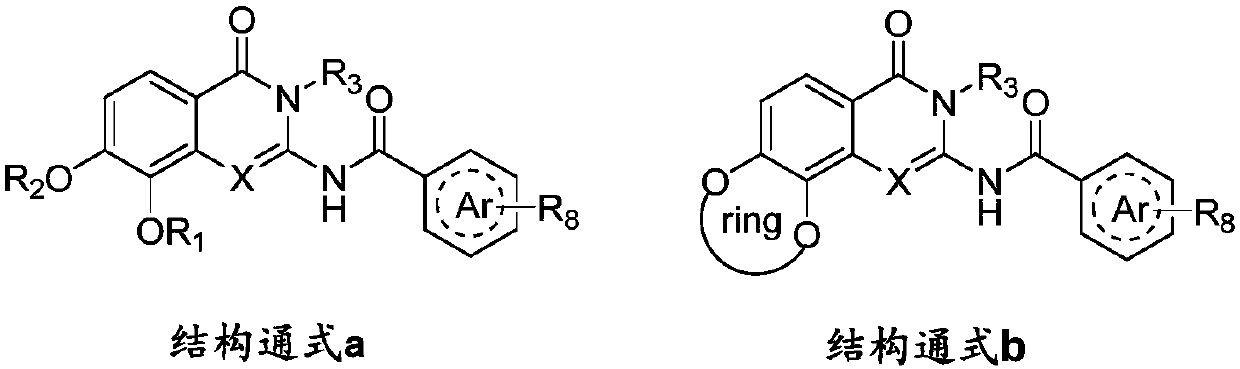
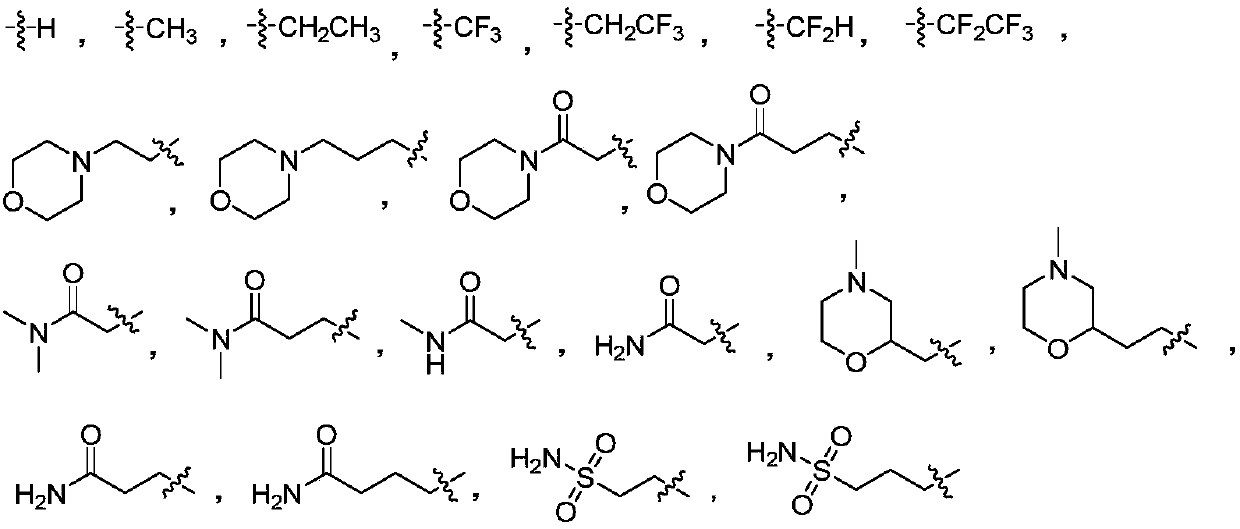
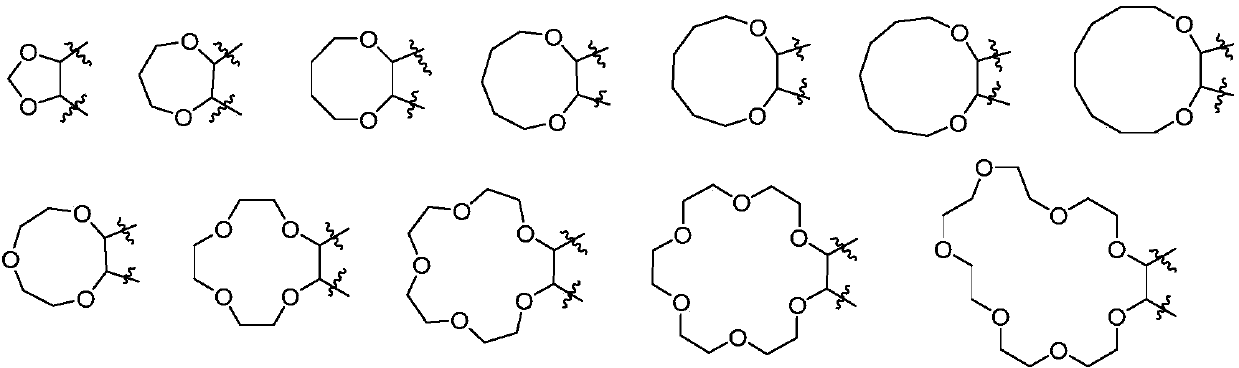
Aminoquinazolinone and aminoisoquinolinone derivatives and application thereof
A technology of aminoisoquinolinone and aminoquinazolinone, which is applied in the fields of aminoquinazolinone and aminoisoquinazolinone derivatives, anti-tumor and anti-inflammatory diseases, and can solve the problem of poor selectivity. , liver toxicity and side effects, poor pharmacokinetic properties and other problems, to achieve the effect of structural optimization, improved pharmacokinetic properties, and good clinical application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0089] Example 1: 2-amino-N-(8-methoxy-3-methyl-7-(3-morpholinopropyl)-4-oxo-3,4-dihydroquinazolone-2 -yl) Preparation of pyrimidine-5-carboxamide (compound 8a-1)
[0090]
[0091] Step 1 Preparation of 2-amino-3-methoxy-4-benzyloxy-N-methylbenzamide (compound 5a-1)
[0092] Dissolve 2-amino-3-methoxy-4-benzyloxybenzoic acid (4,546mg, 2mmol) in 20mL DCM, add methylamine hydrochloride (102mg, 2.4mmol), HOBT (405mg, 3mmol) successively , DIC (378mg, 3mmol), triethylamine (0.83mL, 6mmol), stirred at room temperature for 2h, added water, extracted with dichloromethane, combined organic layers, washed with saturated NaCl, anhydrous Na 2 SO 4 Dry, recover the solvent to get white powder 0.48g, Yield: 80%; ESI-MS: 287.1[M+H] + .
[0093] Step 2. 2-Amino-N-(8-methoxy-3-methyl-7-benzyloxy-4-oxo-3,4-dihydroquinazolinone-2-yl)pyrimidine-5- Preparation of formamide (compound 6a-1)
[0094] 2-Amino-3-methoxy-4-benzyloxy-N-methylbenzamide (5a-1, 0.2mmol), 2-aminopyrimidine-5-acyl i...
Embodiment 2
[0102] Embodiment 2: the synthesis of compound 8a-2~8a-9
[0103] Using compound 7a-1 as raw material, chloroacetamide, chloropropionamide, N-methylchloroacetamide, N,N-dimethylchloroacetamide, 2-(2-chloroethyl)-4-methyl Morpholine, 1-(3-chloro-propionyl)-4-methylpiperazine, chloroacetylmorpholine, 4-chlorobutanamide, difluorochloromethane, chloroethylsulfonamide, TMSCF 3 , Chloropropyl sulfonamide instead of N-(3-chloropropyl) morpholine to prepare compounds 8a-2 to 8a-14. The series of target molecules are shown in Table 1 below.
[0104] Table 1
[0105]
Embodiment 3
[0106] Example 3: Synthesis of compounds 8a-14 to 8a-26 The synthetic route is shown in the following formula
[0107]
[0108] Using compound 5a-1 as a raw material, it was mixed with pyrimidine-5-formyl isothiocyanate, pyridazine-4-formyl isothiocyanate, 2-amino-pyridine-5-formyl isothiocyanate, respectively , 6-amino-pyridazine-3-formyl isothiocyanate, 2-amino-pyrazine-5-formyl isothiocyanate, 5-aminopyridine-2-formyl isothiocyanate, 3-cyano-benzoyl isothiocyanate, pyrazine-2-formyl isothiocyanate, pyrazole-4-formyl isothiocyanate, thiazole-4-formyl isothiocyanate Ester, purine-6-formyl isothiocyanate, benzimidazole-5-formyl isothiocyanate, benzothiophene-5-formyl isothiocyanate, etc. react first, and then under the action of EDC Ring closure to obtain intermediates 6a-2~6a-14, and then catalytic hydrogenation debenzylation with Pd / C to obtain 7a-2~7a-14, and finally they reacted with N-(3-chloropropyl)morpholine to finally obtain Target molecules 8a-14 to 8a-26. Its ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com