Cu-Eu exotic-metal-substituted arsenotungstate as well as preparation method and application thereof
A technology of arsenic tungstate and heterometal, applied in chemical instruments and methods, tungsten compounds, pharmaceutical formulations, etc., to achieve the effect of improving stability and improving reactivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
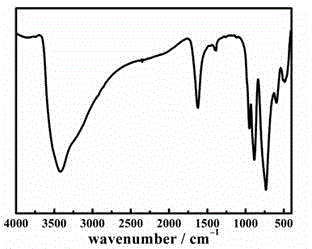
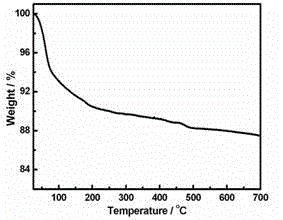
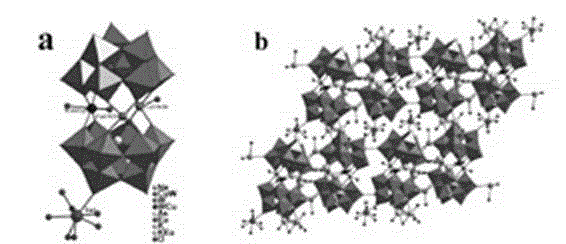
[0035] A kind of copper-europium heterometallic substituted arsenic tungstate, its chemical formula is: Na 2 K 3.5 Eu 0.5 [Eu(H 2 O) 7 ][As 2 W 19 Cu 2 o 67 (H 2 O) 3 ]·22H 2 O.
[0036] The above-mentioned copper-europium heterometallic substituted arsenic tungstate is synthesized by a traditional aqueous solution method, and its preparation method specifically includes the following steps:
[0037] 1) Preparation of arsenotungstate precursor K 14 [As 2 W 19 o 67 (H 2 O)]; details can be found in the literature (U. Kortz, M. G. Savelieff, B. S. Bassil, M. H. Dickman, Angew. Chem. Int. Ed. 2001 , 40 , 3384—3386) for preparation;
[0038] 2) In a 25 mL beaker, 0.263 g (0.050 mmol) of K 14 [As 2 W 19 o 67 (H 2 O)], 0.075 g (0.440 mmol) CuCl 2 2H 2 O, 0.056 g (0.217 mmol) EuCl 3 and 0.060 g (1.027 mmol) of NaCl were dissolved in 15 mL (833 mmol) of distilled water, stirred at room temperature for 2 h to form a homogeneous mixed phase, then placed in a...
Embodiment 2
[0040] A kind of copper-europium heterometallic substituted arsenic tungstate, its chemical formula is: Na 2 K 3.5 Eu 0.5 [Eu(H 2 O) 7 ][As 2 W 19 Cu 2 o 67 (H 2 O) 3 ]·22H 2 O.
[0041] The above-mentioned copper-europium heterometallic substituted arsenic tungstate is synthesized by a traditional aqueous solution method, and its preparation method specifically includes the following steps:
[0042] 1) Preparation of arsenotungstate precursor K 14 [As 2 W 19 o 67 (H 2 O)]; details can be found in the literature (U. Kortz, M. G. Savelieff, B. S. Bassil, M. H. Dickman, Angew. Chem. Int. Ed. 2001 , 40 , 3384—3386) for preparation;
[0043] 2) In a 25 mL beaker, 0.263 g (0.050 mmol) of K 14 [As 2 W 19 o 67 (H 2 O)], 0.025 g (0.147 mmol) CuCl 2 2H 2 O, 0.043 g (0.166 mmol) EuCl 3 and 0.060 g (1.027 mmol) of NaCl were dissolved in 15 mL (833 mmol) of distilled water, stirred at room temperature for 2 h to form a homogeneous mixed phase, then placed in a...
Embodiment 3
[0045] A kind of copper-europium heterometallic substituted arsenic tungstate, its chemical formula is: Na 2 K 3.5 Eu 0.5 [Eu(H 2 O) 7 ][As 2 W 19 Cu 2 o 67 (H 2 O) 3 ]·22H 2 O.
[0046] The above-mentioned copper-europium heterometallic substituted arsenic tungstate is synthesized by a traditional aqueous solution method, and its preparation method specifically includes the following steps:
[0047] 1) Preparation of arsenotungstate precursor K 14 [As 2 W 19 o 67 (H 2 O)]; details can be found in the literature (U. Kortz, M. G. Savelieff, B. S. Bassil, M. H. Dickman, Angew. Chem. Int. Ed. 2001 , 40 , 3384—3386) for preparation;
[0048] 2) In a 25 mL beaker, 0.263 g (0.050 mmol) of K 14 [As 2 W 19 o 67 (H 2 O)], 0.070 g (0.411 mmol) CuCl 2 2H 2 O, 0.048 g (0.186 mmol) EuCl 3 and 0.120 g (2.054 mmol) NaCl were dissolved in 15 mL (833 mmol) distilled water to form a homogeneous mixed phase, then placed in a water bath at 80 °C and heated for 1.5 h, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com