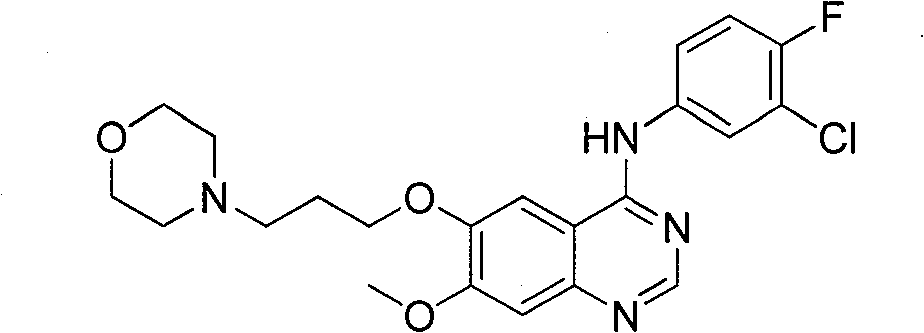
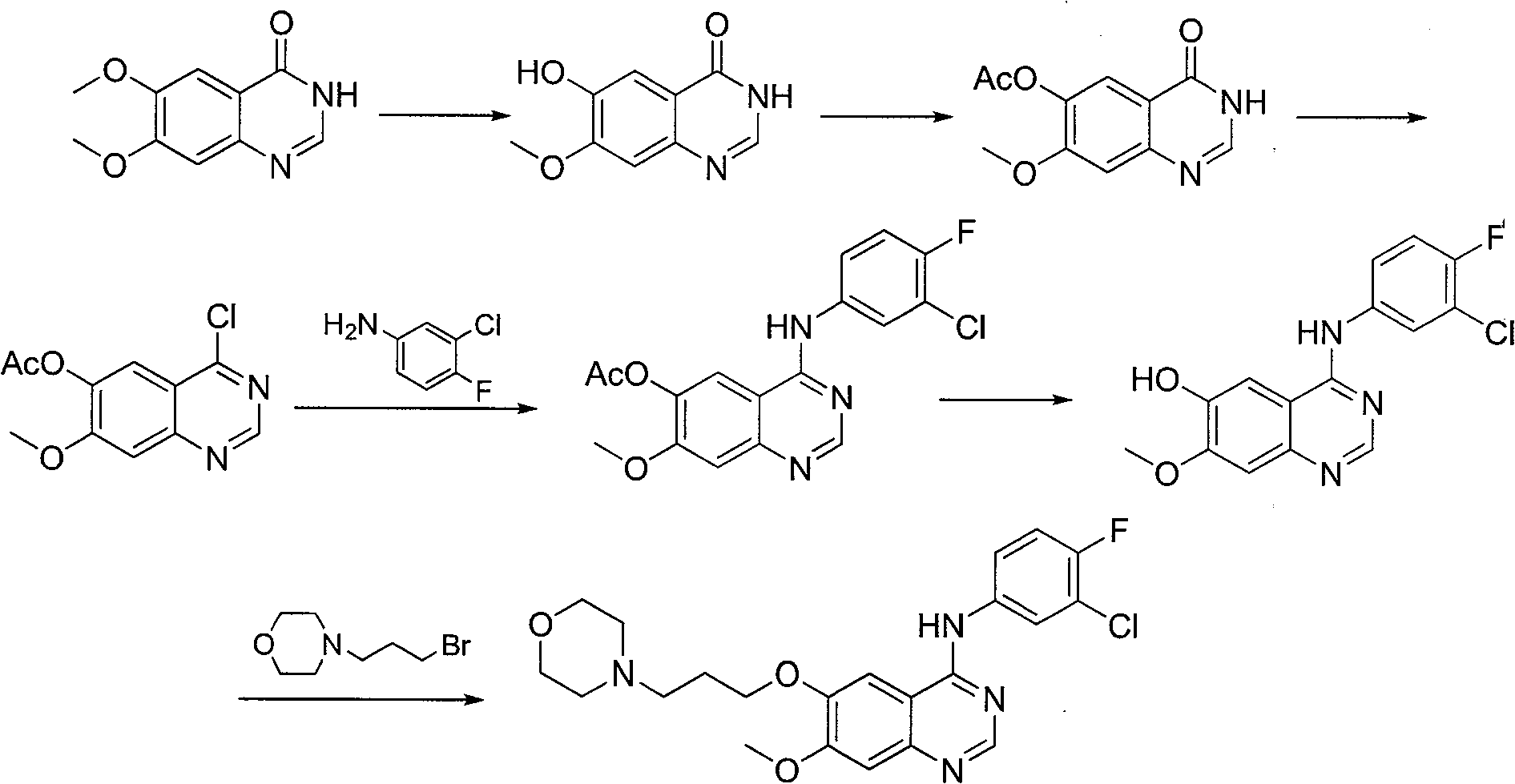
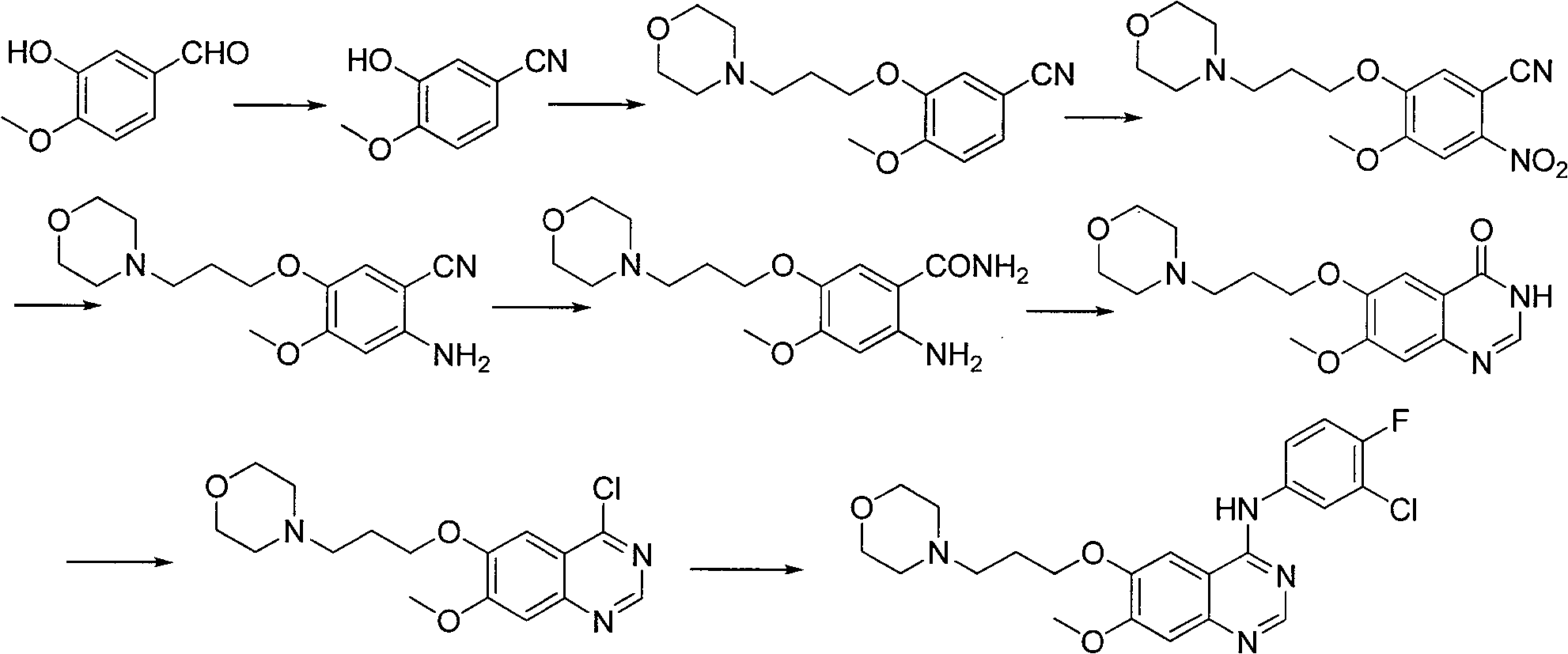
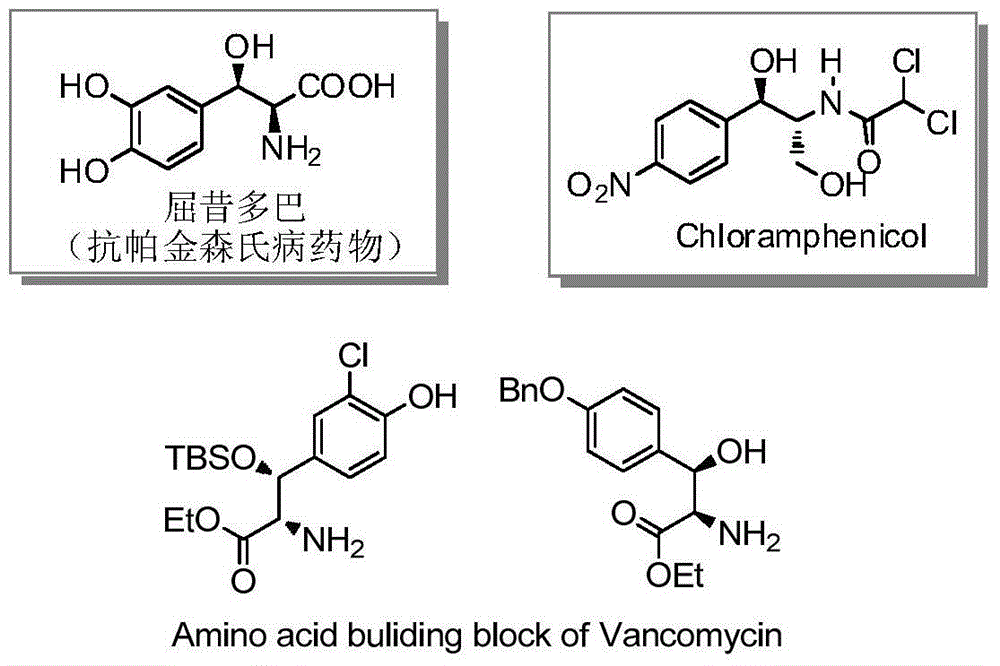
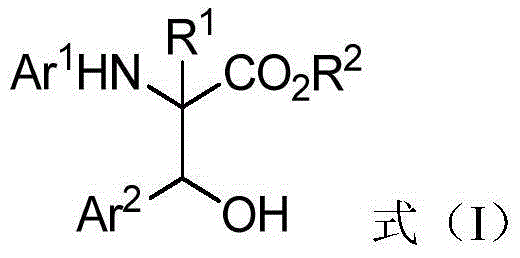
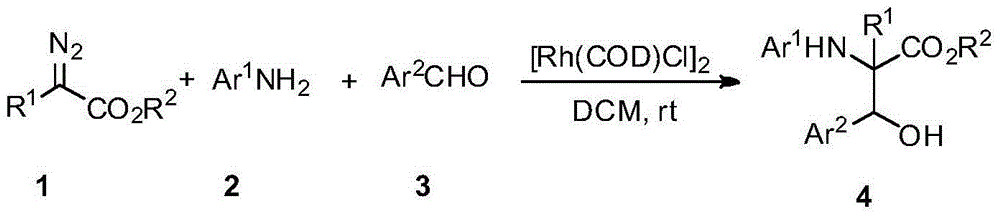
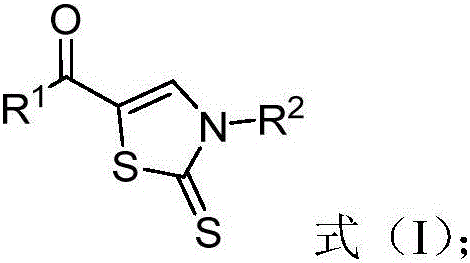
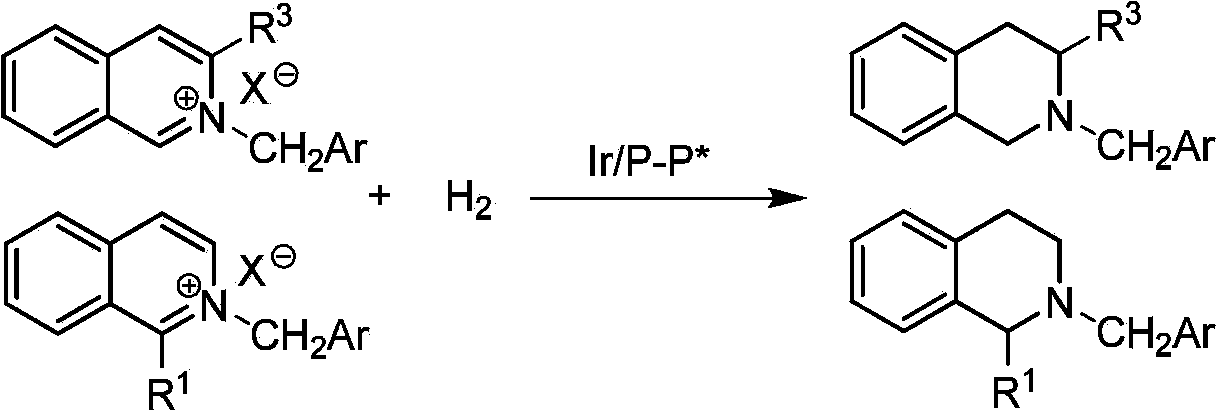Patents
Literature
215results about How to "Atom economy" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Hdrogenation synthesis method for preparing 2-methyl allyl alcohol by using recyclable catalyst
InactiveCN102167657APost-processing is simpleReduce manufacturing costOrganic compound preparationHydroxy compound preparationSolventBoiling point
The invention discloses a method for synthesizing 2-methylacrolein into 2-methyl allyl alcohol by using a catalytic hydrogenation method, belonging to the technical field of synthesis of organic intermediates. A catalyst used in the method is recyclable. The synthesis method comprises the following steps of: (1) adding 2-methylacrolein, a catalyst, a polymerization inhibitor and a high-boiling-point solvent into a high-pressure kettle and introducing hydrogen for reacting; (2) after reacting, exhausting excessive hydrogen and rectifying reaction liquid to obtain 2-methylacrolein; and (3) adding the 2-methylacrolein and the solvent into residual liquid in a rectifying kettle, transferring into the high-pressure kettle and undergoing a reaction for preparing 2-methylacrolein through hydrogenation to realize the recycling of the catalyst. The method has the advantages of atom economy, environmental friendliness, high yield, simple post-treatment and the like.
Owner:武汉凯森化学有限公司
Polyene ether compounds and preparation method thereof
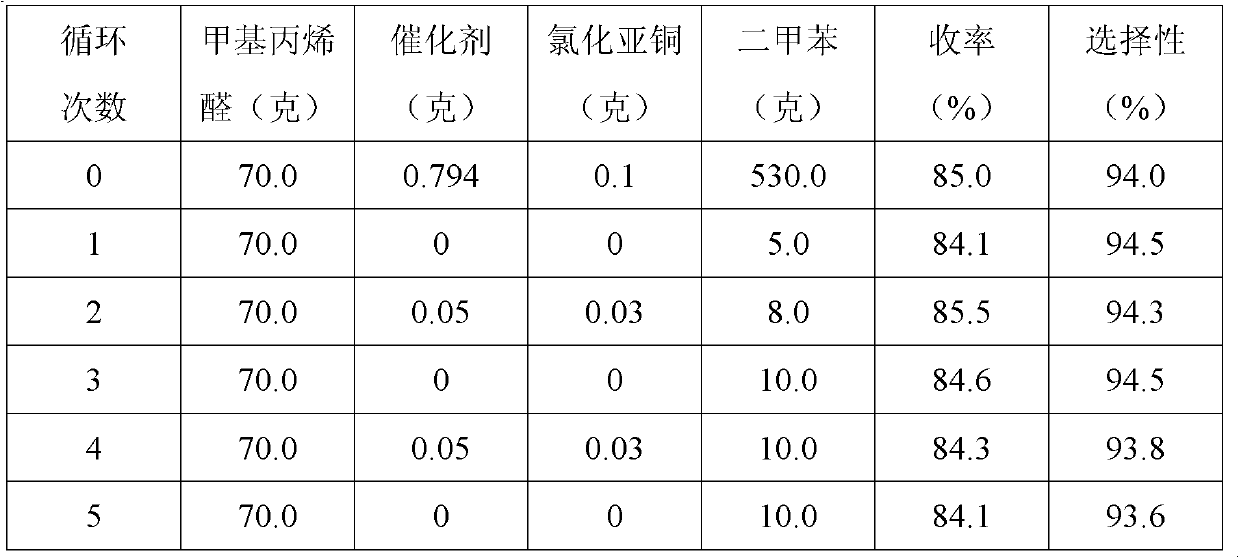
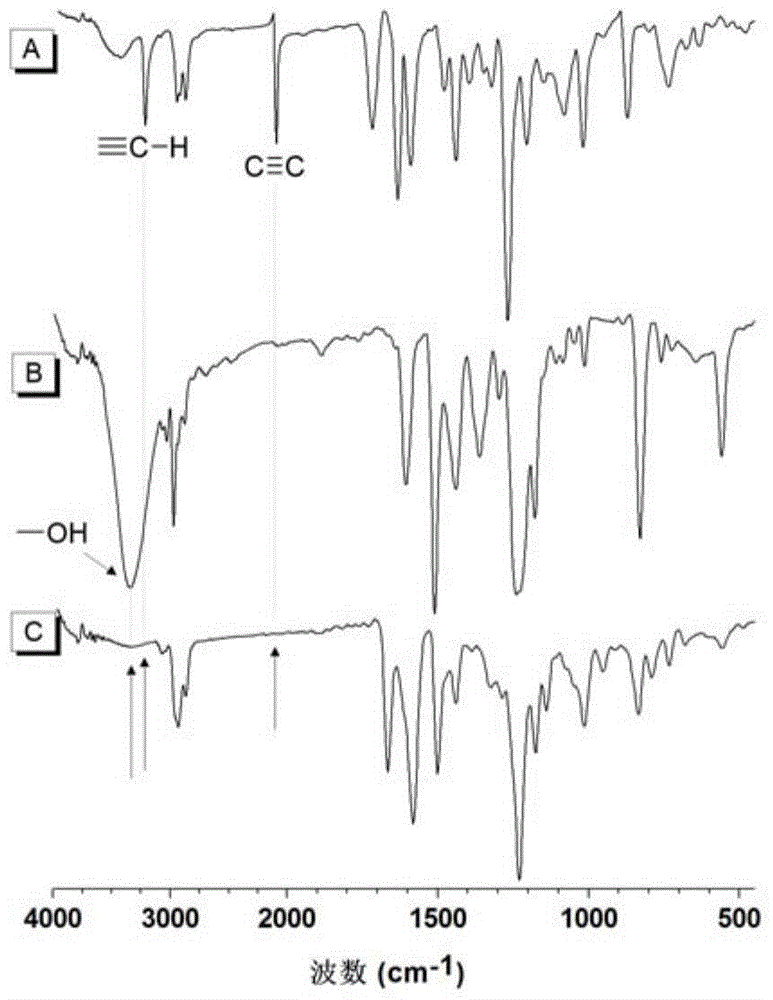
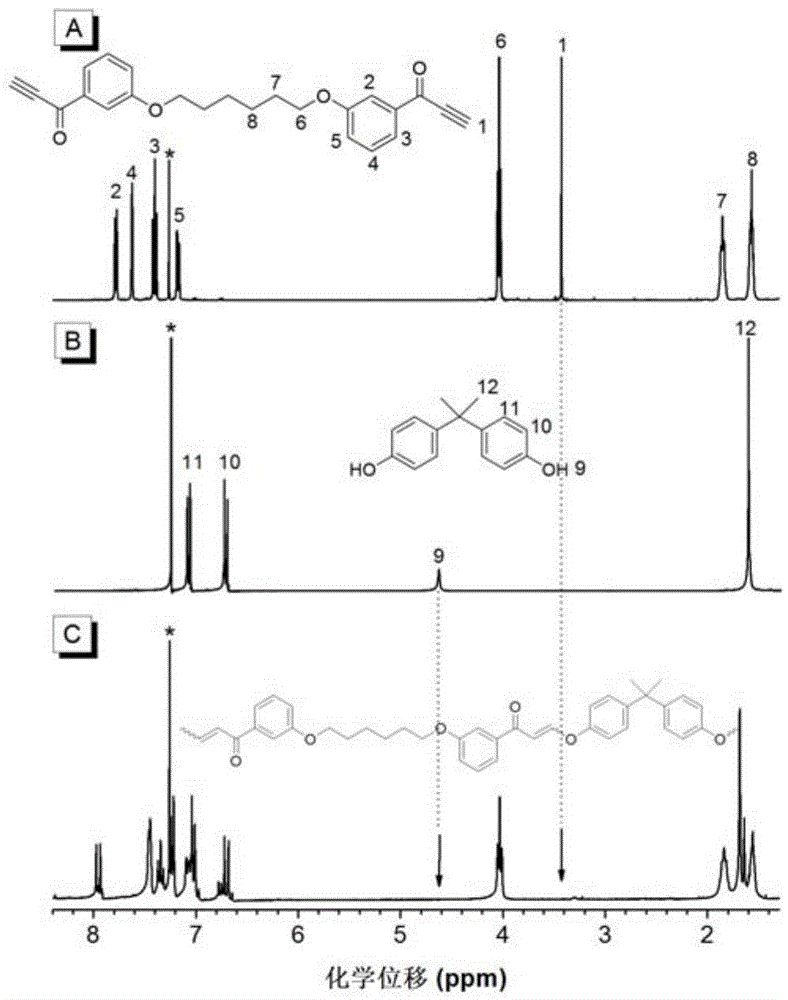
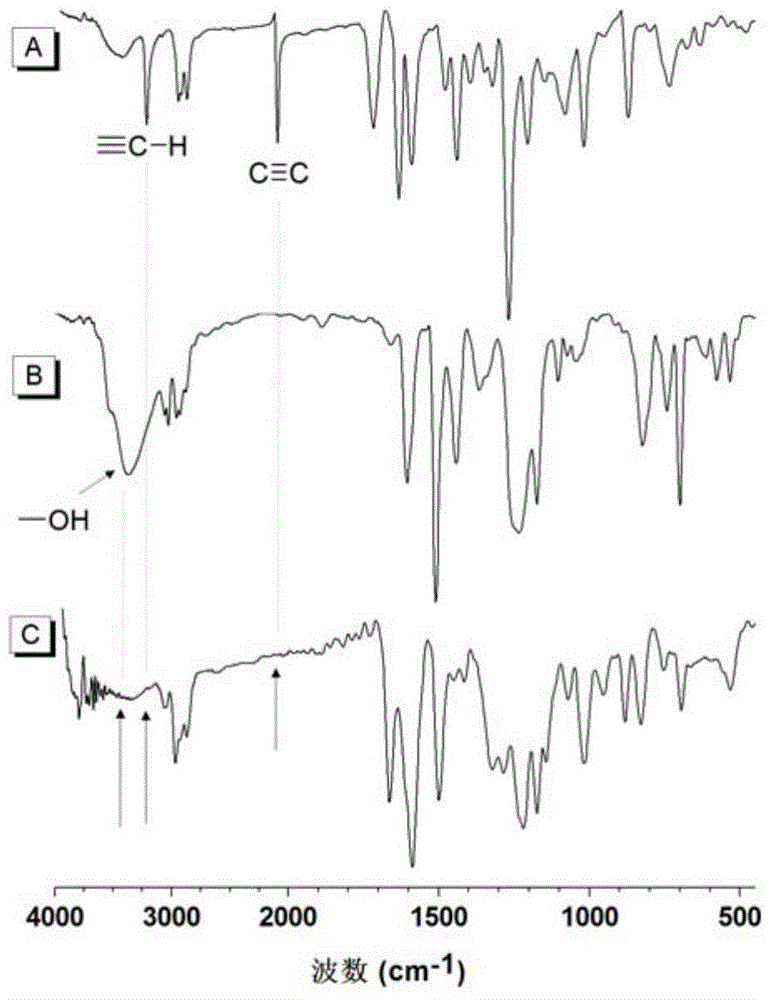
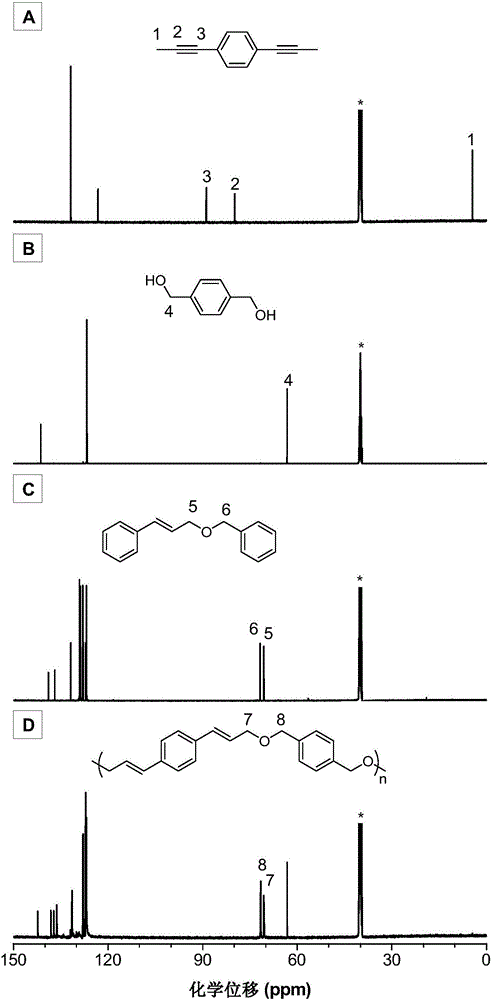
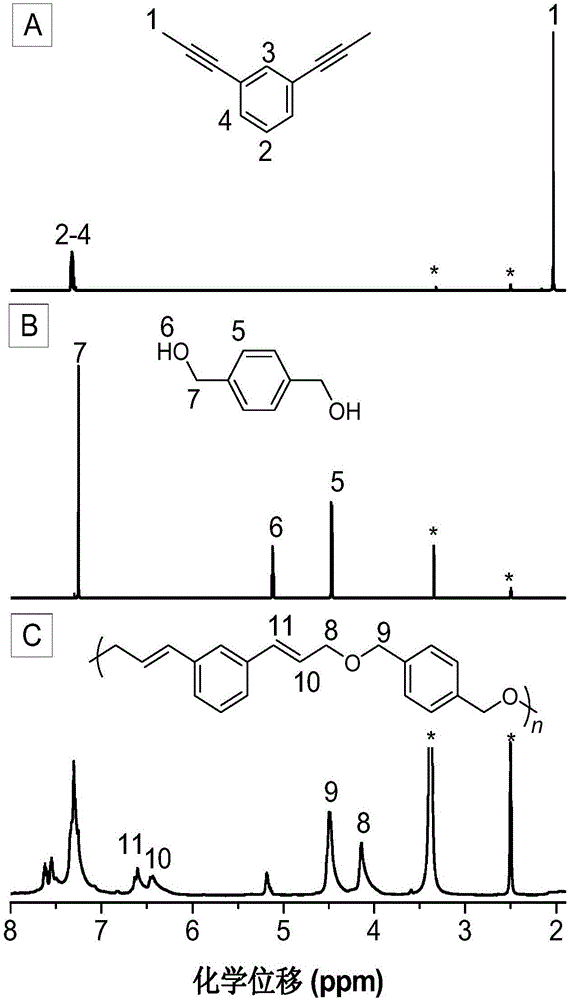
The invention discloses a preparation method of polyene ether compounds. The method includes: organic small-molecule 4-dimethylamino pyridine (DMAP) is used as the catalyst, binary alkynyl compounds and binary hydroxy compounds are mixed with organic solvent, and the polyene ether compounds are obtained by using the click polymerization reaction of alkynyl-hydroxy. The polyene ether compounds comprise an inner unit indicated by formula (I). The polymerization step is showed in the formula (V), wherein n is larger than 1, and R1 and R2 are selected from organic groups. The method has the advantages that water and oxygen do not need to be removed during reaction, polymerization temperature is low, polymerization efficiency is high, and no metal residues exist in products; the prepared polyene ether compounds are high in steric regularity, good in machinability, and high in heat stability, degradability and aggregation-induced emission performance.
Owner:ZHEJIANG UNIV
Method for preparing gefitinib
InactiveCN102030716AThe reaction steps are simpleHigh utilization rate of raw materialsOrganic chemistryMethyl hydroxybenzoateRe crystallization
The invention discloses a novel preparation method for synthesizing gefitinib. 3,4-dimethoxybenzoic acid (2) is taken as a raw material, and the method comprises the following steps of: nitrifying, demethylating and methylating the 3,4-dimethoxybenzoic acid (2) to prepare an intermediate of 2-nitryl-4-methoxy-5-methyl-hydroxybenzoate (5); reacting the intermediate with 4-(3-bromopropyl) morpholine, and introducing an alkyl side chain to prepare an intermediate 6; reducing nitryl, and performing ring formation with methanamide to construct a quinoline matrix ring 8; halogenating 4-carbonyl by using the compound 8 under the action of thionyl chloride to generate an intermediate 9; and reacting with halogen substituted aromatic amine 3-chloro4-fluoroaniline to obtain the target product of gefitinib (1). In the method, the selected initial raw material is low in cost, the synthesizing route is simplified, and the raw material utilization rate and the total yield are greatly improved. The intermediates obtained in the reactions are mostly purified by a re-crystallization method, or directly subjected to the next reaction, so the yield is high, a few three wastes are generated in the reaction process, the cost is low and the method is favorable for industrial production.
Owner:SUN YAT SEN UNIV
Micro spherical hollow structure nickel-based hydrogenation catalyst as well as preparation method thereof
InactiveCN103962140AIncrease the areaHigh strengthOrganic compound preparationCarboxylic acid esters preparationDimethyl terephthalateOpen framework
The invention discloses a micro spherical hollow structure nickel-based hydrogenation catalyst as well as a preparation method thereof in the technical field of catalyst preparation. The method comprises the following steps: controlling the morphology of an aluminum raw material serving as a mold plate which is consumed gradually along with the reaction, inducing hydrotalcite assembly to obtain a micro spherical hollow hydrotalcite assembly body, and carrying out in-situ reduction to obtain the micro spherical hollow nickel-based catalyst. The catalyst material obtained by preparation has high specific surface area, and the internal empty cavity structure and the abundant open-framework structures have great actual application value. The catalyst has relatively high hydrogenation activity and selectivity when being used for catalyzing dimethyl terephthalate (DMT) benzene ring hydrogenation in the reaction for preparing dimethyl 1, 4-cyclohexanedicarboxylate.
Owner:BEIJING UNIV OF CHEM TECH
Hyperbranched polytriazole formate as well as preparation method and application thereof
ActiveCN102585220AHigh StereoselectiveNo protectionFluorescence/phosphorescenceLuminescent compositionsFormateSolvent
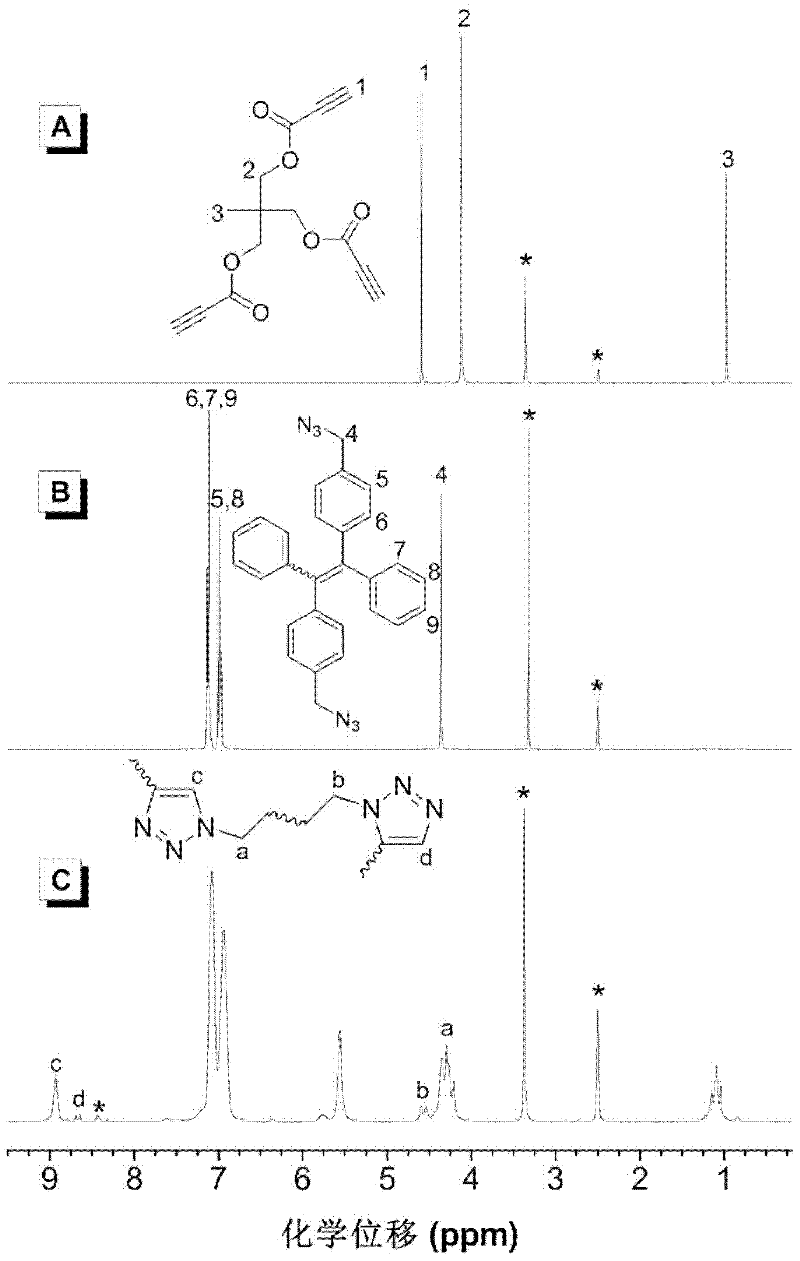
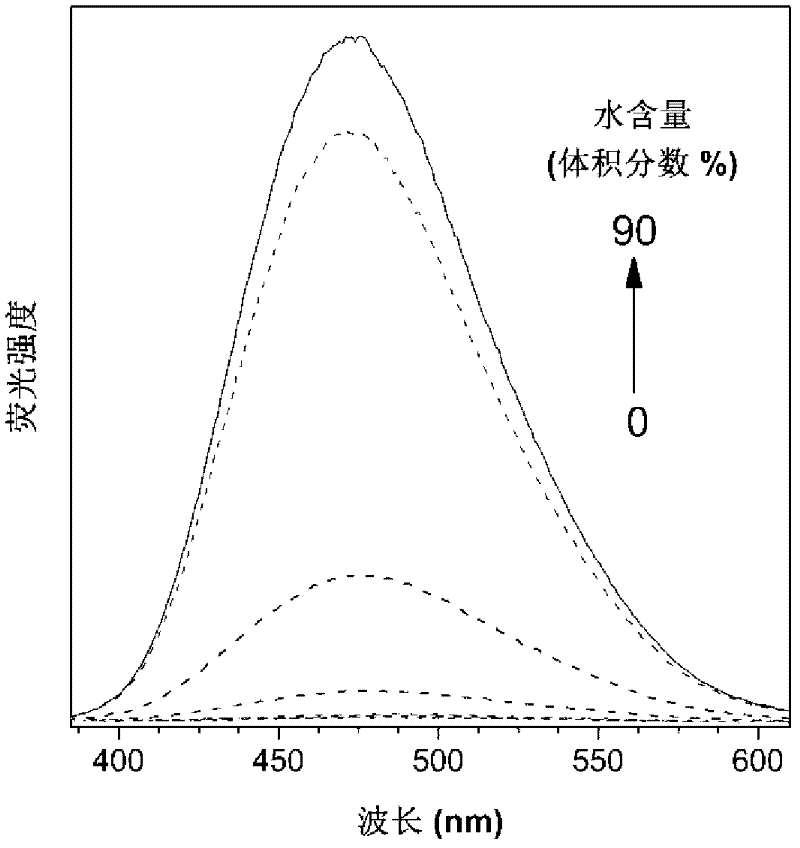
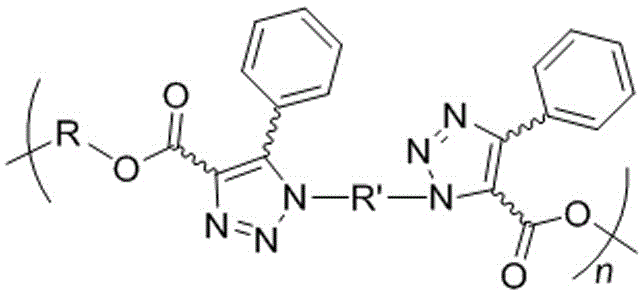
The invention discloses hyperbranched polytriazole formate as well as a preparation method and application thereof. The preparation method of hyperbranched polytriazole formate comprises the following steps: firstly, synthesizing binary azide containing a tetraphenyl ethylene unit; then synthesizing a ternary ester compound containing alkynyl based on ternary alcohol and propiolic acid as raw materials; and finally, carrying out non-metal-catalytic 'click' polymerization reaction under the heating condition in a polar solvent by utilizing the azide and the alkynyl-containing ester compound soas to obtain a target polymer in high yield. The hyperbranched polytriazole formate prepared by using the method is high in 1,4-stereoregularity, good in workability and high in thermal stability, degradability, illumination patterning and aggregation-induced emission property. The invention also discloses application of hyperbranched polytriazole formate in detection of a polynitroarene explosive.
Owner:ZHEJIANG UNIV
C-H Bond Amination and Olefin Aziridination with Beta-Diketiminato Copper Catalysts
ActiveUS20100056806A1Avoid it happening againEconomicalGroup 1/11 element organic compoundsOrganic compound preparationMetal catalystNitrogen
One aspect of the present invention relates to a method for the transition metal (e.g., Cu(I)) mediated amidation of C—H bonds using electron-rich aliphatic azides. In certain embodiments, the methods are useful for the C—H insertion of nitrenes generated and stabilized by a β-diketiminato metal catalyst. In certain embodiments, said nitrenes are generated from organoazides, or by oxidation of the corresponding amine. Another aspect of the present invention relates to olefin aziridination using said β-diketiminato metal catalysts. In addition, the methods of the present invention include stereoselective C—H bond aminations and olefin aziridinatons. In certain embodiments, the methods are conducted in an aerobic environment. In certain embodiments, the present invention relates to the use of O2 as an oxidant, wherein water is the byproduct of oxidation; this fact avoids the generation of toxic byproducts and renders the methods atom economical.
Owner:GEORGETOWN UNIV
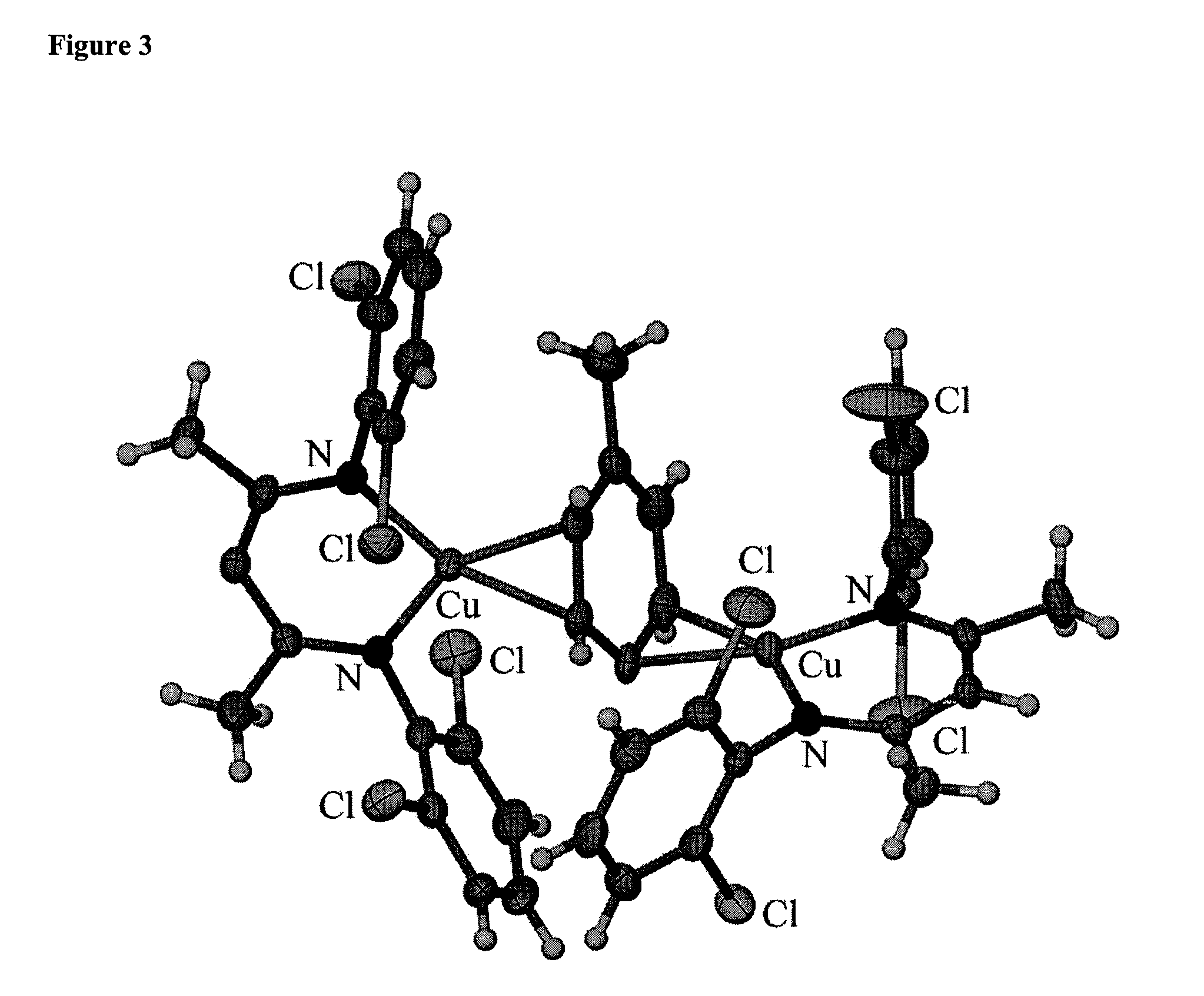
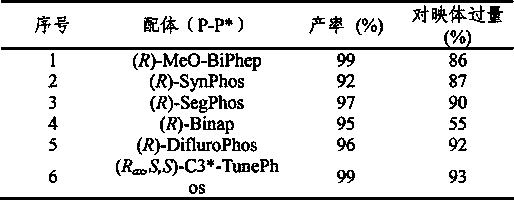
Method for synthesizing chiraltetrahydro naphthalenederivate through asymmetric hydrogenation on isoquinoline by means of iridium catalyst
The invention discloses a method for synthesizing a chiraltetrahydro naphthalenederivate through asymmetric hydrogenation on isoquinoline by means of an iridium catalyst. A catalysis system applied in the method is a chiral bi-phosphine complex of metal iridium. The reaction is carried out under the conditions that the temperature is 25-60 DEG C; the volume ratio of tetrahydrofuran to dichloromethane in a solvent, namely a mixed solvent of tetrahydrofuran and dichloromethane is 1:1; the pressure is 13-50 Mpa; the ratio of a substrate to a catalyst is 50:1; the catalyst is a coordination compound of a (1,5-cyclooctadiene) iridium chloridedipolymer and a chiral bi-phosphine ligand. The corresponding chiral 1-position or 3-position substituted tetrahydro naphthalenederivate through hydrogenation on isoquinoline, and the enantiomeric excess of the derivate can reach 96%. The method is simple and practical in operation, raw materials are easy to obtain, the enantioselectivity is high, the yield is high, the reaction has atom economy, and the environment is friendly.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Preparation method of 2,5-dichlorophenol
ActiveCN107129426AReduce stepsMild conditionsOrganic chemistryOrganic compound preparationAcetic acidPorphyrin
The invention provides a preparation method of 2,5-dichlorophenol, and belongs to the field of preparation of compounds. The preparation method comprises the following steps: taking 1,4-dichlorobenzene as a raw material and taking a combination of one or more of water, methanol, acetonitrile and acetic acid as a solvent under the effects of an oxidizing agent, a metal porphyrin catalyst and a cocatalyst, and reacting at the temperature of 5-80 DEG C for 0.5-20 hours to obtain 2,5-dichlorophenol. By a catalyst system, efficiency and yield of oxidation reaction can be improved remarkably, reaction conditions are gentle relatively, side effects are less, products are separated favorably, the reaction time is greatly shortened, and the 2,5-dichlorophenol can be industrially produced on a large scale.
Owner:NANJING UNIV OF TECH
Method for preparing environment-friendly type plasticizer from low-quality animal and vegetable oil through modification and deep epoxidation
Owner:SICHUAN UNIV
Process for preparing reactive dye and active deep red C-D by micro- reactor
InactiveCN101284950AEnvironmentally friendly"Atom Economy"Disazo dyesReactive dyesMicroreactorCoupling
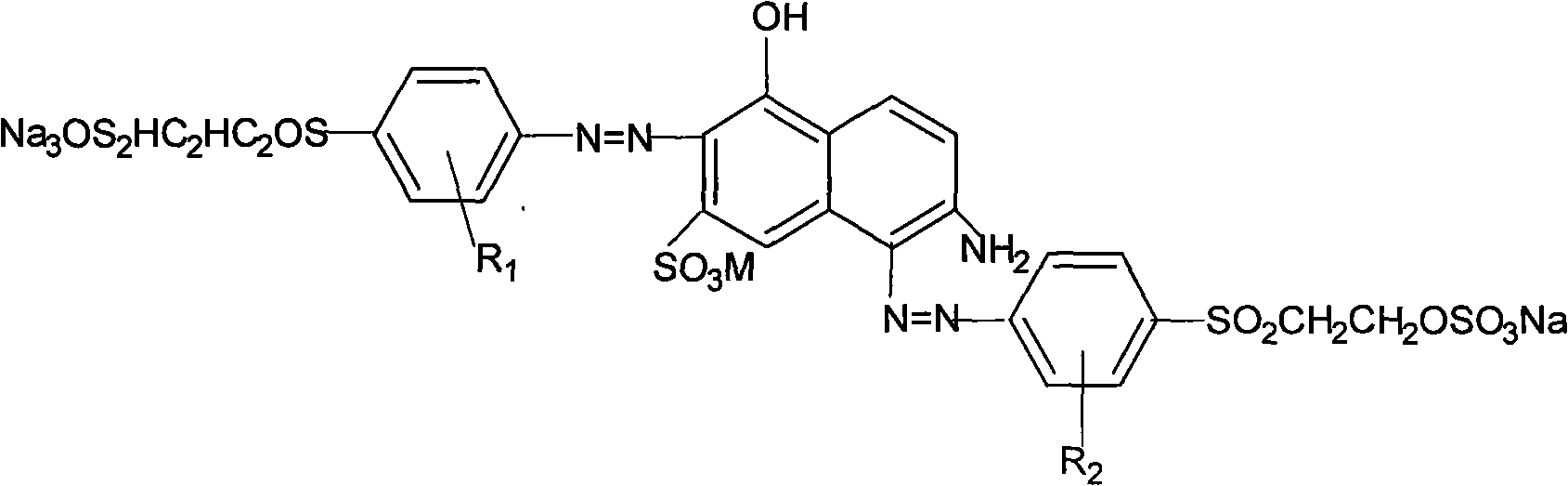
The invention discloses a method for preparing active dye, namely active deep-red C-D by utilization of a microreactor, comprising the following steps that: J acids are coupled with diazonium salts of ethyl sulfone sulfate ester aniline compounds which contain sulfonic group, methyl, methoxyl group, ethyl group or oxyethyl group, and then active deep-red C-D azo dye is obtained; the coupling temperature is between10 and 25 DEG C and the pH value is equal to 1.5 - 6.5; the microreactor is adopted during the reaction process; a plurality of parallelly connected microchannels with diameter of between 10 and 500 micrometers are arranged inside the microreactor and both ends of the microchannels have shell covers. The product has good vividness, stable performance, good reproduction quality, high dye yield, good deep dyeing property and high fixation rate. The structural formula of the active deep-red C-D is as above, wherein, R1 and R2 are equal to H, SO3H, CH3, OCH3, OC2H5 and C2H5.
Owner:赵卫国
Polyphenyltriazole formate, and preparation method and application thereof
ActiveCN106243352APrevent bomb attackImprove solubilityOrganic chemistryFluorescence/phosphorescenceFluorescenceFormate
The invention discloses a polyphenyltriazole formate, and a preparation method and application thereof. A phenylpropargylic acid dibasic ester monomer and a dibasic organic azido monomer used as raw materials are subjected to polymerization reaction in a polar aprotic solvent to obtain the polyphenyltriazole formate. The preparation method has the advantages of accessible reaction raw materials and no byproduct generation in the polymerization reaction process, and conforms to the atom economy. The polymerization reaction has wide substrate applicability and favorable functional group compatibility, and is convenient for introducing multiple functional groups. The polymerization reaction does not use any metal catalyst, and thus, can eliminate the influence of catalyst residues on the biological and photoelectric properties of the polymer material. The polyphenyltriazole formate has the advantages of favorable processability, higher heat stability, higher degradability and higher aggregation-induced luminescence, and has potential application values in the aspects of optical plastics, biomedical materials, fluorescence detection and the like.
Owner:SUZHOU UNIV
Unsymmetrical hydrogen migration synthesizing method for (R, R)-formoterol
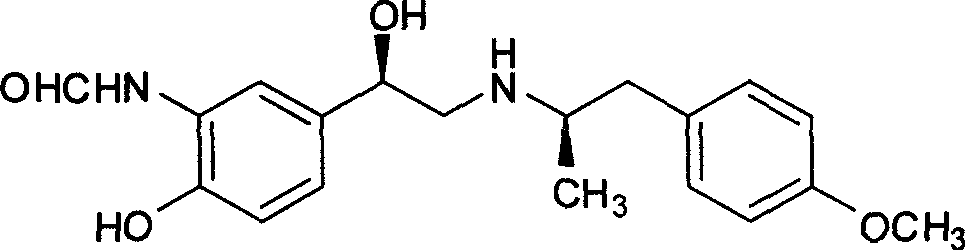
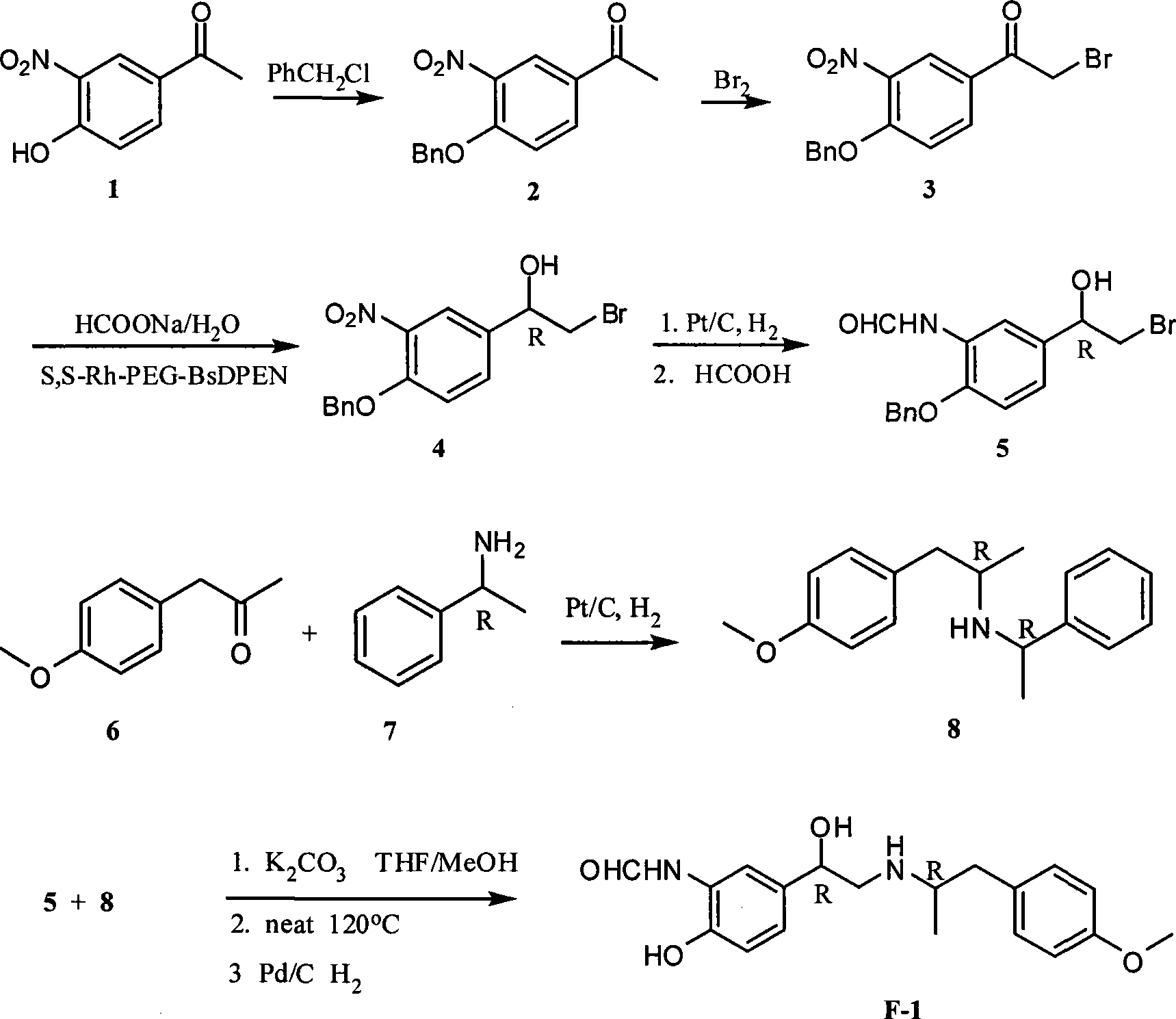
InactiveCN101468954AIn line with the concept of green chemistryLow costOrganic compound preparationCarboxylic acid amides preparationSynthesis methodsPhotochemistry
The invention relates to an asymmetric hydrogen transfer synthesis method for (R,R)-formoterol, and relates to a novel method for synthesizing an optical pure beta 2-adrenoreceptor excitant, namely formoterol. The method comprises: firstly, taking 4-hydroxyl-3 nitroacetophenone as a raw material, using benzyl groups to protect phenolic hydroxyl groups, and obtaining alpha-bromo keto after bromination; secondly, taking (S,S)-Rh-PEG-BsDPEN as a catalyst and formic acid and derivatives of the formic acid as hydrogen sources, and synthesizing chiral alcohol intermediate by an asymmetric hydrogen transfer method; thirdly, using (R)-alpha-methyl phenylethylamine and methoxyl phenylacetone to generate imine compounds, and obtaining chiral amine intermediate through hydrogenation reduction under the catalysis of Pt / C; and fourthly, reacting and coupling the chiral alcohol intermediate and the chiral amine intermediate, removing protective groups, and obtaining the (R,R)-formoterol. The invention uses the asymmetric hydrogen transfer method and a chiral auxiliary reagent to synthesize the (R,R)-formoterol, and has high yield and good ee value. Compared with a method for synthesizing chiral formoterol through chemical splitting, the method has the advantages of high total yield, mild reaction conditions, low cost and so on, and is favorable for industrial production.
Owner:SUN YAT SEN UNIV
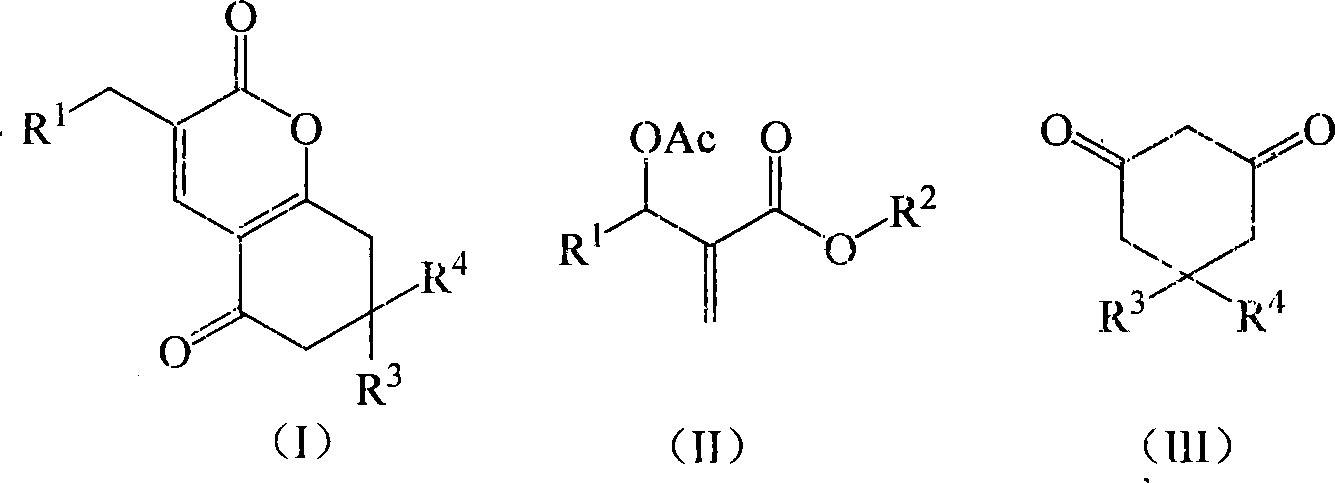
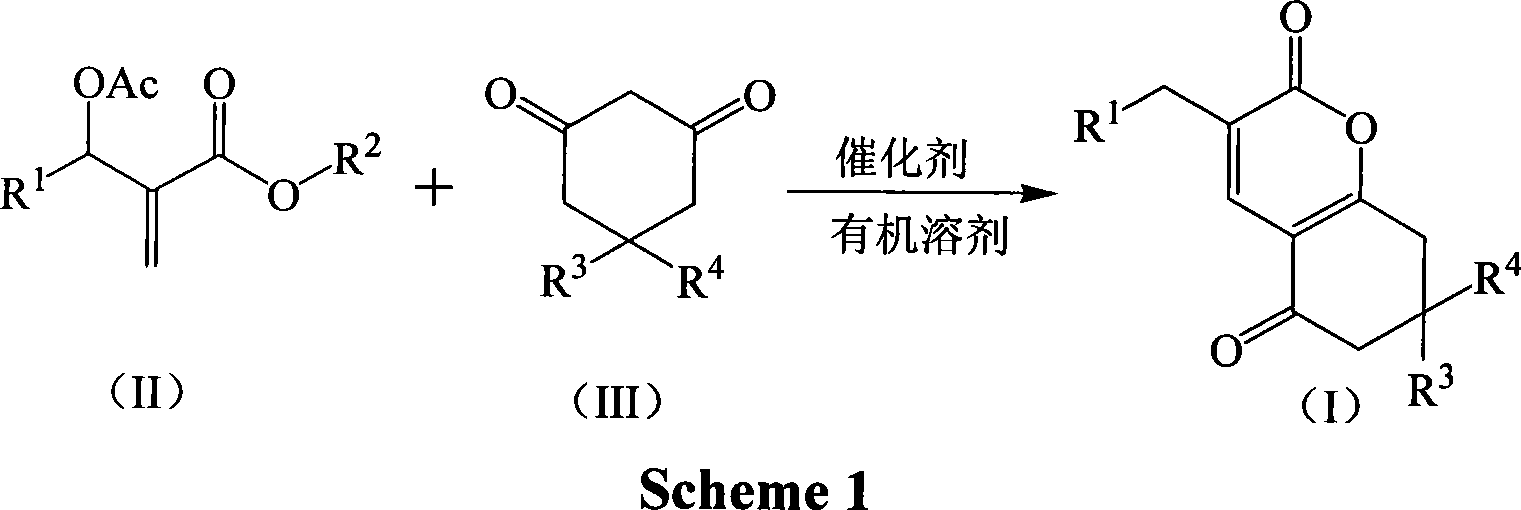
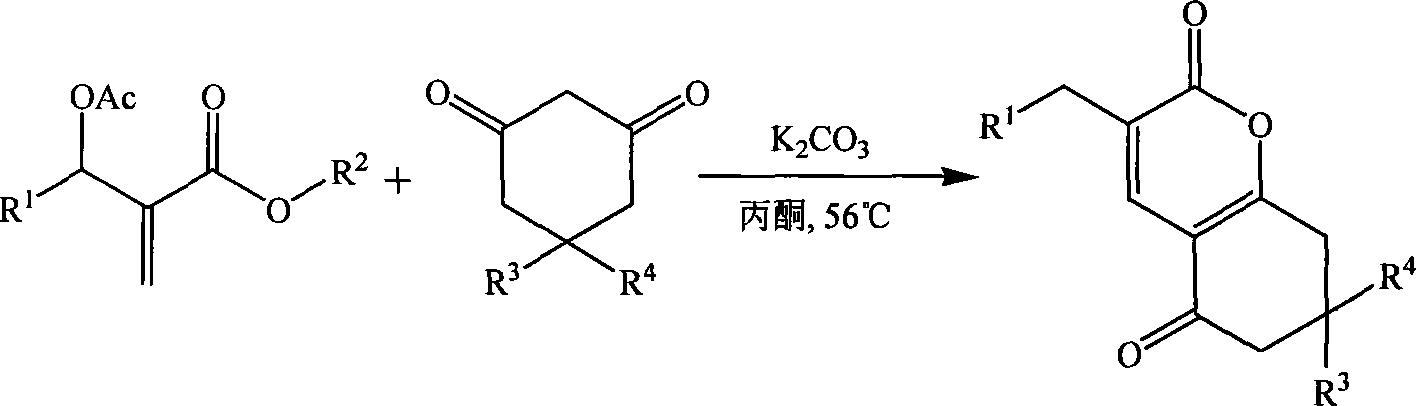
Method for synthesizing alpha-pyranone derivatives
InactiveCN101085769AReduce pollutionSimple reaction conditionsOrganic chemistryCyclohexanoneState of art
The invention discloses a method for synthesizing alpha- pyranone derivant demonstrated in formula (I). It comprises following steps: carrying out cyclic reaction with affixture of Baylis-Hillman and 1, 3- cyclohexanone and its derivant demonstrated in formula (III) under catalytic action of alkali and with existence of organic disslovant or without disslovant at subzero 20 to 10 Deg. C, reacting for 1- 12 hours, post-treating after reaction and getting said alpha- pyranone derivant. The invention is characterized by easy got raw material, simple and safe operation, temperate reaction condition, high productivity, simple post-treatment, little environmental pollution, and great performing value and social and economic benefit.
Owner:ZHEJIANG UNIV OF TECH
Polyimidazole compound, in-situ preparation method and applications thereof
InactiveCN106967217AEfficient Polymerization MethodSimple substrateIn situ polymerizationEnergy conservation
The present invention discloses a polyimidazole compound, an in-situ preparation method and applications thereof, wherein a binary isocyano compound is subjected to a cyclization polymerization reaction in an organic solvent under the action of a cyclization polymerization catalyst to obtain the polyimidazole compound. According to the present invention, the imidazole unit in the prepared polyimidazole compound is formed by carrying out in-situ polymerization on the imidazole-unit-free monomer so as to make up the gaps in the prior art; the cyclization polymerization reaction is the one-component polymerization reaction, the monomer ratio is not required to be considered, the process is simple, the reaction raw materials and the catalyst are easy to obtain, the polymerization reaction conditions are mild, the polymerization can be performed at the room temperature, and the energy source is saved; and the polymerization efficiency of the polymerization reaction is high, the high molecular weight polymer can be obtained after the reaction is performed for a short time, the polymerization reaction has excellent region selectivity and excellent stereoselectivity, no by-product is generated during the polymerization process, and the atom economy is met.
Owner:SOUTH CHINA UNIV OF TECH
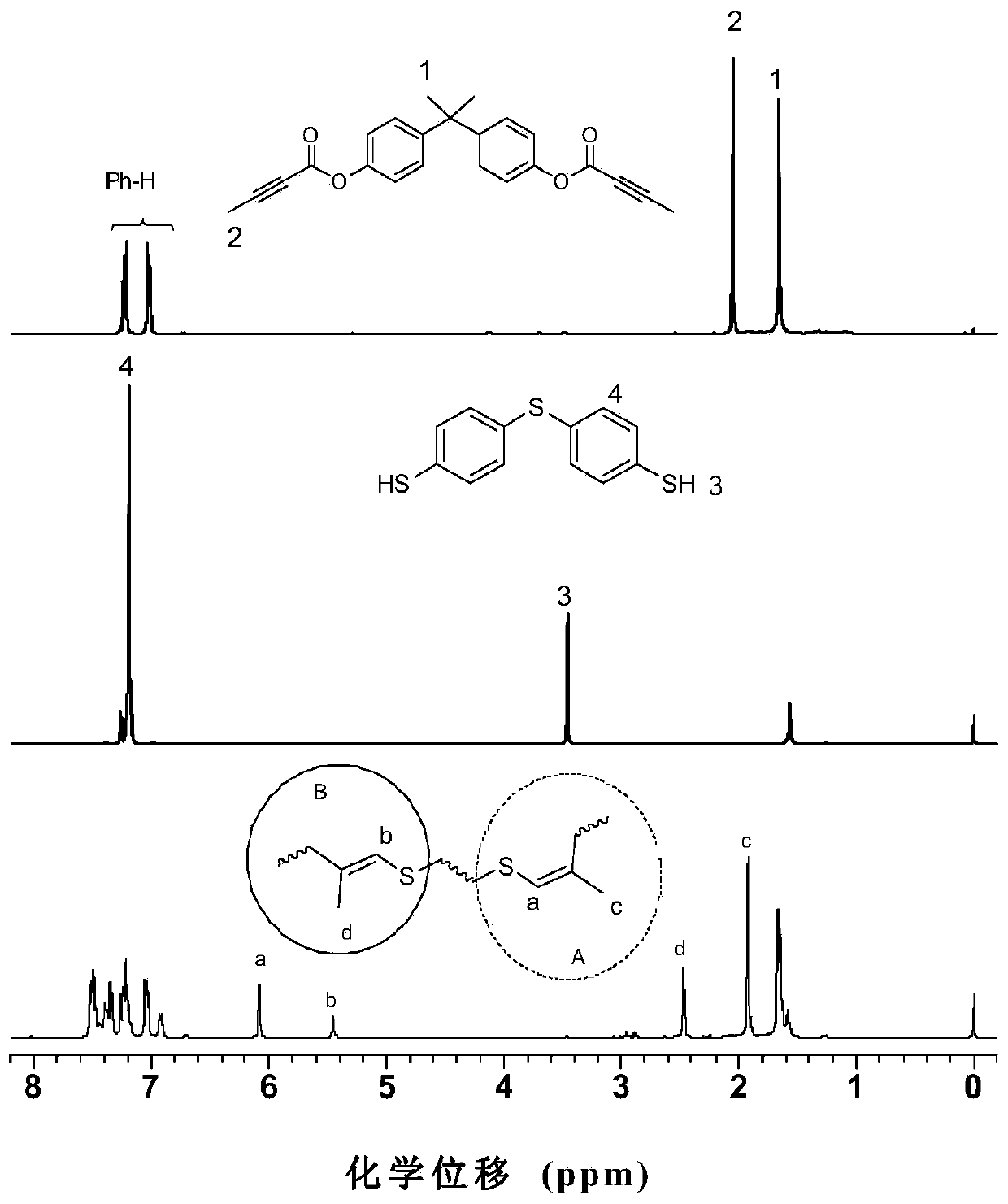
Polyvinylene sulfide and preparation method thereof
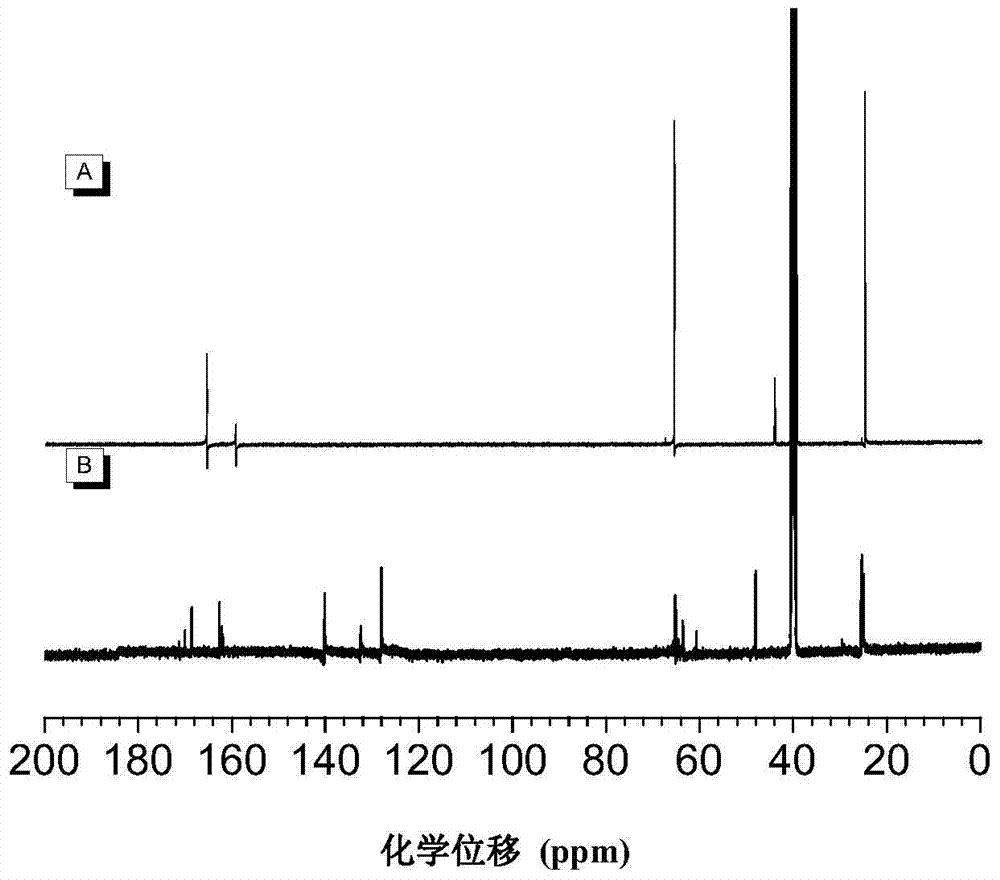
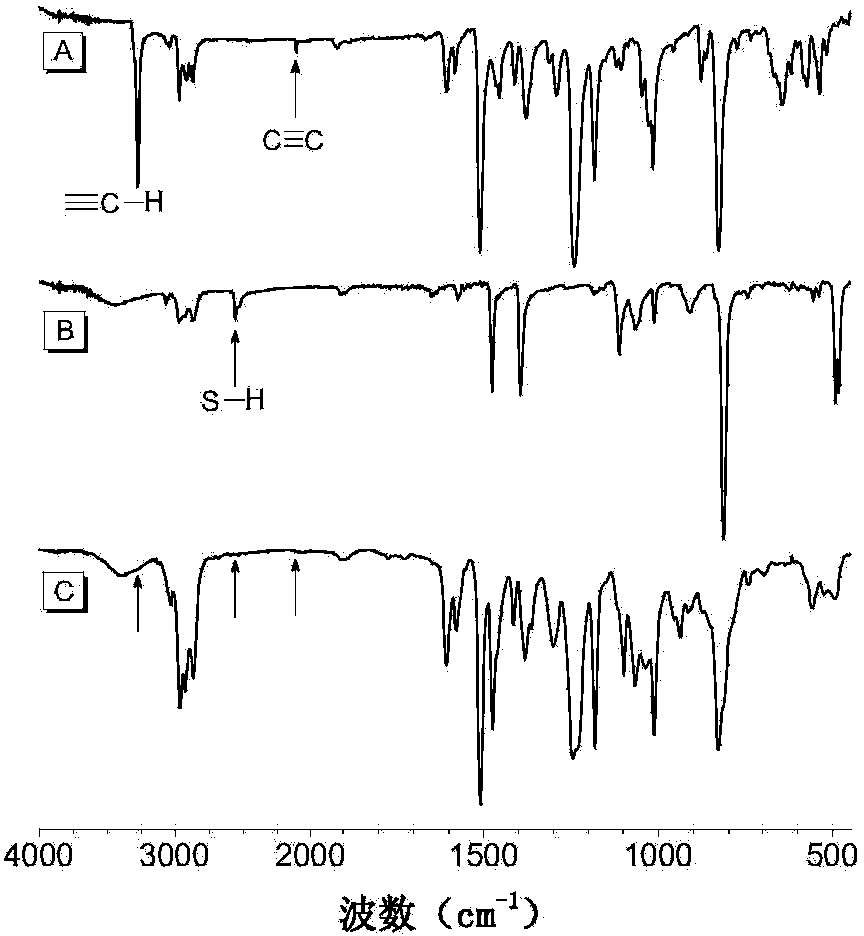
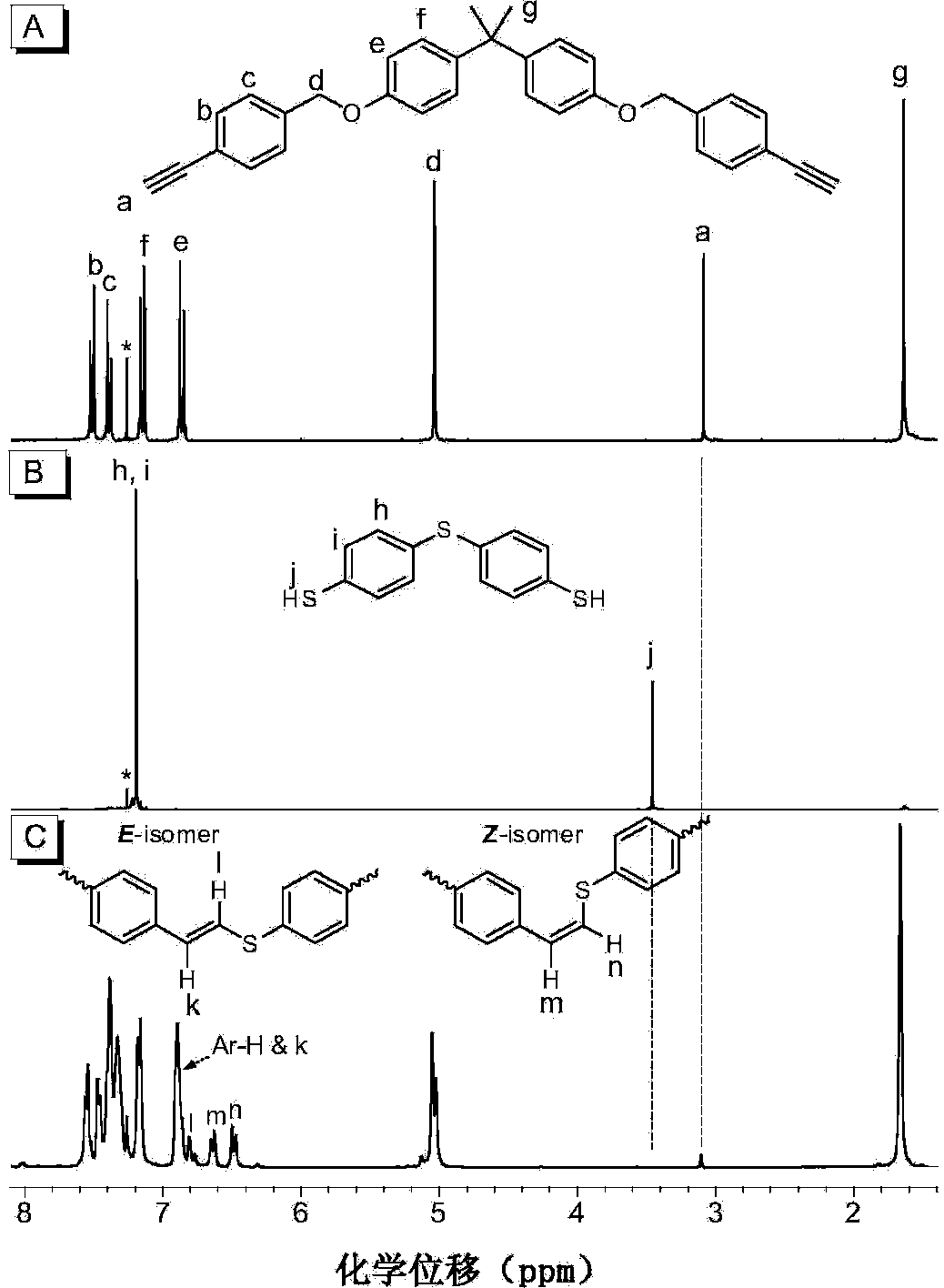
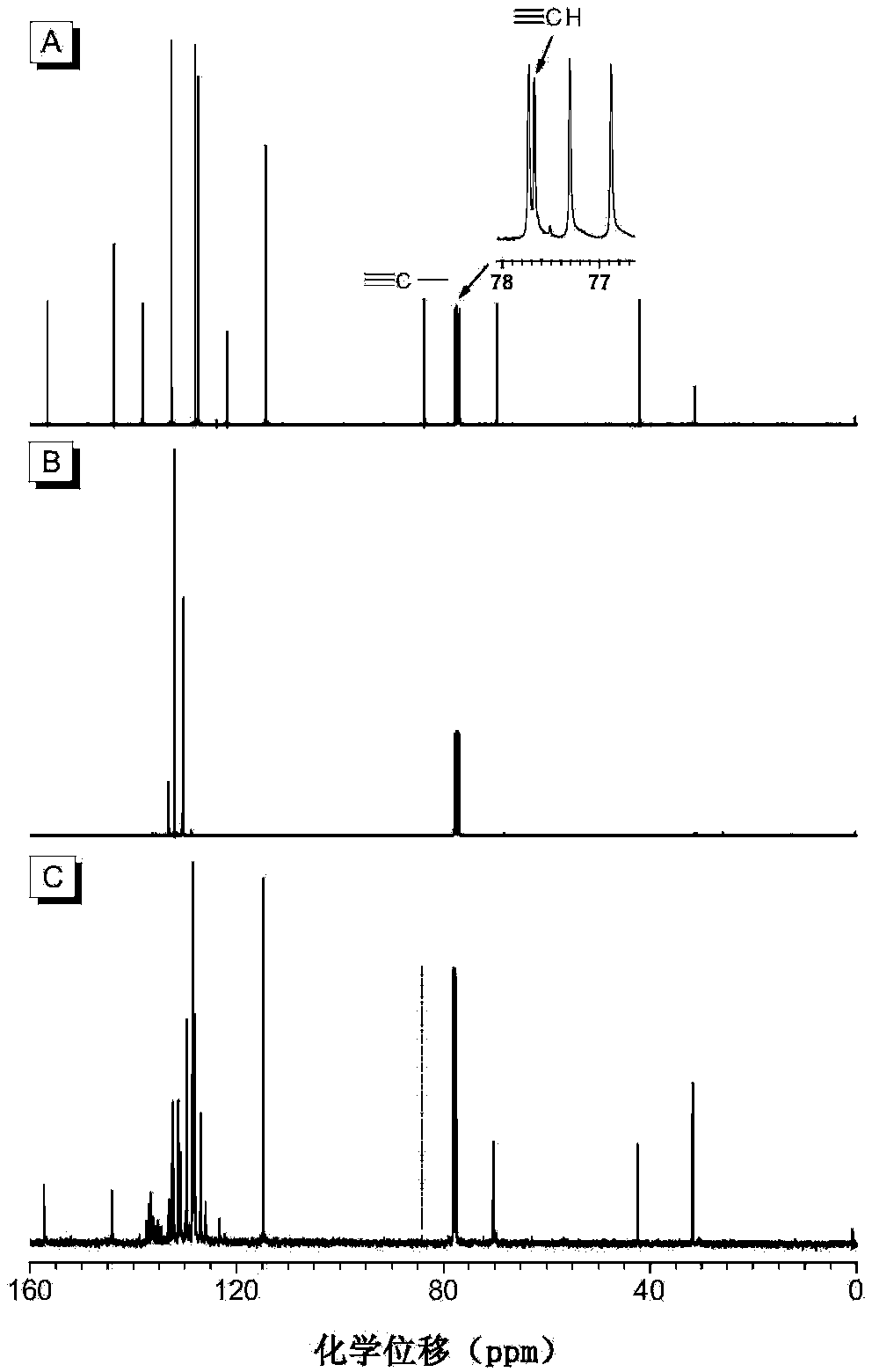
The invention discloses a preparation method of polyvinylene sulfide. The preparation method comprises the following step of performing click polymerization reaction on a binary alkynyl compound and a binary mercapto-compound in an organic solvent to obtain the polyvinylene sulfide. The obtained polyvinylene sulfide comprises an internal unit shown in a formula (I), the step of polymerization is shown in a formula (IV), wherein n is more than 1 and is selected from organic groups. The preparation method does not need heating or any catalyst, is simple and efficient in process, and has excellent space selectivity. The invention also discloses the polyvinylene sulfide obtained through the preparation method, and the polyvinylene sulfide has good workability and film-forming property, high heat stability and refractive index and aggregation induced luminescent property.
Owner:ZHEJIANG UNIV
Alpha-quaternary carbon contained alpha, beta-diamino acid derivative, synthetic method thereof and application thereof
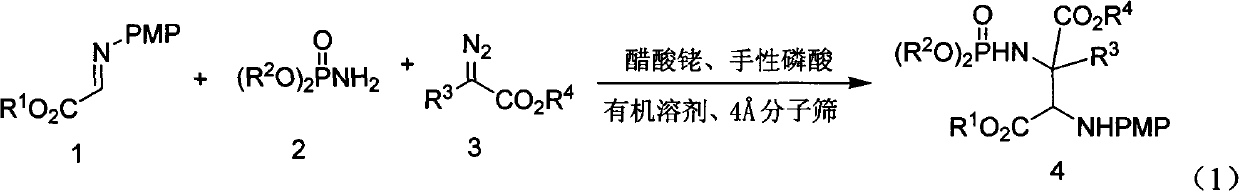
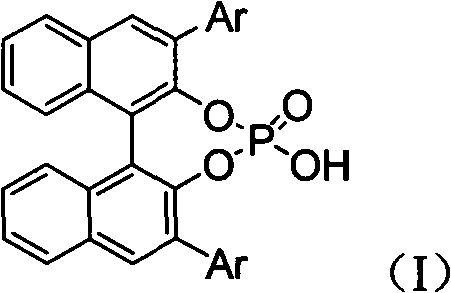
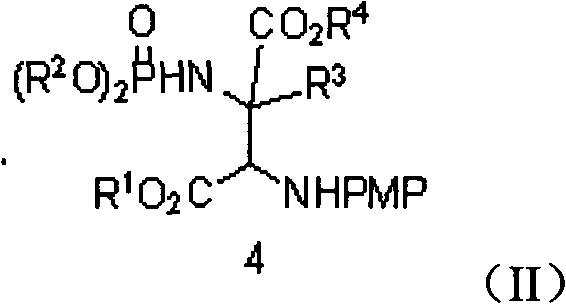
InactiveCN102503976ALow costAtom economyGroup 5/15 element organic compoundsSkeletal disorderSynthesis methodsPhosphoric acid
The invention discloses a synthetic method of an alpha-quaternary carbon-contained alpha, beta-diamino acid derivative. The alpha-quaternary carbon-contained alpha, beta-diamino acid derivative is obtained by treating a diazo compound, an imine and a phosphoramidate as raw materials, chiral phosphoric acid and rhodium acetate as catalysts, an organic solvent as a solvent and a molecular sieve as a water absorbent, and carrying out a catalytic reaction. The synthetic method of the invention has the advantages of simple and easily available raw materials, simple and safe operation, high atom economy, high yield and high selectivity. The alpha-quaternary carbon-contained alpha, beta-diamino acid derivative obtained through the method of the invention, which has a diverse compound skeleton, has a very important meaning to a new medicine screening and preparing technology.
Owner:EAST CHINA NORMAL UNIV
Poly(allyl ether) compound, and preparation method and application thereof
The invention belongs to the fields of high polymer chemistry and material science, and discloses a poly(allyl ether) compound, and a preparation method and application thereof. The method comprises the following steps: (1) performing polymerization reaction on a binary propinyl compound and a binary hydroxyl compound in organic solvent in an inert gas protective atmosphere to obtain a crude product; and (2) performing post-treatment on the crude product to obtain the poly(allyl ether) compound. The poly(allyl ether) compound has a structure shown in Formula (I), wherein n is an integer of 2-200, and R2 is a same or different organic group. The method disclosed by the invention has the advantages of mild conditions, simple and accessible polymerizable monomers, high polymer yield, high molecular weight, high atom economy and excellent regioselectivity; and the poly(allyl ether) compound disclosed by the invention has excellent processability and high thermal stability. The Formula (I) is shown in the specification.
Owner:SOUTH CHINA UNIV OF TECH
Polyvinyl thioether ester as well as preparation method and application thereof
ActiveCN110483773AWide substrate applicabilityImprove compatibilityFluorescence/phosphorescencePolymer scienceAlkyne
The invention discloses polyvinyl thioether ester as well as a preparation method and application thereof. The polyvinyl thioether ester is obtained through solution polymerization reaction by takinga binary acetylene acid ester internal alkyne monomer and a binary sulfydryl monomer as raw materials. The reaction raw materials are easy to obtain, no by-product is generated in the polymerization reaction process, and the atom economy is met; the polymerization reaction has wide substrate applicability and good functional group compatibility, and various functional groups can be conveniently introduced; no catalyst is used in the polymerization reaction, so that the influence of catalyst residues on photoelectric and biological properties of the polymer material can be eliminated. The polyvinyl thioether ester prepared by the invention has good processability, high thermal stability and aggregation-induced emission performance, and has application value in the aspects of optical plastics, biomedical materials, fluorescence sensing and the like.
Owner:SUZHOU UNIV
Process method for preparing (methyl) tert-butyl acrylate through continuous method
ActiveCN104030919AHigh yieldHigh selectivityOrganic compound preparationCarboxylic acid esters preparationMeth-Ptru catalyst
The invention provides a process method for preparing (methyl) tert-butyl acrylate through a continuous method. According to the method, the (methyl) tert-butyl acrylate is continuously produced by utilizing a fixed bed reactor and a rectification device, a silica-loaded phosphorus vanadium and tungsten heteropoly acid serves as a catalyst, and tertiary butanol serves as an olefin polymerization inhibitor. By adoption of the process method, the reaction speed is high, the yield of the product is greatly increased, and the whole set of process is simple in steps, low in cost and pollution-free and accords with atomization economy.
Owner:WUXI ACRYL TECH
Benzo dioxy heterocyclic derivatives with optical activity and preparation method and application thereof
InactiveCN104059049AIncrease profitHigh selectivityOrganic chemistryMetabolism disorderOrtho positionPhosphoric acid
The invention discloses benzo dioxy heterocyclic derivatives with optical activity and a preparation method and application thereof, and the products are synthesized by reaction of four components of a diazo compound, an ortho-substituted aromatic aldehyde, an arylamine and water under catalysis of rhodium acetate, chiral phosphoric acid and an alkali. The raw materials are cheap and easily obtained, three chiral carbons can be simultaneously constructed, the preparation route is short, the operation is simple and safe, the reaction condition is mild, the atom economy is high, the selectivity is high, and the polysubstituted benzo dioxy heterocyclic derivatives can be conveniently obtained, and diverse compound skeletons can be provided for use in screening of new drugs and pharmaceutical fields.
Owner:EAST CHINA NORMAL UNIV
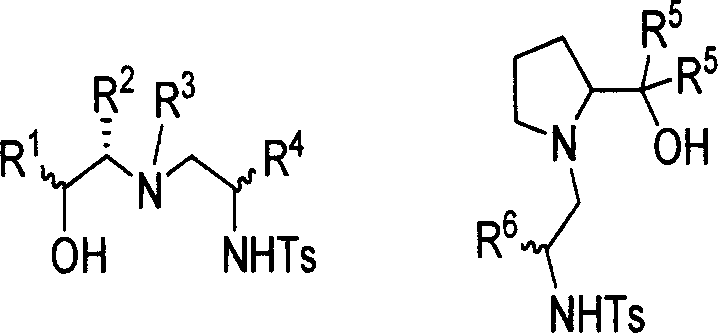
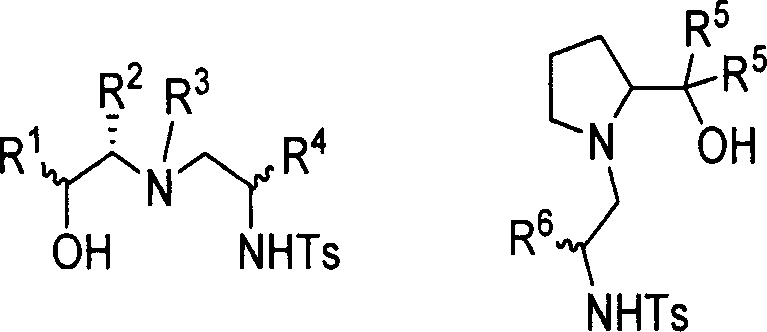
Chirality sulfonamide aminol ligand based on prolinol, its preparing method and application
The invention discloses a making method and application of chiral sulfonamide amide-alcohol ligand based on proamide-alcohol, which is characterized by the following: adopting L-proamide-alcohol as raw material; making each ligand framework possess 1-3 chiral center; proceeding selectivity loop-opening reaction for cycloethyliminum; improving receiving rate obviously; adopting chiral sulfonamide amide-alcohol ligand and similarity as catalyst ligand in the asymmetrical synthetic reaction; fitting for applying in the asymmetrical diethyl zinc additioning reaction, alkynyl zinc additioning reaction for carbonyl compound, reducing reaction and aldehyde alcohol condensing reaction.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Chiral polyacid-based metal organic framework material and preparing method and application thereof
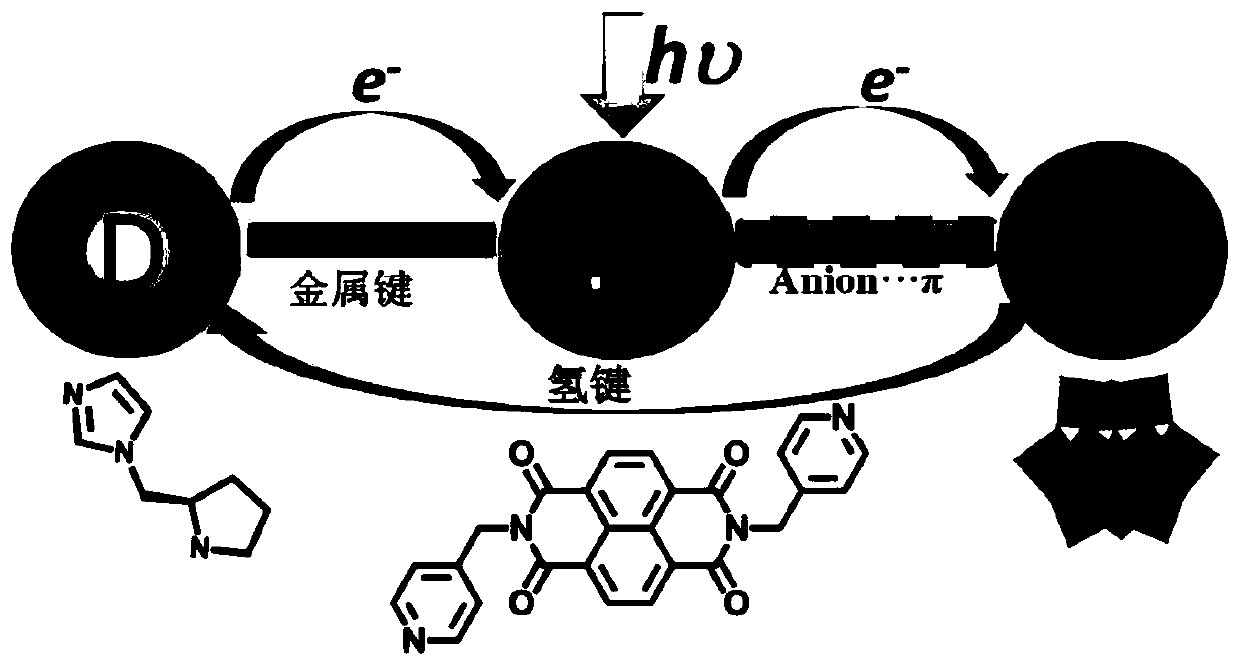
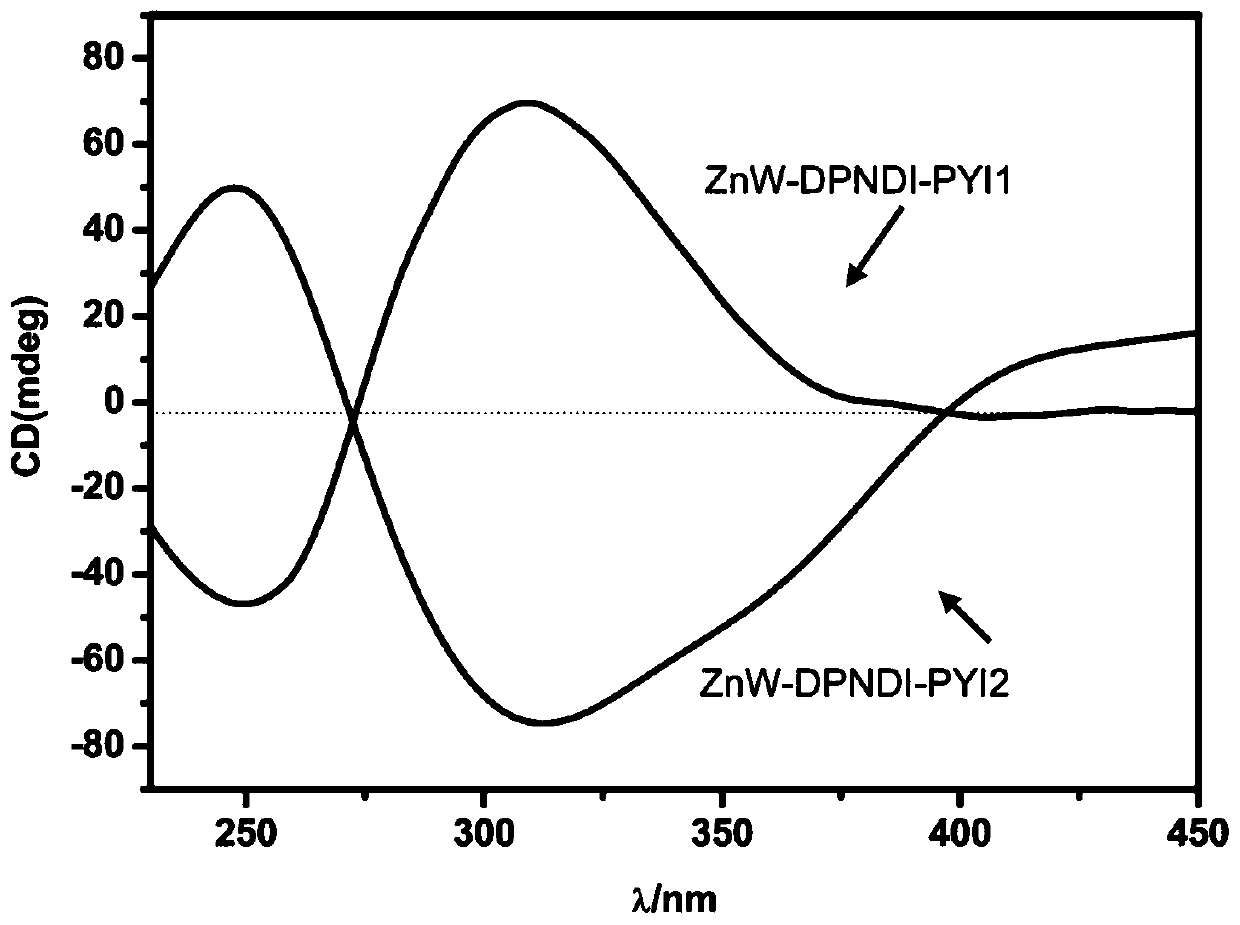
ActiveCN109772459AAccurate understanding of structural featuresUnderstanding Structural FeaturesOrganic chemistryOrganic-compounds/hydrides/coordination-complexes catalystsSpace groupElectron donor
The invention provides a chiral polyacid-based metal organic framework material. The chemical formula of the polyacid-based metal organic framework material is C74H101B2Zn2N22O84W24, and is recorded as ZnW-DPNDI-PYI1 and ZnW-DPNDI-PYI2 according to the chirality, wherein the DPNDI is N,N'-bi(4-picoline) naphthalimide and serves as a bridging ligand and a photosensitizer, and the PYI is L-pyrrolidine-2-imidazole and serves as a chiral ligand and an electron donor; crystals belong to a monoclinic system, and both the space group of the ZnW-DPNDI-PYI1 and the space group of the ZnW-DPNDI-PYI2 areP2(1). The chiral polyacid-based metal organic framework material is a multifunctional inorganic porous material, and has an asymmetric photocatalytic oxidation technology, and in the technology, a photocatalyst is driven by visible light to be an excited state, then the excited-state photocatalyst and substrates are subjected to an oxidation-reduction reaction, and therefore reactions of relatedfree radical types are completed.
Owner:HENAN UNIVERSITY
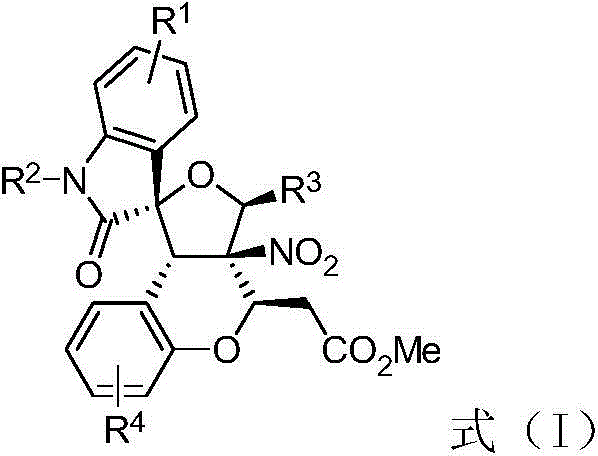
Preparation method of 3, 3-spiro (2-tetrahydrofuranyl)-oxindole polycyclic compound
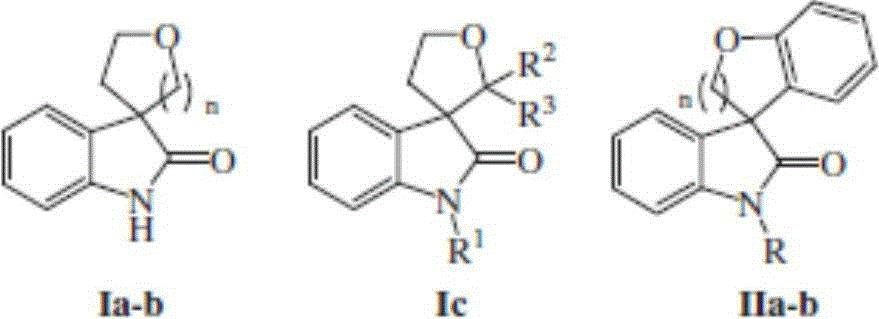
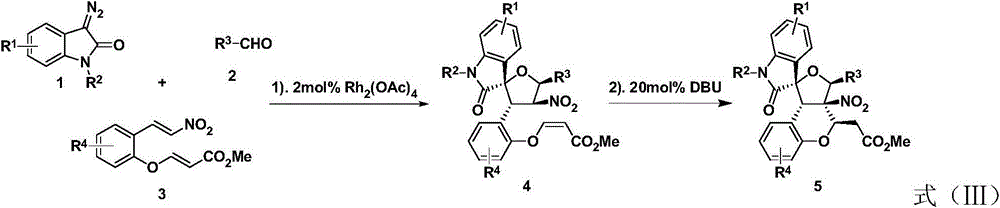
ActiveCN103554120ANo pollution in the processLower synthesis costOrganic chemistryAntineoplastic agentsOrtho positionCycloaddition
The invention discloses a preparation method of a 3, 3-spiro (2-tetrahydrofuranyl)-oxindole polycyclic compound as shown in a formula (I). The preparation method comprises the following steps: performing (3+2) cycloaddition on isatin diazo, aldehyde and ortho-nitro-substituted phenylene under the catalysis of rhodium acetate to construct an intermediate containing a 3, 3-spiro (2-tetrahydrofuranyl)-oxindole structure, adding a base, and then performing intramolecular Michael addition to further perform ring-closure synthesis of a target product. According to the preparation method, raw materials are available, and five cyclic structures are constructed by adopting one-pot method. The preparation method is short in preparation route, simple to operate, mild in reaction conditions and high in yield, and has high atom economy and no environment pollution. The product prepared according to the method can be used for providing various compound frameworks, and has the property of inhibiting the activity of AURKA.
Owner:广东和博制药有限公司
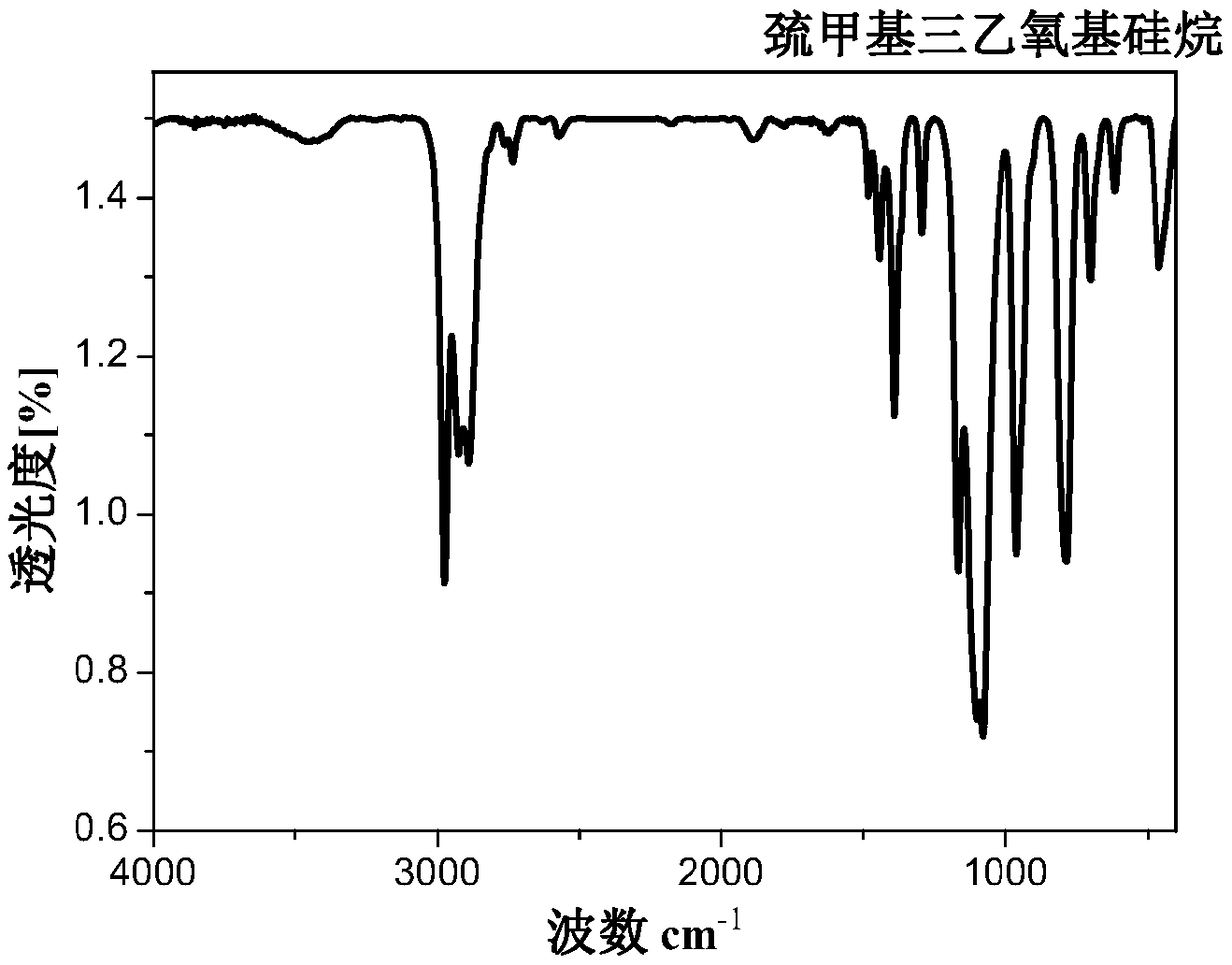
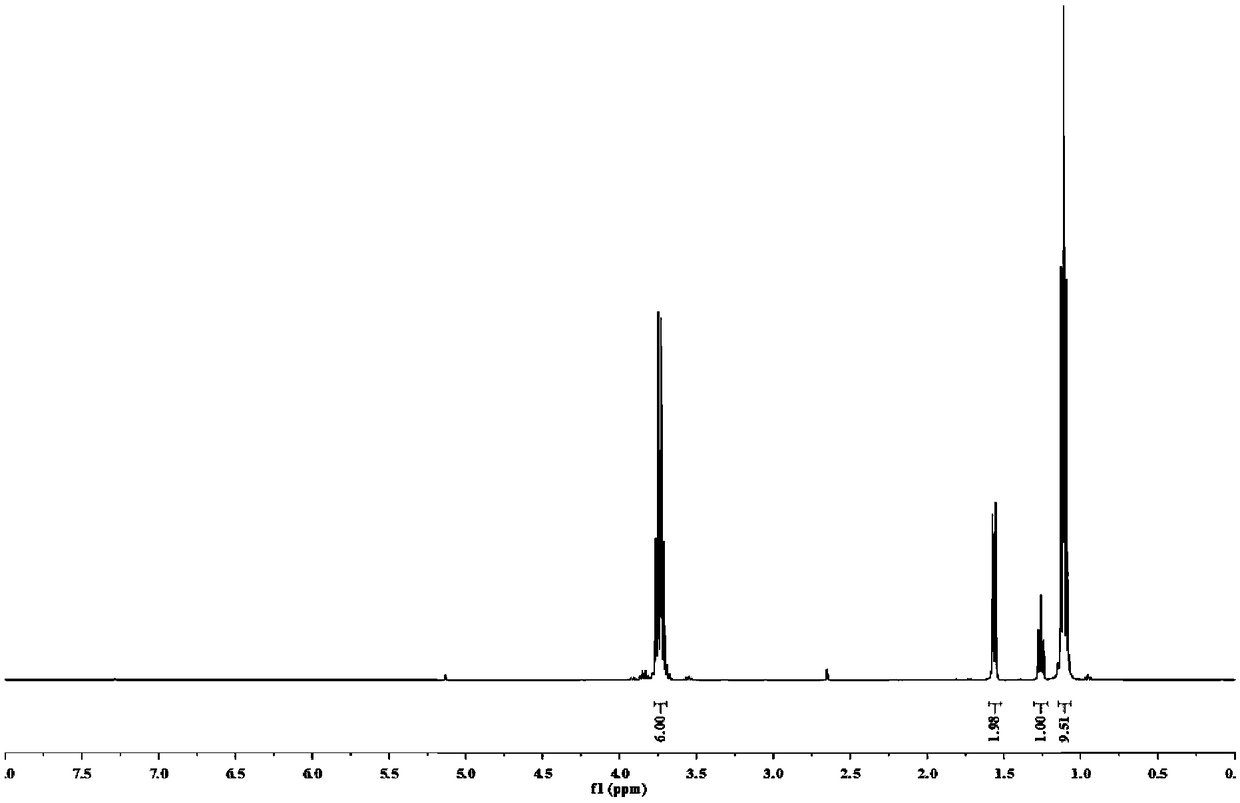
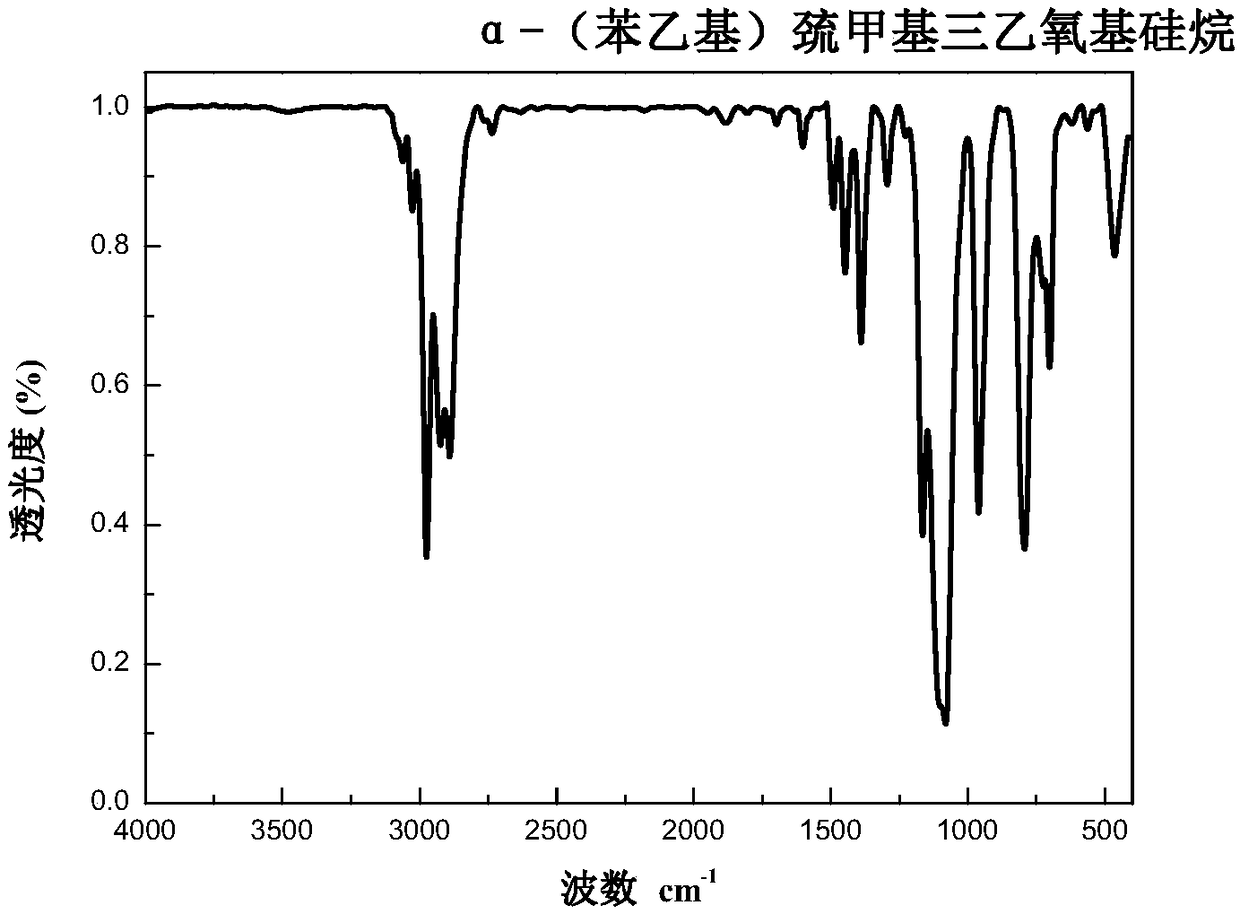
Trialkoxysilanes connected with thioether bond on alpha-carbon and containing different functional groups based on sulfydryl-ene click reaction, and preparation
ActiveCN109053794AMild reaction conditionsRapid responseSilicon organic compoundsChemistryDouble bond
The invention relates to a preparation method of trialkoxysilanes connected with a thioether bond on alpha-carbon and containing different functional groups. The method comprises the steps of reactingchloromethyltrichlorosilane and sodium alkoxide so as to prepare chloromethyltriethoxysilane, then reacting chloromethyltriethoxysilane and thiourea under the action of a catalyst so as to prepare alpha-sulfydrylmethyltriethoxysilane; through performing photo-initiation sulfydryl-ene click reaction, connecting alpha-sulfydrylmethyltriethoxysilane and a double-bond compound, and thus obtaining trialkoxysilanes connected with the thioether bond on the alpha-carbon and containing the different functional groups. According to the method, one of the raw materials is the industrial byproduct chloromethyltrichlorosilane, and the efficient utilization way is opened for chloromethyltrichlorosilane, so that environment protection and energy conversation are realized, the dosage of a toxic catalystis reduced, and the idea of green environment protection is met. According to the preparation method provided by the invention, the raw materials and reaction reagents are easy to get, the product iseasy to separate and purify, and the atom economy is met; the preparation process is simple, the reaction condition is mild, the cost is low, and the preparation method is suitable for large-scale industrial production and application.
Owner:山东时代新材料科技有限公司
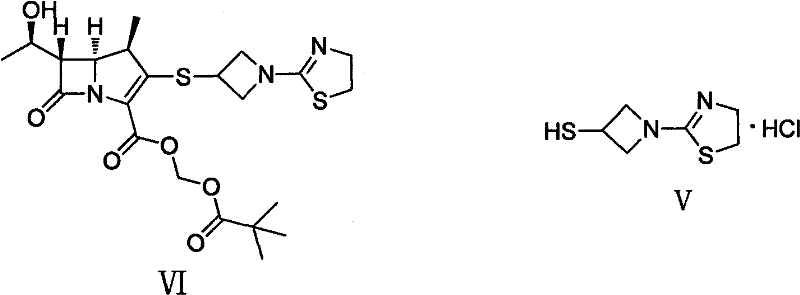
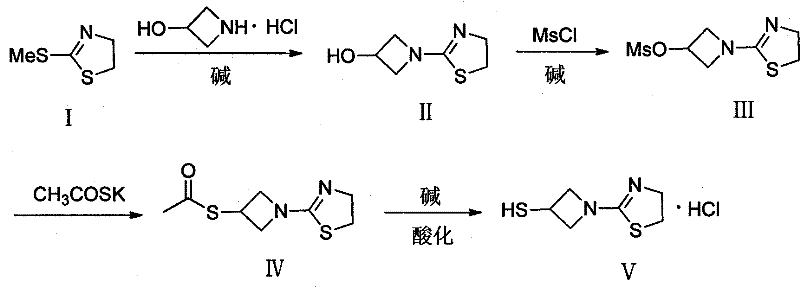
Preparation method of 1-(4, 5-dihydro-2-thiazolinyl)-3-mercaptoazetidine hydrochloride
ActiveCN102250080ARaw materials are easy to getSimple process routeOrganic chemistryHydrogenMethane sulfonate
The invention discloses a method for synthesis of 1-(4, 5-dihydro-2-thiazolinyl)-3-mercaptoazetidine hydrochloride. With 2-methylthio-2-thiazoline (I) as the raw material, the method of the invention comprises the steps of: in the presence of alkali, reacting 2-methylthio-2-thiazoline (I) with 3-hydroxyazetidine hydrochloride so as to obtain 1-(4, 5-dihydro-2-thiazolinyl)-3-hydroxyazetidine (II), and reacting (II) with methyl sulfonylchloride in the presence of alkali so as to obtain 1-(4, 5-dihydro-2-thiazolinyl)-3-methane sulfonate group azetidine (III), subjecting (III) to a reaction with potassium thioacetate so as to obtain 1-(4, 5-dihydro-2-thiazolinyl)-3-thioacetic acid ester group azetidine (IV), in the presence of alkali, subjecting (IV) to hydrolysis and then to acidification with dilute hydrochloric acid, thus obtaining the 1-(4, 5-dihydro-2-thiazolinyl)-3-mercaptoazetidine hydrochloride (V). With easily available and inexpensive starting material, the method of the invention simplifies the synthesis route, improves the raw material utilization ratio and the overall yield. An intermediate obtained in the reaction can be subjected to refinement by a recrystallization method or to a next reaction directly, so that the yield is high and the "three wastes" produced during the reaction process are few. In addition, with low cost, the method of the invention is beneficial for industrial production.
Owner:GUANGZHOU BAIYUNSHAN PHARMA HLDG CO LTD BAIYUNSHAN PHARMA GENERAL FACTORY
Environment-friendly crude oil decalcifying agent
ActiveCN105713646AThe preparation process is green and environment-friendlyEasy to operateRefining with non-metalsHydrocarbon oils treatmentCalcium EDTAOrganic acid
The invention discloses an environment-friendly crude oil decalcifying agent. The decalcifying agent is an uniform transparent mixture with the solid content being 20-40 wt.% formed by mixing water and an organic compound at the room temperature. The organic compound is formed by mixing 30-50 wt.% of a chelating agent, 5-20 wt. / % of organic acid, 5-20 wt.% of a dispersing agent and 10-30 wt.% of a neutralizing agent at the room temperature. The prepared environment-friendly crude oil decalcifying agent is applicable to crude oil with the middle and high calcium content, calcium in crude oil can be effectively removed, and the decalcifying agent contains no phosphate and is environmentally friendly.
Owner:SOUTHEAST UNIV
Beta-hydroxy-alpha-amino acid derivative, and synthesis method and application thereof
ActiveCN104803864ALow costThe synthetic route is simpleCarboxylic acid nitrile preparationMetabolism disorderChemical synthesisSynthesis methods
The invention relates to a beta-hydroxy-alpha-amino acid derivative represented by formula (I), and a chemical synthesis method thereof. The method comprises the following steps: carrying out a one-step reaction on raw materials comprising alpha-aryldiazoester, arylamine and arylaldehyde at room temperature with monovalent metal rhodium as a catalyst and an organic solvent as a solvent, and carrying out column chromatography purification to obtain a product. The preparation method has the advantages of high step economy, strong atom economy, high diastereoselectivity, high yield, mild reaction conditions, and simple and safe operation. The beta-hydroxy-alpha-amino acid novel derivative is an important compound for preparing various bioactive natural products and medicines. The invention also discloses an application of the beta-hydroxy-alpha-amino acid derivative in the inhibition of activity of protein tyrosine phosphatase. The derivative has a wide application prospect in the field of pharmaceutical and chemical engineering.
Owner:广东和博制药有限公司
Thiazole derivative, and synthesis method and application thereof
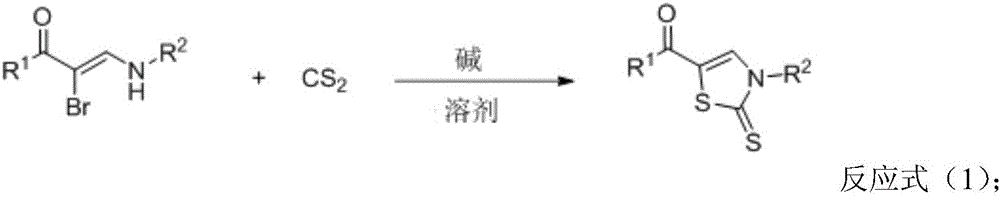
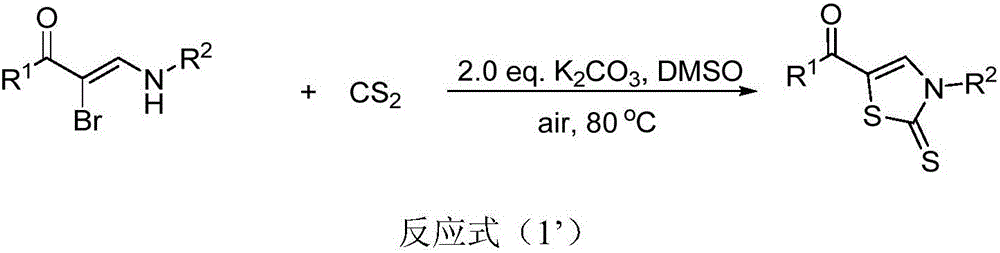
InactiveCN105859651AImprove universalityImprove responseOrganic chemistryAntiinfectivesSynthesis methodsAntibacterial activity
The invention discloses a thiazole derivative represented by formula (I), and a synthesis method thereof. The thiazole derivative represented by formula (I) is synthesized through a reaction of an alpha-bromoenaminone compound and carbon disulfide used as raw materials in a solvent under the action of an alkali. Like compounds have good bioactivities, such as antibacterial activity and anti-breast cancer activity. The synthesis method has the advantages of simple and easily available raw materials, good universality, simple post-treatment, good yield and environmental protection. The invention also discloses an application of the thiazole derivative represented by formula (I) in the field of medicines.
Owner:EAST CHINA NORMAL UNIV
Reaction method for selectively synthesizing aromatic aldehyde or aromatic carboxylic acid
ActiveCN111960936ATo achieve selective regulationAtom economyOrganic compound preparationCarbonyl compound preparationRecyclable catalystPtru catalyst
The invention provides a reaction method for selectively synthesizing aromatic aldehyde or aromatic carboxylic acid. Toluene aromatic hydrocarbon without substituent or with substituent on a benzene ring is used as a raw material, an inorganic salt of ferric iron is used as a catalyst, air or oxygen is used as an oxidizing agent, a mixed solution of acetonitrile and water is used as a solvent, theraw material is oxidized by adjusting the dosage of the catalyst to obtain aromatic aldehyde or aromatic carboxylic acid, and the aromatic aldehyde or aromatic carboxylic acid is irradiated by ultraviolet light for 10-16 hours. Aromatic carboxylic acid obtained under the condition that the dosage of the catalyst is 5-50% mol of aromatic hydrocarbon is used as a main product, wherein the use amount of the catalyst is 70-200% mol of aromatic hydrocarbon. The reaction method provided by the invention has the characteristics of atom economy and high selectivity, uses the metal iron salt with richearth content for catalysis, and has the advantages of mild conditions, recyclable catalyst and solvent and the like.
Owner:NANJING UNIV OF TECH
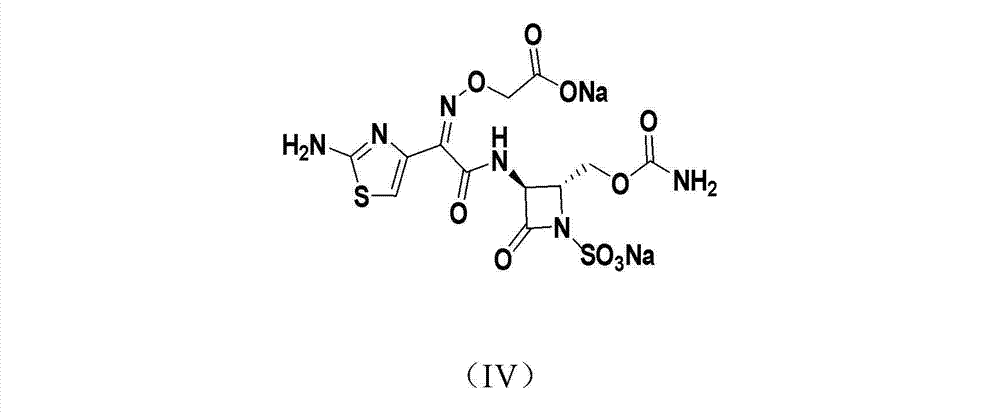
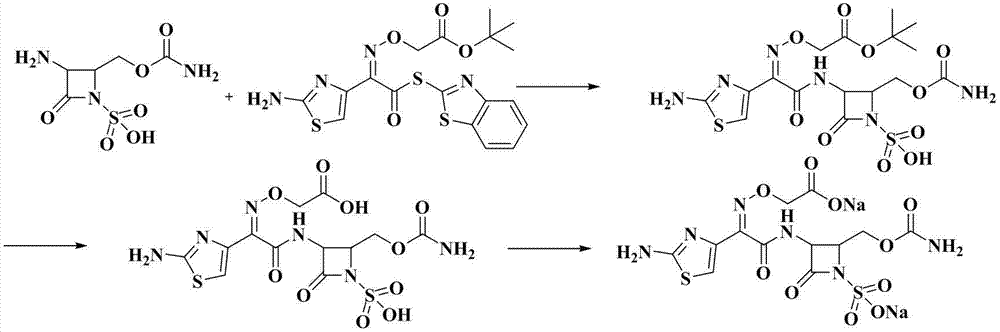
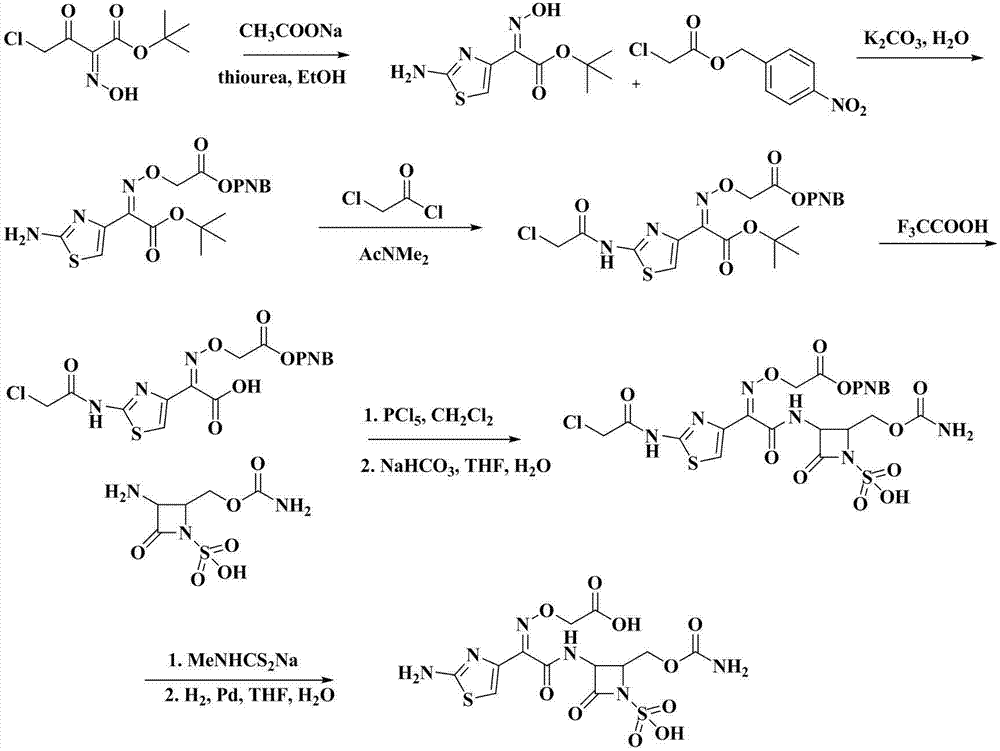
Synthetic method of Carumonam sodium
The invention provides a synthetic method of Carumonam sodium, which can be specifically expressed as condensing 2-[[(Z)-1-(2-amino-4-thiazolyl)-2-chlorine-2-oxygen generation ethylidene]amino]oxygen-2-methyl acrylic acid hydrochloride and (3S-anti-form)-3-amino-2-(carbamoyl group oxygen methyl)-4-nitrogen heterocyclic oxygen generation butane sulfonic acid to obtain the Carumonam sodium directly, and further reacting the Carumonam sodium with inorganic base to obtain sodium salt. Compared with a traditional technique, the synthetic method has the following characteristics: (1), the damage of strong acid to beta-lactam ring in the traditional technique is avoided from the source by adopting chloride method; (2), the residual of 2-mercapto benzothiazole (M) in the product is eliminated from the source; and (3), the product yield of the Carumonam sodium is high, and the purity is more than 99 percent, so that the synthetic method has an important industrial application prospect.
Owner:ZHEJIANG HUAFANG PHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com