In vitro culture-amplified human liver progenitor cell and preparation thereof
A technology of hepatic progenitor cells and in vitro culture, which is applied in the field of in vitro expansion and preparation of human hepatic progenitor cells, and can solve the problem of inability to overcome animal protein pollution, human hepatic stem/progenitor cells difficult to satisfy, and human fetal liver non-hepatic parenchymal cell technology. Difficulty in quality control of indicators
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0056] The preparation of example 1 human fibrin glue
[0057] Thrombin (Shanghai Xinxing Pharmaceutical, China) was added to the M199 medium containing 3-5 mg / ml human fibrinogen (Shanghai Xinxing Pharmaceutical, China) to a final concentration of 0.02 units / ml. Quickly add 0.5 to 2 ml of the above solution to one well of a six-well plate to cover the entire well. The six-well plate was placed in a cell culture incubator at 37°C. After human fibrinogen was degraded for at least two hours, aprotinin (Hangzhou Aoya, China) was added to serum-free medium to a final concentration of 20 units / ml to inhibit thrombin activity for at least two hours. The prepared human fibrin glue can be sealed and stored at 4° C. for long-term storage. When human fibrin glue is used together with other extracellular matrices (such as laminin, fibronectin or both), it can be added to the M199 medium of human fibrinogen first, and then added to thrombin for degradation. If an acidic solution contai...
example 2
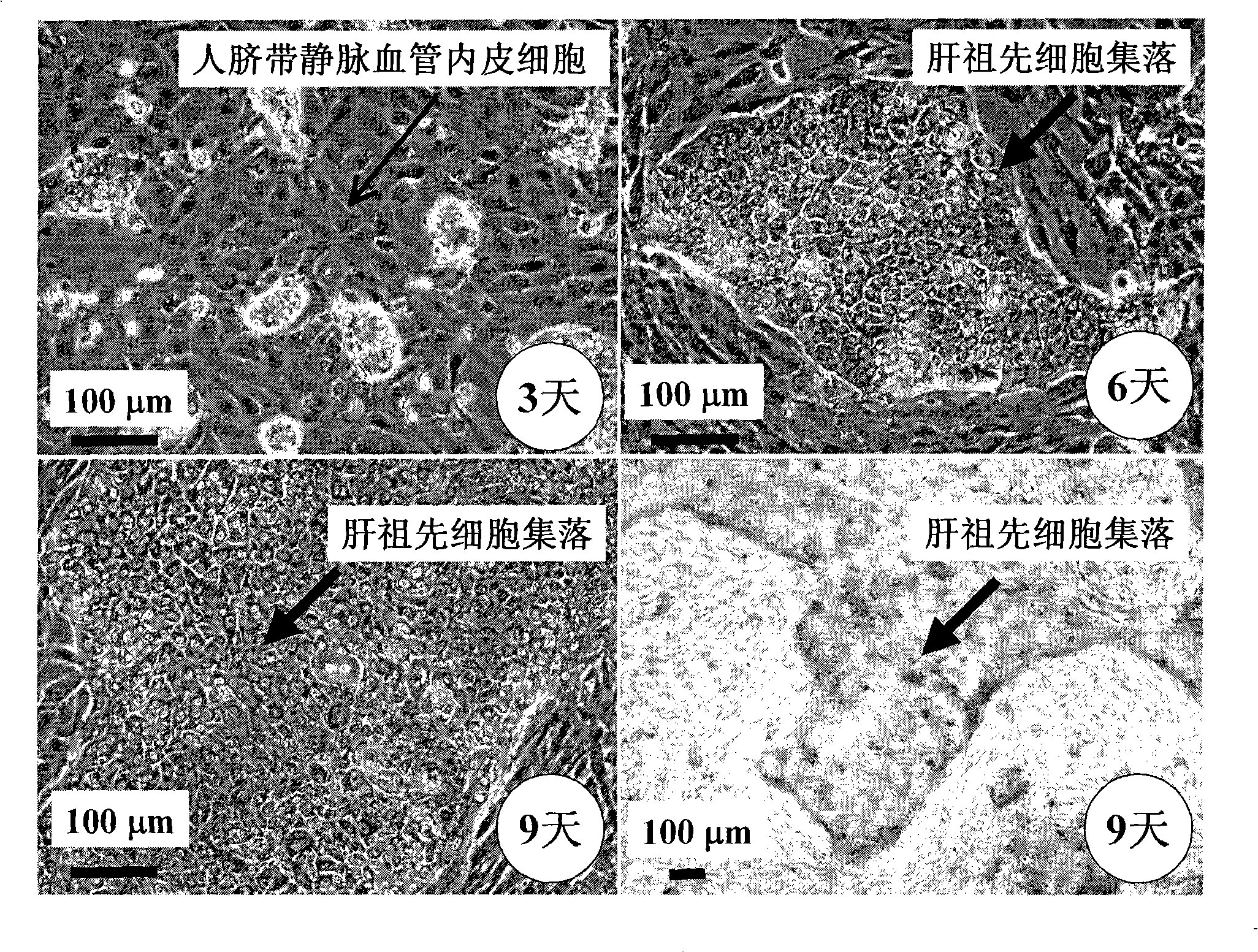
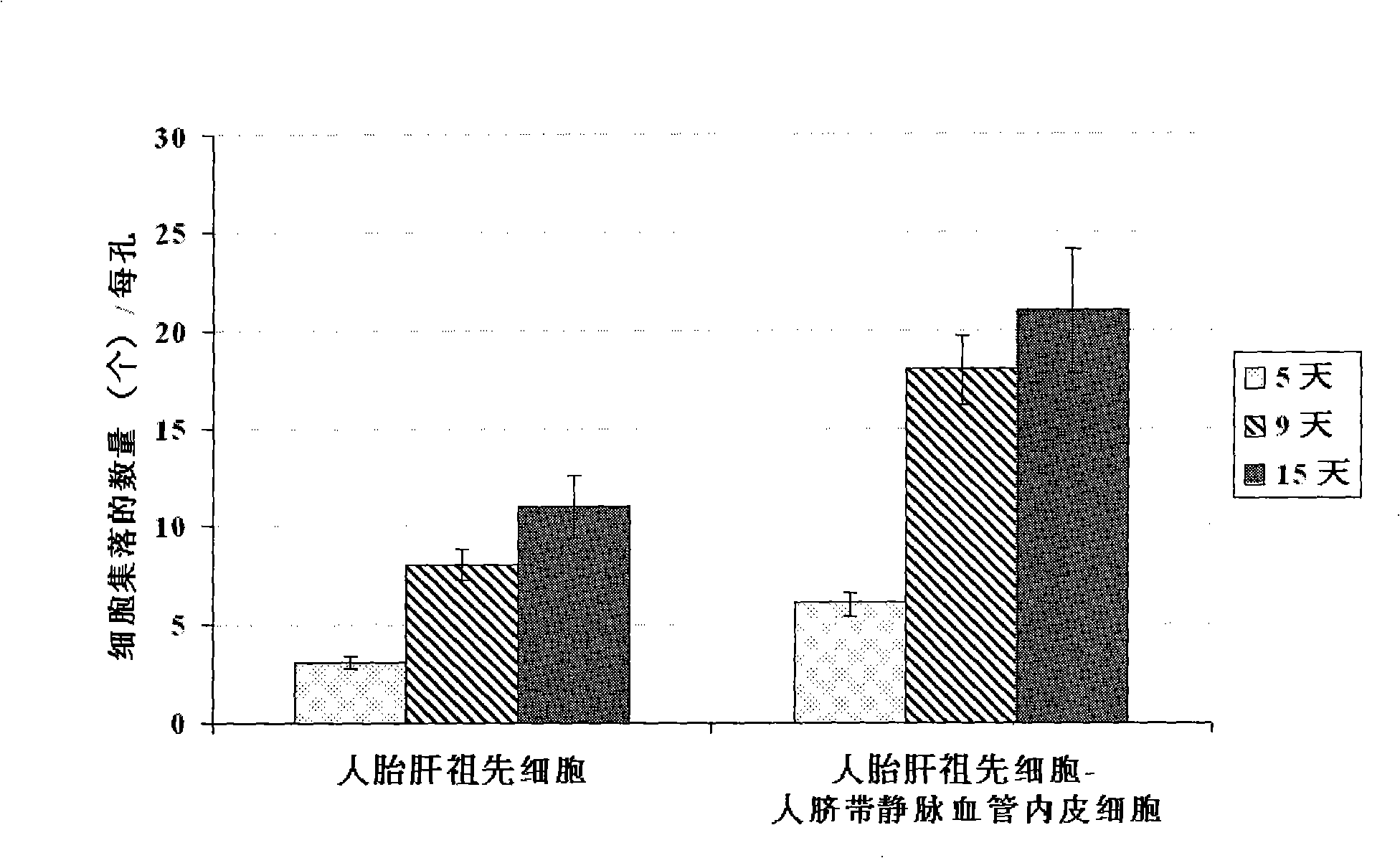
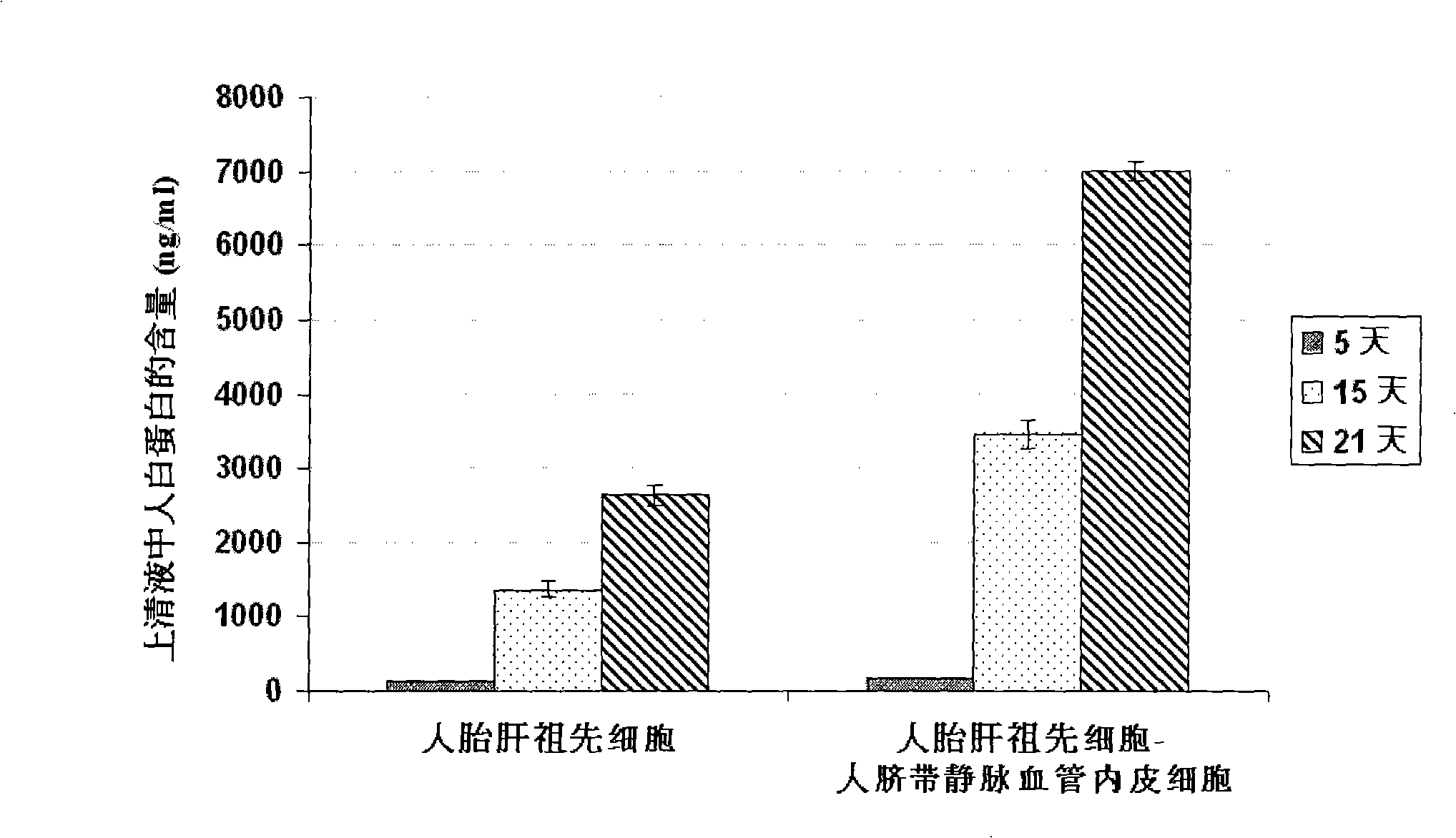
[0059] Example 2 Co-culture of human fetal liver progenitor cells and human umbilical cord vein endothelial cells on human fibrin glue
[0060] According to the method reported in the literature, the fetal liver or adult liver of 16-22 weeks of gestation was preheated with 0.2 mg / ml deoxyribonuclease I and 0.1% collagenase H or 120 μg / ml highly purified collagenase liberase Blendzyme 3 (Roche Applied Sciences, USA) Hank's buffer (containing Ca 2+ and Mg 2+ Ionic Hank's Balanced Salt Solution) was degraded and prepared as a human liver single cell suspension. Rich in EpCAM + or / and CD44 + but CD45 - 、NCAM - 、HLA - / low Human fetal liver progenitor cells can be isolated and purified by FACS or immunomagnetic bead sorting using the method described by Schmelzer et al. (16) , or with Dan et al. (17) , Herraza, etc. (18) , Turner, etc. (19) The described methods are enriched. In order to increase the purity of human fetal liver progenitor cells, the human fetal liver prog...
example 3
[0061] Example 3 Preparation of human liver progenitor cells from the co-culture of human fetal liver progenitor cells and human vascular endothelial cells
[0062] After washing the co-culture of human fetal liver progenitor cells and human vascular endothelial cells twice with 1× phosphate (PBS) buffer, add 0.5 ml containing 0.1% collagenase H or 120 μg / ml highly purified collagenase liberase Blendzyme 3 ( Roche Applied Sciences, the United States) Hank's buffer (containing Ca 2+ and Mg 2+ Ionic Hank's Balanced Salt Solution) was added to each well, degraded at 37°C for 1-4 minutes with slight shaking, until most of the culture was detached from the surface of human fibrin glue, and another 2ml of Hank's buffer was added to From each well, collect the culture into a sterile tube and centrifuge at low speed (100 xg) for 10 minutes to pellet the cell culture. The cell pellet was suspended in a Ca-free 2+ and Mg 2+Add an equal volume of trypsin-EDTA to the ionic Hank's buff...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com