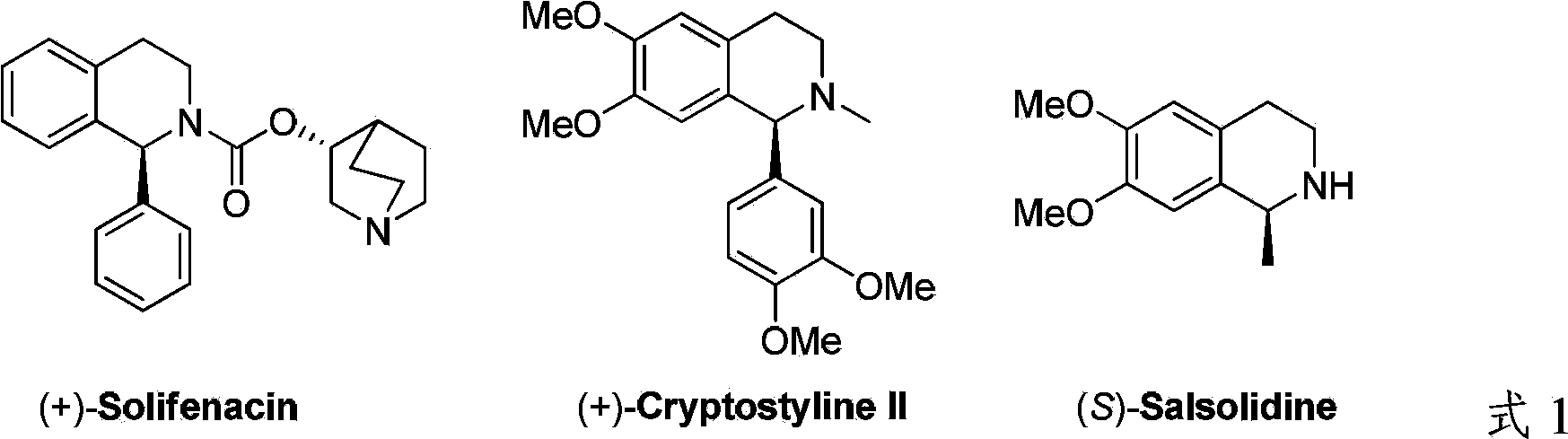
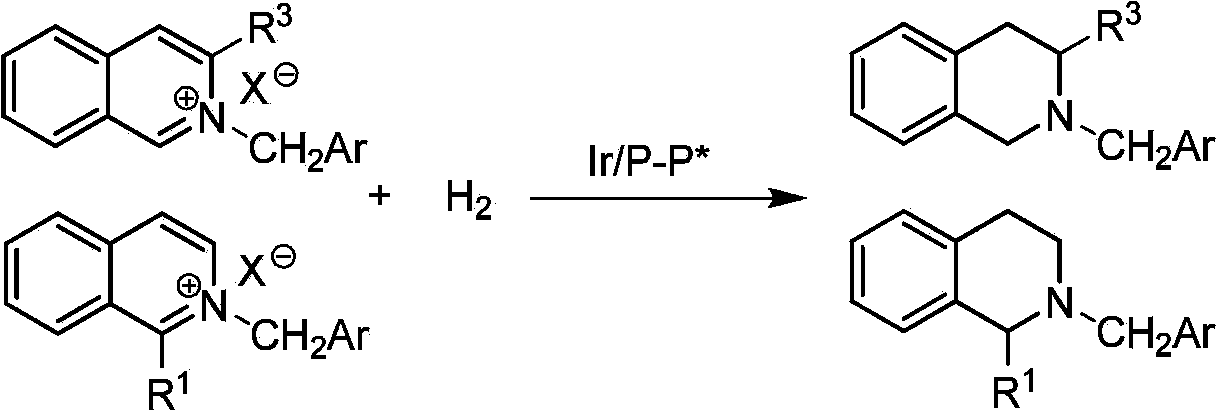Method for synthesizing chiraltetrahydro naphthalenederivate through asymmetric hydrogenation on isoquinoline by means of iridium catalyst
A technology of tetrahydroisoquinoline and isoquinoline, applied in the direction of organic chemistry, can solve the problems of limited application range of substrates, low hydrogenation reaction activity, low enantioselectivity, etc., and achieve simple and practical reaction operation, raw materials Easy access and high enantioselectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
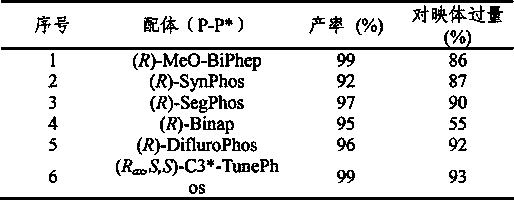
[0033] Example 1: Optimization of conditions
[0034] In a glove box filled with nitrogen, place a reaction flask containing (1,5-cyclooctadiene) iridium chloride dimer (0.0025 mmol, 1.7 mg) and chiral ligand (0.0055 mmol) Add 1mL tetrahydrofuran / dichloromethane (v / v=1:1) mixed solvent, stir at room temperature for 10-30 minutes, and then transfer the prepared catalyst to another containing raw material isoquinoline salt (0.25 mmol ), add 2 mL of tetrahydrofuran / dichloromethane (v / v=1:1) mixed solvent to make the total solvent volume reach 3 mL. The reaction flask was put into a stainless steel autoclave, hydrogen gas was introduced at 600 psi, and the reaction was conducted at room temperature for 20-24 hours. Slowly release hydrogen, add saturated NaHCO to the reaction system 3 The aqueous solution was stirred for 10 minutes, and then extracted three times with dichloromethane. The organic phases were combined and dried, and the solvent was removed by a rotary evaporator, and ...
Embodiment 2
[0039] Example 2: Synthesis of various chiral tetrahydroisoquinoline derivatives by asymmetric hydrogenation of isoquinoline catalyzed by iridium
[0040] In a glove box filled with nitrogen, the (1,5-cyclooctadiene) iridium chloride dimer (0.0025 mmol, 1.7 mg) and the chiral ligand (R ax ,S, S)-C3 * -Add 1mL of mixed solvent tetrahydrofuran / dichloromethane (v / v=1:1) to the TunePhos (0.0055 mmol) reaction flask, stir at room temperature for 10-30 minutes, and then transfer the prepared catalyst to another container with a needle In the reaction flask with the raw material isoquinoline salt (0.25 mmol), share 3mL solvent mixed solvent tetrahydrofuran / dichloromethane (v / v=1:1). The reaction flask was put into a stainless steel autoclave, hydrogen gas was introduced at 600 psi, and the reaction was conducted at room temperature for 20-24 hours. After the reaction is over, slowly release hydrogen, add saturated NaHCO to the system 3 The aqueous solution was stirred for 10 min, and th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com