A chiral polyacid-based metal-organic framework material and its preparation method and application
An organic framework and metal-based technology, applied in the field of chiral polyacid-based metal-organic framework materials and their preparation, can solve the problems of high process cost, toxicity, danger, etc., and achieve stable three-dimensional structure, good performance, and atom economy. green effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
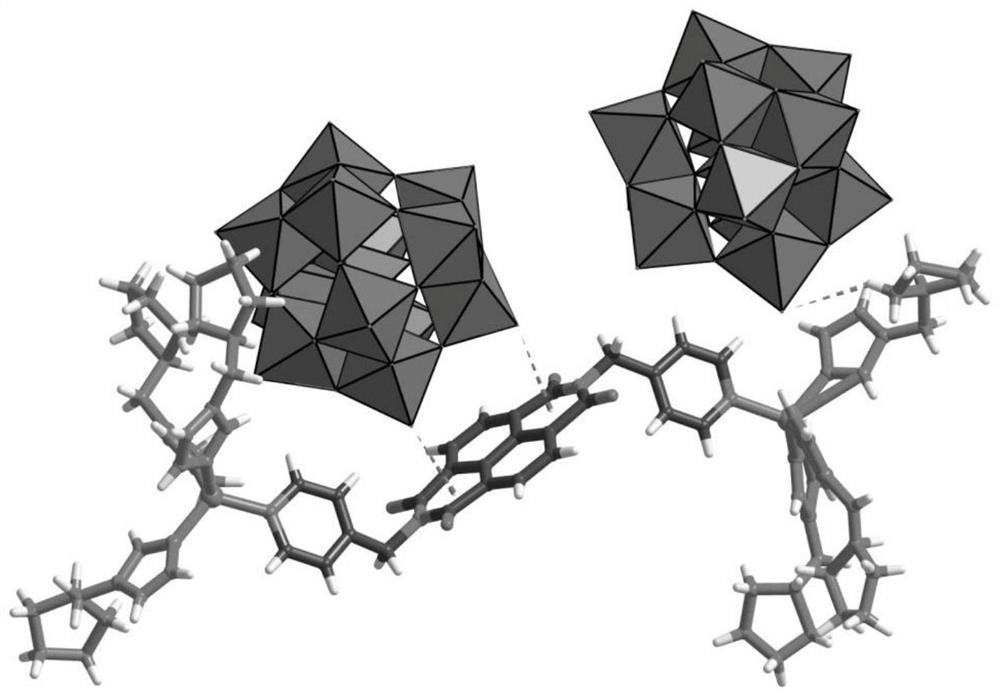
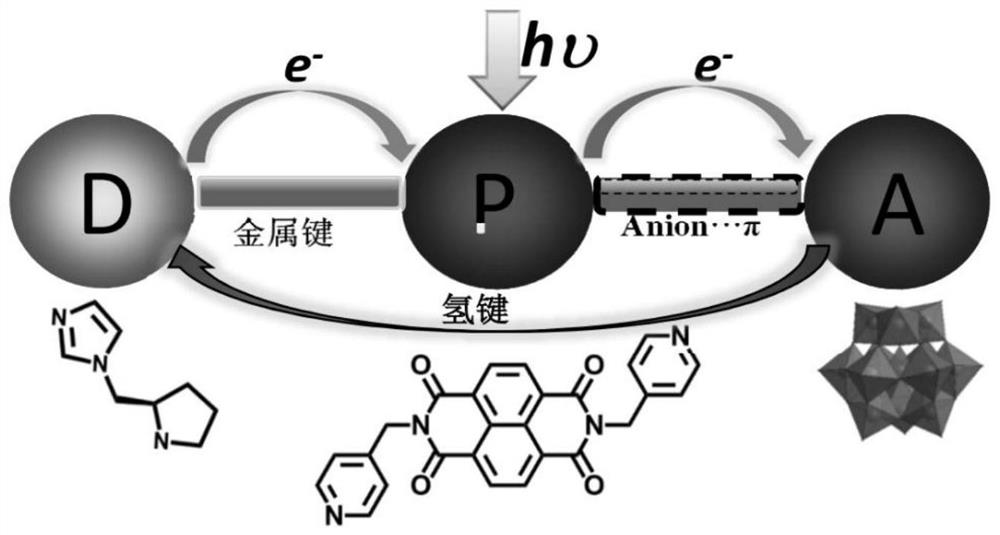
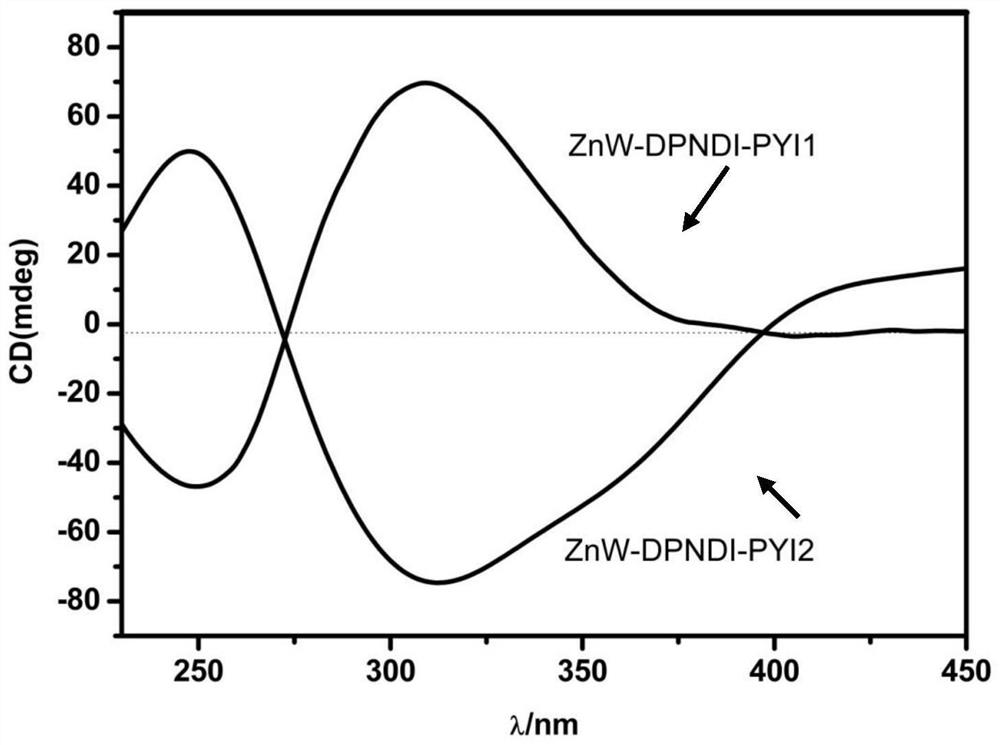
[0027] The chemical formula of the chiral polyacid-based metal-organic framework material in this example is: C 74 h 101 B 2 Zn 2 N 22 o 84 W 24 , recorded as ZnW-DPNDI-PYIs, according to the material chirality and recorded as ZnW-DPNDI-PYI1 and ZnW-DPNDI-PYI2.
[0028] The preparation method of ZnW-DPNDI-PYI1 in this example is as follows: Weigh 45mg and 0.15mmol of Zn(NO 3 ) 2 ·6H 2 O, 157.8 mg, 0.05 mmol of K 5 [BW 12 o 40 ]·5H 2 O, 23.4mg, 0.15mmol of DPNDI and 24.5mg, 0.1mmol of L-BCIP in a small glass bottle, add 4.0mL of distilled water, 2.0mL of methanol to dissolve, stir overnight to obtain a suspension solution, transfer the suspension solution Put it into a 25mL autoclave, put it into an oven, heat it to 120°C and react for five days, take out the reaction product after cooling to room temperature, and wash it repeatedly with distilled water to obtain white-yellow rod-shaped crystal ZnW-DPNDI-PYI1, the ZnW-DPNDI- The yield of PYI1 was 60%;
[0029] The...
Embodiment 2
[0038] This embodiment is substrate with styrene, O 2 As an oxidant, add 0.1% mol of ZnW-DPNDI-PYI1 or ZnW-DPNDI-PYI2, and react for 12 hours under visible light irradiation to realize the heterogeneous asymmetric catalytic olefin epoxidation reaction. The reaction equation is as follows Figure 6 As shown, the reaction conditions and reaction results are shown in Table 1, the selective oxidation yield of styrene reaches 92%, and is converted into product styrene oxide, while the ee value is as high as 45%, and the substrate can be extended to styrene derivatives, Such as 4-chlorostyrene, 2-chlorostyrene, 4-methylstyrene.
Embodiment 3
[0040] Select multiple substrates as in table 1 to carry out catalytic oxidation reaction, wherein select 3,5-di-tert-butyl-4-vinylbiphenyl (i.e. tert-butylstyrene in table 1) as substrate, when with Under the same conditions of Example 2, the yield of the asymmetric catalytic epoxidation reaction is less than 10%. We speculate that this is because the substrate is too bulky to enter the pores of ZnW-DPNDI-PYI1 for reaction, and only a small amount of substrate is adsorbed on the surface of ZnW-DPNDI-PYI1 for reaction. This phenomenon not only reflects the advantages of POMOFs shape-selective catalysis, but also proves that the asymmetric catalytic epoxidation reaction in this experiment does occur in the pores of POMOFs. result of the action.
[0041] Table 1 Conversion and selectivity of aryl olefin epoxidation
[0042]
[0043]
[0044] The reaction equation in Table 1 can be passed Figure 6 Reaction conditions: substrate olefin 5mmol, ZnW-DPNDI-PYI1 (10mg, 12.5μmol...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com