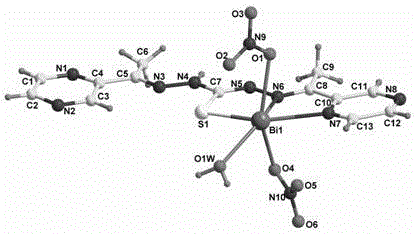
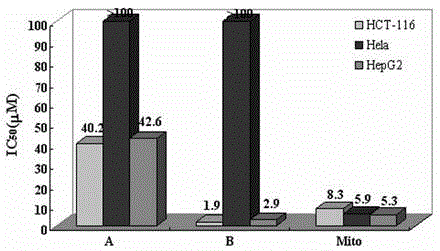
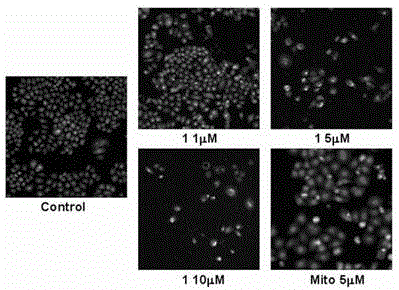
Bis(2-acetylpyrazine) thiocarbonohydrazone and preparation method and application of bismuth (III) complex thereof
A technology based on thiobisemicarbazone and acetylpyrazine, which is applied in the fields of bismuth organic compounds, drug combinations, organic chemistry, etc., can solve the problems of few literature reports, difficult synthesis, difficult cultivation, etc., and achieve a strong growth inhibitory effect Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] The preparation method of two (2-acetylpyrazinyl) thiobisemicarbazones, it comprises the steps:
[0039] 1) Synthesize the required 1.3-symmetric dithiosemicarbazide according to the literature method (see Sheng Mei, Jiang Yanqing, Lu Zhengwei, Journal of Jiangsu Institute of Technology , 2004, 16(1) : 35-39);
[0040] 2) Add 1.3-symmetric dithiosemicarbazide (1.00 g, 10 mmol) and 2-acetylpyrazine (2.40 g, 20 mmol) into a flask containing 50 mL of ethanol at room temperature, and then add 0.35 mL of glacial acetic acid dropwise , Mixed and then stirred and refluxed for 4h, cooled, filtered, and the precipitate was recrystallized with ethanol and dried in vacuum to obtain an orange-red powder with a yield of about 75%. Elemental analysis results measured value: C 49.87, H 4.18, N 35.78; calculated value C 13 h 13 N 8 S (%): C 49.97, H 4.09, N 35.90; IR (KBr, cm -1 ): ν (C=N) 1614 cm -1 , ν (C=S) 852 cm -1 , ν (N=N) 1104 cm -1 .
[0041] The preparation m...
Embodiment 2
[0044] The preparation method of two (2-acetylpyrazinyl) thiobisemicarbazones, it comprises the steps:
[0045] 1) Synthesize the required 1.3-symmetric dithiosemicarbazide according to the literature method (see Sheng Mei, Jiang Yanqing, Lu Zhengwei, Journal of Jiangsu Institute of Technology , 2004, 16(1) : 35-39);
[0046] 2) Add 1.3-symmetric dithiosemicarbazide (1.00 g, 10 mmol) and 2-acetylpyrazine (3.00 g, 25 mmol) into a flask containing 50 mL of methanol at room temperature, and then add 0.35 mL of glacial acetic acid dropwise , Mixed and then stirred and refluxed for 4h, cooled, filtered, and the precipitate was recrystallized with methanol and then vacuum-dried to obtain an orange-red powder with a yield of about 81%. Elemental analysis results measured value: C 49.87, H 4.18, N 35.78; calculated value C 13 h 13 N 8 S (%): C 49.97, H 4.09, N 35.90; IR (KBr, cm -1 ): ν (C=N) 1614 cm -1 , ν (C=S) 852 cm -1 , ν (N=N) 1104 cm -1 .
[0047] The preparati...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com