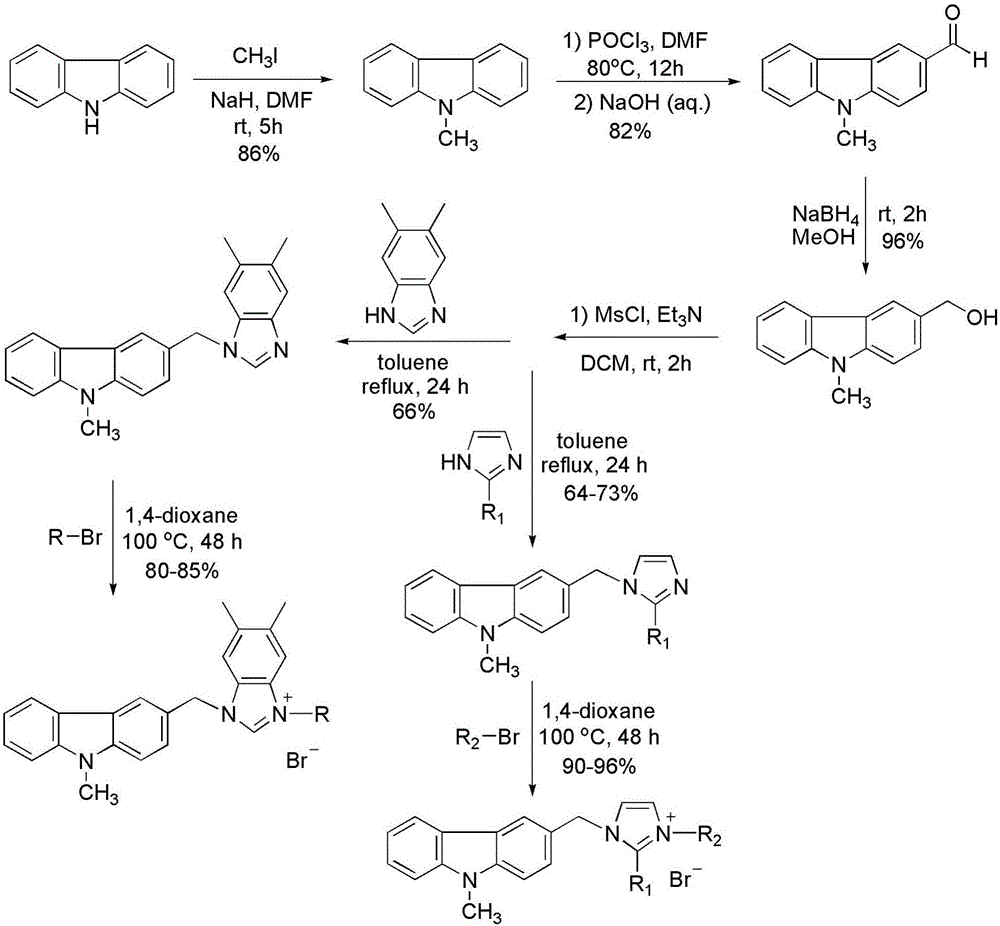
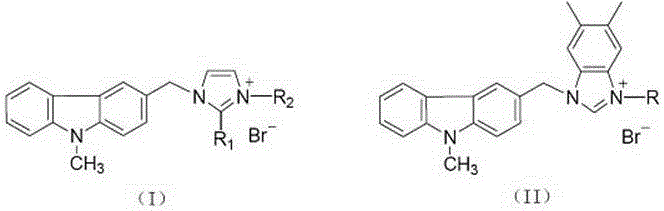
Substituted carbazole-imidazolate or benzimidazolium salt compounds, and preparation method thereof
A salt compound, benzimidazole technology, applied in the field of anti-tumor application, can solve the problems that have not been reported in the literature
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
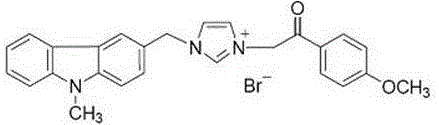
[0021] Example 1 1-(N-methylcarbazole-3-methyl)-3-(4-methoxyphenacylmethyl)imidazolium bromide
[0022]
[0023] Preparation process: see the above preparation methods A, B, C, D, E for details.
[0024] White solid, 93% yield, m.p. 223-224 o c.
[0025] IR ν max (cm -1 ): 3080, 3016, 2322, 1682, 1600, 1566, 1474, 1443, 1322,1244, 1156, 1120, 1065, 1016, 990, 846, 815, 752, 718, 625.
[0026] 1 H-NMR (400 MHz, DMSO) δ: 9.29 (1H, s), 8.35 (1H, s), 8.14 (1H, d, J =7.7 Hz), 8.02-7.98 (3H, m), 7.76 (1H, s), 7.68 (1H, d, J = 8.4 Hz), 7.62 (2H, d, J = 7.9 Hz), 7.50 (1H, t, J = 7.5 Hz), 7.25 (1H, t, J = 7.5 Hz) , 7.15-7.12 (2H, m), 6.02 (2H, s), 5.70 (2H, s), 3.89 (3H, s), 3.87 (3H, s).
Embodiment 2
[0027] Example 2 1-(N-methylcarbazole-3-methyl)-3-(4-bromophenacylmethyl)imidazolium bromide
[0028]
[0029] Preparation process: see the above preparation methods A, B, C, D, E for details.
[0030] White solid, yield 96%, m.p. 208-209 o c.
[0031] IR ν max (cm -1 ): 3148, 3072, 2325, 1700, 1586, 1565, 1491, 1361, 1335, 1251, 1229, 1120, 1068, 991, 855, 819, 750, 714, 621.
[0032]1 H-NMR (400 MHz, DMSO) δ: 9.20 (1H, s), 8.33 (1H, s), 8.13 (1H, d, J =7.7 Hz), 7.97-7.95 (3H, m), 7.89-7.85 ( 2H, m), 7.72-7.69 (2H, m), 7.65-7.60(2H, m), 7.53-7.49 (1H, m), 7.26 (1H, t, J = 7.5 Hz), 6.02 (2H, s) , 5.69 (2H,s), 3.90 (3H,s).
Embodiment 3
[0033] Example 3 1-(N-methylcarbazole-3-methyl)-3-(2-naphthoylmethyl)imidazolium bromide
[0034]
[0035] Preparation process: see the above preparation methods A, B, C, D, E for details.
[0036] White solid, yield 92%, m.p. 200-201 o c.
[0037] IR ν max (cm -1 ): 3077, 1692, 1627, 1599, 1566, 1494, 1472, 1438, 1362, 1334, 1253, 1159, 1125, 1041, 860, 812, 725, 624.
[0038] 1 H-NMR (400 MHz, DMSO) δ: 9.37 (1H, s), 8.82 (1H, s), 8.38 (1H, s), 8.20-8.10 (3H, m), 8.06-8.03 (3H, m), 7.84-7.82 (1H, m), 7.75-7.62 (5H, m), 7.52-7.49 (1H, m), 7.27-7.24 (1H, m), 6.25 (2H, s), 5.74 (2H, s), 3.90 (3H, s).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com