaldehyde fluorescent probe, preparation method therefor and use of ratio-type two-photon formaldehyde fluorescent probe
ActiveCN106946773ALow fluorescence quantum yieldSimple structureOrganic chemistryFluorescence/phosphorescenceMicro imagingCytotoxicity testing
The invention discloses a ratio-type two-photon formaldehyde fluorescent probe, a preparation method therefor and use of the ratio-type two-photon formaldehyde fluorescent probe. The ratio-type two-photon formaldehyde fluorescent probe has a structure as follows shown in the description. Molecules of the fluorescent probe disclosed by the invention show relatively high selectivity and sensitivity to formaldehyde in a coexisting system of molecules of the formaldehyde and other aldehydes, amino acids and the like. Proven by cytotoxicity tests, the molecules of the fluorescent probe disclosed by the invention are almost free of toxic action on cells; and shown by two-photon fluorescence microscopy imaging experiments, the molecules of the fluorescent probe disclosed by the invention have good cell permeability to MCF-7 cells, so that the fluorescent probe is applicable to the detection of presence of formaldehyde molecules in the cells.
Owner:ANHUI UNIVERSITY
Test method for detecting cytotoxicity of buccal tobacco products
ActiveCN104789633AEasy to handleAccurate and effective detectionMicrobiological testing/measurementFiberPretreatment method
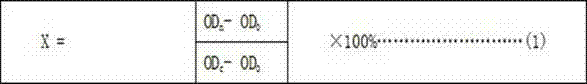
The invention provides a test method for detecting cytotoxicity of buccal tobacco products. The test method comprises the following steps: sample pretreatment, single-cell suspension preparation, cell concentration calculation, cell inoculation, test substance addition, test substance incubation, dye incubation, light absorption value measurement, cell inhibition rate calculation and cell half lethal dose calculation. According to the test method provided by the invention, the exposure means and action manners of the buccal tobacco products are comprehensively surveyed, a sample pretreatment method of the buccal tobacco products is established, human oral mucosa fibroblasts hoMF are selected as the cells for cytotoxicity test of the buccal tobacco products according to the acting target cells, the sample detection dosage is determined and a cytotoxicity test method suitable for the buccal tobacco products is formed.
Owner:CHINA TOBACCO YUNNAN IND
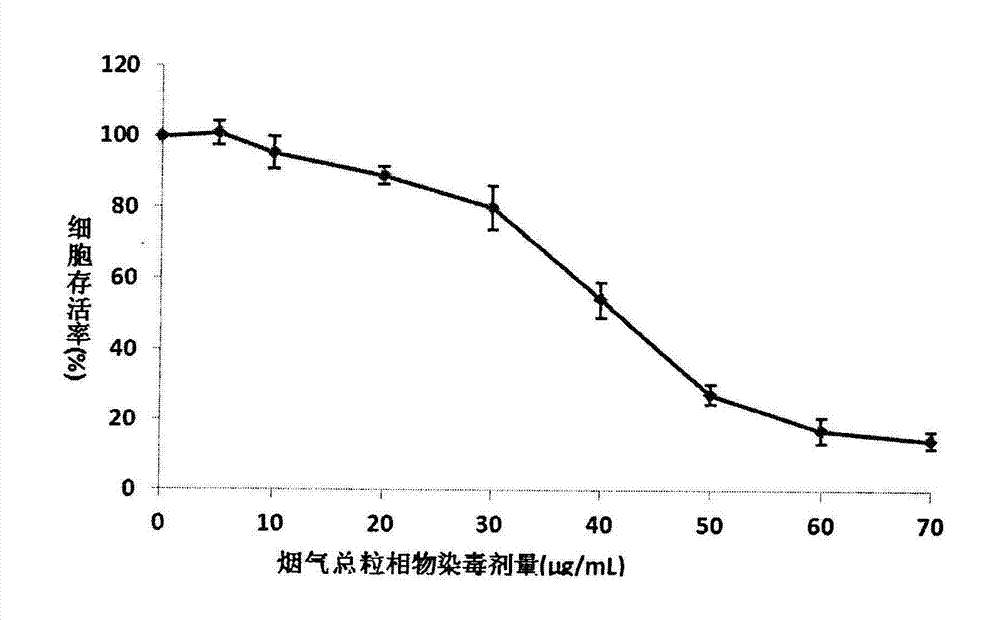
Detection method for cytotoxicity of gas-liquid contact type cigarettes under full-smoke exposure
ActiveCN102495183ASimple methodLow costAir quality improvementMaterial analysisMethyl thiazolyl tetrazoliumEngineering
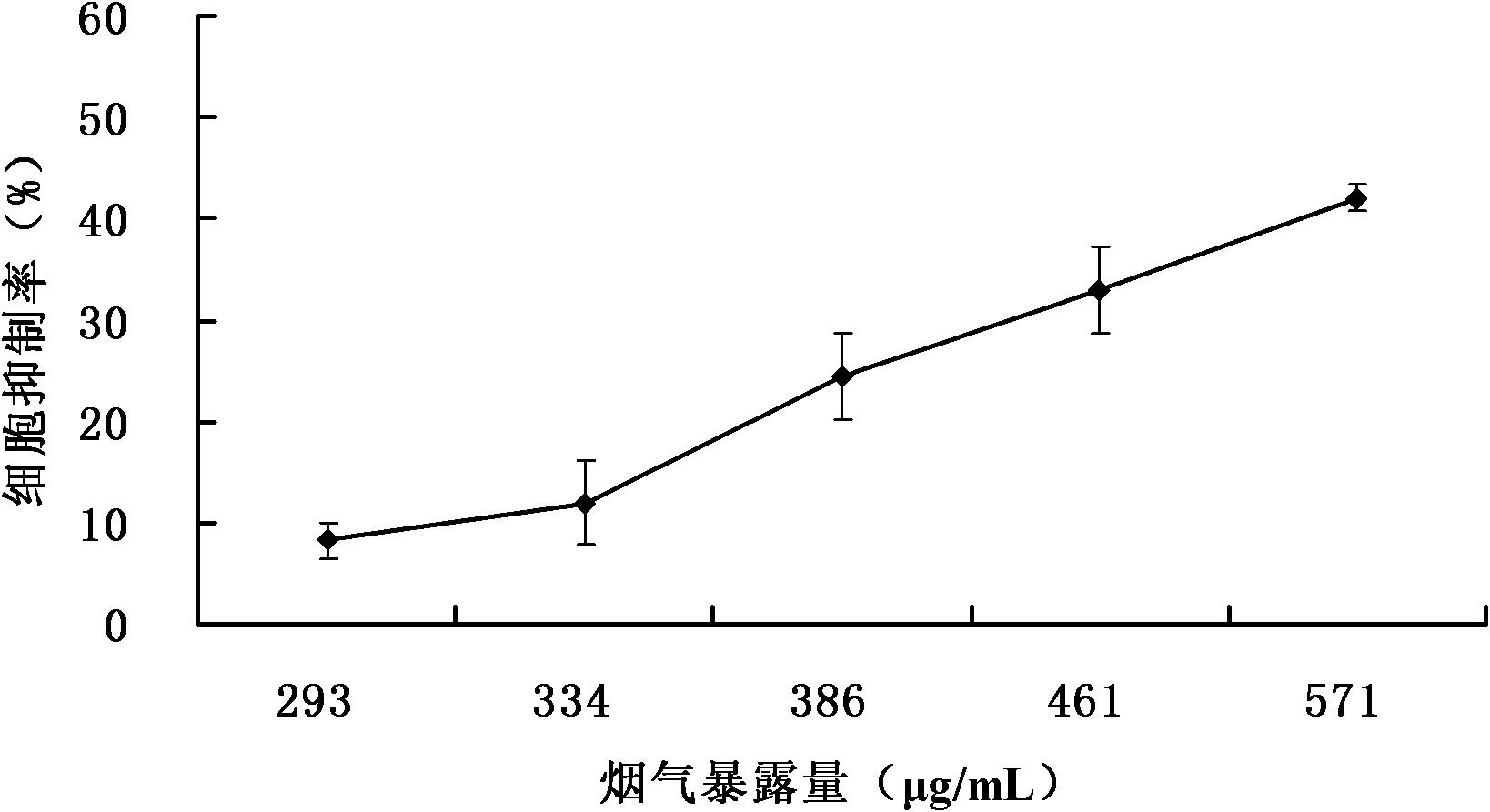
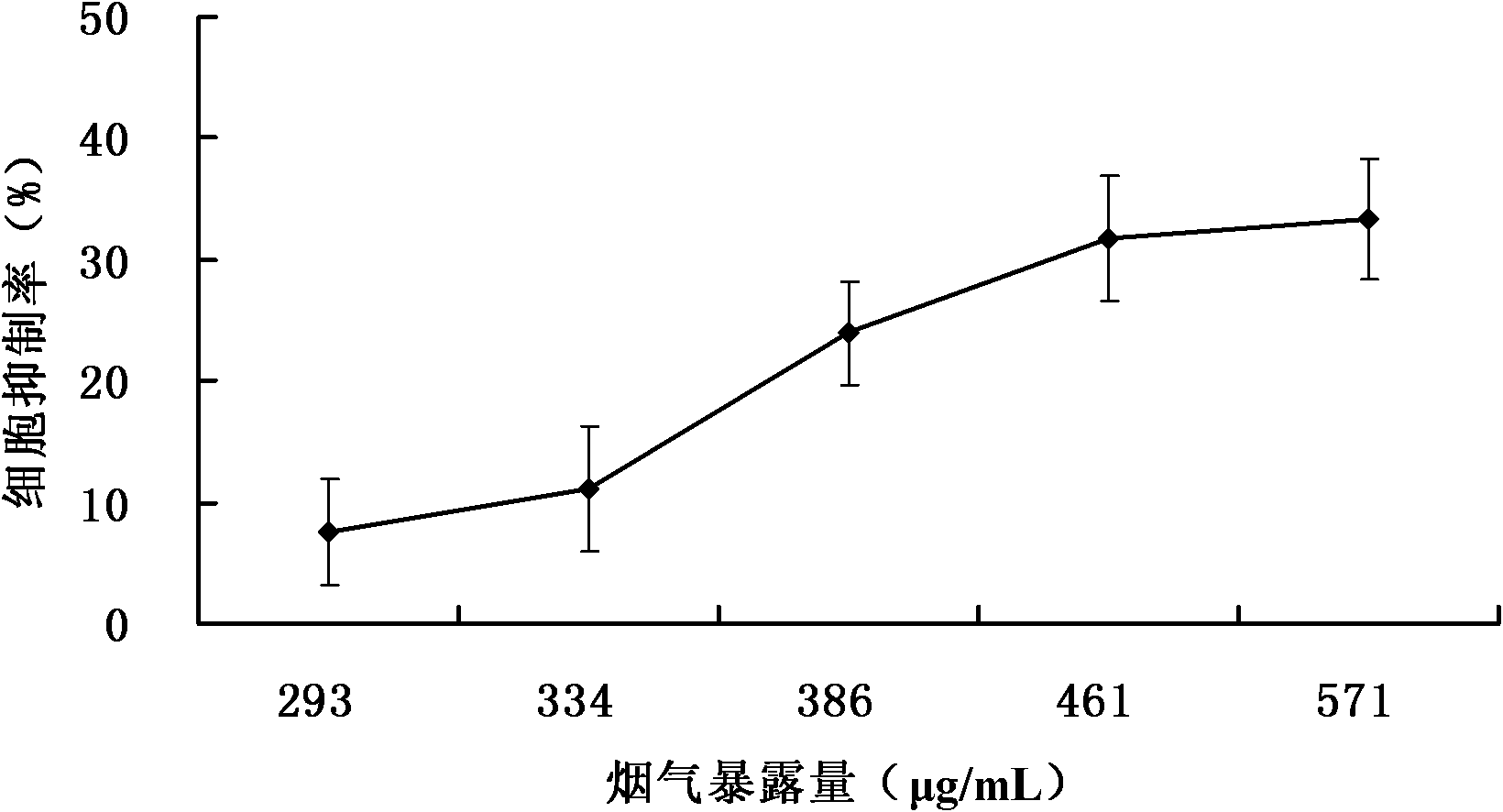
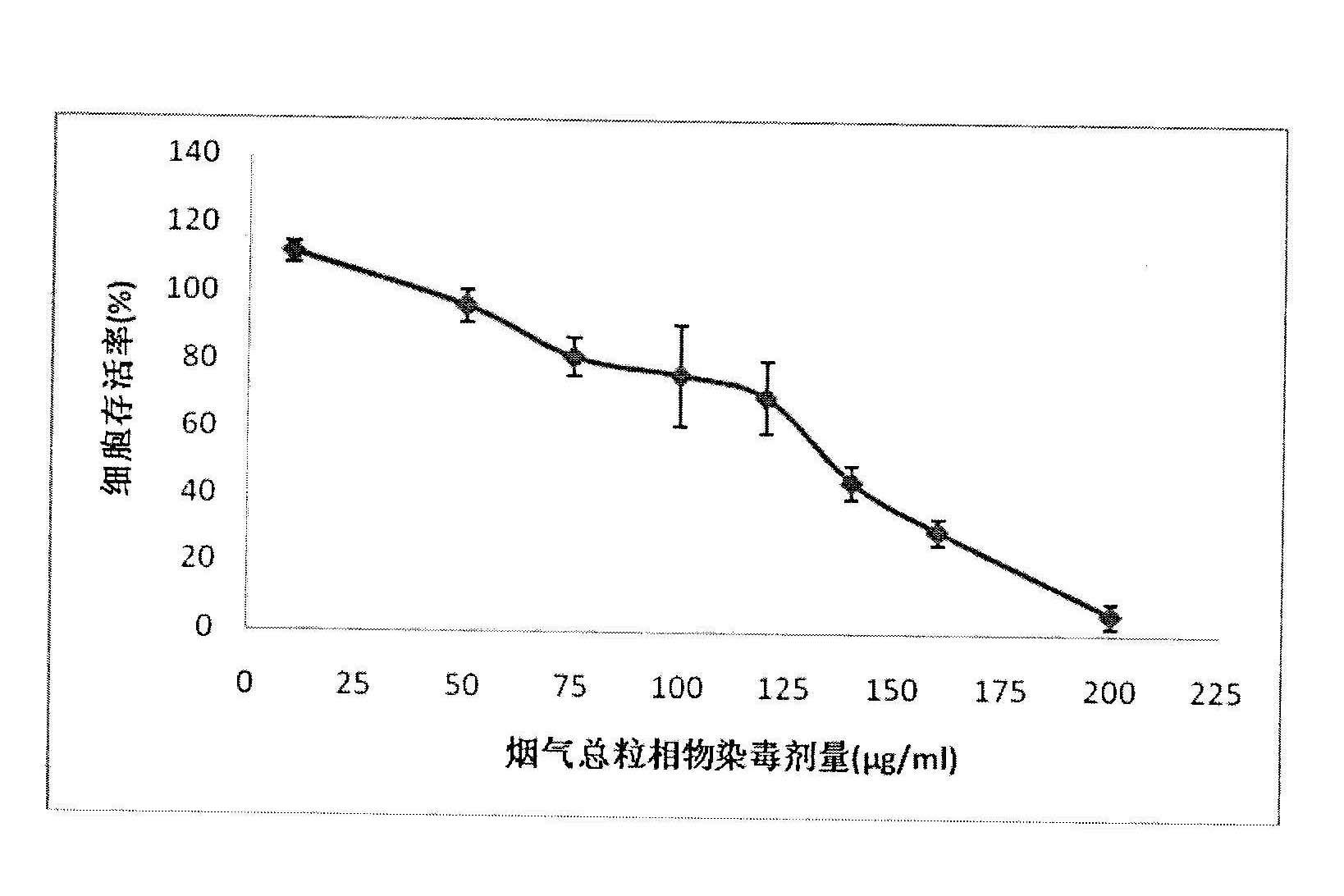
The invention relates to a detection method for the cytotoxicity of gas-liquid contact type cigarettes under full-smoke exposure, belonging to the technical field of safety evaluation of cigarette smoke. The detection method provided by the invention comprises the following steps of: firstly, setting cigarette suction parameters to carry out suction on a cigarette; diluting the smoke by clean air with the flow speed of 0-1000 mL / min and transferring the diluted cigarette full smoke into a full-smoke exposure bottle disclosed in the patent with the patent number of ZL201120063251.6 to come into contact and exposure with suspension cells in the bottle, wherein the exposure time is 5 min; after the exposure, carrying out neutral erythrocyte toxicity test or MTT (Methyl Thiazolyl Tetrazolium) cytotoxicity test according to a regular method; analyzing a dosage reaction relation between smoke exposure amount and cytotoxicity according to the following formula: full-smoke dilution multiple=( flow speed of smoke + flow speed of clean air) / flow speed of smoke; smoke exposure amount under exposure of 10 mL of a cell suspension solution for 5 min=TPM (Total Particulate Matter) amount of single cigarette / suction times of single cigarette*6 suctions / 10 mL; and representing the extent of cytotoxicity by a cell inhibition ratio CI. The detection method provided by the invention has the advantages of simplicity, feasibility, low cost and the capability of providing technical supports for the safety evaluation of cigarette smoke.
Owner:YUNNAN RES INST OF TOBACCO SCI
MTT (thiazolyl blue) cell toxicity test method of biological assessment of total particle matter in cigarette smoke
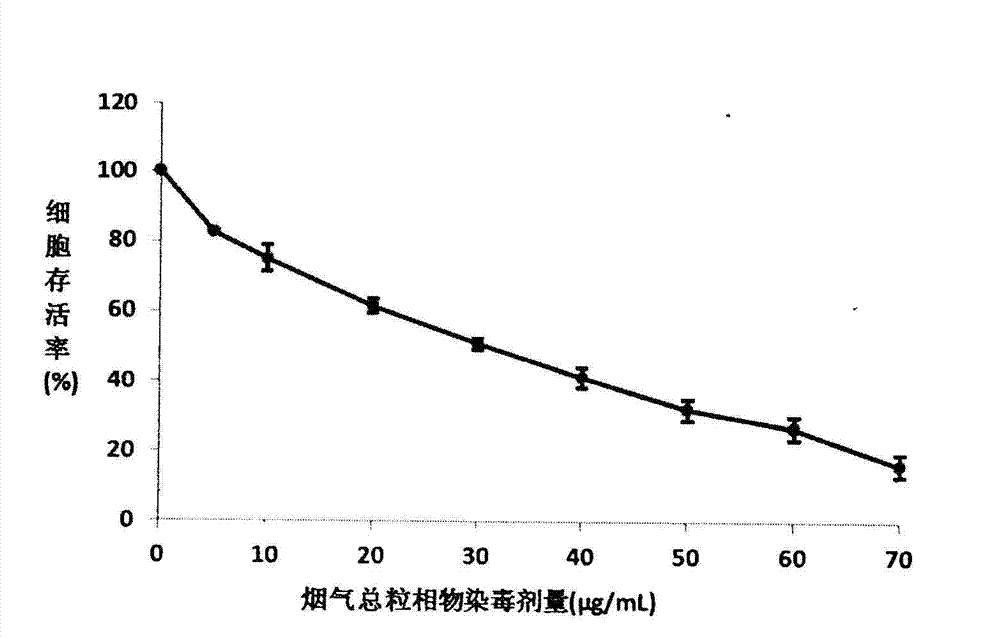
InactiveCN103088105AReduce the number of steps to wash cellsStrong targetingMicrobiological testing/measurementColor/spectral properties measurementsBronchial epitheliumCell system
The invention relates to an MTT (thiazolyl blue) cell toxicity test method of biological assessment of a total particle matter in cigarette smoke and belongs to the technical field of safety assessment of tobacco and cigarette smoke. The MTT cell toxicity test method is characterized by comprising the following steps of: inoculating and culturing immortalized human bronchial epithelial cells (BEAS-2B cells) and contaminating the total particle matter in the cigarette smoke; detecting the cell survival rate by adopting an MTT method; and analyzing and evaluating the cell toxicity of the total particle matter in the cigarette smoke according to a test result. Compared with the prior art, the MTT cell toxicity test method has the following characteristics that BEAS-2B cells are human cells and are target organ source cells acting on a human body by the smoke; a BEAS-2B cell system is used for carrying out cell toxicity assessment on the cigarette smoke and has the strong pertinence; in a testing process, steps of washing the cells for a plurality of times are reduced; and a formaldehyde fixing step does not need to be carried out so that a testing period is shortened, the operation is rapid and convenient, the sensitivity is high and the result is stable and reliable. Moreover, the MTT test method disclosed by the invention is further applicable to a smoke cell toxicity test of various cell systems and the commonality is strong.
Owner:ZHENGZHOU TOBACCO RES INST OF CNTC
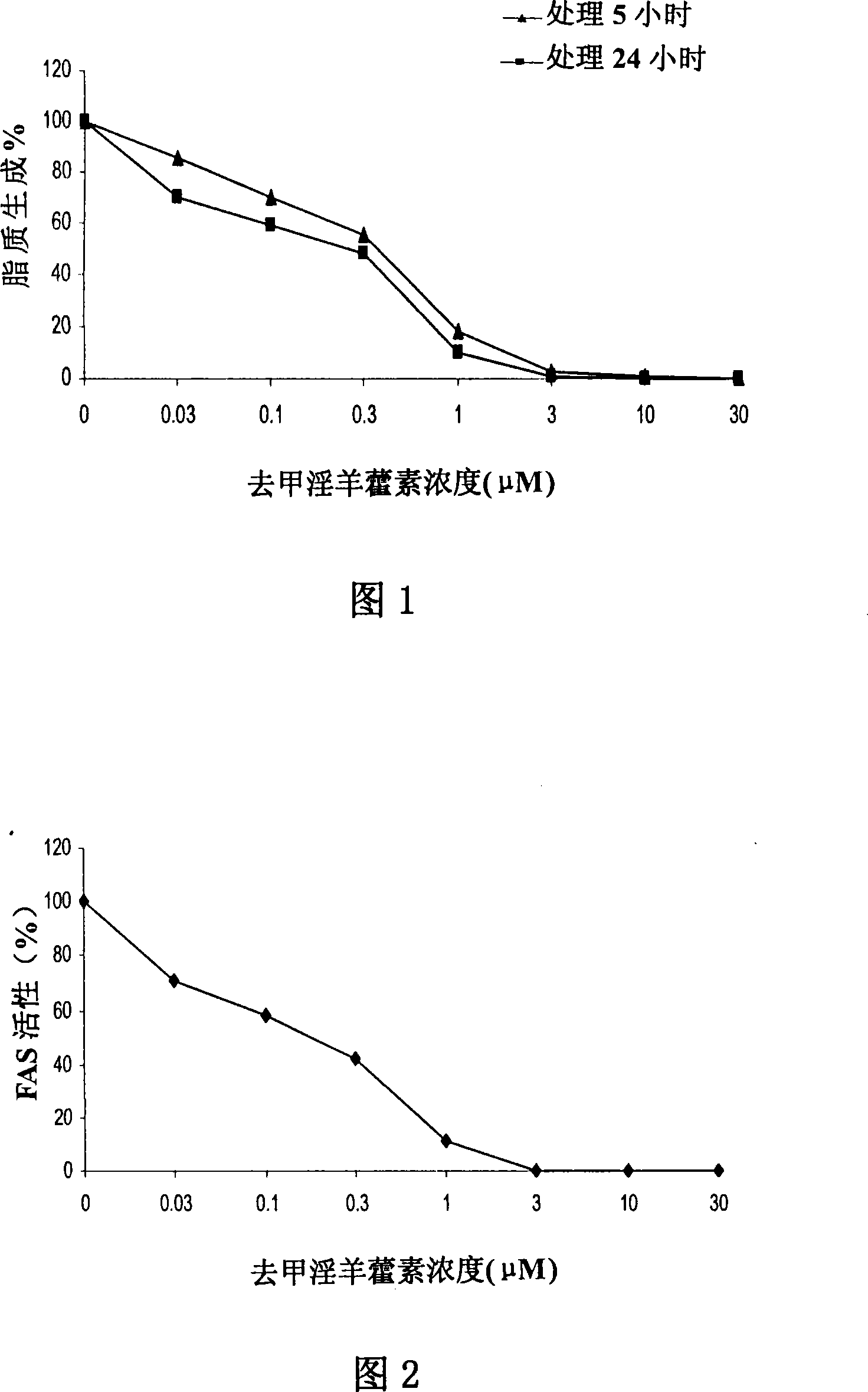
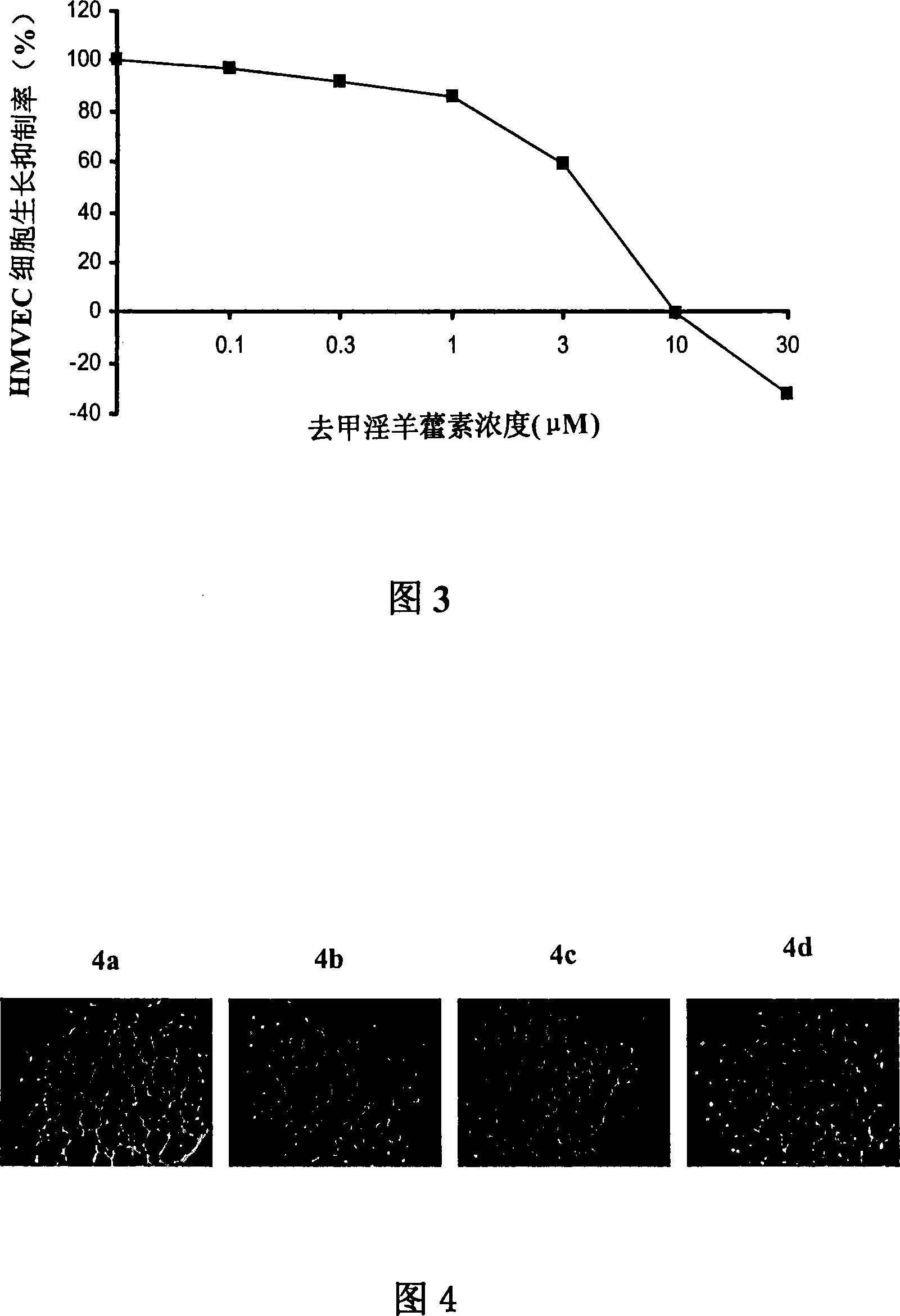
Anti-tumor medicine
InactiveCN101103973AOrganic active ingredientsAntineoplastic agentsAbnormal tissue growthProstate cancer cell
Disclosed is an anti-tumor drug with nor-icariin as the effective ingredient. Pharmacological test effect proves that the nor-icariin has the activity to inhibit the production of cellular lipid, can reduce the production of cellular fatty acid by inhibiting the activity of FAS, has a function of inhibiting the production and causing withering of microvascular endothelial cells, shows a broad-spectrum anti-tumor function in a cytotoxicity test of thirty-three tumor cell strains of a human body, and shows a function in strongly inhibiting the production of tumor, such as prostate cancer cells LnCAP, lung cancer cells NCI-H23 and colon cancer cells HCT-116 of a human body in a tumor transplantation nude mice model test. A pharmacokinetics test of the nor-icariin in the body of a little mouse proves that the nor-icariin can be kept with a certain concentration, good for better anti-humor effect. The acute toxicity test of the nor-icariin in the body of the mouse shows nontoxicity; therefore the nor-icariin is proved to be a newly-developed broad spectrum anti-humor drug with broad development prospect.
Owner:殷正丰 +1
Two-photon fluorescent probe for detecting polarity and viscosity through two channels as well as preparation method and application thereof
ActiveCN111253935ALow cytotoxicityImprove permeabilityOrganic chemistryFluorescence/phosphorescenceStructural formulaCytotoxicity testing
The invention discloses a two-photon fluorescent probe for detecting polarity and viscosity through two channels as well as a preparation method and application thereof. The two-photon fluorescent probe can monitor the real-time change of polarity and viscosity in cell mitochondria in two different channels at the same time, and the structural formula of the two-photon fluorescent probe is shown in the specification. The two-photon fluorescent probe molecule can perform fluorescence response on polarity (short wave with the wave length of 410 nm) and viscosity (long wave with the wave length of 580 nm) at the same time by utilizing two different fluorescence bands. Furthermore, a cytotoxicity test shows that the probe has almost no toxic or side effect on cells; a two-photon confocal fluorescence microimaging experiment shows that the probe has good permeability to HeLa cells, can effectively locate mitochondria (the locating coefficient is 0.97) in the cells, and is suitable for two-channel two-photon fluorescence imaging and detection of polarity and viscosity in the cell mitochondria.
Owner:ANHUI UNIVERSITY
Antiseptic dressing containing nanogold and preparing method thereof
InactiveCN106139224AImprove antibacterial propertiesLow toxicityPharmaceutical delivery mechanismAbsorbent padsNanoparticleMedicine
The invention provides antiseptic dressing containing nanogold. The antiseptic dressing comprises a base material and nanogold particles which adhere to the base material and have an antiseptic effect. The invention further provides a preparing method and application of the antiseptic dressing containing nanogold. The gram-negative bacterium and gram-positive bacterium resisting performance of the antiseptic dressing containing nanogold is higher; furthermore, cytotoxicity tests and animal experiments prove that the toxicity of the antiseptic dressing containing nanogold is far lower than that of medical nanosilver dressing using nanosilver.
Owner:THE NAT CENT FOR NANOSCI & TECH NCNST OF CHINA
Andrographolide and its derivatives as TNF-alpha antagonists
InactiveUS20060106098A1Inhibition releaseInhibiting and antagonizing TNFαBiocideOrganic chemistryPaw edemaCytotoxicity test
The present invention relates to andrographolide and its derivatives of the general formula (I), as well as the stereoisomers and salts of andrographolide and the derivatives. Andrographolide and its derivatives represented by general formula (I) defined above are useful as TNFα (tumor necrosis factor alpha) antagonists or inhibitors which have inhibitory effect on the binding of TNFα to TNF-RI. Andrographolide exhibited inhibitor activity with IC50 values 60 μM on L929 cell proliferation / cytotoxicity assay without cell cytotoxicity. In addition, in the animal model test of collagen-induced arthritis, andrographolide exhibited 50% paw edema. Andrographolide and its derivatives are promising sources with high TNFα-inhibiting or antagonizing activity.
Owner:HERBCOPOEIA PHARMA
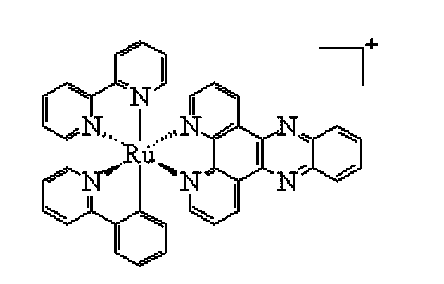
Cyclometalated ruthenium complex, and preparation method and application thereof
InactiveCN103509059AStrong growth inhibitory effectImprove developmentGroup 8/9/10/18 element organic compoundsAntineoplastic agentsCancer cellBiology
The invention aims at developing a novel DNA (deoxyribonucleic acid) inserting reagent with good antitumor activity. A cyclometalated single-core ruthenium (II) complex is synthetized. The complex is stable in structure; the water-solubility is better than that of a common organic small molecular reagent at present; good DNA transcription inhibitory activity is represented. A cytotoxicity test indicates that the cyclometalated ruthenium (II) complex has significant growth inhibition effects on cancer cells of 11 different tissue parts of a human body; the inhibitory activity is much better than that of cis-platinum.
Owner:SUN YAT SEN UNIV
Structure and preparation method of liver-targeted platinum-loaded nanometer prodrug
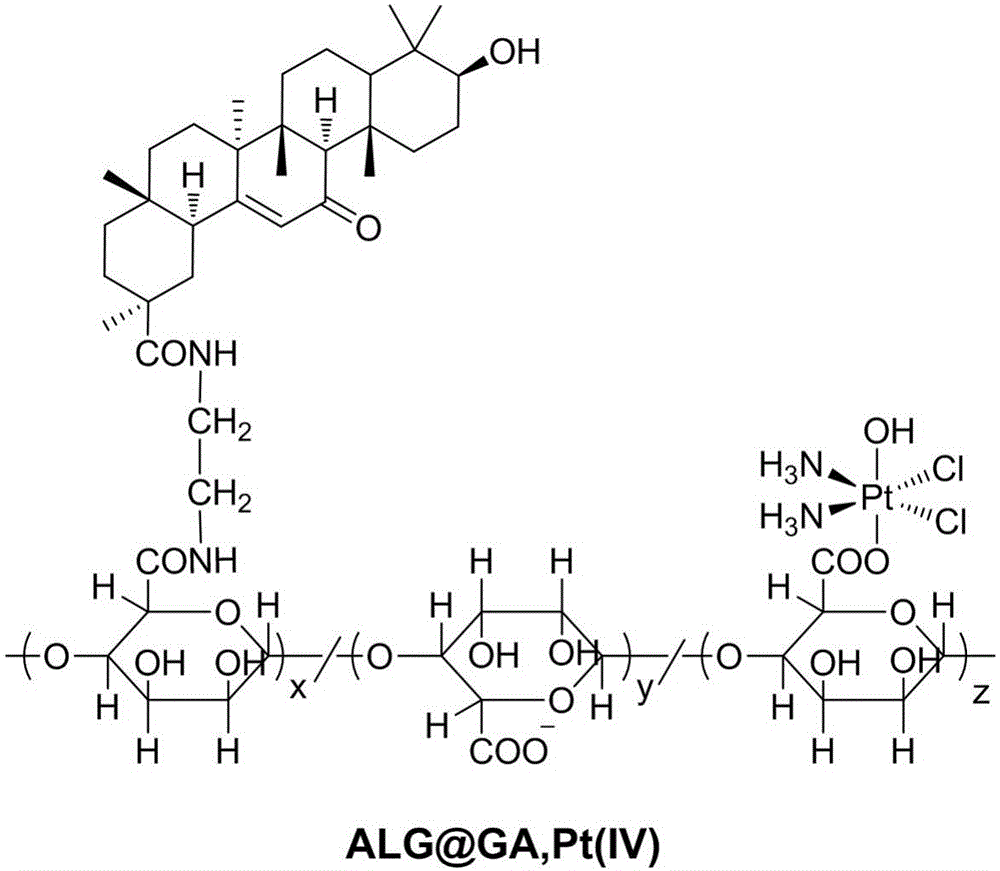
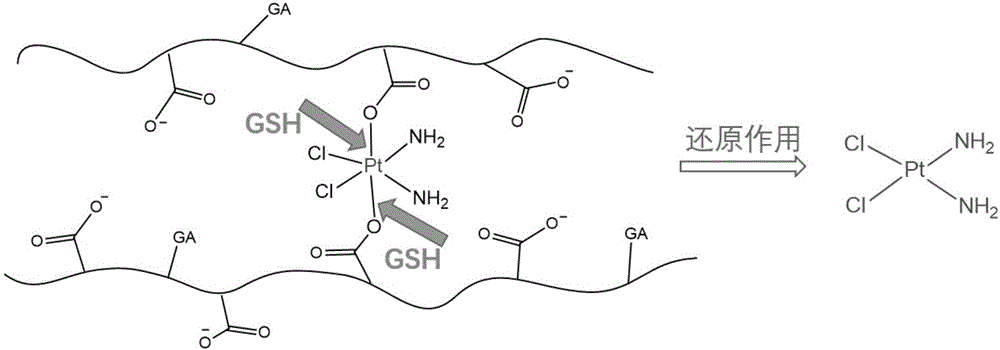
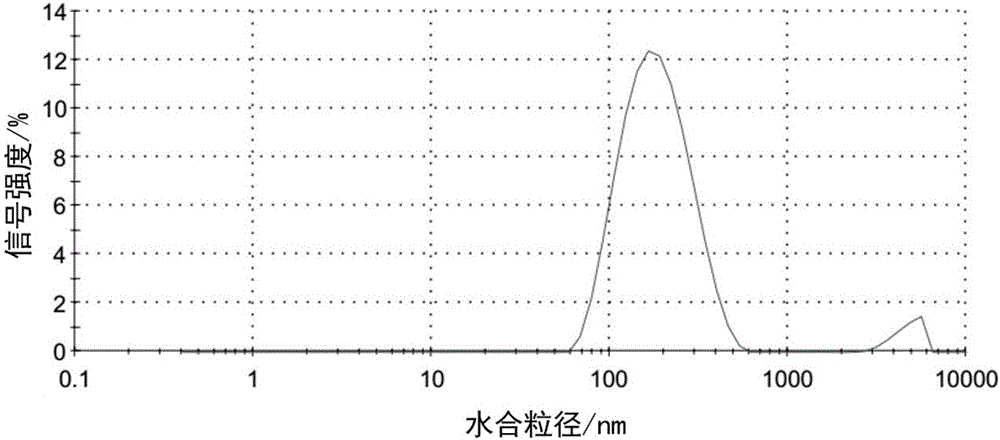
ActiveCN106267229AAvoid non-specific adsorptionReduce the binding forceHeavy metal active ingredientsDigestive systemBiocompatibility TestingPt element
The invention discloses a liver-targeted platinum-loaded nanometer prodrug for treating liver cancers and a preparation method thereof. The liver-targeted platinum-loaded nanometer prodrug is prepared by taking sodium alginate with the good biocompatibility as a framework to be modified with glycyrrhetinic acid (GA) with a liver cancer cell targeting capacity and a tetravalent platinum compound (PtCl2(OH)2(NH3)2) through a self-assembly technique. In-vitro drug releasing and cytotoxicity tests prove that the platinum-loaded drug nanoparticles have the good blood stability and the intracellular slow-release effect and also have the tumor cell killing capacity. The nanometer prodrug is expected to be used for targeting treatment on the liver cancers and has a good application prospect in the biomedical field.
Owner:NANKAI UNIV
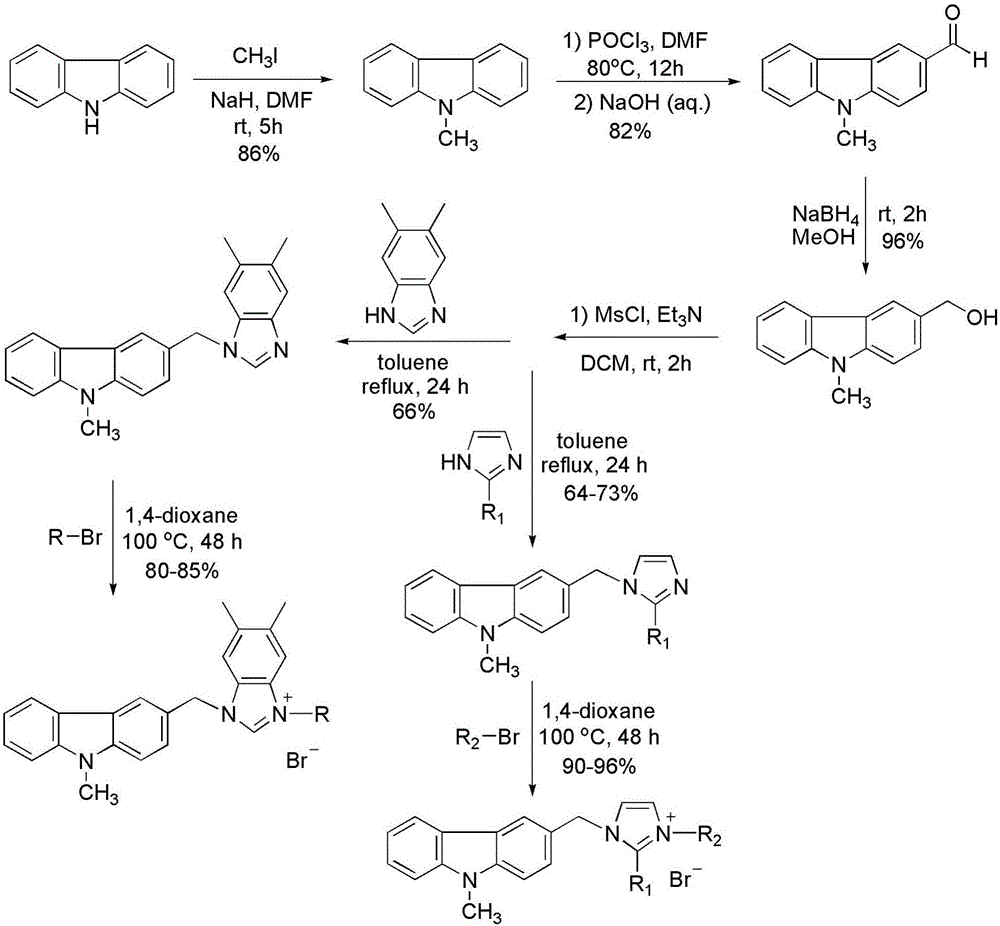
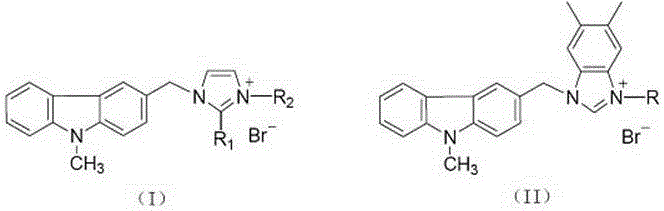
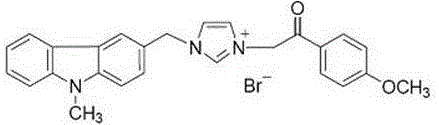
Substituted carbazole-imidazolate or benzimidazolium salt compounds, and preparation method thereof
The invention discloses a series of substituted carbazole-imidazolate or benzimidazolium salt compounds with a general structure represented by formula I or II, and a preparation method thereof. According to the preparation method, carbazole is taken as a raw material, and the target compound is obtained via five steps including N-methylation, acylation, reduction, combination with imidazole, and salifying. It is shown by results of in vitro antitumor activity cytotoxicity test that the substituted carbazole-imidazolate or benzimidazolium salt compounds possess excellent cytotoxic activity.
Owner:YUNNAN UNIV
Strong signal and low toxicity composite nanometer material and preparation method thereof
InactiveCN103642491AHigh quantum yieldSolve the problem of water solubilityMaterial nanotechnologyInorganic non-active ingredientsCancer cellBiological imaging
The invention relates to a strong signal and low toxicity composite nanometer material and a preparation method thereof, and belongs to the technical field of material preparation, wherein Au-Cu2S quantum dots are adopted as an inner core, and ZnS is adopted as a shell to wrap the Au-Cu2S quantum dot inner core to prepare the Au-Cu2S / ZnS quantum dots. The preparation method comprises: preparing Au nanoparticles, preparing Au-Cu2S composite nanoparticles, and preparing Au-Cu2S / ZnS quantum dots. According to the present invention, the particle size of the obtained the composite nano-particles can be controlled in several nanometers to tens of nanometers, and the obtained the composite nano-particles can be used for cytotoxicity tests and biological labeling carriers or targeted drug delivery carriers, can be provided for observing fluorescence signals through a biological imaging system, can be combined with folic acid so as to provide biocompatibility, can further enter in vivo cells and be combined with the cells so as to be adopted as the biological probe marker to perform biological labeling, and can form the durg complex, wherein the durg complex can reside on the cancer cell surface.
Owner:CHANGCHUN UNIV OF SCI & TECH
Improved cigarette smoke in-vitro contamination method
InactiveCN101671733AComprehensive real biological effectsComprehensive and true reflection of biological effectsMicrobiological testing/measurementAmes testGas phase
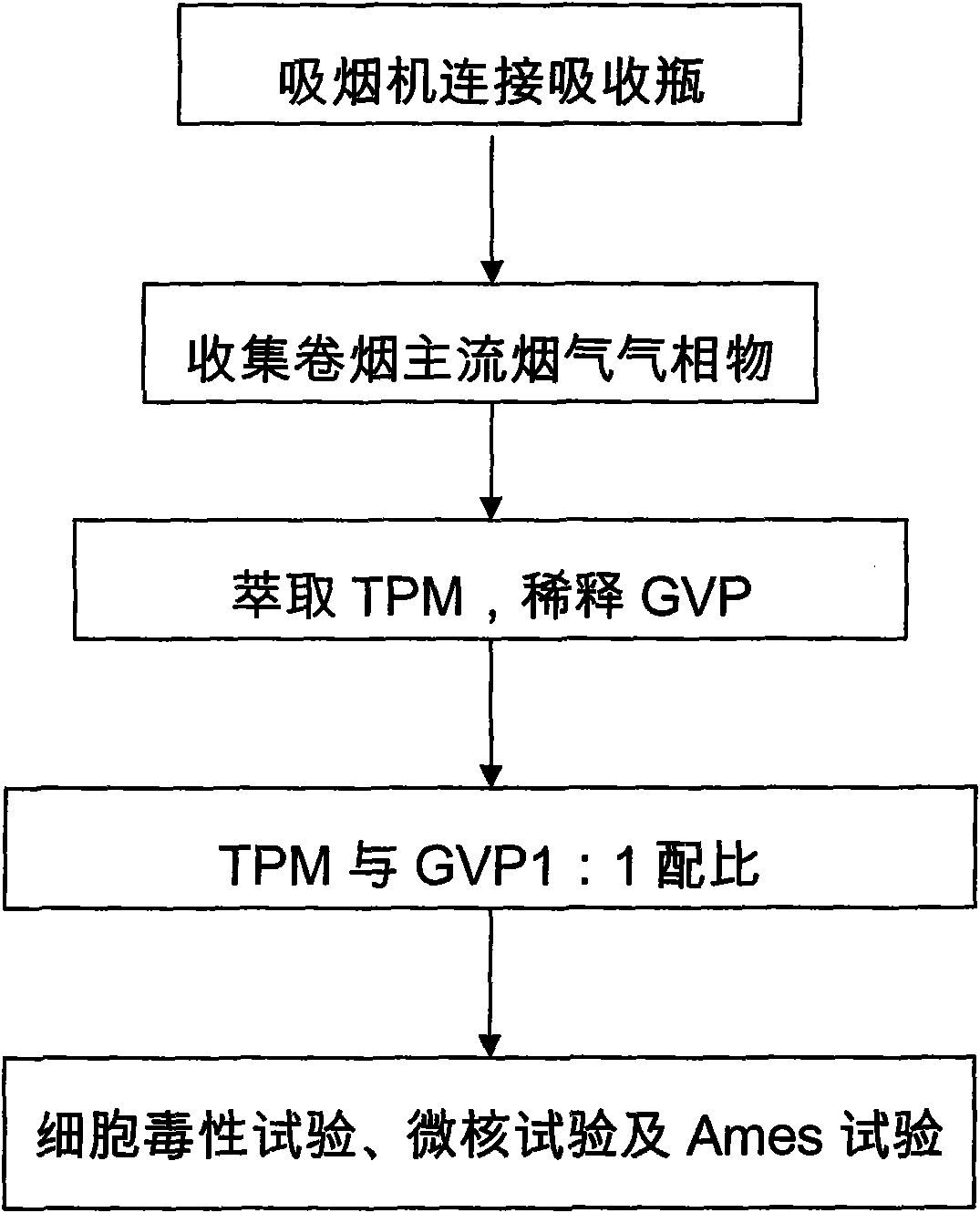
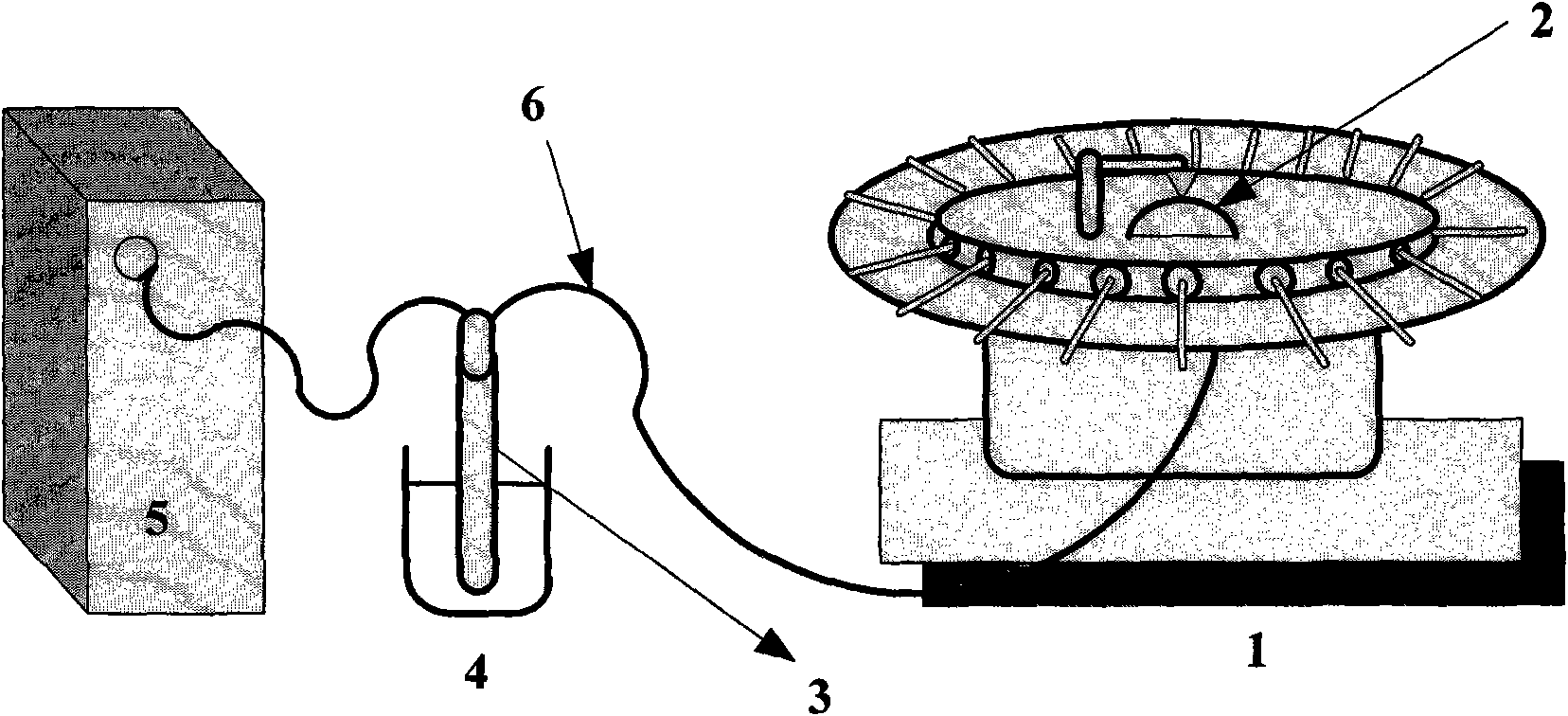
The invention relates to an improved cigarette smoke in-vitro contamination method. The biological effect of the cigarette smoke is evaluated by mixing the gaseous phase part (GVP) and the particle phase part (TPM) of the cigarette smoke to carry out the in-vitro contamination method. Specifically, the improved cigarette smoke in-vitro contamination method comprises the following steps: a. collection of the particle phase part and the gaseous phase part of smoke; b. mixing of the particle phase part and the gaseous phase part; and c, in-vitro contamination: diluting TPM+GVP with a proportion of 1:1 into different concentration gradients, and carrying out a cytotoxicity test, a micronucleus test and an Ames test by in-vitro contamination method. The invention has the advantages that the gaseous phase part of cigarette smoke is collected by an absorption bottle; the in-vitro contamination way of combining GVP and TPM can comprehensively and truly reflect the biological effect of the cigarette smoke; based on the conventional TPM contamination way, the contamination of the gaseous phase part of cigarette smoke is added, so that the in-vitro contamination method is more close to the actual cigarette smoke exposure condition; besides, the contamination concentration is easy to control and the operation is simple.
Owner:ZHENGZHOU TOBACCO RES INST OF CNTC
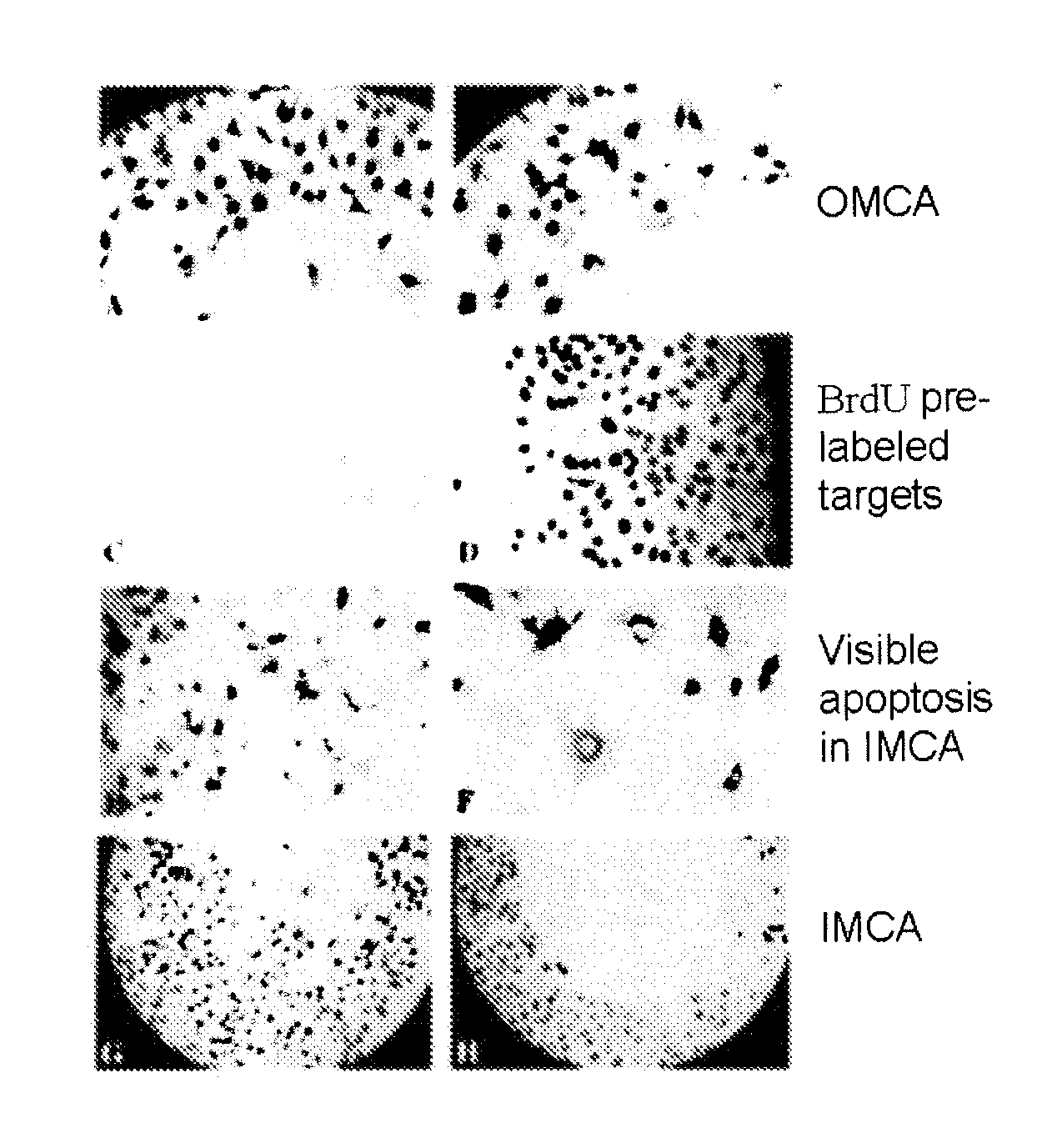
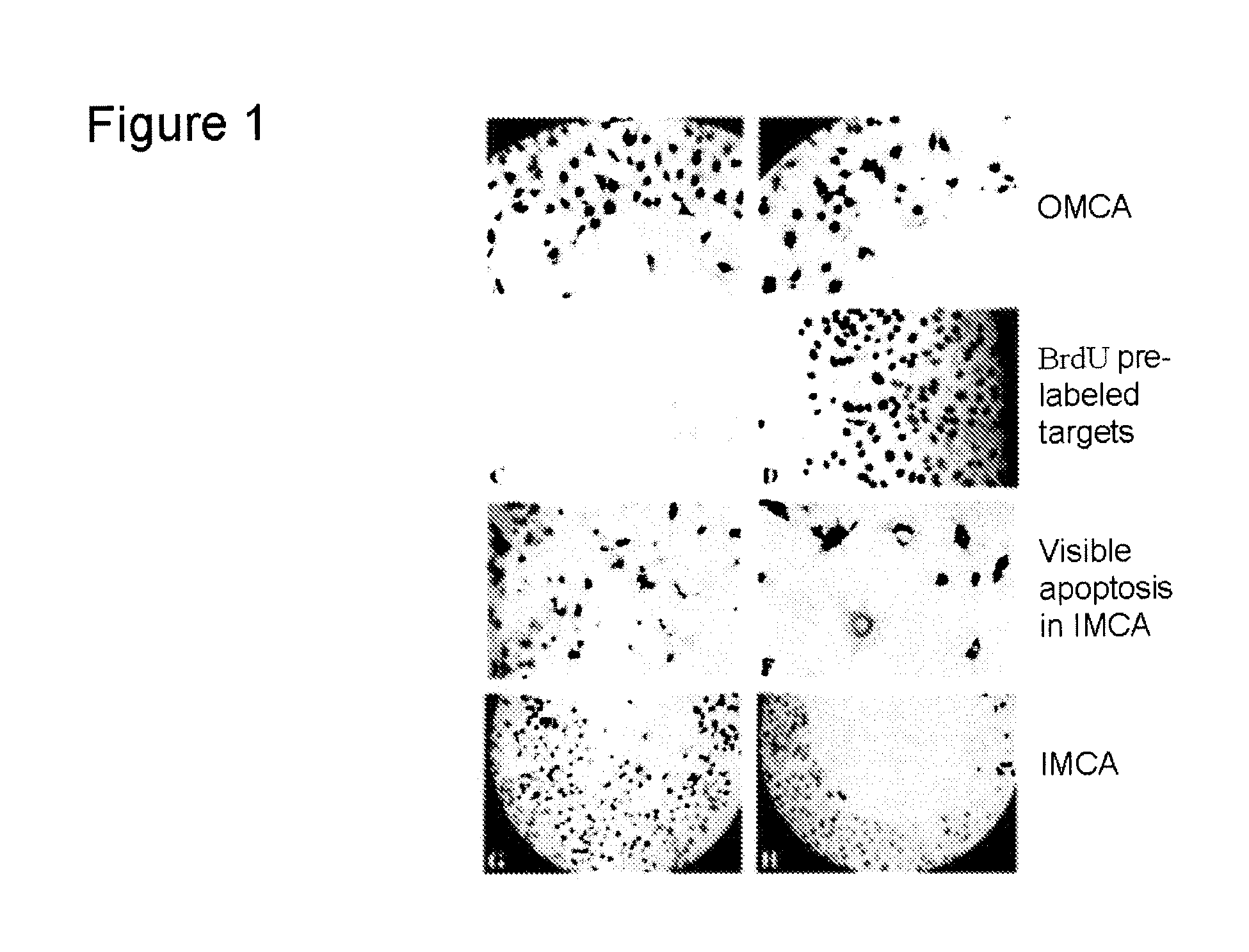
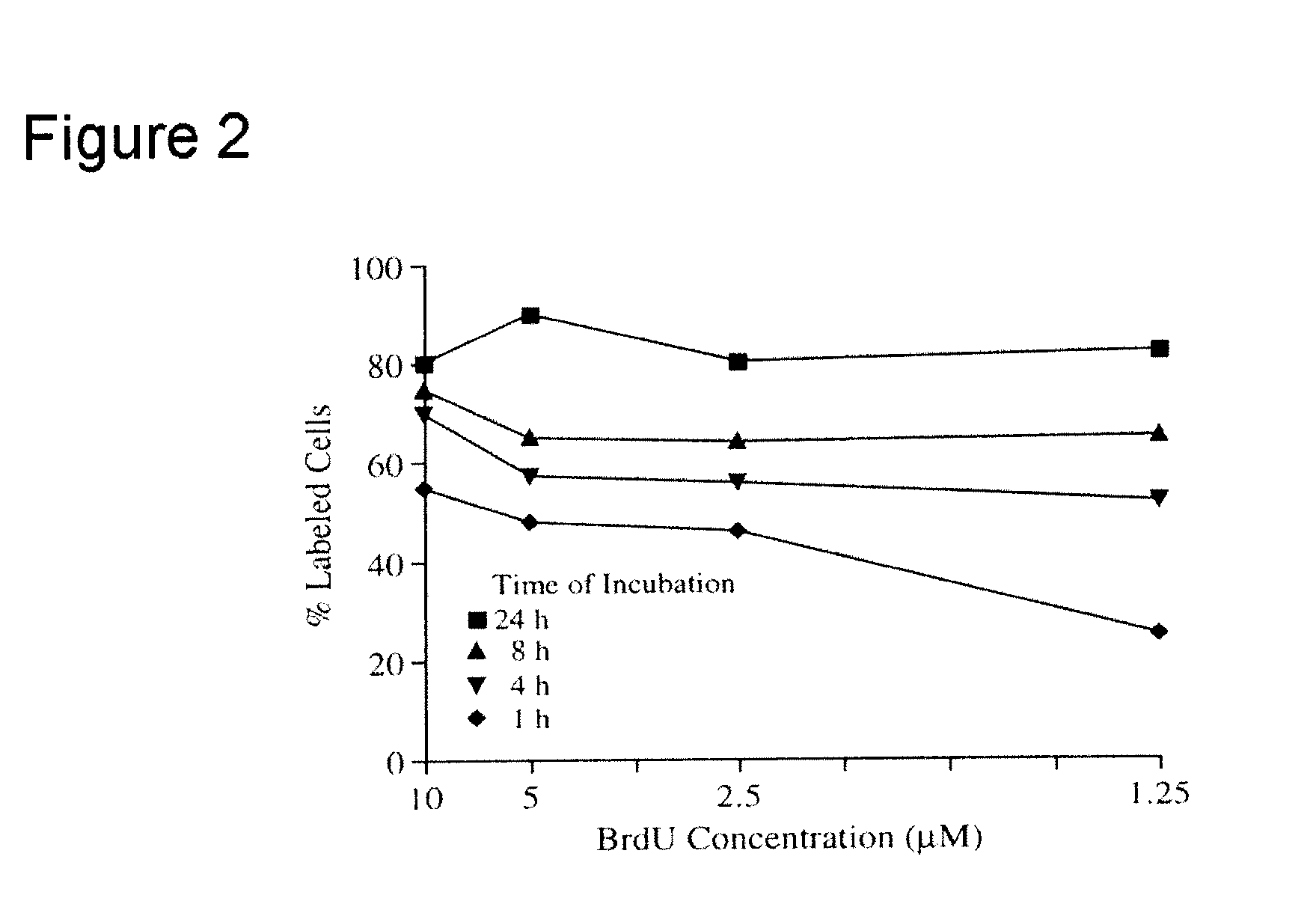
Microcytoxicity assay by pre-labeling target cells
The standard or original microcytotoxicity assay (OMCA) has significant advantages over other cytotoxicity assays, since it is able to detect both cell necrosis and apoptosis and it is simpler, safer, more practical, and more economical. OMCA has serious weaknesses, however, such as low accuracy, low selectivity, and low sensitivity. These drawbacks are ameliorated or eliminated by pre-labeling of target cell nuclei, for instance, with 5-bromo2′-deoxyuridine. This improved microcytotoxicity assay (IMCA) is readily adapted to a wide range of applications, such as screening of cytotoxicity drug candidates, selecting an anticancer cytotoxic therapy, detecting abnormalities including reduced tumor cell killing ability of NK cells in cancer patients, predicting outcome of cytokine therapy and immunotherapy, determining effectiveness of cytokine therapy and immunotherapy in follow up studies following treatment, determining effectiveness of anticancer cytotoxic therapy during and following therapy and ascertaining cytotoxic T cell activity during anticancer vaccination therapy.
Owner:UNIVERSITY OF PITTSBURGH
Method for testing in vitro cytotoxicity of cigarette smoke
ActiveCN102618619AWide linear rangeHigh sensitivityMicrobiological testing/measurementBiotechnologyStaining
The invention discloses a method for testing in vitro cytotoxicity of cigarette smoke. The method is characterized by comprising the following steps of: 1) preparing a laboratory reagent; 2) performing cell inoculation culture; 3) performing cigarette smoke contamination; 4) dyeing with water-soluble tetrazolium-1 (WST-1); and 5) obtaining a result and analyzing. Compared with the prior art, the method is characterized in that a test step for washing cells for multiple times is eliminated in the whole test process, so that the method is easy and convenient to operate; a step of replacing a nutrient solution is not required during dyeing with the WST-1, and a diluted nutrient solution can be directly added for reacting; formazan produced by the WST-1 is water-soluble, so that a subsequent dissolution step is eliminated; and absorbance detection can be finished in 2 hours after WST-1 dyeing, so that the test period is shortened, and the method is quick in comparison with the conventional testing method. The measuring method is quick and convenient to operate, and also has the advantages of high sensitivity and stable result; and the method can be applied to smoke cytotoxicity test of multiple cell lines.
Owner:ZHENGZHOU TOBACCO RES INST OF CNTC
Disulfide bond containing poly(beta-amino ester) polymer gene carrier, and synthesis method and applications thereof
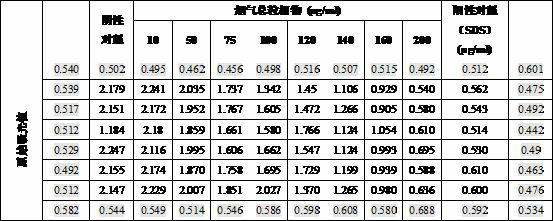
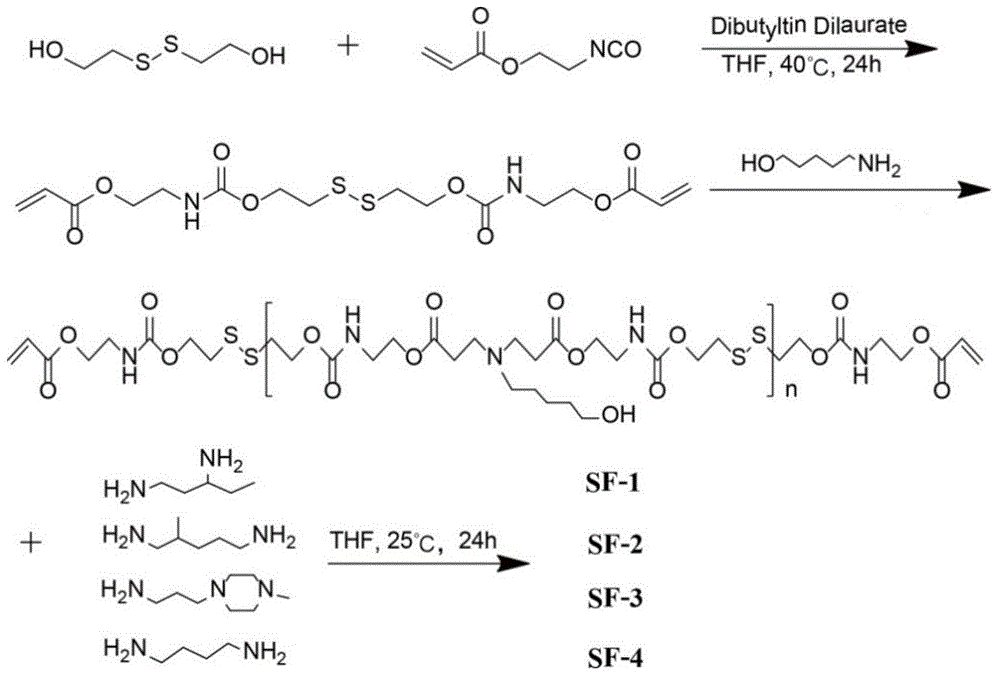
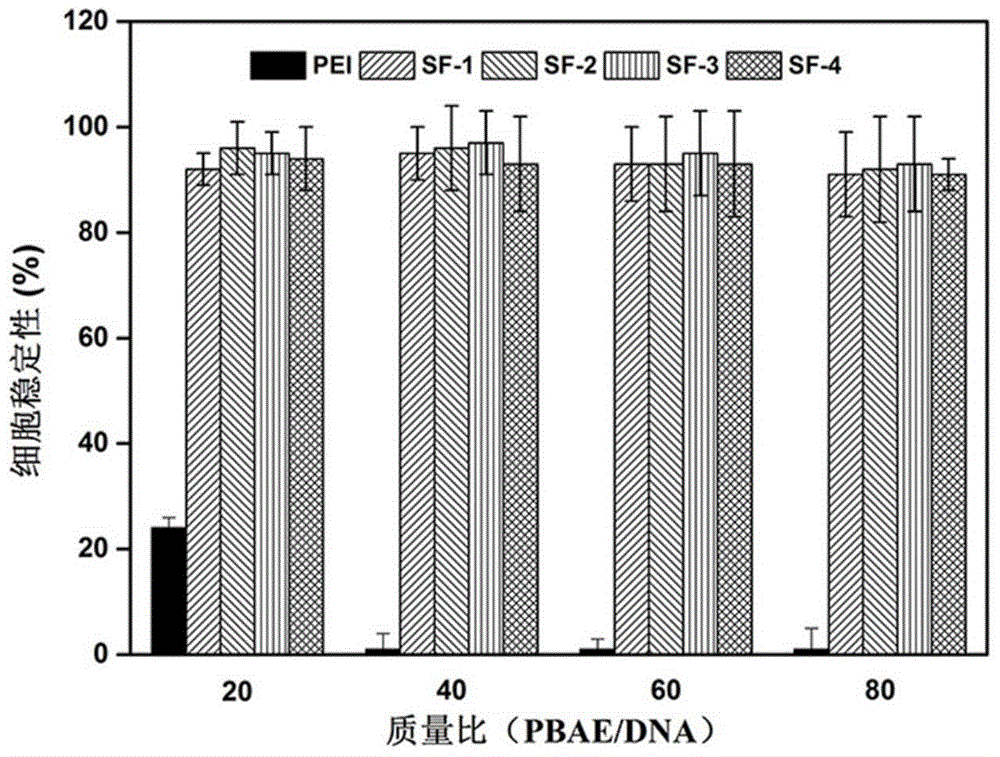
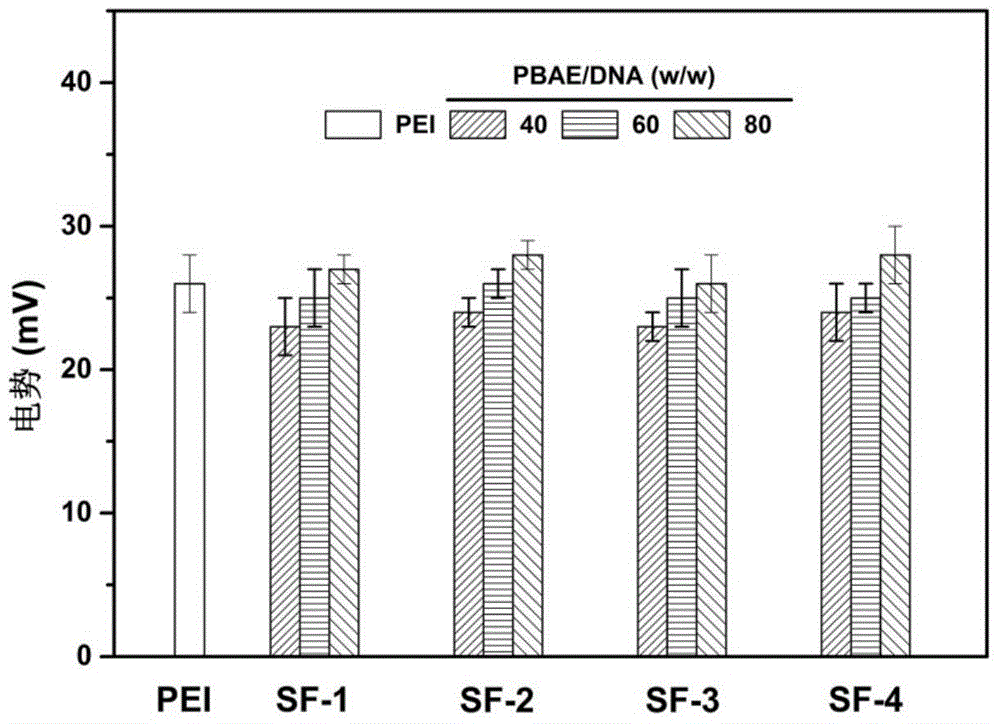
ActiveCN106554499APromote degradationLow toxicityGenetic material ingredientsGene therapyGene carrierCytotoxicity test
The invention discloses a disulfide bond containing poly(beta-amino ester) polymer gene carrier. The poly(beta-amino ester) polymer is a cationic polymer prepared through addition polymerization, is capable of being combined with plasmid DNA with negative charges through the electrostatic interaction, and thus delivers DNA into cells to realize genetic transmission. By introducing disulfide bonds into the poly(beta-amino ester) polymer, the poly(beta-amino ester) polymer has an oxidation-reduction responding mechanism in cells, thus the polymer degradation is assisted, and DNA can be fully released in the cells. The results of cytotoxicity tests and cell transfection experiments show that the disulfide bond containing poly(beta-amino ester) polymer gene carrier has low cytotoxicity and high transfection efficiency, which is obviously higher than that of common commercial gene transfection reagents. The provided poly(beta-amino ester) polymer gene carrier is a low toxic and high efficient non-virus gene carrier, and has a very good application prospect.
Owner:NANJING UNIV OF SCI & TECH
Two-photon fluorescent probe as well as preparation method and application thereof
ActiveCN105859733ASimple structureSelectivityOrganic chemistryFluorescence/phosphorescenceMicro imagingCytotoxicity testing
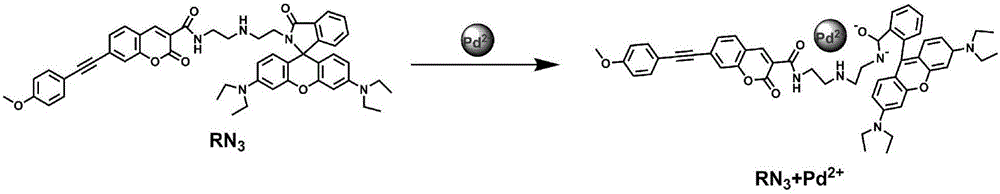
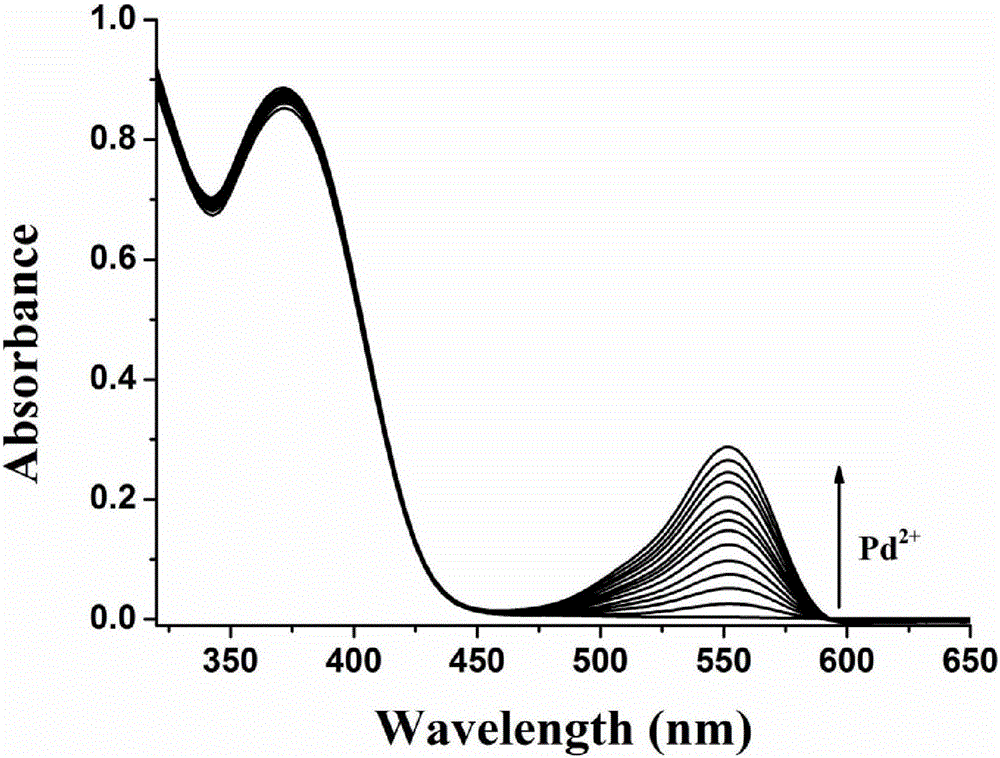
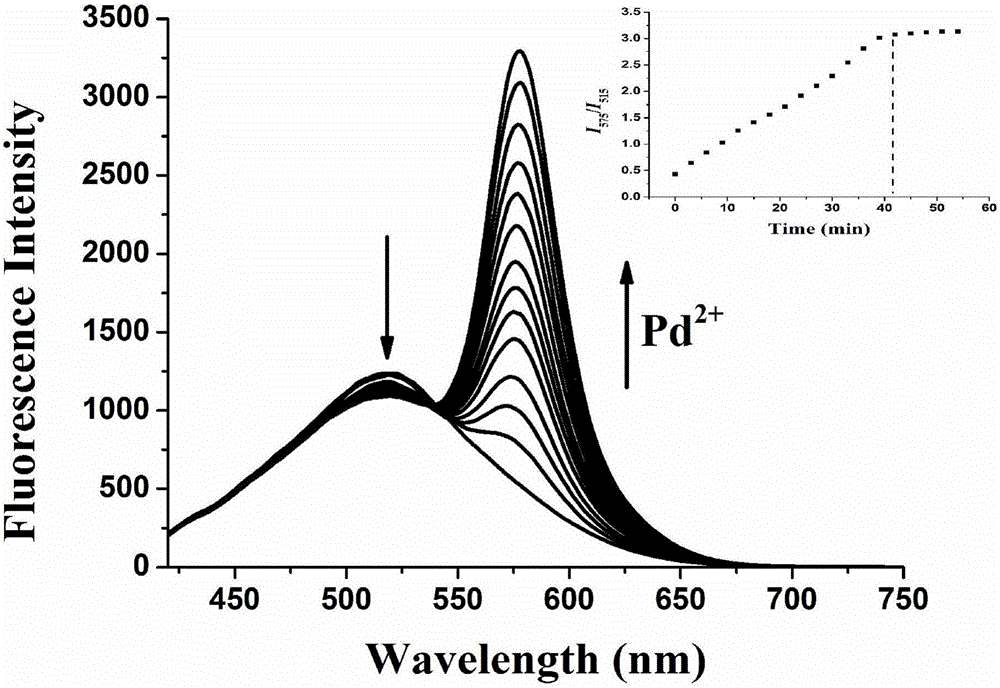
The invention discloses a two-photon fluorescent probe as well as a preparation method and application thereof. The two-photon fluorescent probe takes coumarin and rhodamine as a matrix. The structure of the two-photon fluorescent probe is described in the specification. Molecules of the two-photon fluorescent probe show relatively high selectivity and sensitivity in a system in which palladium ions (II) and other positive ions coexist. A cytotoxicity test shows that the two-photon fluorescent probe disclosed by the invention has hardly any toxic effect on cells, and a two-photon fluorescence microscopy imaging experiment shows that the two-photon fluorescent probe has good HeLa cell permeability and is applicable to detection of distribution of palladium ions (II) in cells.
Owner:ANHUI UNIVERSITY
Method for preparing polypeptide copolymer porous nanofiber by using electrostatic spinning
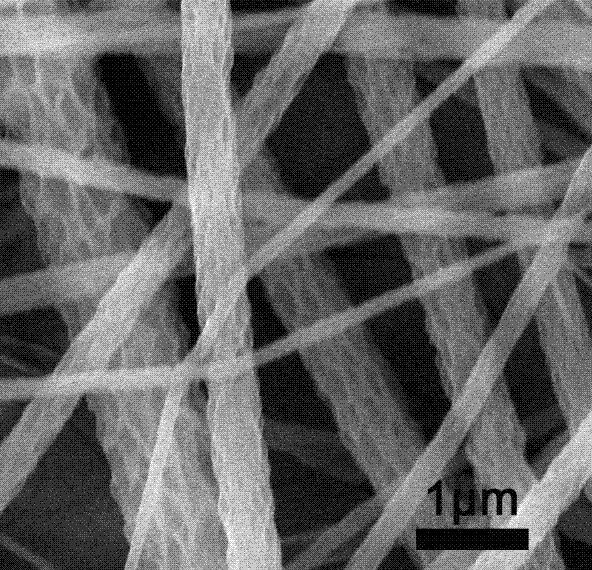
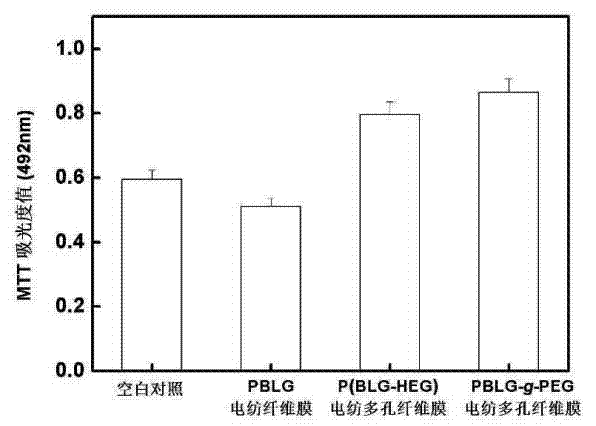
InactiveCN103590133AGood biocompatibilityPromotes Adhesive GrowthFilament/thread formingMonocomponent copolyamides artificial filamentYarnPolymer science
The invention relates to a method for preparing polypeptide copolymer porous nanofiber by using electrostatic spinning. The method comprises the following steps: firstly, carrying out hydrophilic modification on a hydrophobic polypeptide homopolymer to prepare a poly(gamma-benzyl L-glutamate-hydroxyethyl glutamine) or poly(gamma-benzyl L-glutamine)-g-polyethylene glycol grafted copolymer; secondly, preparing a compound solvent by using solvents with different volatilization properties, and then preparing into a spinning solution; thirdly, spinning by using a high voltage electrostatic spinning method; and finally, placing spun yarns into a vacuum drying chamber and drying for more than 3 hours at room temperature to obtain the porous nanofiber with high specific surface area. A cytotoxicity test indicates that the prepared porous nanofiber with high specific surface area is free of cytotoxicity and good in biocompatibility, can be used as a porous scaffold for cell growth, has the function of promoting cell migration and amplification and has a relatively good application value in the aspect of preparation of scaffolds for tissue engineering; besides, the polypeptide copolymer porous nanofiber has an application prospect in the fields of medical dressing, medicine released control, artificial organs, sewage treatment and the like.
Owner:EAST CHINA UNIV OF SCI & TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com