Patents
Literature
450 results about "Thiazole derivative" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Thiazole, or 1,3-thiazole, is a heterocyclic compound that contains both sulfur and nitrogen; the term 'thiazole' also refers to a large family of derivatives. Thiazole itself is a pale yellow liquid with a pyridine-like odor and the molecular formula C 3H 3NS.
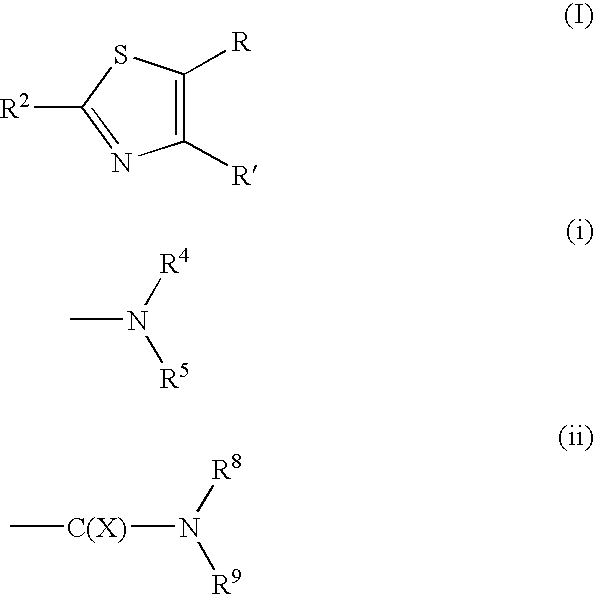
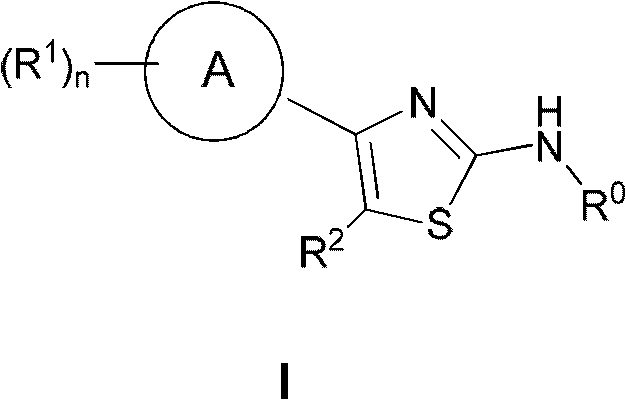
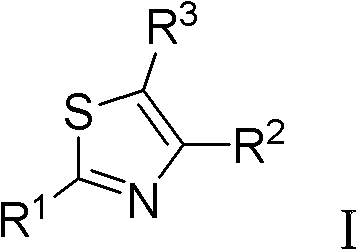
Thiazole derivative and pharmaceutical use thereof
InactiveUS20050004134A1High reactivityImprove solubilityNervous disorderOrganic chemistryArylHydrogen
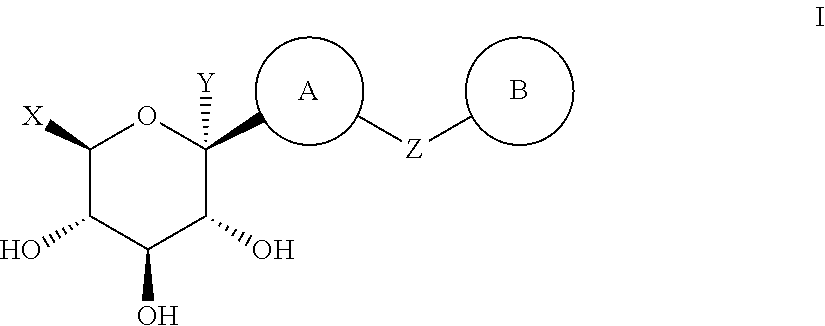
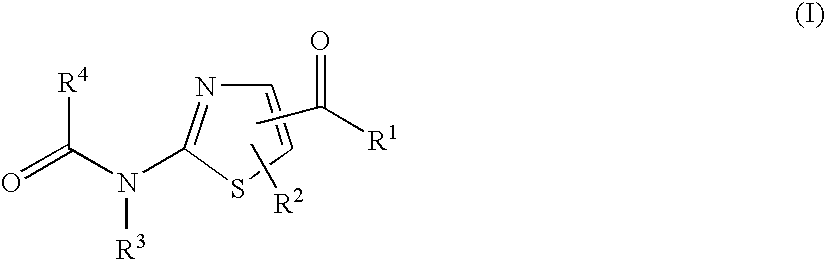
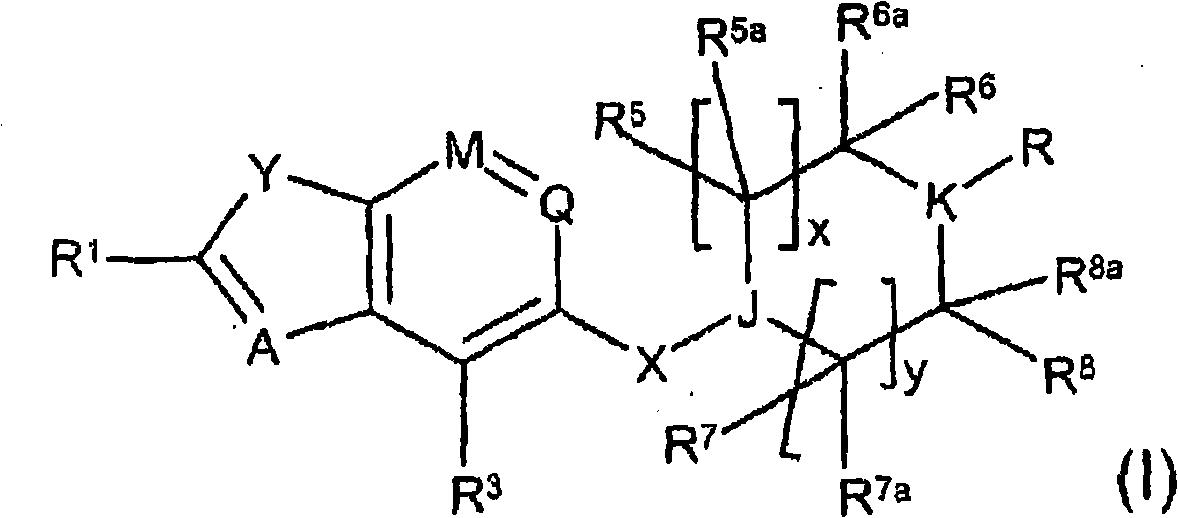
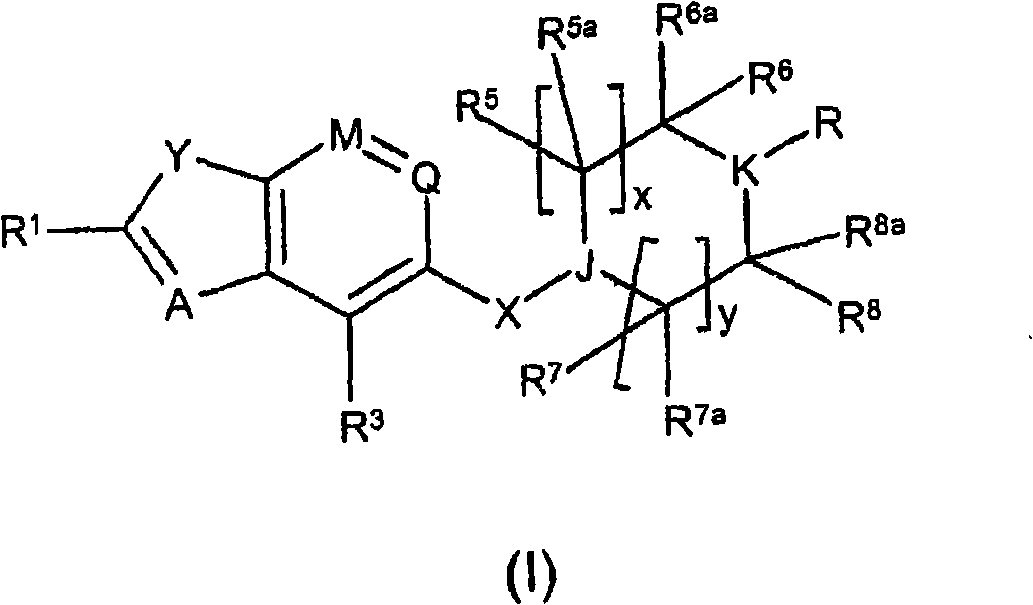
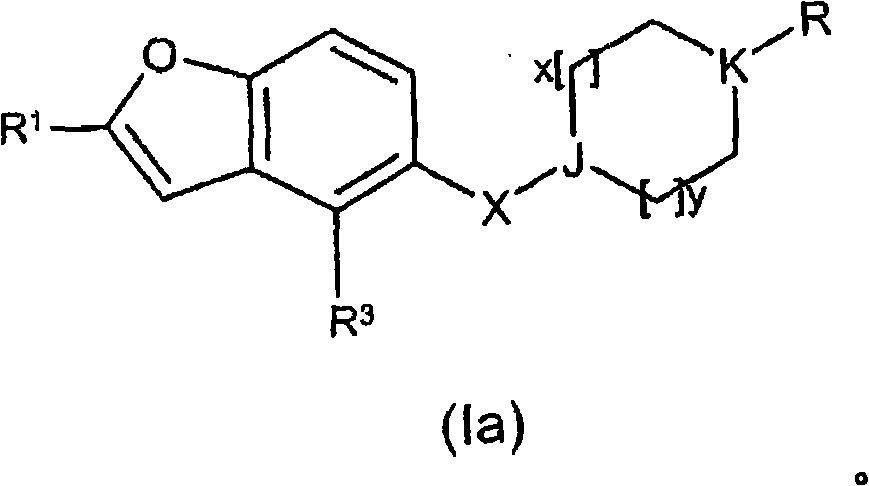
A thiazole derivative of the formula (I): wherein R is a 1-optionally substituted-6-oxo-1,6-dihydro-3-pyridazinyl, R′ is an optionally substituted phenyl, and R2 is hydrogen, a group of the formula (i): wherein R4 is hydrogen, lower alkyl or lower alkenyl, and R5 is hydrogen, optionally substituted lower alkyl, acyl, cyclo(lower)alkyl, lower alkenyl, optionally substituted aryl or heterocyclic, or a group of the formula (ii): wherein X is oxygen or sulfur, R8 is hydrogen or lower alkyl, R9 is hydrogen, optionally substituted lower alkyl, cyclo(lower)alkyl, lower alkoxy or mono- or di-lower alkylamino or R8 and R9 may combine together to form optionally substituted saturated N-containing heterocyclic, or a salt thereof.
Owner:ASTELLAS PHARMA INC
Indole derivative having piperidine ring
InactiveUS20050256103A1High affinityEnhanced inhibitory effectBiocideNervous disorderHydrogen atomSerotonin 1A Receptor
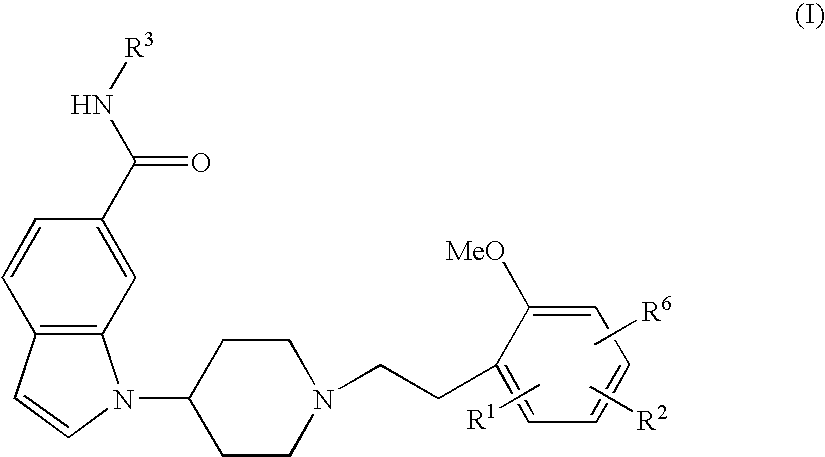
The present invention relates to a compound represented by the following formula, a pharmacologically acceptable salt thereof, or a use thereof as a pharmaceutical: wherein R1 and R2 are substituents adjacent to each other, and together with two carbon atoms to each of which they attach, form a 5- to 7-membered non-aromatic carbocyclic group or the like, which may be substituted by 1 to 4 substituents selected from (1) an oxo group, (2) a hydroxyl group, and the like; R3 represents a hydrogen atom or the like; and R6 represents a hydrogen atom or the like. It is an object of the present invention to discover an agent for treating or preventing lower urinary tract symptoms, and particularly symptoms regarding urinary storage, which has a superior strength of binding to a 5-HT1A receptor and an antagonism to the receptor.
Owner:EISIA R&D MANAGEMENT CO LTD
Composition and use
The present invention relates to a composition comprising: (i) an anti-microbial agent comprising a polymeric biguanide, alone or in combination with at least one other microbiologically active component selected from the group consisting of quaternary ammonium compounds, monoquaternary heterocyclic amine salts, urea derivatives, amino compounds, imidazole derivatives, nitrile compounds, tin compounds or complexes, isothiazolin-3-ones, thiazole derivatives, nitro compounds, iodine compounds, aldehyde release agents, thiones, triazine derivatives, oxazolidine and derivatives thereof, furan and derivatives thereof, carboxylic acids and the salts and esters thereof, phenol and derivatives thereof, sulphone derivatives, imides, thioamides, 2-mercapto-pyridine-N-oxide, azole fungicides, strobilurins, amides, carbamates, pyridine derivatives, compounds with active halogen groups, and organometallic compounds; and (ii) an amphoteric co-polymer of the Formula (1): wherein:[A] is of Formula (9), [B] is of Formula (10), [C] is of Formula (12), [D] is of Formula (13), and X is of Formula (11), wherein [A], [B], [C] and [D] may occur in any order; T is an optionally substituted substituent; L, G and Z each independently is an optionally substituted linking group; R1, R2 and R3 are each independently H, optionally substituted C1-20-alkyl or optionally substituted C3-20-cycloalkyl; R4 and R5 are each independently H or C1-4-alkyl; q is 15 to 1000; p is 3 to 50; J is an optionally substituted hydrocarbyl group; F is an acidic substituent; E is a basic substituent; m is 0 to 350; n is 1 to 75; v is 0 to 100; y is 1 to 100; b is 0, 1 or 2; s is 0 or 1; w is 1 to 4; and provided that at least one of R4 and R5 is H.
Owner:ARCH UK BIOCIDES LTD
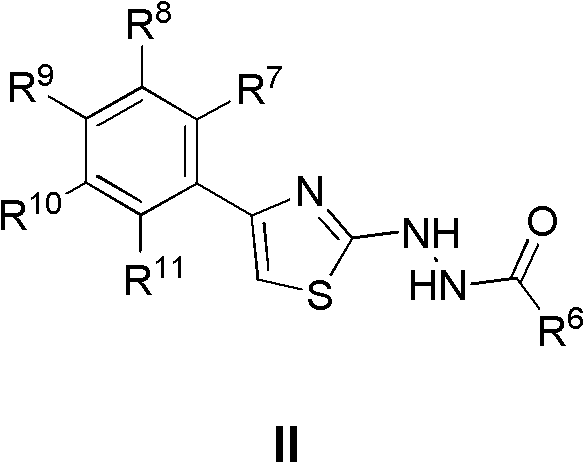
Use of thiazole derivatives and analogues in the treatment of cancer
There is provided a use of a compound of formula (I), wherein X, Y, T, W, A1, A2 R1, R5 and R6 have meanings given in the description for the manufacture of a medicament for the treatment of cancer.
Owner:BETAGENON AB
Polishing solution based on metal Co for polishing process
InactiveCN102304327AOther chemical processesSemiconductor/solid-state device manufacturingCopperCobalt
The invention belongs to the technical field of microelectronic process, in particular to a polishing solution based on metal Co for a polishing process. The polishing agent comprises 0-5% of oxidizing agent, 0.1-25% of grinding particles, 0.001-10% of chelating agent, 0.01-10% of inhibitor and the balance of water according to proportions. The inhibitor is 2-mercaptothiazoline, 2-mercaptobenzothiaozole or other thiazole derivatives or a mixture of several of the 2-mercaptothiazoline, 2-mercaptobenzothiaozole or other thiazole derivatives. The range of the pH value of the polishing solution is 3-5. The inhibitor used for the polishing liquid of the invention can effectively inhibit the static corrosion of copper and cobalt and reduce the polishing rate of copper and cobalt, thereby effectively reducing the defects generated after polishing.
Owner:FUDAN UNIV
Thiazole derivative
InactiveUS6140330AEasily be converted into saltEasy to convertBiocideOrganic chemistryThreoninePhosphoprotein phosphatase
PCT No. PCT / JP97 / 02609 Sec. 371 Date Mar. 24, 1998 Sec. 102(e) Date Mar. 24, 1998 PCT Filed Jul. 29, 1997 PCT Pub. No. WO98 / 04536 PCT Pub. Date Feb. 5, 1998A thiazole compound of the formula: wherein T is lower alkylene; u is 0 or 1; R1 and R2 are the same or different and are each H, or lower alkyl, etc.; R3 is R4 is H or lower alkanoyloxy-lower alkyl, which shows inhibitory activity on protein kinase C (PKC, Ca2+ / phospholipid-depending serine / threonine protein phosphatase), and are useful as a protein kinase C inhibitor.
Owner:OTSUKA PHARM CO LTD
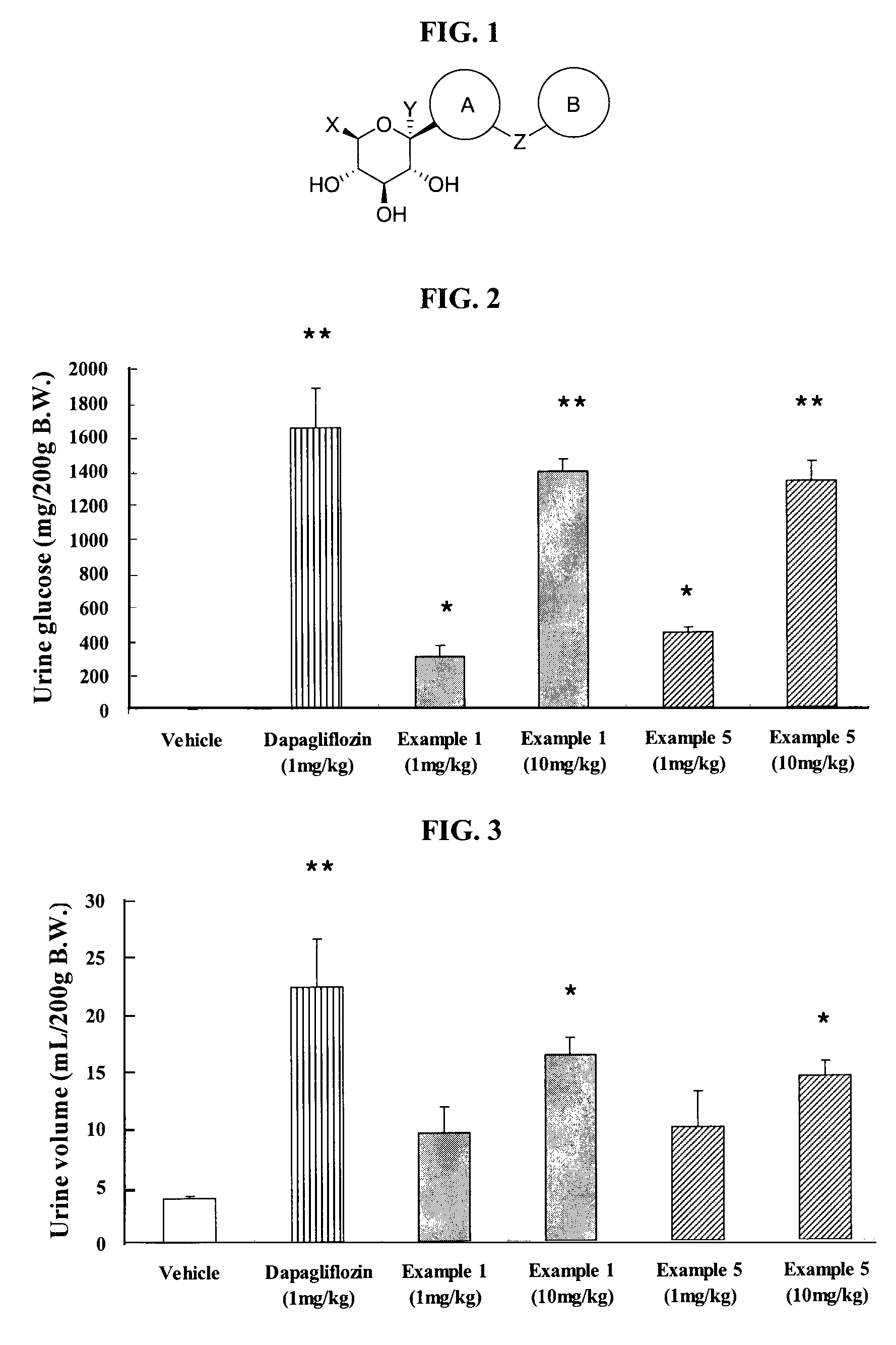
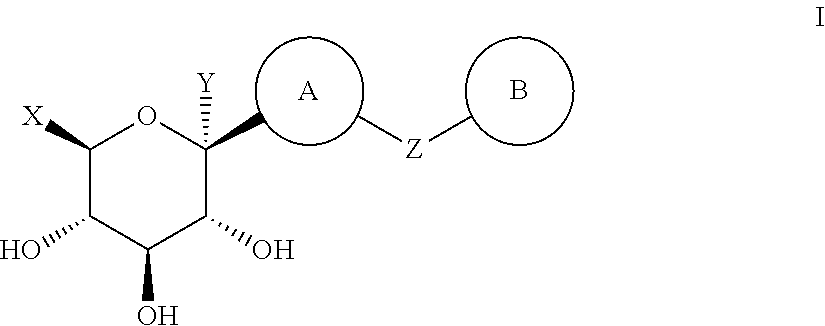
Thiazole derivatives as SGLT2 inhibitors and pharmaceutical composition comprising same
ActiveUS8586550B2Prevention and/or treatment of metabolic disordersReduce usageBiocideSaccharide with heterocyclic radicalsSodium dependentSGLT2 Inhibitor
The present invention relates to a novel compound with thiazole ring having an inhibitory activity against sodium-dependent glucose cotransporter 2 (SGLT2) being present in the intestine and kidney, and a pharmaceutical composition comprising the same as an active ingredient, which is useful for preventing or treating metabolic disorders, particularly diabetes.
Owner:THE GREEN CROSS CORP
Applications of 2,4-disubstituted thiazoles derivatives being taken as DHODH (dihydroorotate dehydrogenase) inhibitor
The invention relates to applications of a 2,4-disubstituted thiazoles compound in pharmaceutical chemistry and pharmacotherapy. Specifically, the invention relates to applications of a compound shown in formula I (described in the specification) and a medicinal composition of the compound in preparation of medicines used for treating diseases related to DHODH (dihydroorotate dehydrogenase).
Owner:EAST CHINA UNIV OF SCI & TECH
Thiazole derivative
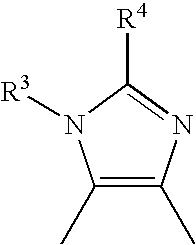
InactiveUS20070154428A1Inhibit functioningInhibit phosphorylationCosmetic preparationsBiocideHydrogen atomStimulant
A thiazolylimidazole derivative represented by the formula or a pharmaceutically acceptable salt thereof, and an ALK5 inhibitor, an therapeutic agent for alopecia or a hair growth agent having the above as an active ingredient, wherein: X1 and X2 are different from each other and represent a sulfur atom or a carbon atom; R1 represents a phenyl group; a substituted phenyl group; a phenyl group condensed with a hetero aromatic ring; a pyridyl group; or a pyridyl group condensed with a hetero aromatic ring; R2 represents a hydrogen atom, a halogen atom, an alkyl group having 1 to 6 carbon atoms, an alkyl group having 1 to 6 carbon atoms substituted with 1 to 5 halogen atoms, an alkoxy group having 1 to 6 carbon atoms, an alkanoyl group having 1 to 5 carbon atoms, or a hydroxyalkyl group having 1 to 6 carbon atoms, A represents a group which is represented by the formula. The present invention provides an inhibitory substance against ALK5 which is a TGF-β type I receptor and provides a hair growth stimulant or a hair growth agent based on its novel activities.
Owner:TAISHO PHARMACEUTICAL CO LTD
3-hydroxy-5-arylisothiazole derivative
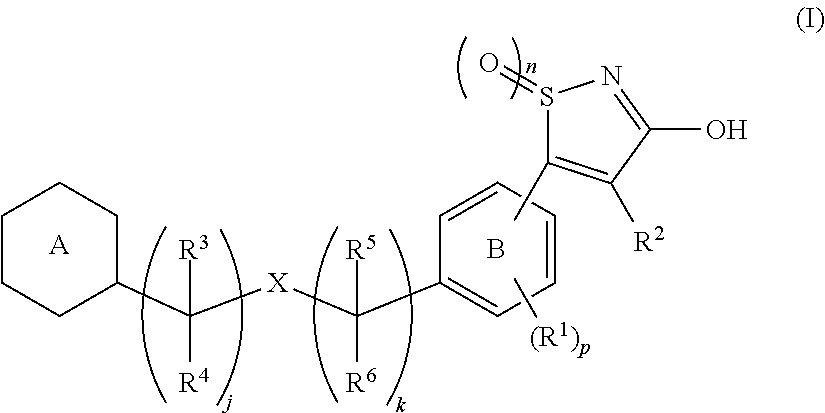
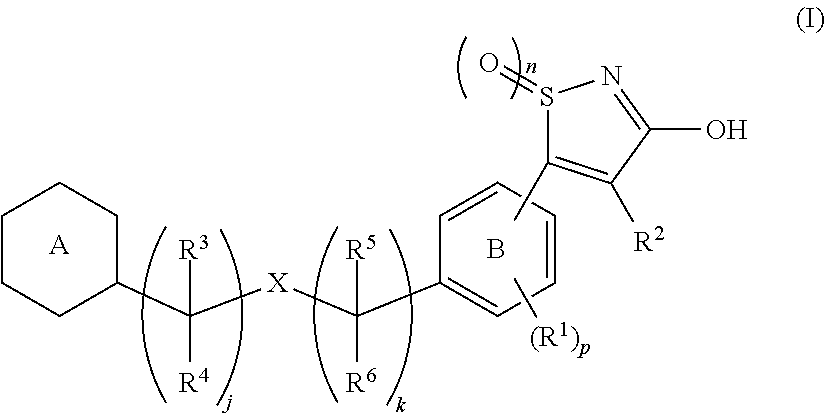
[Problem]To provide a GPR40 activating agent having, as an active ingredient, a novel compound having a GPR40 agonist action, a salt of the compound, a solvate of the salt or the compound, or the like, particularly, an insulin secretagogue and a prophylactic and / or therapeutic agent against diabetes, obesity, or other diseases.[Means of solving the problem]A compound of Formula (I):(where n is 0 to 2; p is 0 to 4; j is 0 to 3; k is 0 to 2; a ring A is an aryl group which is optionally substituted with L or a heterocyclic group which is optionally substituted with L; a ring B is a benzene ring, a pyridine ring, or a pyrimidine ring; X is O, S, —NR7—; and R1 to R7 are specific groups),a salt of the compound, or a solvate of the salt or the compound.
Owner:MOCHIDA PHARM CO LTD
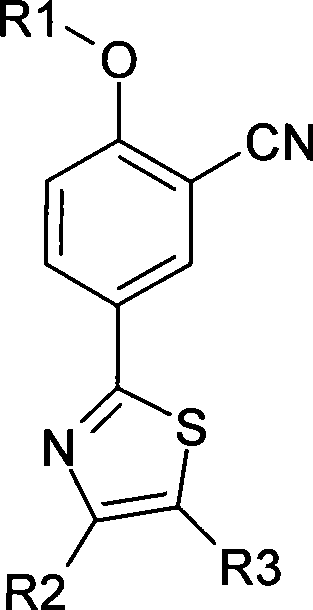
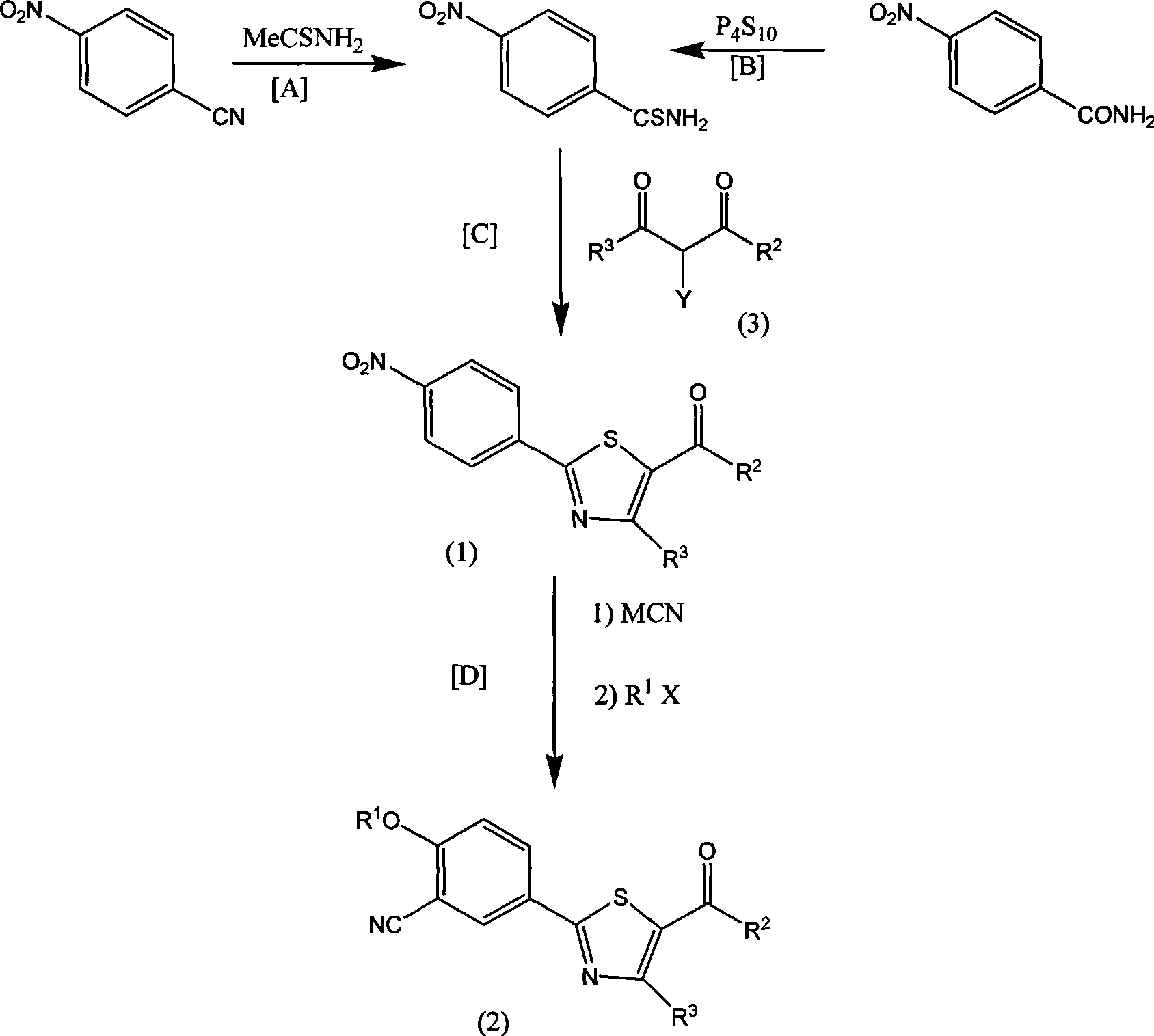
Aromatic nitrile-base thiazole derivatives for inhibiting xanthine oxidase activity, preparation method and application
ActiveCN101386604AEasy to operateHigh yieldOrganic active ingredientsOrganic chemistryCyclobutaneLithium formate
The invention disclosed an aryl nitrile group thiazole derivative for inhibiting the activity of xanthine oxidase, a method for preparing the same and application thereof. In the aryl nitrile group thiazole, R1 is methyl, ethyl, propyl, isopropyl, isobutyl, methyl cyclopropane, methyl cyclobutane, isoamyl, methyl cyclopentane, methyl cyclohexane or aromatic ring methyl, R2 is methyl or trifluoromethyl, and R3 is formic acid, sodium formate, potassium formate, lithium formate, methyl formate, or ethyl formate. Simultaneously, the invention discloses a method for synthesizing the aryl nitrile group thiazole derivative by using inexpensive raw materials, which has the advantages of simple operation, high yield, easy purification of products, application to industrial production and the like, and can obtain an efficient compound with low toxicity through screening; besides, the effective compound is expected to be widely applied to inhibit the activity of the xanthine oxidase required on animals and humans, and to become a new generation of antigout drugs and hyperuricemia drugs with special effect.
Owner:HANGZHOU ADAMERCK PHARMLABS INC
Thiazole derivatives
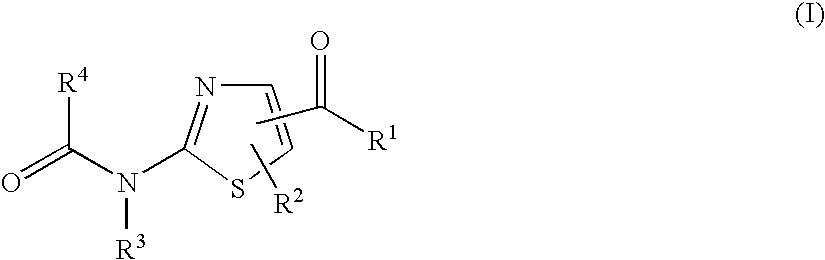
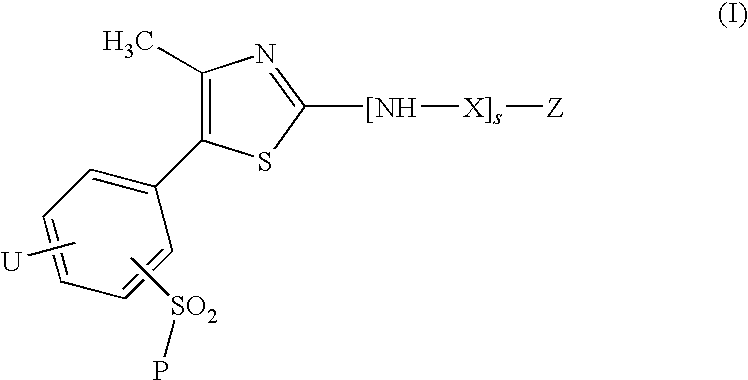
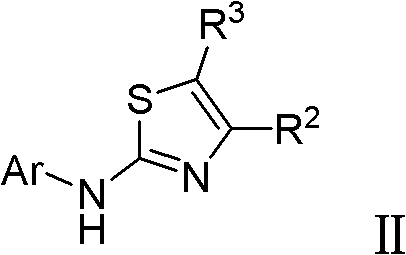
A compound of the formula (I): R<1>-NH-X-Y-Z (I) wherein each symbol is as defined in the specification, or a pharmaceutically acceptable salt thereof useful as a vascular adhesion protein-1 (VAP-1) inhibitor, a pharmaceutical composition, a method for preventing or treating a VAP-1 associated disease, especially macular edema, which method includes administering an effective amount of the compound or a pharmaceutically acceptable salt thereof to a mammal, and the like.
Owner:R TECH UENO
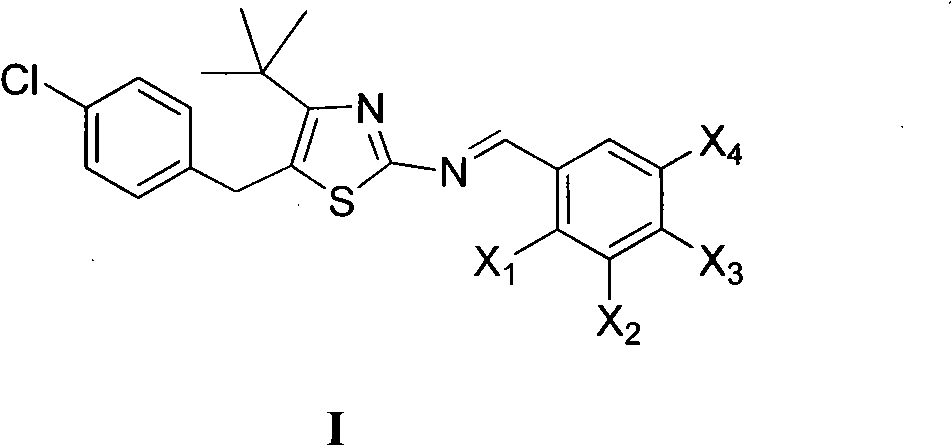
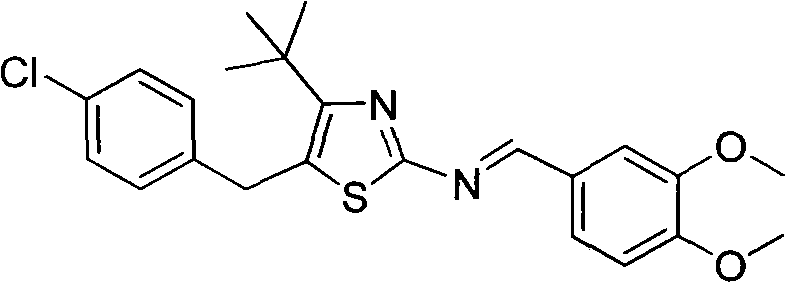
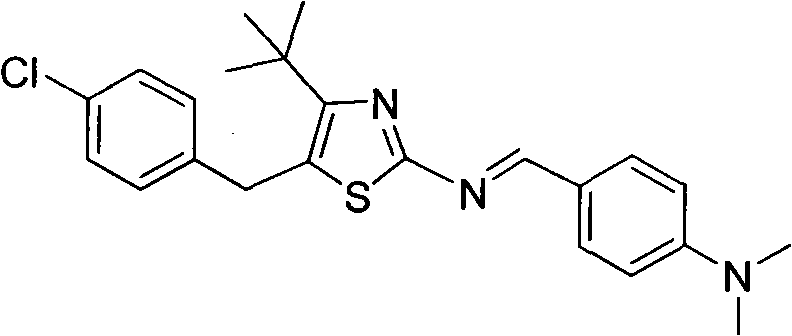
5-(4-chlorophenylmethyl)-4-tertiary butyl thiazole derivatives and preparation method and application thereof
InactiveCN101845026AHas antitumor activityOrganic active ingredientsOrganic chemistryHydrogenBromine
The invention discloses 5-(4-chlorophenylmethyl)-4-tertiary butyl thiazole derivatives (I), which has the following structural formula as shown in the specification of the invention, wherein X1 in a formula I is selected from hydrogen, hydroxyl group, methoxy group, nitro group, amino group and chlorine; X2 is selected from hydrogen, nitro group, amino group, methoxy group, chlorine, bromine and iodine; X3 is selected from hydrogen, methyl group, ethyl group, nitro group, methoxy group, chlorine, bromine, amino group and dimethylamino group; and X4 is selected from hydrogen, nitro group, amino group, methoxy group, chlorine, bromine and iodine. The 5-(4-chlorophenylmethyl)-4-tertiary butyl thiazole derivatives (I) have favorable inhibition activity on human cervical cancer cells, human liver cancer cells, human nasal and oral cancer cells and the like and can be used for preparing anti-tumor medicines.
Owner:HUNAN UNIV
Thiazole Derivatives as SGLT2 Inhibitors and Pharmaceutical Composition Comprising Same
ActiveUS20130090298A1Reduce usageUseful in treatmentBiocideSaccharide with heterocyclic radicalsIntestinal structureBULK ACTIVE INGREDIENT
The present invention relates to a novel compound with thiazole ring having an inhibitory activity against sodium-dependent glucose cotransporter 2 (SGLT2) being present in the intestine and kidney, and a pharmaceutical composition comprising the same as an active ingredient, which is useful for preventing or treating metabolic disorders, particularly diabetes.
Owner:THE GREEN CROSS CORP
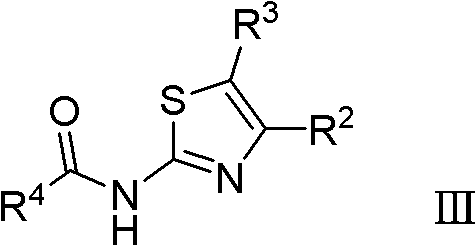
Thiazole derivatives
The present invention relates to compounds of formula (I) wherein R1, R2, R3 and R4 are as defined in the description and claims, and pharmaceutically acceptable salts thereof, for use as therapeutically active substances. The compounds are useful for the treatment and / or prophylaxis of diseases which are associated with the modulation of CB1 receptors, including obesity.
Owner:F HOFFMANN LA ROCHE INC
Thiazole derivatives as inhibitors of p13 kinase
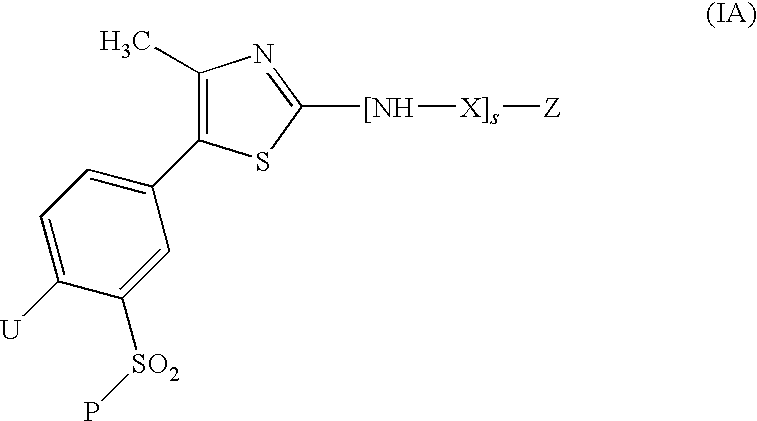
Compounds of formula (I) are inhibitors of P13 kinase activity, and useful in treatment of, inter alia, autoimmune, inflammatory and proliferative diseases: wherein: s is 0 or 1; U is hydrogen or halogen; X is —(C═O), an optionally substituted divalent phenylene, pyridinylene, pyrimidinylene, or pyrazinylene radical, or a bond; P is optionally substituted C1-C6 alkyl and Z is —(CH2)Z—X1-L1-NHCHR1R2; or Z is optionally substituted C1-C6 alkyl and P is —(CH2)Z—X1-L1-NHCHR1R2; R1 is a carboxylic acid group (—COOH), or an ester group which is hydrolysable by one or more intracellular carboxylesterase enzymes to a carboxylic acid group; R2 is the side chain of a natural or non-natural alpha amino acid; X1 is (i) a bond; —NR4C(═O)NR5— or —NR4S(═O)2—; or except when X is —(C═O)— (ii) —C(═O)—, —S(═O)2—, or —S(═O)2NR4— wherein R4 and R5 are independently hydrogen or optionally substituted C1-C6 alkyl; and z and L1 are as defined in the specification.
Owner:CHROMA THERAPEUTICS
Thiazole derivatives
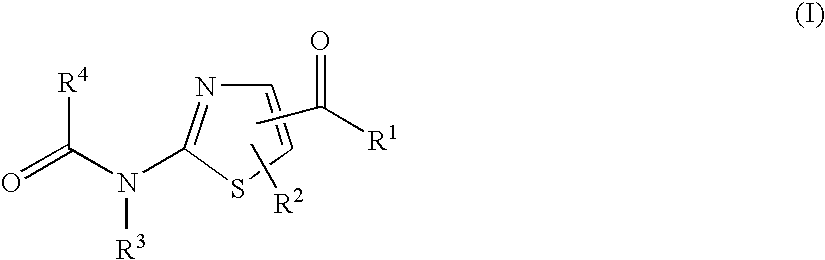
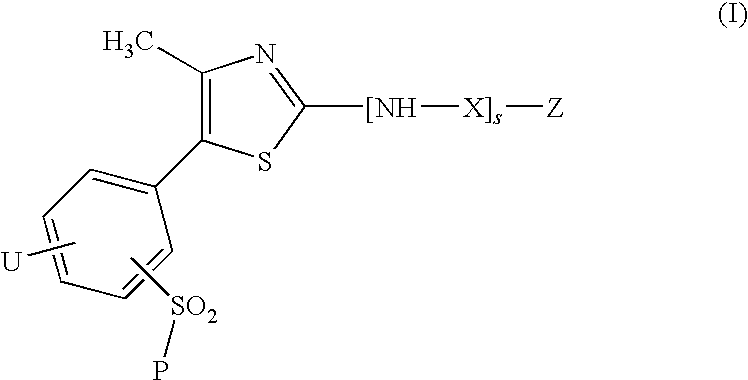
A compound of the formula (I): R1—NH—X—Y-Z (I) wherein each symbol is as defined in the specification, or a pharmaceutically acceptable salt thereof useful as a vascular adhesion protein-1 (VAP-1) inhibitor, a pharmaceutical composition, a method for preventing or treating a VAP-1 associated disease, especially macular edema, which method includes administering an effective amount of the compound or a pharmaceutically acceptable salt thereof to a mammal, and the like.
Owner:ASTELLAS PHARMA INC
Benzofurane, benzothiophene, benzothiazol derivatives as fxr modulators
The present invention relates to compounds of formula (I) wherein the substituents are as defined in the claims, including pharmaceutical compositions thereof and for their use in the treatment and / or prevention and / or amelioration of one or more symptoms of disease or disorders related to the activity of FXR. The invention is also directed to intermediates and to a method of preparation of compounds of formula (I).
Owner:MERCK PATENT GMBH
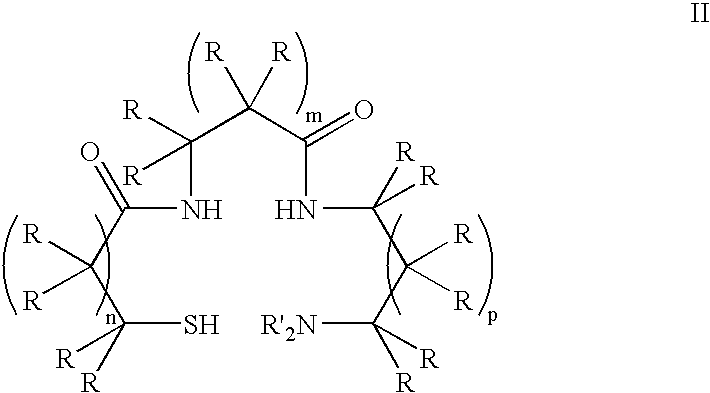
Monoamine, diamide, thiol-containing metal chelating agents
InactiveUS7238340B1Effective therapeutic amountOrganic chemistryOther chemical processesDiseaseMetallole
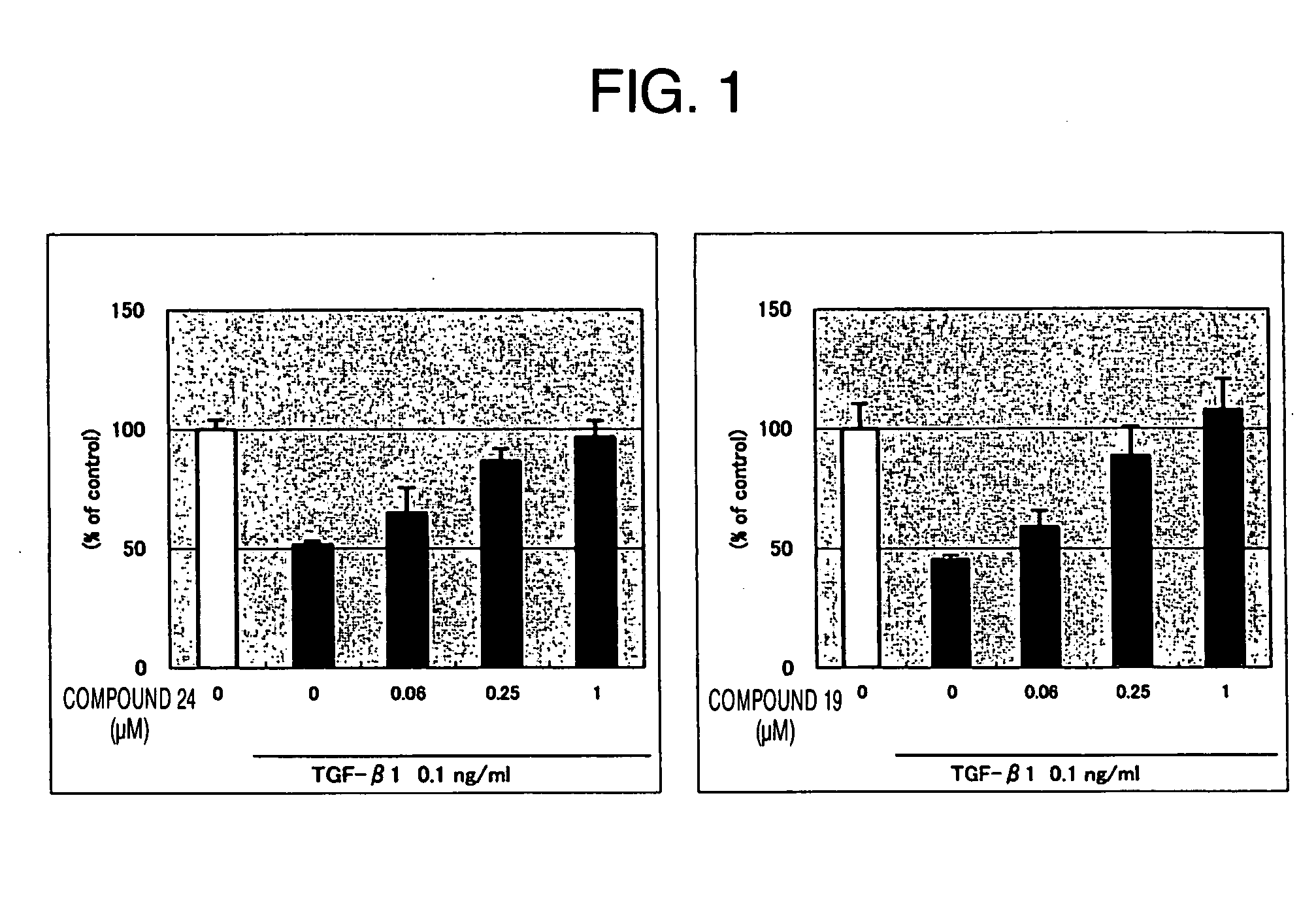
Therapeutically active thiazole derivatives of formula (I) wherein R1-R2, X and X′ as are defined in the specification, processes for the preparation thereof, the use thereof in therapy, particularly in the treatment or prophylaxis of disorders characterised by overexpression of transforming growth factor • (TGF-•), and pharmaceutical compositions for use in suchtherapy.
Owner:CIS BIO INT
Indole Derivative Having Piperidine Ring
InactiveUS20070219179A1Enhanced inhibitory effectIncrease frequencyBiocideNervous disorderHydrogen atomSerotonin 1A Receptor
The present invention relates to a compound represented by the following formula, a pharmacologically acceptable salt thereof, or a use thereof as a pharmaceutical: wherein R1 and R2 are substituents adjacent to each other, and together with two carbon atoms to each of which they attach, form a 5- to 7-membered non-aromatic carbocyclic group or the like, which may be substituted by 1 to 4 substituents selected from (1) an oxo group, (2) a hydroxyl group, and the like; R3 represents a hydrogen atom or the like; and R6 represents a hydrogen atom or the like. This compound has a superior strength of binding to a 5-HT1A receptor and an antagonism to the receptor, and is useful as an agent for treating or preventing lower urinary tract symptoms, and particularly symptoms regarding urinary storage.
Owner:EISIA R&D MANAGEMENT CO LTD
Benzoselenadiazole derivatives with anti-tumor and anti-oxidation activities and their preparation and application
ActiveCN102285944AGood antitumor activityImproves antioxidant activityOrganic chemistryAntinoxious agentsChemical structureDiamine
The invention discloses benzoselenadiazole derivatives with antitumor and antioxidation activities, and a preparation method and application thereof. The chemical structure of the benzoselenadiazole derivatives is disclosed as Formula I, wherein R1 is -H or -NO2, R2 is -H, -NO2 or a group shown in specification, and R1 and R2 can not be H at the same time. The benzoselenadiazole derivatives are synthesized by reacting ortho-diamine compounds with selenium dioxide in a liquid phase or solid phase. The benzoselenadiazole derivatives have favorable antitumor and antioxidation activities, and have the advantages of simple preparation method and high yield. The benzoselenadiazole derivatives disclosed by the invention can be used for preparing antitumor drugs and / or antioxidation drugs. Formula I is shown in the specification.
Owner:GUANGDONG JINAN ESTABLISHED SELENIUM SOURCE NANO TECH RES INST CO LTD
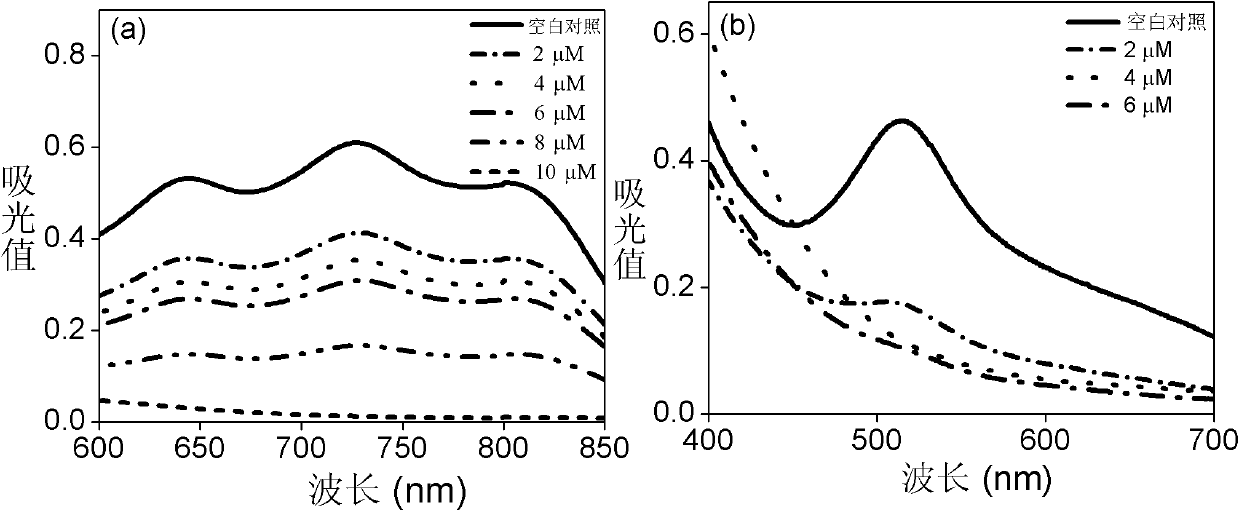
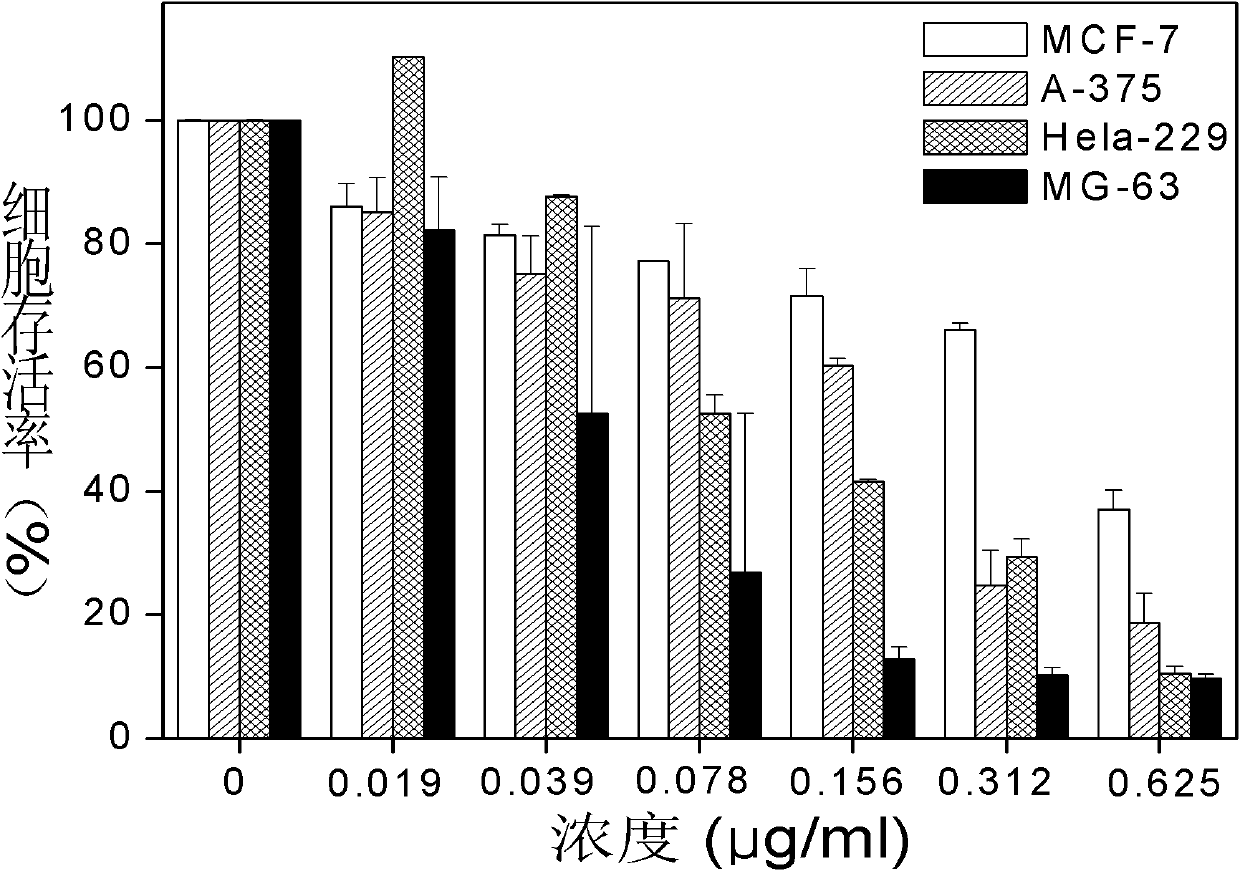
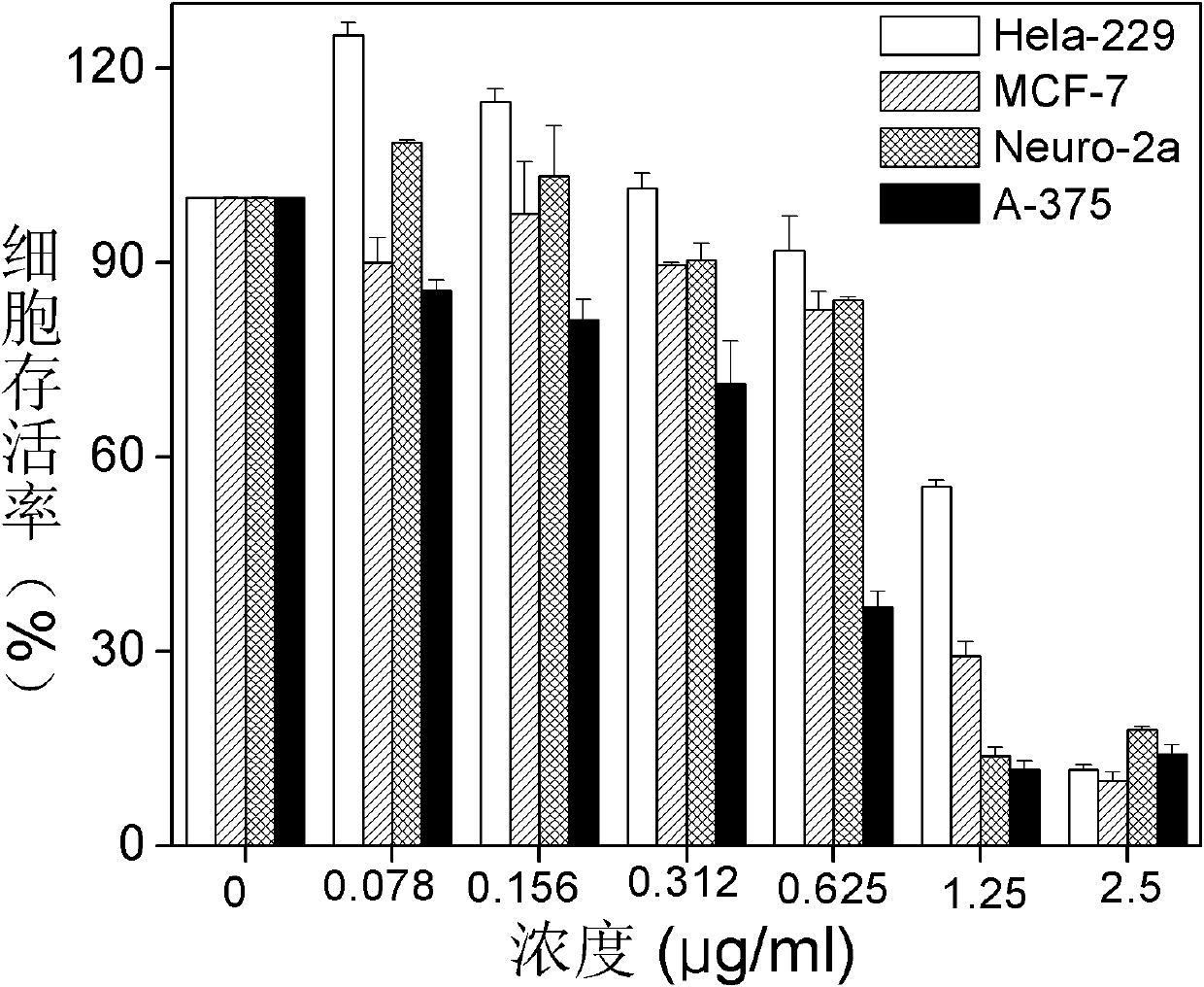
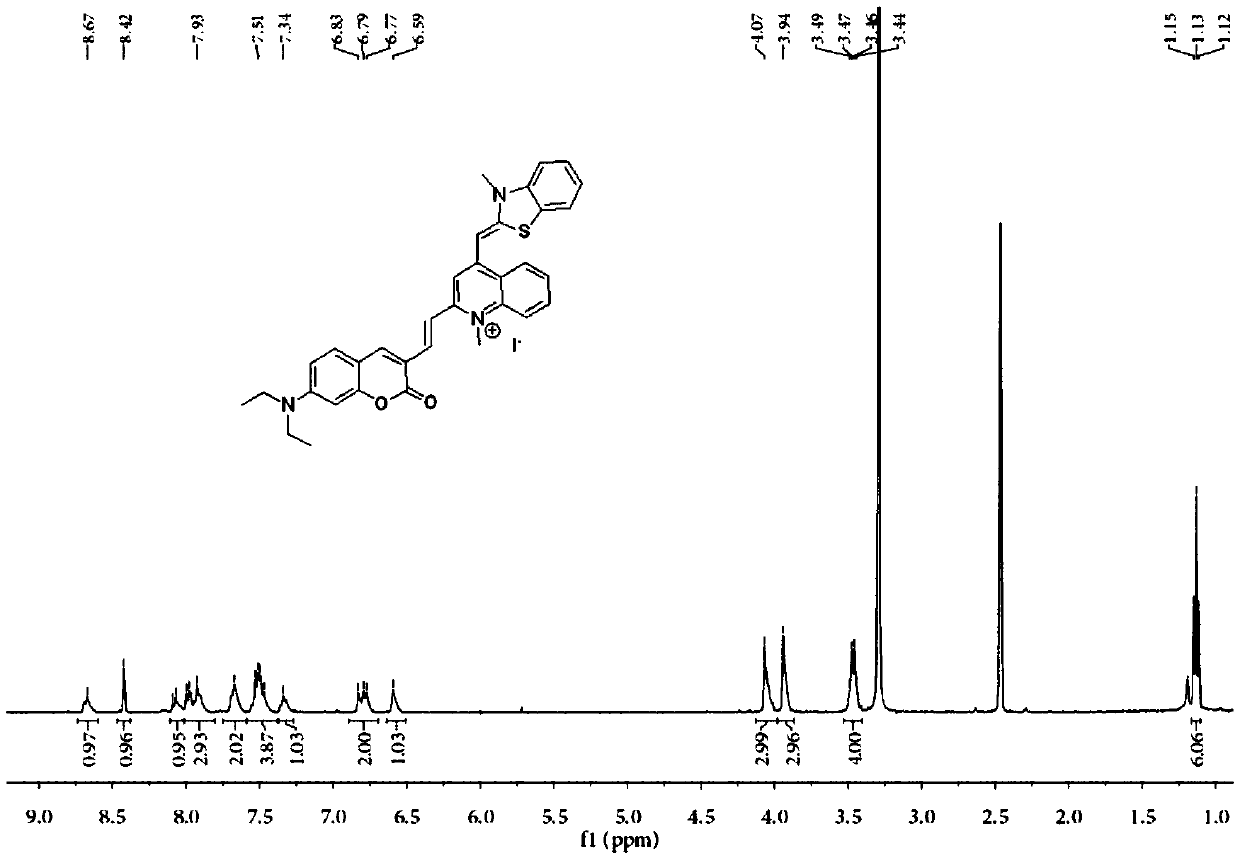
Substituted coumarin-thiazole orange derivative, preparation method therefor and use of substituted coumarin-thiazole orange derivative
ActiveCN105367566AGood selective binding abilityGood choiceOrganic chemistryMicrobiological testing/measurementStructural formulaPerylene derivatives
The invention belongs to the field of biochemistry and particularly relates to a substituted coumarin-thiazole orange derivative, a preparation method therefor and use of the substituted coumarin-thiazole orange derivative. The substituted coumarin-thiazole orange derivative provided by the invention has a structural formula represented by a formula I shown in the description. The invention also provides the preparation method for the substituted coumarin-thiazole orange derivative and use of the substituted coumarin-thiazole orange derivative in fluorescent recognition of G-quadruplex. The substituted coumarin-thiazole orange derivative provided by the invention has the advantages that the toxicity is low, the selectivity is good, the sensitivity is high, raw materials are simply and easily obtained, the whole synthesis route is high in operability, reaction conditions are relatively mild, the overall cost is relatively low, and the like, thereby undoubtedly having better market competitiveness compared with the existing inefficient G-quadruplex fluorescent probes.
Owner:SICHUAN UNIV
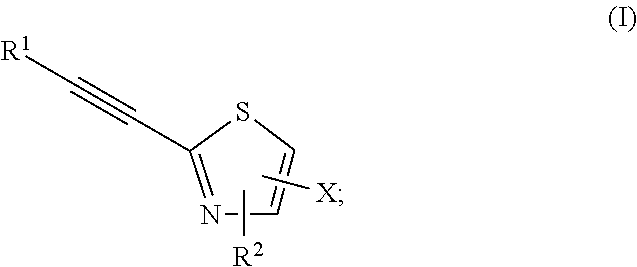
2-substituted-ethynylthiazole derivatives and uses of same
Owner:H LUNDBECK AS
Thiazole derivative acting as DHODH inhibitor and its application
The invention relates to a thiazole derivative which is shown as general formula I and acts as a DHODH (dihydroorotate dehydrogenase) inhibitor, and its application. The compound provided in the invention can be used for treatment or prevention of various DHODH-mediated diseases, including but not limited to rheumatoid arthritis, tumors, organ transplantation rejection, psoriasis and other autoimmune diseases.
Owner:EAST CHINA UNIV OF SCI & TECH
Glaucocalyxin A thiazole derivative, preparation method and application thereof
ActiveCN104356090AImprove bioavailabilityGood water solubilityOrganic active ingredientsOrganic chemistryHuman tumorHydrogen
The invention relates to a glaucocalyxin A (GLA) thiazole derivative. The structure II of the derivative is shown in the description, wherein R is hydrogen, alkyl, alkylene and aromatic hydrocarbon. Based on cytotoxic activity evaluation experiment, the GLA thiazole derivative disclosed by the invention has very strong human tumor cell proliferation inhibitory activity which is better than that of parent compound GLA.
Owner:XINXIANG MEDICAL UNIV
Methods respectively for producing optically active compound having agonistic activity on thrombopoietin receptors and intermediate of said compound
InactiveCN105992761AHigh chemical purityOrganic compound preparationCarboxylic acid esters preparationBiochemistryPerylene derivatives
The present invention relates to: a method for producing an intermediate having high chemical purity and / or high optical purity; a crystal of an intermediate having high chemical purity and / or optical purity; a method for producing an intermediate, in which two reactions can be carried out sequentially substantially in a single step; and a method for producing an optically active 1,3-thiazole derivative having an agonistic activity on thrombopoietin receptors.
Owner:SHIONOGI & CO LTD
Thiazole derivative
A thiazolylimidazole derivative represented by the formula (I) or a pharmaceutically acceptable salt thereof, and an ALK5 inhibitor, an therapeutic agent for alopecia or a hair growth agent having the above as an active ingredient, wherein: X 1 and X 2 are different from each other and represent a sulfur atom or a carbon atom; R 1 represents a phenyl group; a substituted phenyl group; a phenyl group condensed with a hetero aromatic ring; a pyridyl group; or a pyridyl group condensed with a hetero aromatic ring; R 2 represents a hydrogen atom, a halogen atom, an alkyl group having 1 to 6 carbon atoms, an alkyl group having 1 to 6 carbon atoms substituted with 1 to 5 halogen atoms, an alkoxy group having 1 to 6 carbon atoms, an alkanoyl group having 1 to 5 carbon atoms, or a hydroxyalkyl group having 1 to 6 carbon atoms, A represents a group which is represented by the formula (II). The present invention provides an inhibitory substance against ALK5 which is a TGF- type I receptor and provides a hair growth stimulant or a hair growth agent based on its novel activities.
Owner:TAISHO PHARMACEUTICAL CO LTD
Process for the preparation of biologically active tetrahydrobenzthiazole derivative
InactiveUS20070123573A1Shorten the setting timeOrganic active ingredientsBiocideCyclohexanoneThiourea
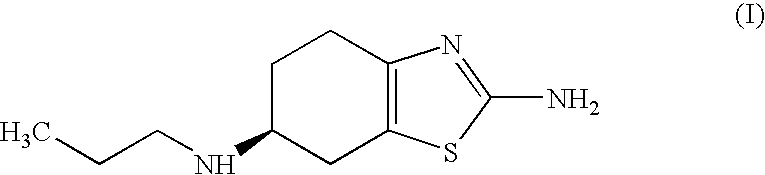
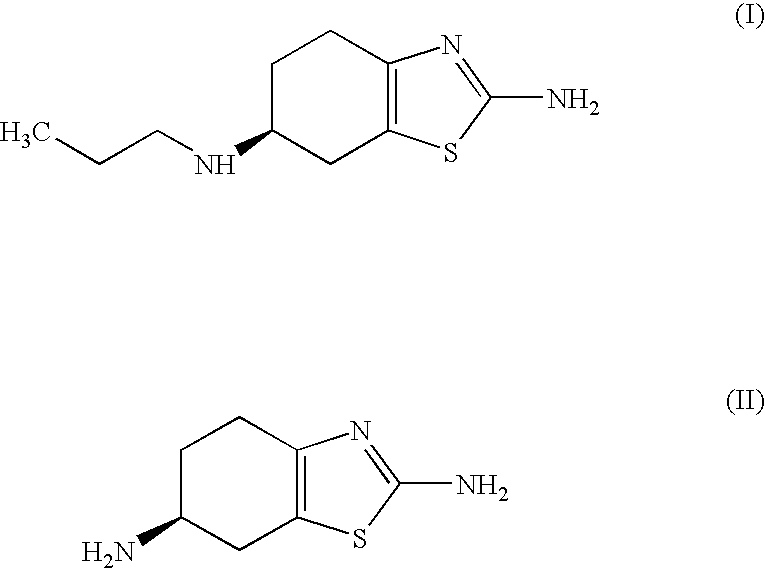
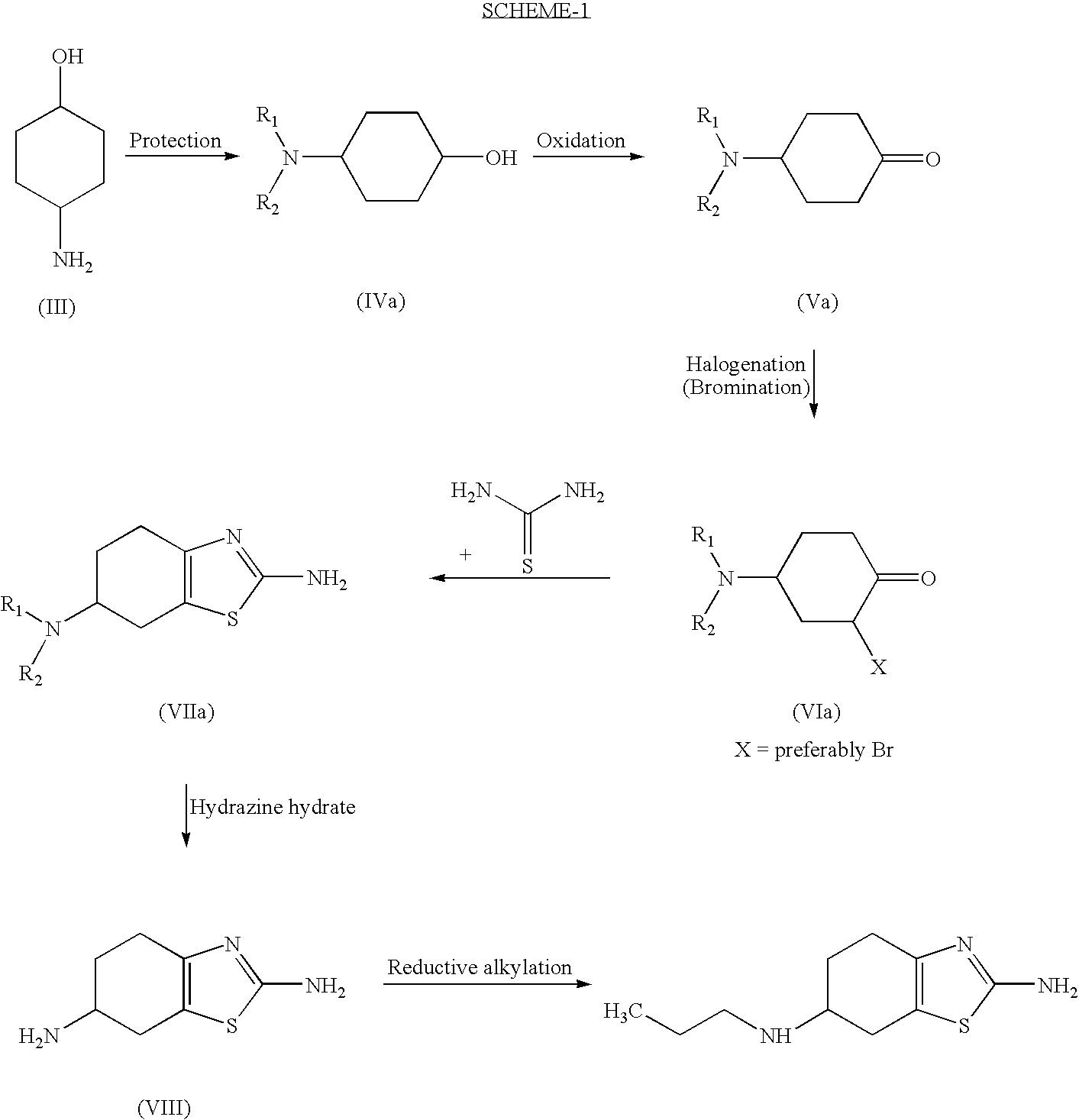
Improved process for the preparation of the intermediate compound of formula II for formation of biological active tetrahydrobenzothiazole compound of formula (I) as well as the biological active tetrahydrobenzothiazole compound of formula (I) and / or its pharmaceutically acceptable salts or solvates. The process comprises reacting 4-amino cyclohexanol of formula (III) or its acid addition salts with phthalic anhydride in presence of acid catalyst and their salts, in polar aprotic solvent or its mixture with organic solvent, capable of removing water azeotropically to give 4-(phthalimido)-cyclohexanol of formula (IV); oxidizing 4-(phthalimido)-cyclohexanol of formula (IV) to give 4-(phthalimido)-cyclohexanone of formula (V); brominating 4-(phthalimido)-cyclohexanone of formula (V) with brominating agent in organic solvent in presence of Lewis acid catalyst to prepare 2-bromo-4-(phthalimido)-cyclohexanone of formula (VI); treating 2-bromo-4-(phthalimido)-cyclohexanone of formula (VI) with thiourea in organic solvent in presence of base to give 2-amino-6-phthalimido-4,5,6,7-tetrahydro benzothiazol of formula (VII); reacting compound of formula (VII) with hydrazine hydrate and base in polar solvent to give racemic 2,6-diamino-4,5,6,7-tetrahydro-1,3-benzothiazole of formula (VIII); resolving racemic 2,6-diamino-4,5,6,7-tetrahydro-1,3-benzothiazole of formula (VIII) to prepare (6S)-2,6-diamino-4,5,6,7-tetrahydro-1,3-benzothiazole of formula (II). To form the compound of Formula I and if desired its salts / solvates the above process is carried out with further steps of coupling (6S)-2,6-dimino-4,5,6,7-tetrahydro-1,3-benzothiazole of formula (II) with propionaldehyde in presence of mineral acid in polar organic solvent and reducing agent to prepare (S)-(−)-2-Amino-6-(n-propylamino)-4,5,6,7-tetrahydrobenzothiazole of formula (I);and if desired converting (S)-(−)-2-Amino-6-(propylamino)-4,5,6,7-tetrahydrobenzothiazole to its pharmaceutically acceptable salts or solvates.
Owner:ALEMBIC LTD
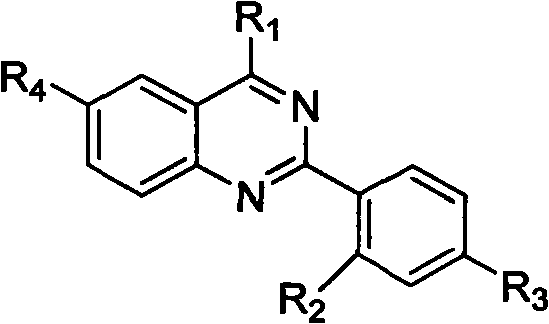
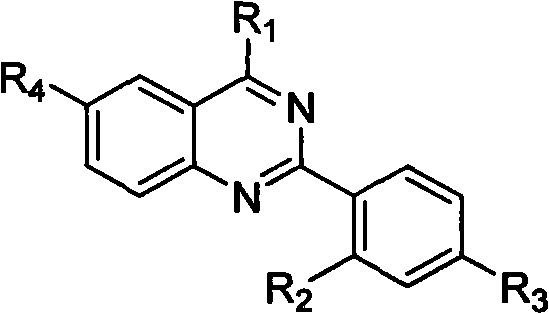
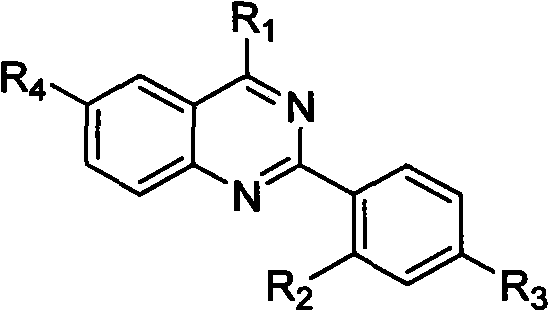
Quinazoline derivative and preparation method thereof and application of quinazoline derivative for preparing anticancer drugs
InactiveCN101967127AStrong interactionStrong inhibitory activityOrganic active ingredientsOrganic chemistryChemical industryCancer cell
The invention belongs to the fields of drugs and chemical industry, and discloses a quinazoline derivative and a preparation method thereof and an application of the quinazoline derivative used as an anticancer drug. The structural formula of the quinazoline derivative is shown in the specifications, wherein R1 is NH(CH2)mNR5 or NH(CH2)m-Ar; R2 is NHCO(CH2)n NR5, NHCO(CH2)n-Ar, NHCO(CH2)nNH(CH2)nNR5 or NHCO(CH2)nNH(CH2)n-Ar; R3 is F, Cl, Br, I, H, CH3, SO2CH3 or OCH3; R4 is H, NHCO(CH2)nNR5, NHCO(CH2)n-Ar, NHCO(CH2)nNH(CH2)nNR5 or NHCO(CH2)nNH(CH2)n-Ar; -Ar represents various aromatic rings comprising various aromatic heterocyclic rings; m is equal to 2, 3 or 4; n is equal to 1, 2, 3, 4 or 5; and R5 represents alkyl of C1-6, cycloalkyl of C3-6, piperidyl, morpholinyl, piperazinyl or quinoxalinyl. The invention simultaneously discloses the preparation method of the quinazoline derivative and the application of the quinazoline derivative used as the anticancer drug. The experiment proves that the quinazoline derivative of the invention has strong inhibiting effect on telomere DNA expression, has obvious inhibiting effect on various kinds of cancer cell strains, has low toxicity to normal cells, and has wide application prospects in preparation of anticancer drugs.
Owner:SUN YAT SEN UNIV
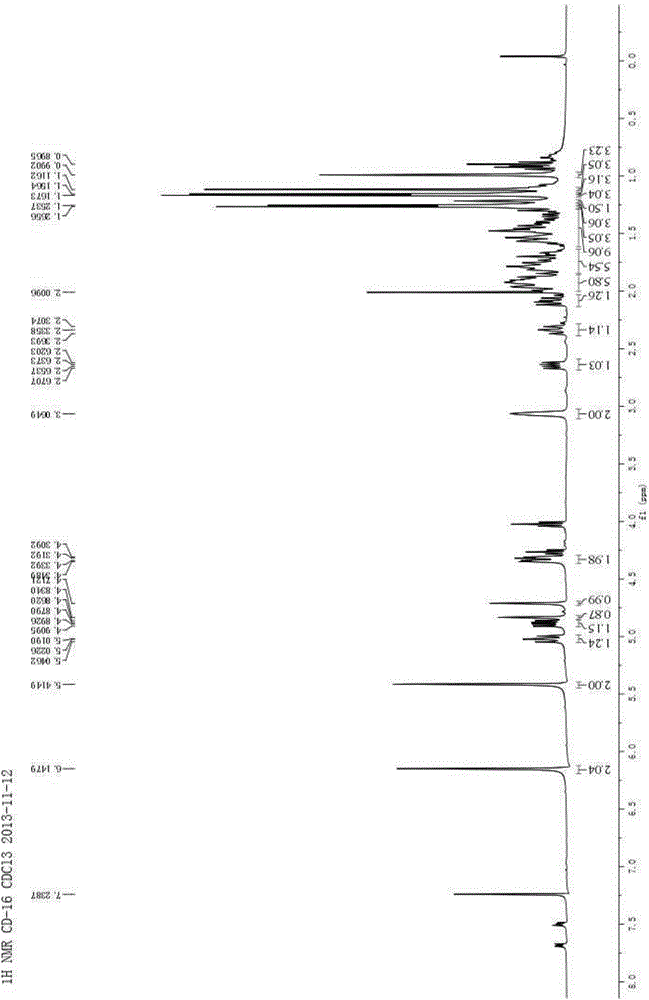
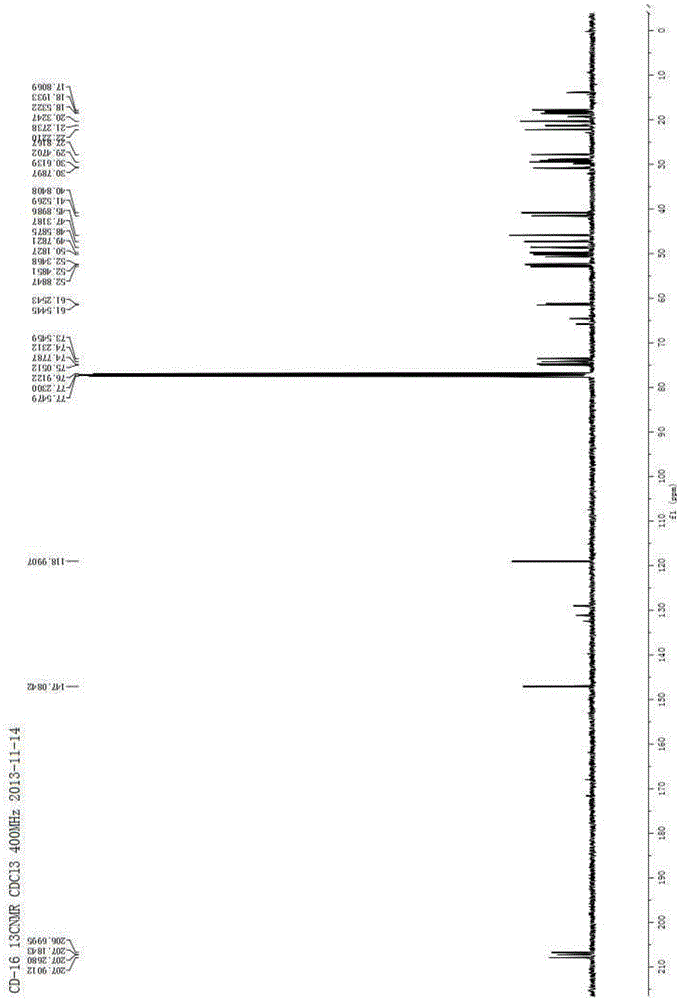
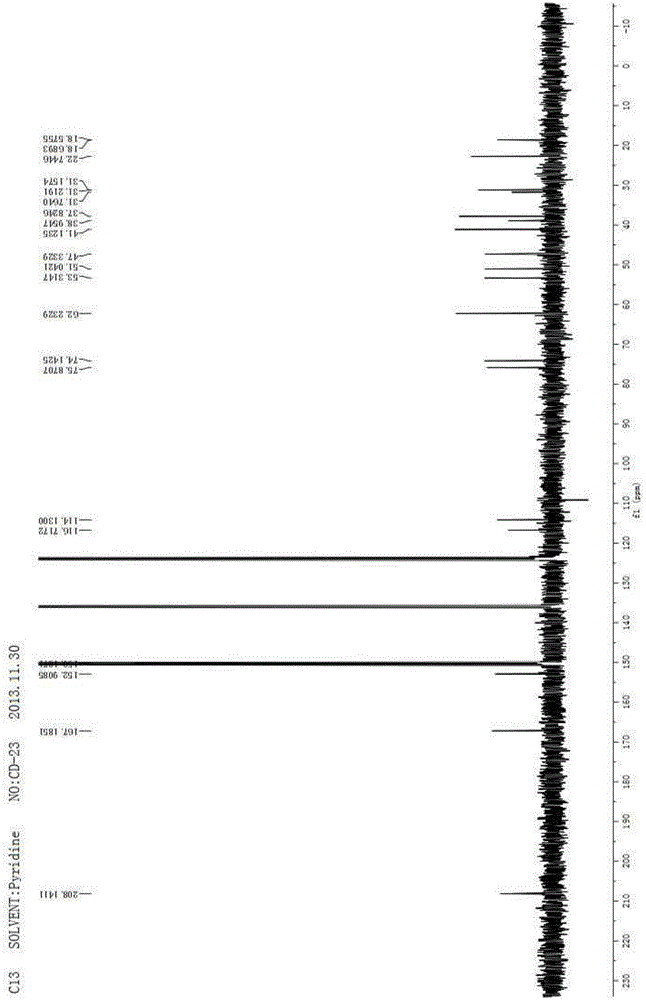
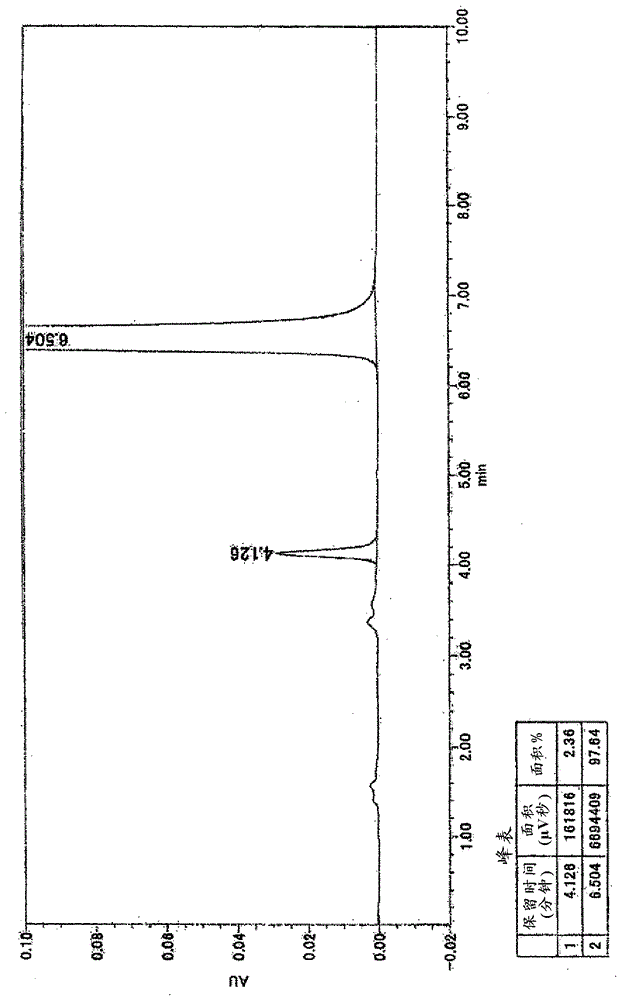
Method for preparing imidazo[2,1-b]thiazole derivative by catalysis of copper salt
The invention discloses a method for preparing an imidazo[2,1-b]thiazole derivative by catalysis of a copper salt. In an environment of an organic solvent, the copper salt is taken as a catalyst, 1,1-dibromo-1-olefin or alkynyl bromide and a 2-mercaptoimidazole compound are taken as substrates to synthesize the imidazo[1,5-b]thiazole derivative under the action of alkali by one step, wherein the molar ratio of the 2-mercaptoimidazole compound to the 1,1-dibromo-1-olefin or alkynyl bromide is 1:1-1:5; the molar ratio of the 2-mercaptoimidazole compound to the alkali is 1:3-1:8; based on the 2-mercaptoimidazole compound, the using amount of the copper salt is 0.1 percent to 3.0 mol percent; the molar ratio of a ligand to the copper salt is 1:2-2:1; and the reaction temperature is 20 to 110 DEG C. The preparation method is simple and mild in reaction conditions, and has wide application prospect in fine chemical industry and pharmacy industry.
Owner:ZHEJIANG UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com

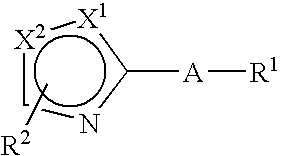
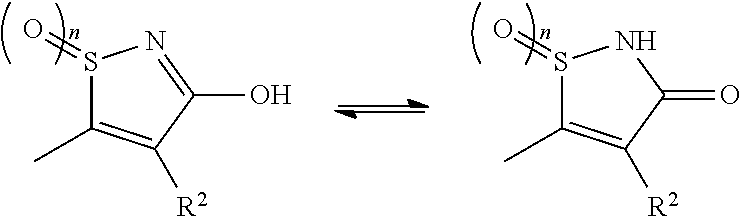
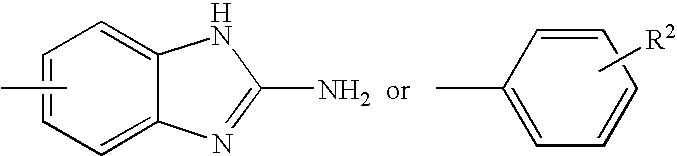
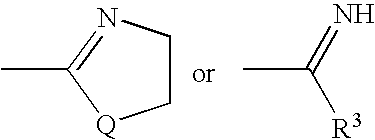
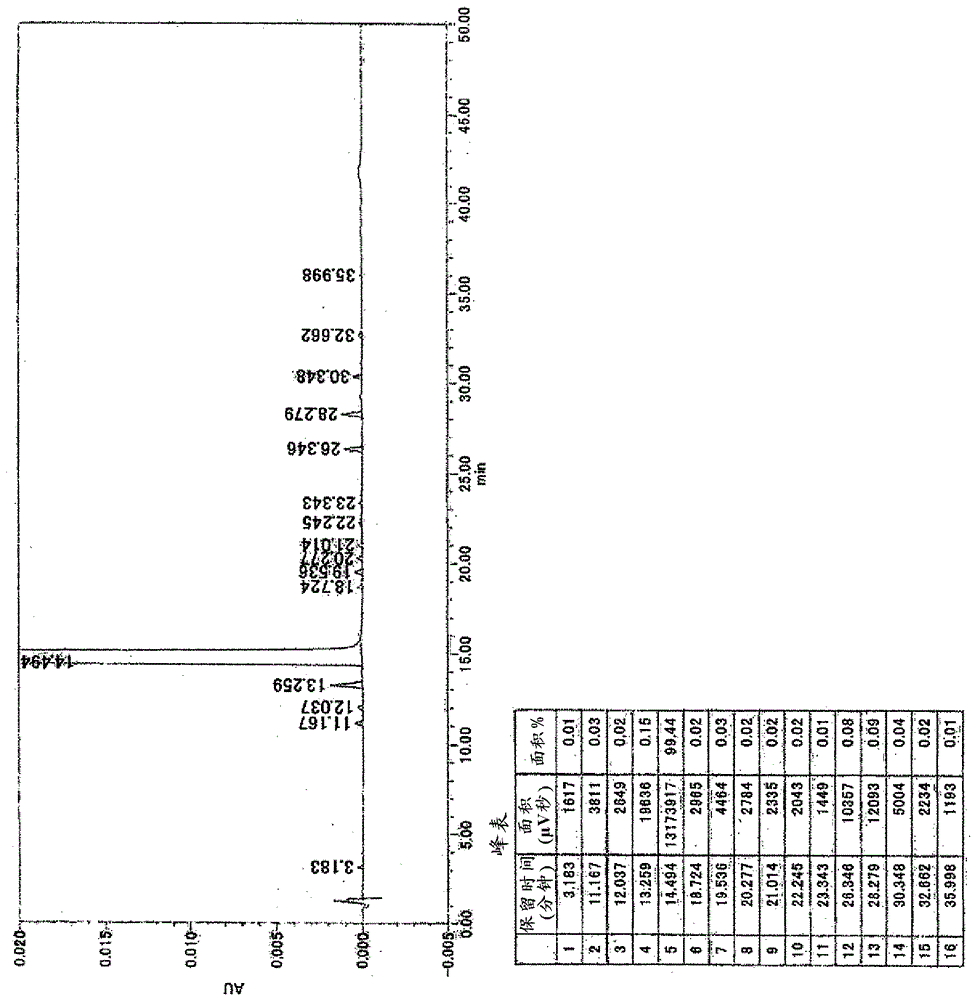
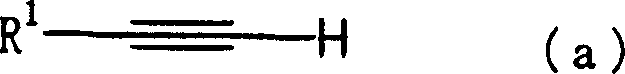
![Method for preparing imidazo[2,1-b]thiazole derivative by catalysis of copper salt Method for preparing imidazo[2,1-b]thiazole derivative by catalysis of copper salt](https://images-eureka.patsnap.com/patent_img/90c63b3c-fbda-43aa-be80-a12c680840dd/GSA00000059252400021.PNG)
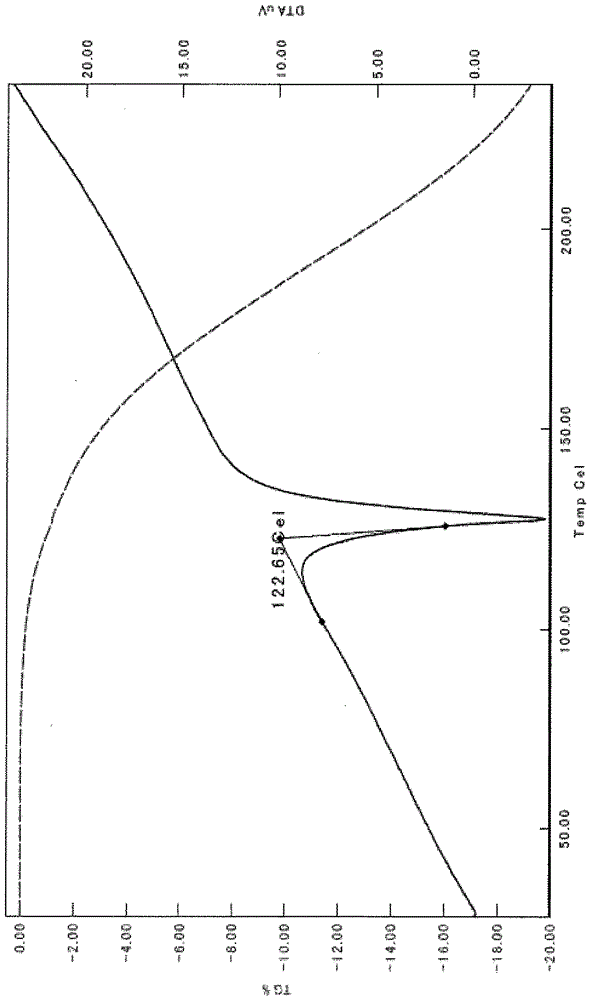
![Method for preparing imidazo[2,1-b]thiazole derivative by catalysis of copper salt Method for preparing imidazo[2,1-b]thiazole derivative by catalysis of copper salt](https://images-eureka.patsnap.com/patent_img/90c63b3c-fbda-43aa-be80-a12c680840dd/GSA00000059252400022.PNG)
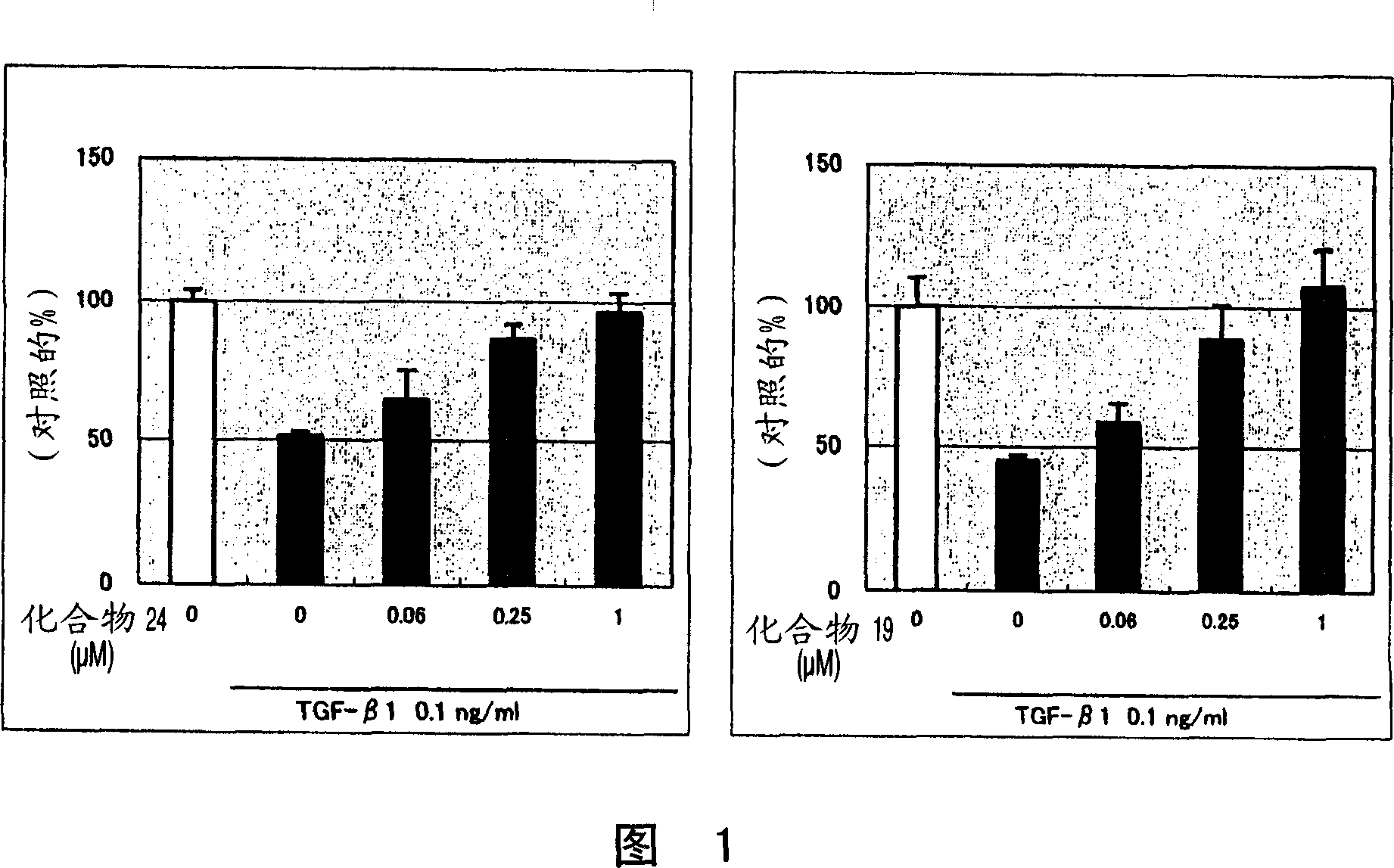
![Method for preparing imidazo[2,1-b]thiazole derivative by catalysis of copper salt Method for preparing imidazo[2,1-b]thiazole derivative by catalysis of copper salt](https://images-eureka.patsnap.com/patent_img/90c63b3c-fbda-43aa-be80-a12c680840dd/GSA00000059252400031.PNG)