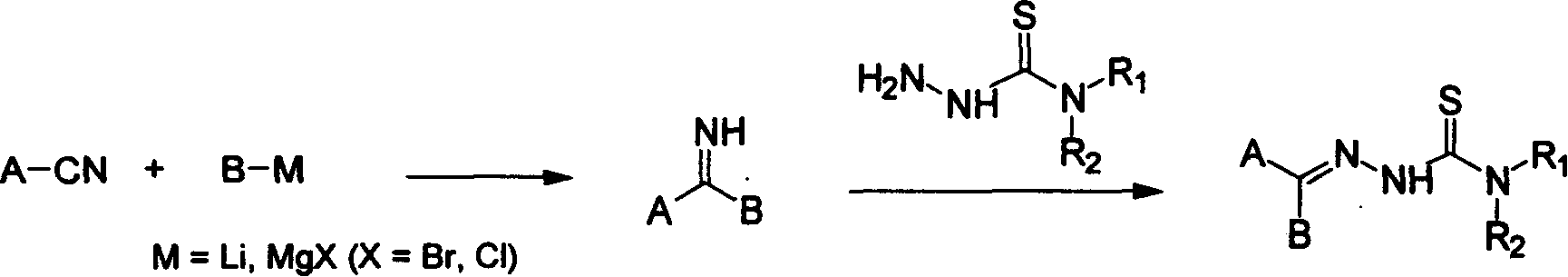
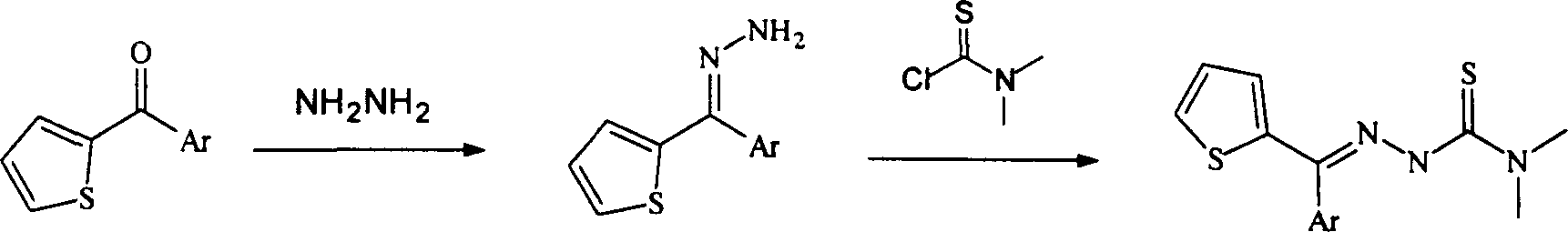
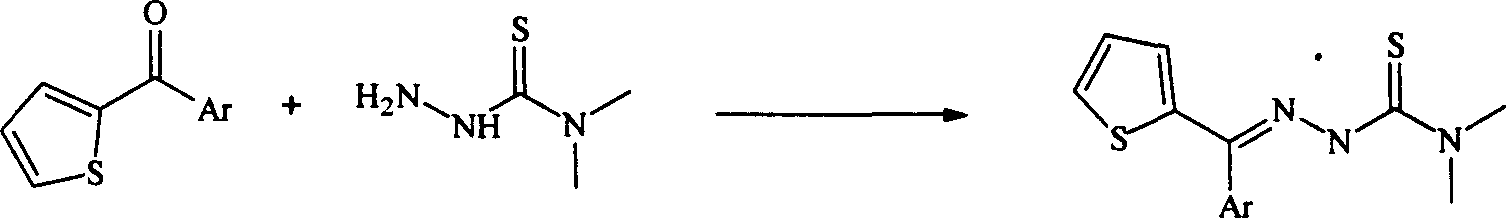Method for synthesizing heteroaryl thiosemicarbazone antineoplastic
A technology of thiosemicarbazones and synthetic methods, which is applied in the direction of antineoplastic drugs, drug combinations, organic chemistry, etc., can solve the problems of low yield, inability to synthesize thiosemicarbazone compounds, difficult purification, etc., and achieve less purification times , The process steps are short and the raw materials are easy to obtain
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Example 1 Synthesis of 2-pyridyl-phenylimine
[0027] A solution of 2-cyanopyridine (104 g, 1 mol) in tetrahydrofuran (200 ml) was added dropwise to a freshly prepared phenyl Grignard reagent (1.5 liters of ether-tetrahydrofuran solution, 1 mol) at room temperature, and the mixture was stirred for 5 hours . 20% aqueous ammonium chloride (600 mL) was added and stirred for another 30 minutes, then extracted with ethyl acetate (3 x 500 mL). The extract was dried with anhydrous sodium sulfate, filtered and concentrated to dryness to obtain the product which could be directly used in the next step (Example 7).
Embodiment 2
[0028] Example 2 Synthesis of 2-pyridyl-2-thienyl imine
[0029] As in Example 1, the newly prepared 2-thienyl Grignard reagent (prepared from 2-bromothiophene by a conventional method) was reacted with 2-cyanopyridine to obtain the product (directly used in Example 8).
Embodiment 3
[0030] Example 3 Synthesis of 2-pyridyl-3-pyridyl imine
[0031] A solution of 2-bromopyridine (15.8 g, 0.1 mol) in tetrahydrofuran (100 ml) was dropped into a solution of butyllithium (0.1 mol) in hexane (60 ml) and tetrahydrofuran (140 ml) at -40°C and Stir for 30 minutes to generate the 2-pyridyllithium reagent. A solution of 3-cyanopyridine (10.4 g, 0.1 mol) in THF (200 mL) was dropped into freshly prepared 2-pyridyllithium reagent at low temperature, and the mixture was stirred for 5 hours and slowly warmed to room temperature. 20% aqueous ammonium chloride (600 mL) was added and stirred for 2 hours, then extracted with ethyl acetate (3 x 500 mL). The extract was dried with anhydrous sodium sulfate, filtered and concentrated to dryness to obtain the product which could be directly used in the next step.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com