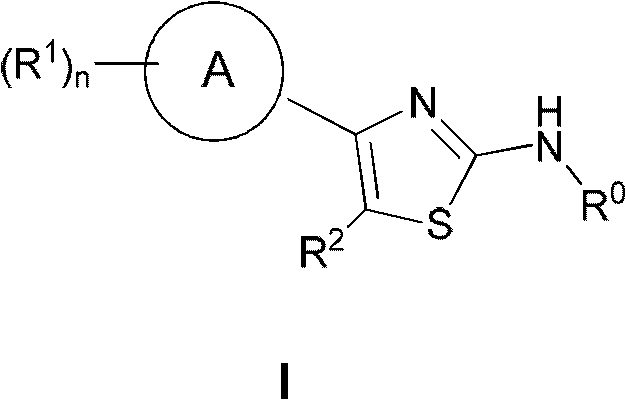
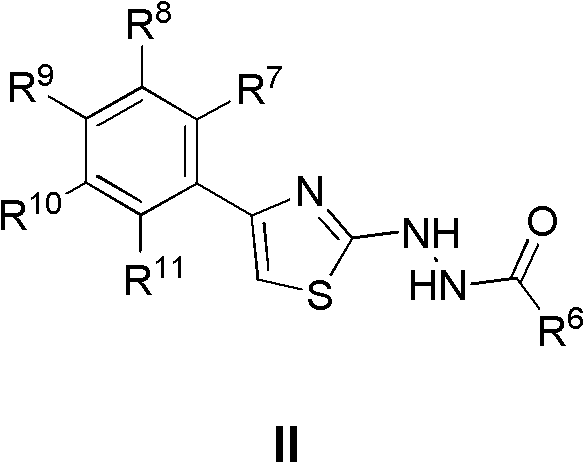
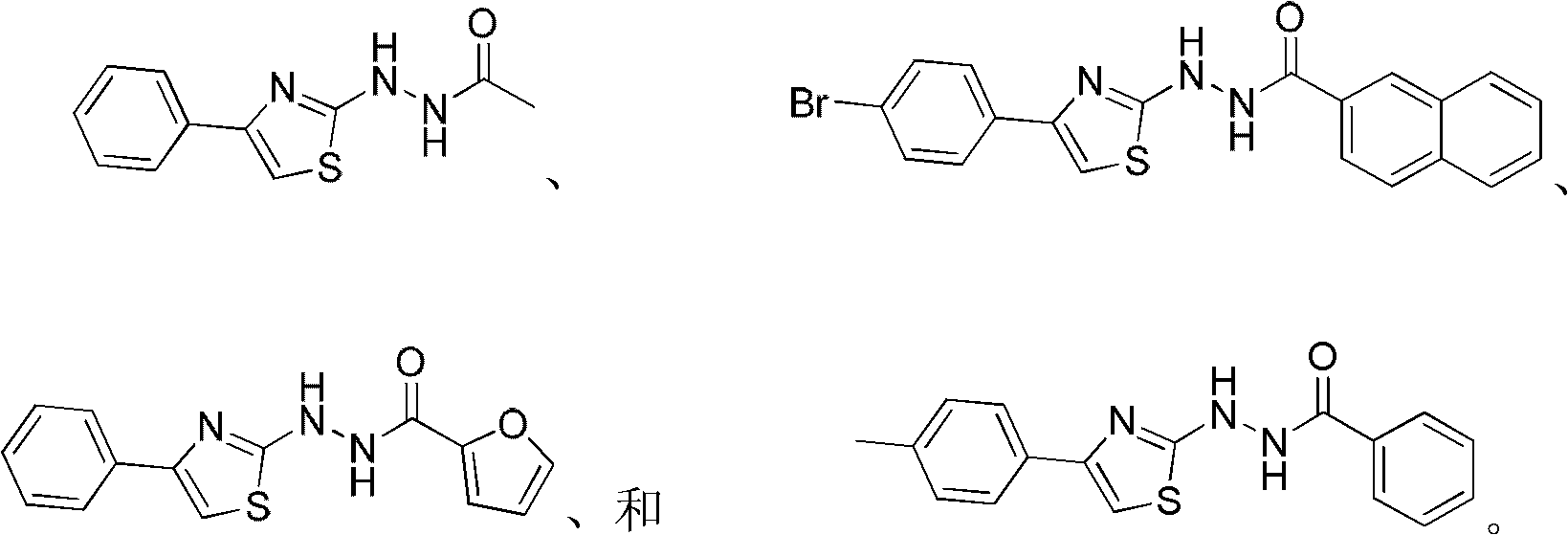
Applications of 2,4-disubstituted thiazoles derivatives being taken as DHODH (dihydroorotate dehydrogenase) inhibitor
A disubstituted, thiazole-based technology, used in the application field of 2,4-disubstituted thiazole derivatives as DHODH inhibitors
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0070] Example 1: The compounds provided by the present invention were obtained through virtual screening by computer-aided drug design method.
specific example 1 to 21
[0072] Using the complex crystal structure of DHODH and its small molecule inhibitor Brequinar analogue (PDB database number 1D3G) as the receptor, the 230,000 compound structures in the SPECS compound database were docked into the receptor using the Glide docking software, and the docking mode was successively adopted Glide's "standard precision mode" (SP mode) and "extreme precision mode" (XP mode), and then according to the GlideGscore to score and sort the docking mode of each compound, carefully observe and analyze the docking binding mode of each compound and DHODH and finally Select 21 compound structures to carry out the in vitro test experiment of inhibiting DHODH activity, and all compounds are purchased from Holland SPECS company ( www.specs.net ).
Embodiment 2
[0073] Embodiment 2: The compound provided by the invention is to the in vitro inhibitory effect of dihydroorotate dehydrogenase (DHODH) activity
[0074] Materials: The plasmid containing the full-length human DHODH gene was donated by Prof.Jon Clardy (Harvard Medical School) (J.Bio.Chem.2008, 283(50), 35078-35085) or can be obtained from Aurui Dongyuan Biotechnology Co., Ltd. purchased. The pET-19b vector, E.coli DH5α and E.coli BL21(DE3) strains were purchased from Novagen. Restriction enzymes Nde I and Bam HI were purchased from NEB Company. Primers were synthesized by Shanghai Yingjun Biotechnology Co., Ltd. Other reagents were purchased from sigma. 〔The materials owned by individuals or organizations are not available to the public. Therefore, the above-mentioned plasmids may lead to insufficient disclosure of the instructions, and ultimately lead to the failure of the application to be authorized. Suggestions: (1) Provide the source of the literature, and at the same...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com