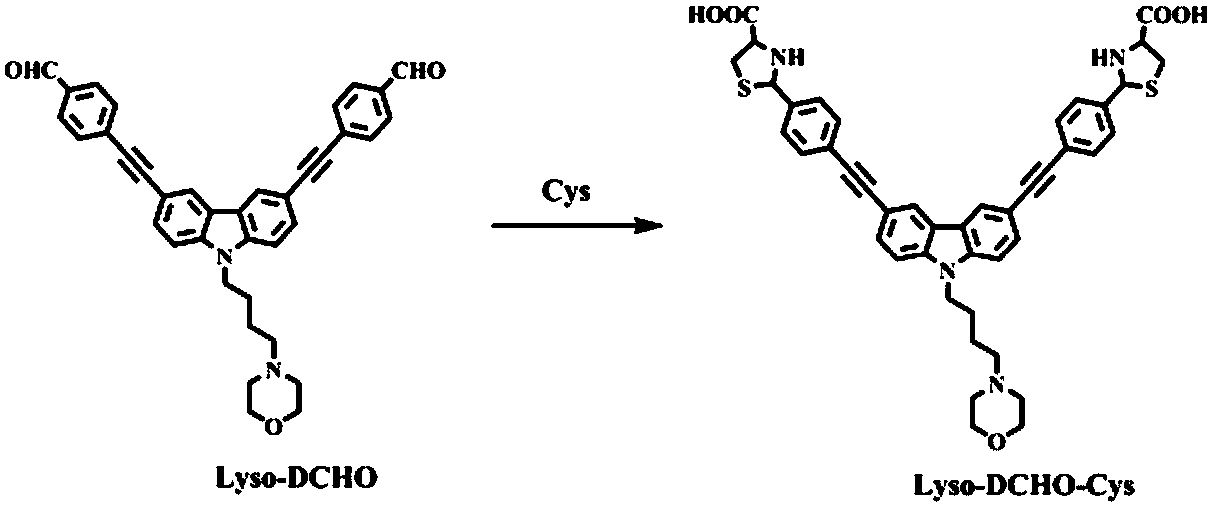
Carbazolyl two-photon fluorescent probe and preparation method and application thereof
A two-photon fluorescence, carbazole-based technology, used in fluorescence/phosphorescence, chemical instruments and methods, luminescent materials, etc., can solve the problem of few reports of two-photon fluorescent probes, and achieve simple structure, high sensitivity, and detection limit. low effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Embodiment 1: the synthesis of fluorescent probe molecule Lyso-DCHO
[0029] 1. Synthesis of Intermediate 1
[0030] A mixture of KOH (2.0g, 35.8mmol), KI (0.4g, 2.39mmol) and 1,4-dibromobutane (7.73g, 35.8mmol) was heated to 60°C, and 3,6 -diiodocarbazole (10g, 23.9mmol), continue to heat and reflux for 12 hours; cool to room temperature after the reaction, add 200mL H 2O, the mixture was extracted with dichloromethane (100 mL×2), and the organic phase was evaporated to give a crude residue, which was purified by column chromatography (petroleum / dichloromethane = 10:1 as eluent) to give intermediate 1, 6.8 g, yield 51.4%.
[0031] 2. Synthesis of Intermediate 2
[0032] Intermediate 1 (1g, 1.8mmol), 4-ethynylbenzaldehyde (0.7g, 5.4mmol), Pd 2 (PPh 3 ) 2 Cl 2 (5.1mg, 0.007mmol), CuI (2.7mg, 0.014mmol) and Et 3 N (5 mL) was stirred and reacted at 30° C. for 12 hours under anhydrous and anaerobic conditions. After the reaction was completed, it was cooled to room ...
Embodiment 2
[0036] Embodiment 2: the spectroscopic test of fluorescent probe molecule
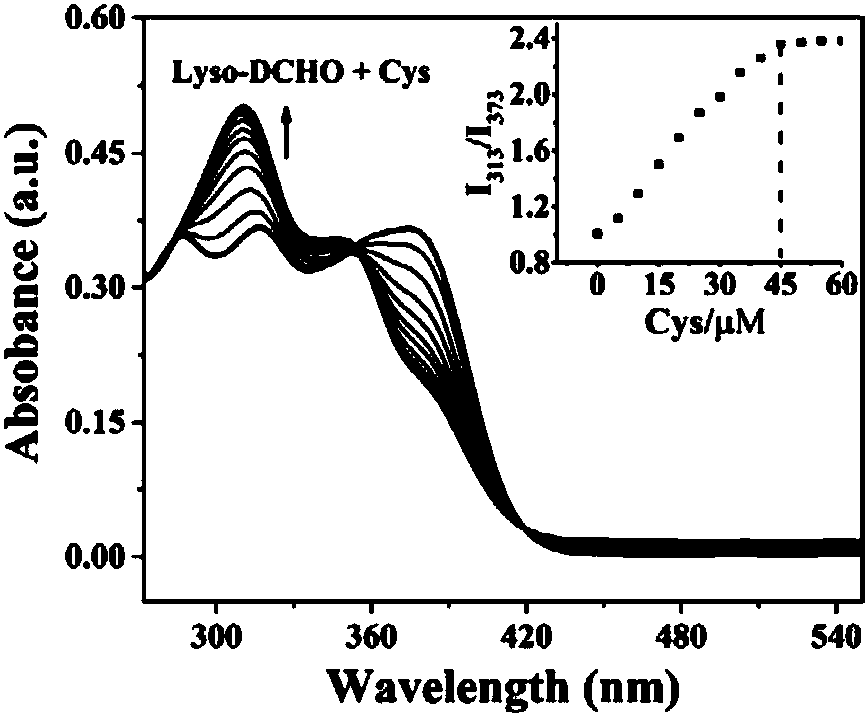
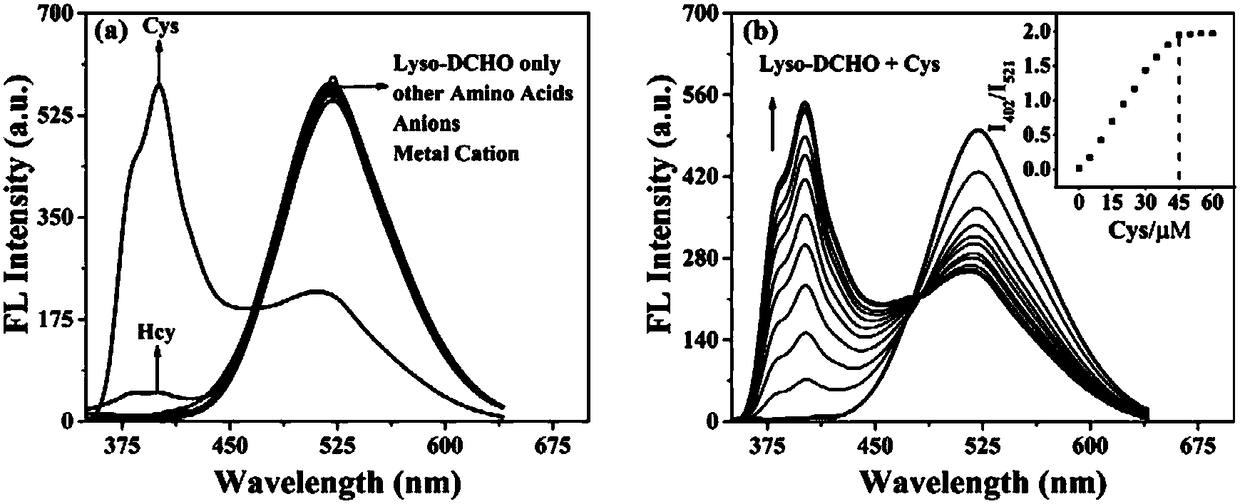
[0037] Dissolve the two-photon fluorescent probe of the present invention in DMSO to prepare a 1mM mother solution, take 100 μL of the mother solution in a 10mL volumetric flask, and then use a solvent of DMSO:PBS (v / v)=1:1 to make up the volume to prepare 10 μM detection reagent. The detection reagents have absorption peaks at 313nm and 373nm respectively; with the addition of Cys, the absorption peak of Lyso-DCHO at 373nm gradually decreases, and the absorption peak at 313nm gradually increases. When Cys is added to 4.5 times the equivalent, the absorbance at 313nm reaches saturation ( figure 2 ). 10μM detection reagent added 8 times the equivalent of other amino acids, various anions and metal cations for 20min ( image 3 a), detection of fluorescence spectrum changes in the range of 350-700nm, it can be seen that Lyso-DCHO only has obvious fluorescence changes to Cys, and has a specific response...
Embodiment 3
[0038] Example 3: Two-photon performance test of fluorescent probe molecules before and after adding Cys
[0039] Using the two-photon induced fluorescence measurement technique, the two-photon effective absorption cross-section of the fluorescent probe molecule (Lyso-DCHO) was tested. Lyso-DCHO has a maximum two-photon effective absorption cross-section of 58GM at 760nm, and the maximum at 720nm after adding Cys The value becomes 45GM. It shows that the probe can be applied to two-photon confocal imaging.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com