Two-photon fluorescent probe as well as preparation method and application thereof
A two-photon fluorescence and probe technology, applied in fluorescence/phosphorescence, chemical instruments and methods, luminescent materials, etc., can solve the problem of low sensitivity and detection concentration, and achieve the effect of low detection concentration, high sensitivity and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
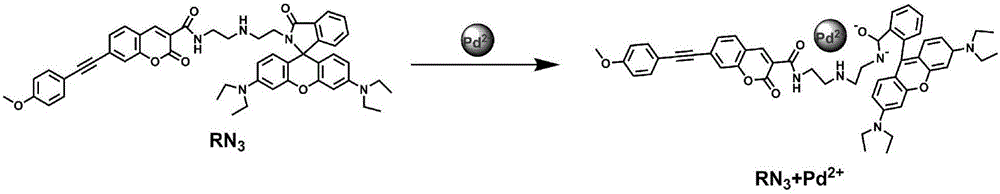
[0021] Example 1: Fluorescent probe molecule RN 3 Synthesis
[0022] 1. Mix 7-((4-methoxyphenyl)acetylene)-3-carboxylic acid-coumarin (1g, 3mmol), 1-hydroxybenzotriazole (0.65g, 4.5mmol) and EDC·HCl (0.65g, 3.3mmol) into the Shrek bottle, add 30mL N,N-dimethylformamide under anhydrous and anaerobic conditions, stir at room temperature for 24h; after the reaction, add 50mL dichloromethane to dissolve the reactant , and then extracted 3 times with 30 mL of water to obtain an organic phase, which was distilled under reduced pressure and spin-dried to obtain intermediate 1;
[0023] 2. Put rhodamine B (2g, 4.16mmol) in a three-necked flask, add 100ml of ethanol and heat to reflux, after the rhodamine is completely dissolved, slowly add diethylenetriamine (0.646g, 6.26mmol) dropwise, and heat to reflux reaction for 24h; after the reaction is completed and down to room temperature, the intermediate 1 prepared in step 1 is added to the reaction solution, and stirred at room tempera...
Embodiment 2
[0024] Embodiment 2: Fluorescence test of fluorescent probe molecule
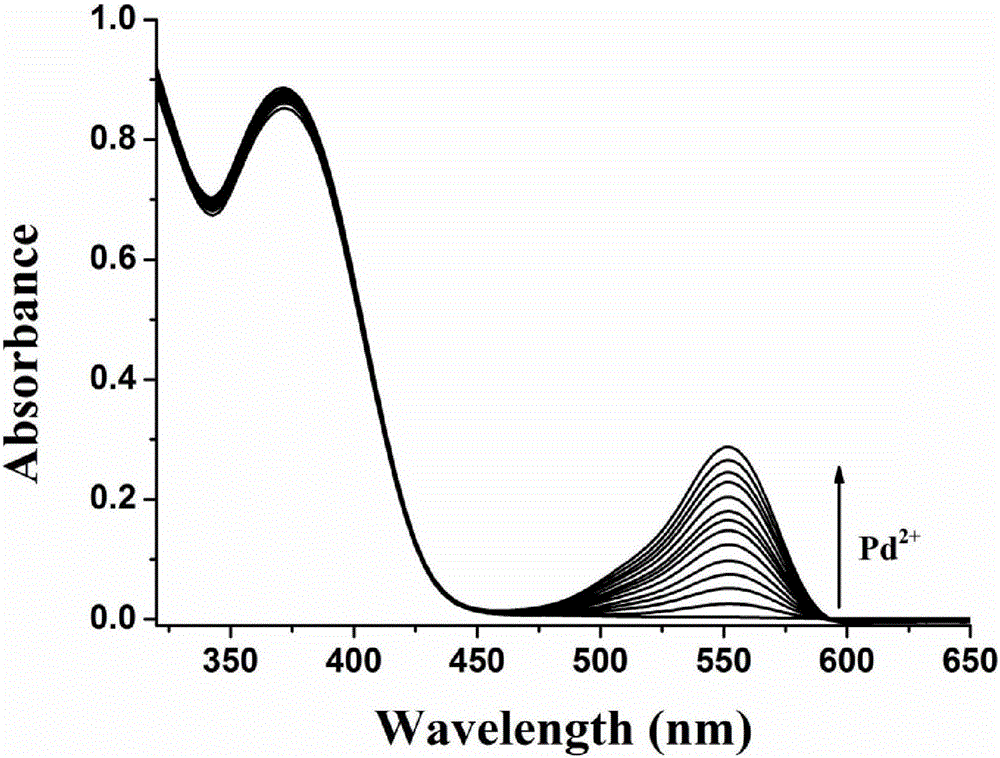
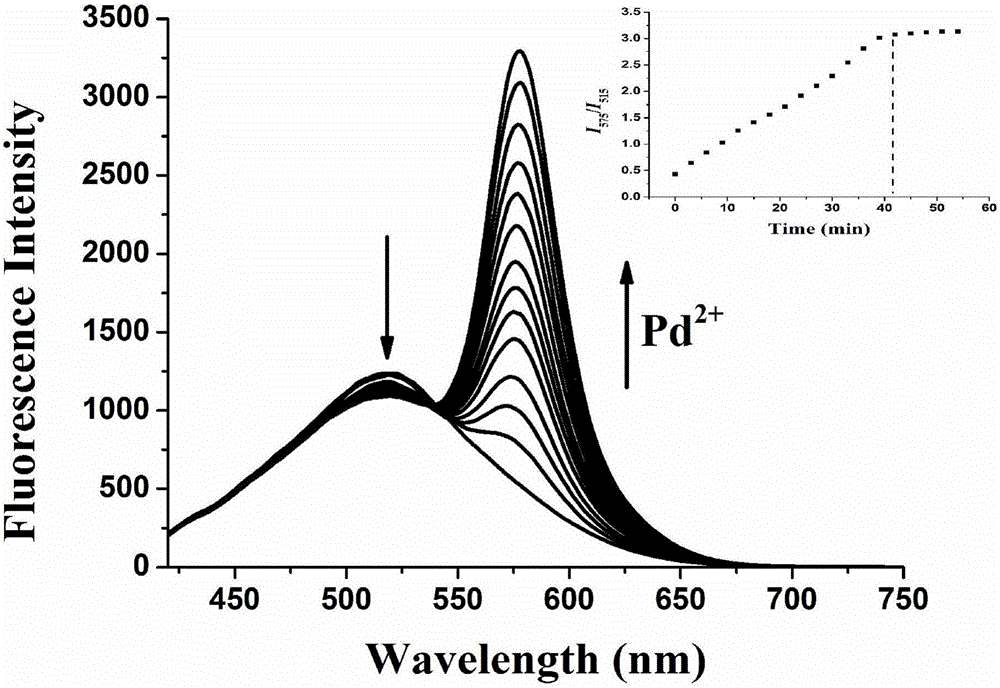
[0025] Dissolve the fluorescent probe molecule of the present invention in DMSO to prepare a 1 mM mother solution, take 250 μL of the mother solution in a 10 mL volumetric flask, and then dilute to volume with DMSO to prepare a 25 μM detection reagent. Add 1 times the equivalent of palladium ion (II) to the detection reagent, and visible ultraviolet light has an obvious absorption at about 550nm ( figure 2), detect the change of fluorescence spectrum in the range of 415-700nm, as time changes, it can be observed that the emission peak at 515nm is gradually weakened, and the emission peak at 575nm is gradually enhanced ( image 3 ).
[0026] When the flexible chain region is not chelated with palladium ion (II), the excitation wavelength of 395nm can provide energy for the coumarin group to fluoresce at 515nm. After chelating with palladium ion (II), the rhodamine group undergoes a ring-opening reaction du...
Embodiment 3
[0027] Example 3: Cell Imaging Test
[0028] 293FT cells were cultured with DEME (invitrogen) medium. One day before imaging, HeLa cells were placed in a flat-bottomed surface dish. During imaging, HeLa cells and 10 μM fluorescent probe RN 3 DMSO solution at 37°C, containing 5% CO 2 Incubate in a cell culture box for 0.5 hours, wash fully with neutral PBS buffer solution or culture medium, and use fluorescent confocal imaging to obtain Figure 4 . Add (10 μM) palladium ion (II) solution to the above-mentioned cell culture medium containing the fluorescent probe, at 37°C, containing 5% CO 2 Incubate in a cell culture incubator for 0.5 hours, wash thoroughly with neutral PBS buffer solution or culture medium, and perform two-photon fluorescence confocal imaging. It can be seen from the figure that before adding palladium ion (II), there is fluorescence at 515nm; after adding palladium ion (II), the fluorescence at 575nm is obviously enhanced, and the maximum emission peak of ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com