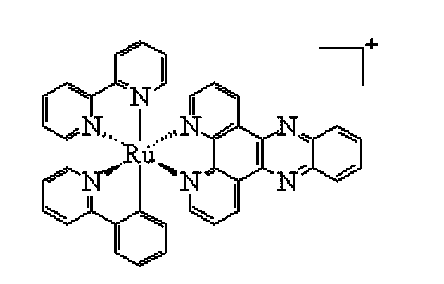
Cyclometalated ruthenium complex, and preparation method and application thereof
A technology of cyclometalation and ruthenium complexes, which is applied in the field of DNA insertion reagents and anticancer drugs, to achieve the effects of high-efficiency anticancer drugs, overcoming the defects of tumor treatment, and strong growth inhibition ability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] The preparation method of embodiment 1 ligand and complex
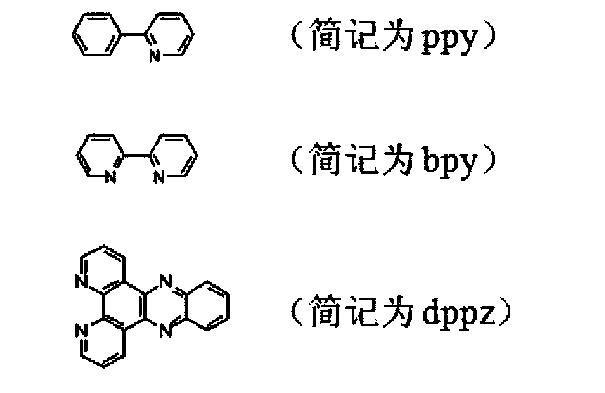
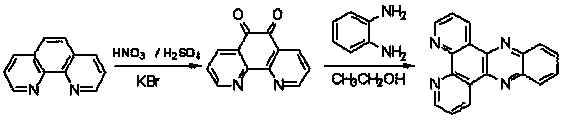
[0043] (1) Synthetic method of ligand dppz:
[0044] a) Synthesis method of 1,10-phenanthrolene-5,6-dione:
[0045] The synthesis of 1,10-phenanthroline-5,6-dione is according to (J.Am.Chem.Soc.1993,115,3448) method: stir 4.0g phenanthroline and 4.0g potassium bromide And placed in a 250mL round bottom flask, then dropwise added cold concentrated sulfuric acid (40mL) and concentrated nitric acid (20mL) mixed acid. After the dropwise addition, heat and reflux at 100°C for 3h, cool to room temperature, pour it into 500mL ice water, and then use 10molL -1 The NaOH solution was carefully neutralized to neutral, and the solution was extracted with 3×100 mL of chloroform. After combining the organic phases, they were washed with 50 mL of water, and finally dried overnight with anhydrous sodium sulfate. Filtrate, remove chloroform under reduced pressure, recrystallize the solid product from ethanol to obtain orange...
Embodiment 2
[0056] The ultraviolet-visible spectroscopic titration experiment of embodiment 2 complex
[0057] When the transition metal complex interacts with DNA, the absorption spectrum wavelength and absorption intensity change due to the change of the environment in which the complex resides, and the wavelength and absorption intensity change in different ways when the complex interacts with DNA. When small molecules are DNA-bonded in an intercalation manner, the corresponding absorption peaks produce obvious hypochromicity, accompanied by a certain degree of red shift (red shift). This is due to the π-electron stacking between the insertion ligand and the base pair of DNA, making it π * After the empty orbital is coupled with the p-electron orbital of the base pair, the energy level decreases, resulting in π→π * The transition energy decreases, resulting in a redshift. Simultaneously coupled π * Empty orbitals are partially filled with electrons, making π→π * The transition chan...
Embodiment 3
[0058] Transcription inhibition experiment of the complex of embodiment 3
[0059] Complexes with large planar ligands can be inserted into the double strands of DNA, preventing T7 RNA polymerase from binding to template DNA during DNA transcription, thus effectively preventing DNA transcription. In transcription inhibition experiments, varying the content of the complex and keeping the other components constant, the final content of RNA produced was used as a measure of repressive capacity. Depend on Figure 5 As can be seen from the figure below, the IC of the transcriptional inhibition ability of the complex 50 The value is 7.5 μM.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com