Carbazole-based two-photon fluorescent probe as well as preparation method and application thereof
A two-photon fluorescence and probe technology, applied in the direction of fluorescence/phosphorescence, chemical instruments and methods, luminescent materials, etc., can solve the problems of high cell phototoxicity, large fluorescence interference, and small excitation wavelength, and achieve low detection concentration and high sensitivity. High, easy-to-operate effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Embodiment 1: the synthesis of fluorescent probe molecule MBCB
[0025]Add 3-benzothiazole-6-formyl-N-methylcarbazole (0.53g, 1.5mmol) into a 100mL round bottom flask, add 15mL of methanol, heat and stir until the raw material is dissolved, then add 2,3-di Tolylbenzothiazol-3-ium iodide (0.44g, 1.5mmol), and 2-3 drops of piperidine were added dropwise, and the reaction was refluxed at 70°C for 4h; after the reaction was completed, it was left to cool, and after separation, it was filtered to obtain a red crude The product was recrystallized twice with a mixed solvent of dichloromethane:methanol=4:1 (v / v) to obtain 0.63 g of the target product with a yield of 67%.
[0026] 1 H NMR (400MHz, DMSO-d 6 ): δ9.22(s,1H),9.04(s,1H),8.42(m,2H),8.30–8.19(m,3H),8.15(t,J=12.6Hz,2H),8.07(d, J=8.1Hz, 1H), 7.89(m, 3H), 7.79(t, J=7.6Hz, 1H), 7.57(t, J=7.4Hz, 1H), 7.47(t, J=7.4Hz, 1H) ,4.60(q,J=6.6Hz,2H),4.41(s,3H),1.42(t,J=7.0Hz,3H).δ171.87,167.93,150.04,142.82,142.02,141.94,134.29,...
Embodiment 2
[0027] Embodiment 2: the spectroscopic test of fluorescent probe molecule
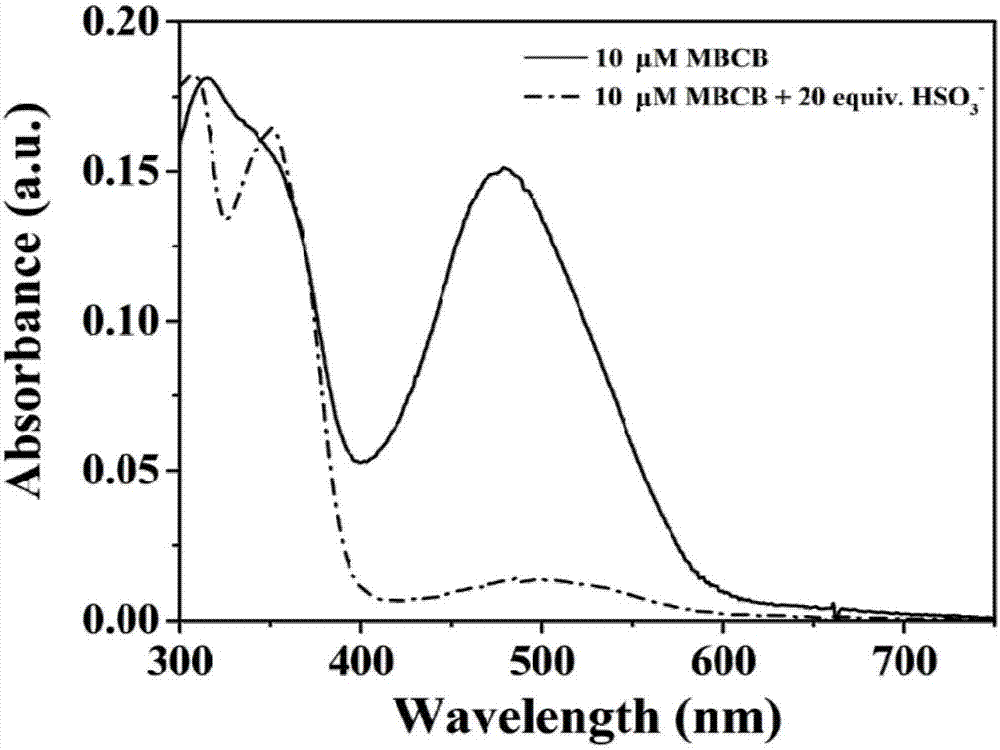
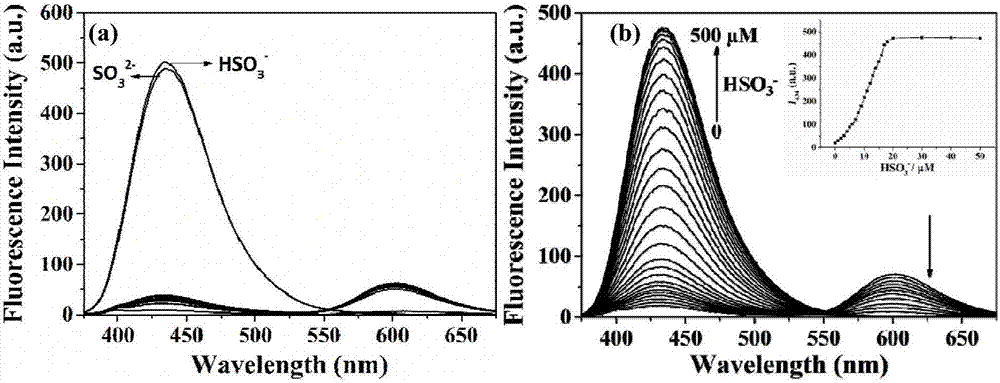
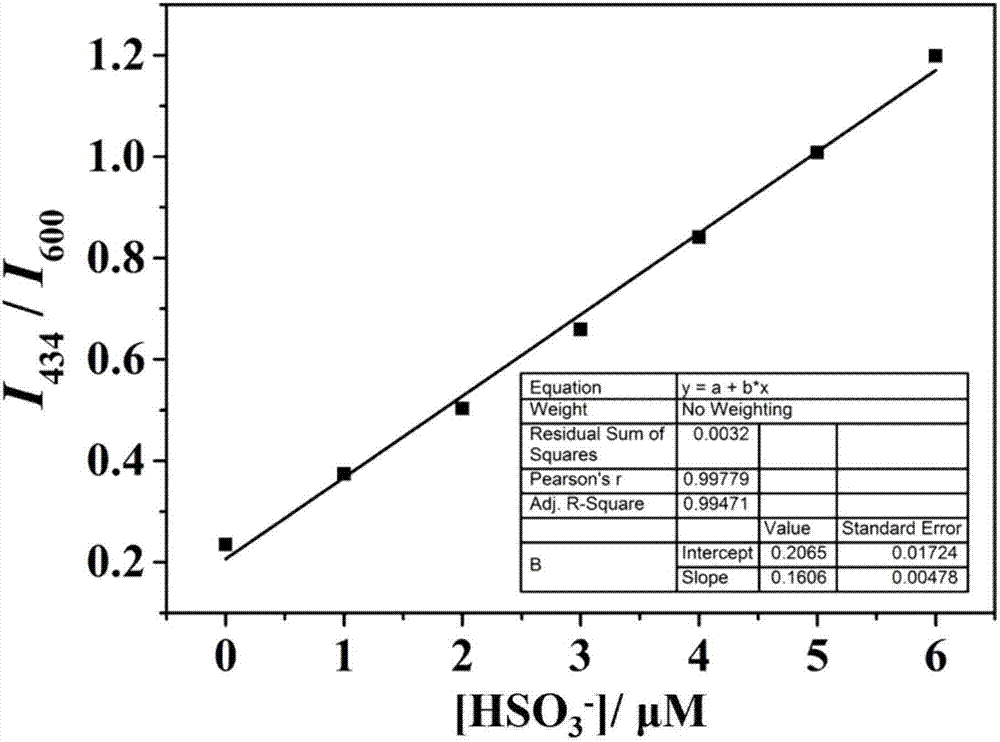
[0028] Dissolve the two-photon fluorescent probe of the present invention in DMSO to prepare 1 mM mother solution, take 100 μL of the mother solution in a 10 mL volumetric flask, and then use glycerol:PBS=2:8 solvent to make up a 10 μM detection reagent. The detection reagents have absorption peaks at 316nm and 480nm respectively; add 20 times the equivalent of HSO 3 - After that, the absorption peaks of MBCB at 316nm and 480nm decreased gradually, and two new absorption peaks appeared at 306nm and 351nm ( figure 1 ). After adding 20 times the equivalent of various anions, reactive oxygen species and amino acids to 10 μM detection reagent ( figure 2 a), detection of fluorescence spectrum changes in the range of 375-675nm, it can be seen that MBCB is only for HSO 3 - and SO 3 2- There is an obvious fluorescence enhancement phenomenon, with a specific response; when accompanied by HSO 3 - (0-50...
Embodiment 3
[0029] Example 3: Two-photon performance test of fluorescent probe molecules
[0030] Using the two-photon induced fluorescence measurement technique, the two-photon absorption cross section of the fluorescent probe molecule (MBCB) was tested, from Figure 4 It can be seen that when the two-photon excitation wavelength is 740nm, the maximum absorption cross section of the fluorescent probe molecule is 138GM. It shows that the probe can be applied to two-photon bioimaging.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com