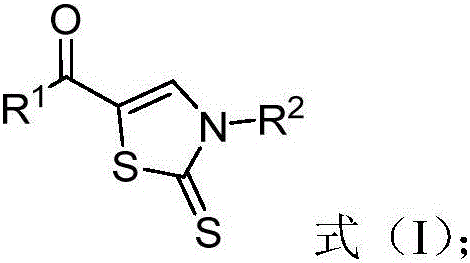
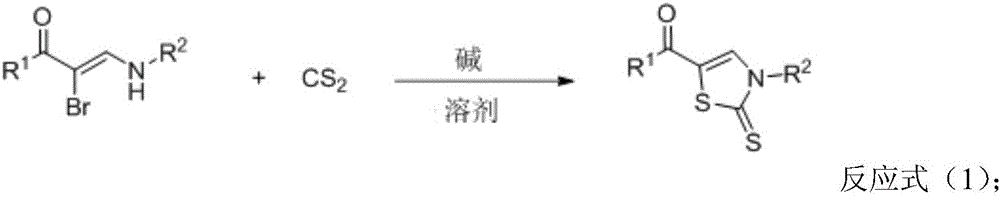
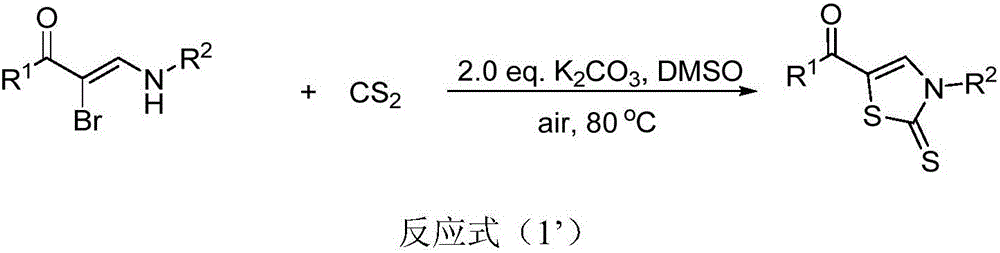
Thiazole derivative, and synthesis method and application thereof
A synthesis method and technology of derivatives, applied in the fields of drug combination, organic chemistry, antitumor drugs, etc., can solve the problems of low yield, dangerous reagents, high temperature, etc., and achieve the effects of good yield, environmental friendliness, and simple reaction process.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Example 1: Synthesis of 3-benzyl-2-thio-2,3-dihydro-thiazole-5-benzophenone
[0044]
[0045] α-Bromoamine enone compound, base and solvent are respectively selected from (Z)-3-(benzylamino)-2-bromo-1-phenyl-2-en-1-one, K 2 CO 3 , dimethyl sulfoxide (DMSO), the amount of raw material is (Z)-3-(benzylamino)-2-bromo-1-phenyl-2-en-1-one 0.3mmol, 2.0eq.K 2 CO 3 , solvent 3mL, and reacted at 80° C. for 1.5 hours to obtain the target product formula (IA), brown liquid, and the separation yield was 76%.
[0046] NMR data: 1 H NMR (400MHz, CDCl 3 , TMS) δ5.38(s,2H),7.30-7.39(m,5H),7.43-7.47(m,2H),7.52(s,1H),7.56-7.58(m,1H),7.68(d, J = 7.2Hz, 2H); 13 CNMR (100MHz, CDCl 3 , TMS): δ52.93, 126.76, 128.33, 128.63, 128.99, 129.03, 129.41, 133.25, 134.52, 136.82, 138.02, 184.46, 191.04; HRMS (EI) calcd for C 17 h 13 ONS 2 m + :311.0439,found 311.0443.
[0047] High-resolution mass spectrometry data: HRMS(EI)calcd for C 17 h 13 ONS 2 m + :311.0439,found 311.0443.
...
Embodiment 2
[0052] Example 2: Synthesis of 3-benzyl-2-thio-2,3-dihydro-thiazole-5-(4-bromophenyl)-methanone
[0053]
[0054]
[0055] α-Bromoamine enone compound, base, and solvent are respectively selected from (Z)-3-(benzylamino)-2-bromo-1-(4-bromophenyl)-2-en-1-one, K 2 CO 3 , dimethylsulfoxide (DMSO), the amount of raw material is (Z)-3-(benzylamino)-2-bromo-1-(4-bromophenyl)-2-en-1-one 0.3mmol, 2.0 equivalent K 2 CO 3 , solvent 3mL, and reacted at 80° C. for 2.5 hours to obtain the target product formula (IB), a brown liquid, and the separation yield was 62%.
[0056] NMR data: 1 H NMR (400MHz, CDCl 3 , TMS) δ5.38(s, 2H), 7.32-7.41(m, 5H), 7.48(s, 1H), 7.55(d, J=8.8Hz, 2H), 7.60(d, J=8.4Hz, 2H ); 13 CNMR (100MHz, CDCl 3 , TMS): δ53.06, 126.30, 128.38, 128.47, 129.19, 129.57, 130.16, 132.44, 134.51, 135.65, 137.97, 183.34, 191.11;
[0057] High-resolution mass spectrometry data: HRMS(EI)calcd for C 17 h 12 Brons 2 m + :388.9544, found 388.9547.
Embodiment 3
[0058] Example 3: Synthesis of 3-benzyl-2-thio-2,3-dihydro-thiazole-5-(4-chlorophenyl)-methanone
[0059]
[0060] α-Bromoamine ketene compound, base and solvent are respectively selected from (Z)-3-(benzylamino)-2-bromo-1-(4-chlorophenyl)-2-en-1-one, K 2 CO 3 , dimethyl sulfoxide (DMSO), the amount of raw material is (Z)-3-(benzylamino)-2-bromo-1-(4-chlorophenyl)-2-en-1-one 0.3mmol, 2.0 equivalent K 2 CO 3 , solvent 3mL, reacted at 80°C for 3.5 hours to obtain the target product formula (IC), a brown liquid, and the separation yield was 69%;
[0061] NMR data: 1 H NMR (400MHz, CDCl 3 , TMS) δ5.38(s, 2H), 7.31-7.44(m, 7H), 7.51(s, 1H), 7.63(d, J=8.4Hz, 2H); 13 CNMR (100MHz, CDCl 3 ,TMS): δ53.00, 126.31, 128.41, 129.11, 129.40, 129.50, 130.04, 134.47, 135.13, 137.99, 139.78, 183.14, 191.00;
[0062] High-resolution mass spectrometry data: HRMS(EI)calcd for C 17 h 12 ClONS 2 m + :345.0049, found 345.0045.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com