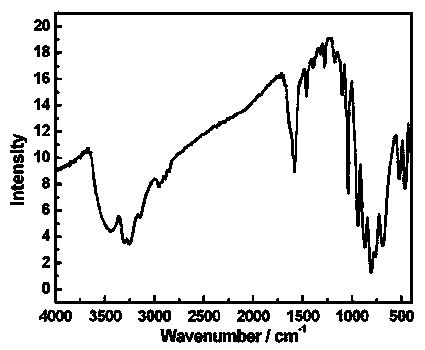
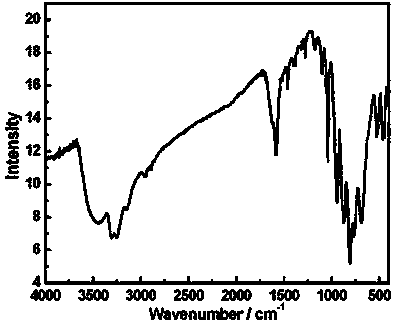
Organic-inorganic hybridization transition-rare earth dissimilar metal substituted germanium tungstate crystal hydrogen storage material and preparation method thereof
A technology of germanium tungstate and hydrogen storage materials, which is applied in chemical instruments and methods, other chemical processes, hydrogen production, etc., can solve problems that have not been reported, and achieve a clear structure, improved stability, and simple preparation methods Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Example 1: Na 3 h 7 [Cu(en) 2 ] 5 [Cu(en) 2 (H 2 O)] 2 [(α-GeW 11 o 39 Gd) 2 (α-GeW 11 o 39 Gd(H 2 O))
[0036] (α-GeW 11 o 39 Gd (H 2 O) 2 ) (WO 4 ) 2 ]·13H 2 Preparation of O crystalline hydrogen storage materials:
[0037] 1) Synthesize the required triple-deficient germanium tungstate precursor K according to the literature method 8 Na 2 [A-α-GeW 9 o 34 ]·25H 2 O, see L. H. Bi, U. Kortz, S. Nellutla, A. C. Stowe, J. van Tol, N. S. Dalal, B. Keita, L. Nadjo, Inorg. Chem. 2005, 44, 896);
[0038] 2) 3.62 g (0.01 mol) rare earth oxide Gd 2 o 3 Dissolve in 6.70 mL, 12 mol L under heating –1 Concentrated hydrochloric acid (0.08mol), and then heated and evaporated to dryness at 80°C (about 40 min) to obtain anhydrous GdCl 3 , cooled and sealed for storage;
[0039] 3) Under stirring conditions, 330mg (0.107mmol) K 8 Na 2 [A-α-GeW 9 o 34 ]·25H 2 O, 68 mg (0.399 mmol) CuCl 2 2H 2 O, 68 mg (0.258 mmol) GdCl 3 and 0.1mL (1.480mmol) of e...
Embodiment 2
[0040] Example 2: Na 3 h 7 [Cu(en) 2 ] 5 [Cu(en) 2 (H 2 O)] 2 [(α-GeW 11 o 39 Gd) 2 (α-GeW 11 o 39 Gd(H 2 O))
[0041] (α-GeW 11 o 39 Gd (H 2 O) 2 ) (WO 4 ) 2 ]·13H 2 Preparation of O crystalline hydrogen storage materials:
[0042] 1) Synthesize the required triple-deficient germanium tungstate precursor K according to the literature method 8 Na 2 [A-α-GeW 9 o 34 ]·25H 2 O, see L. H. Bi, U. Kortz, S. Nellutla, A. C. Stowe, J. van Tol, N. S. Dalal, B. Keita, L. Nadjo, Inorg. Chem. 2005, 44, 896);
[0043] 2) 3.62 g (0.01 mol) rare earth oxide Gd 2 o 3 Dissolve in 6.70 mL, 12 mol L under heating –1 Concentrated hydrochloric acid (0.08mol), then heated and evaporated to dryness at 90°C to obtain anhydrous GdCl 3 , cooled and sealed for storage;
[0044] 3) Under stirring conditions, 330mg (0.107mmol) K 8 Na 2 [A-α-GeW 9 o 34 ]·25H 2 O, 68 mg (0.399 mmol) CuCl 2 2H 2 O, 68 mg (0.258 mmol) GdCl 3 and 0.05mL (0.740mmol) of ethylenediamine wer...
Embodiment 3
[0045] Example 3: Na 3 h 7 [Cu(en) 2 ] 5 [Cu(en) 2 (H2 O)] 2 [(α-GeW 11 o 39 Y) 2 (α-GeW 11 o 39 Y(H 2 O))(α-GeW 11 o 39 Y (H 2 O) 2 ) (WO 4 ) 2 ]·13H 2 Preparation of O crystalline hydrogen storage materials:
[0046] 1) Synthesize the required triple-deficient germanium tungstate precursor K according to the literature method 8 Na 2 [A-α-GeW 9 o 34 ]·25H 2 O, see L. H. Bi, U. Kortz, S. Nellutla, A. C. Stowe, J. van Tol, N. S. Dalal, B. Keita, L. Nadjo, Inorg. Chem. 2005, 44, 896);
[0047] 2) 2.26 g (0.01 mol) rare earth oxide Y 2 o 3 Dissolve in 6.70 mL, 12 mol L under heating –1 Concentrated hydrochloric acid (0.08mol), and then heated and evaporated to dryness at 80°C (about 30min) to obtain anhydrous YCl 3 , cooled and sealed for storage;
[0048] 3) Under stirring conditions, 431mg (0.140mmol) K 8 Na 2 [A-α-GeW 9 o 34 ]·25H 2 O, 63 mg (0.370 mmol) CuCl 2 2H 2 O, 98mg (0.502mmol) YCl 3 and 0.1mL (1.480mmol) of ethylenediamine were ad...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thermal stability | aaaaa | aaaaa |
| porosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com