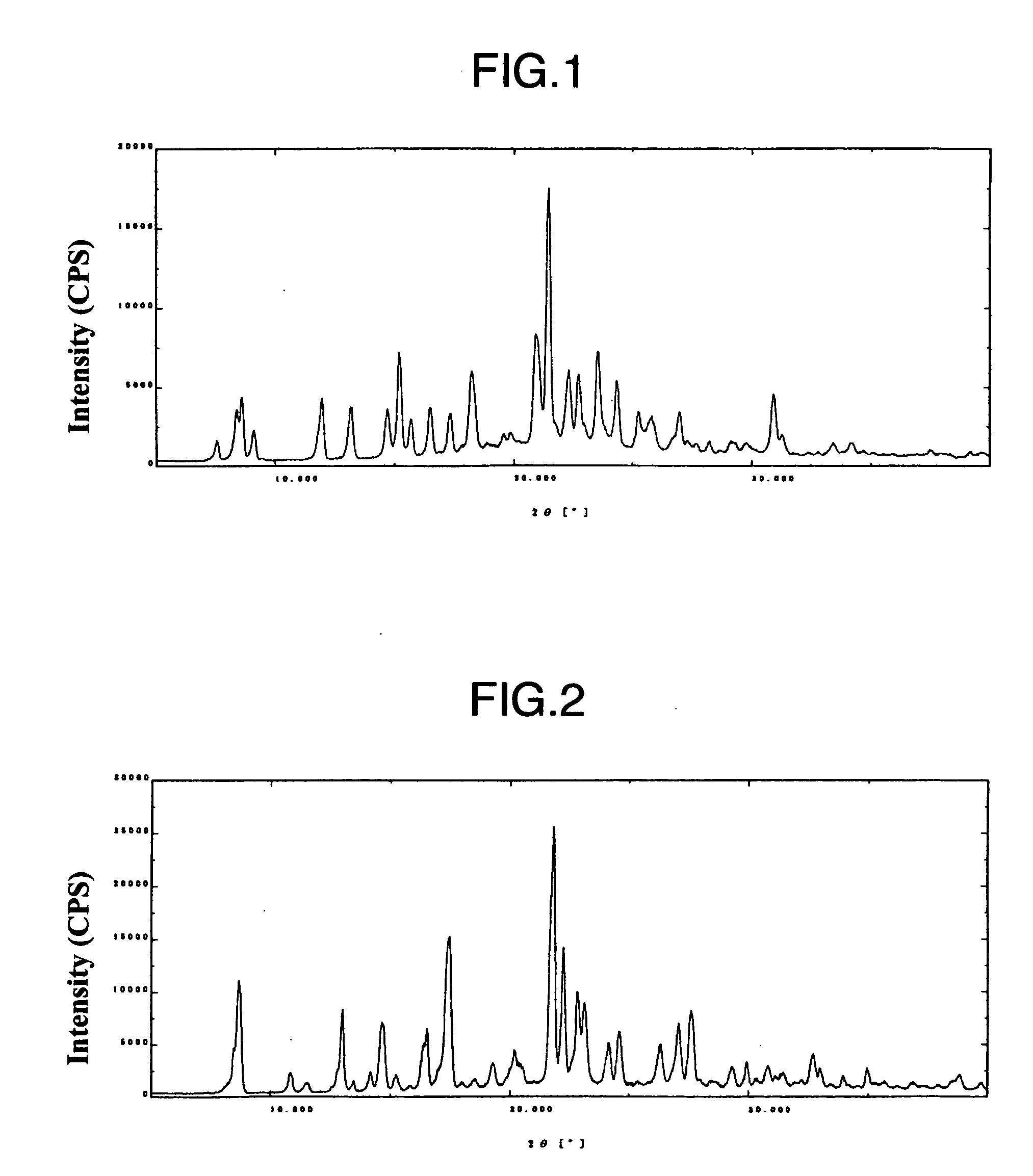
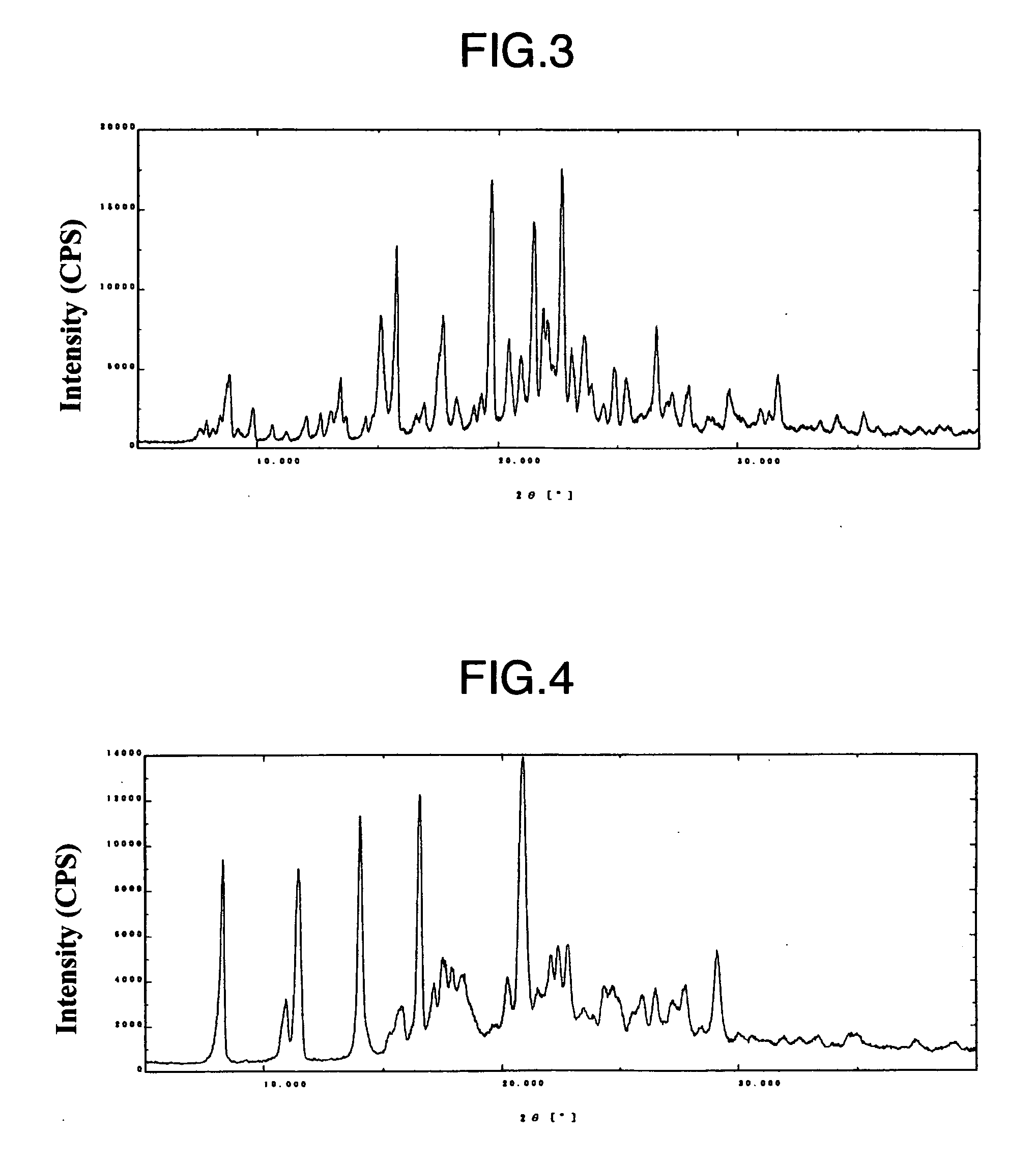
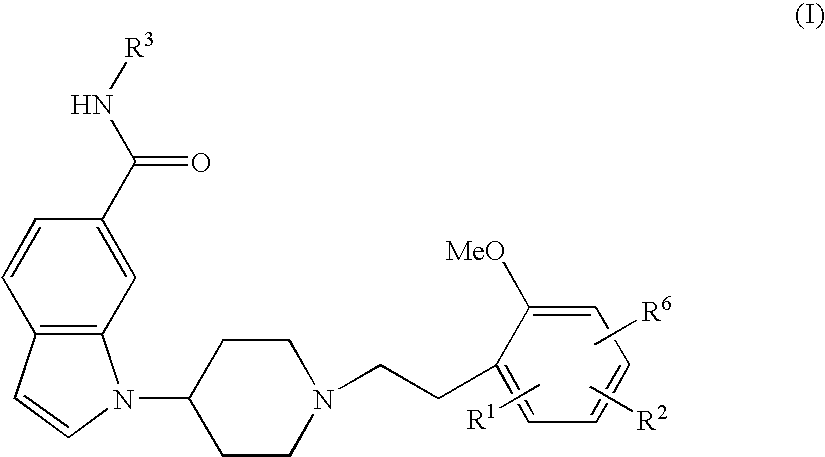
Indole derivative having piperidine ring
a technology of piperidine and indole, which is applied in the field of indole derivatives having piperidine rings, to achieve the effect of superior binding affinity to a 5-ht1a receptor and superior clinical action to treat or prevent lower urinary tract symptoms
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
production example 1
Synthesis of 1-(piperidin-4-yl)-1H-indole-6-carboxamide
[0602]
(1) Synthesis of methyl 1-(1-benzyloxycarbonylpiperidin-4-yl)-1H-indole-6-carboxylate
[0603] 44.3 g of methyl 3-amino-4-(2,2-dimethoxyethyl)benzoate synthesized according to the publication (Tetrahedron Letters, Vol. 37, No. 34, pp. 6045-6048) and 64.9 g of benzyl 4-oxo-1-piperidinecarboxylate were dissolved in 485 ml of acetic acid, followed by stirring at room temperature. Approximately 20 minutes later, 58.9 g of sodium triacetoxyborohydride was added to the reaction solution. Then, the reaction solution was further stirred for 2 hours. Thereafter, 485 ml of water was added to the reaction solution, and the obtained mixture was heated to a temperature between 100° C. and 115° C. Approximately 3 hours later, the reaction solution was cooled, and then concentrated under a reduced pressure. Thereafter, water and ethyl acetate were added thereto, so as to separate an organic layer. The obtained organic layer was washed wit...
production example 2
Synthesis of N-methyl-1-(piperidin-4-yl)-1H-indole-6-carboxamide
[0611]
(1) Synthesis of N-methyl-1-(1-benzyloxycarbonylpiperidin-4-yl)-1H-indole-6-carboxamide
[0612] 2.00 g of 1-(1-benzyloxycarbonylpiperidin-4-yl)-1H-indole-6-carboxylic acid was dissolved in 20 ml of tetrahydrofuran, and 1.03 g of 1,1′-carbonylbis-1H-imidazole was then added thereto. The obtained mixture was stirred at room temperature for 1.5 hours, and 4.11 ml of a 40% methylamine aqueous solution was added thereto. After completion of the reaction, the reaction solution was extracted with ethyl acetate. The organic layer was washed with a saturated sodium bicarbonate aqueous solution, a saturated ammonium chloride aqueous solution, and a saturated sodium chloride solution. Thereafter, the organic layer was dried over anhydrous magnesium sulfate. After removing the drying agent by filtration, the organic layer was concentrated under a reduced pressure, and the residue was then purified by NH silica gel column chro...
production example 3
Synthesis of 3-amino-4-(2,2-dimethoxyethyl)benzamide
[0616]
(1) Synthesis of 3-nitro-4-methylbenzamide
[0617] 20.0 g of 3-nitro-4-methylbenzoic acid was dissolved in 400 ml of tetrahydrofuran. Thereafter, 21.5 g of 1,1′-carbonyldiimidazole and 0.1 ml of dimethylformamide were added thereto. The obtained mixture was stirred for 45 minutes. Thereafter, 20 ml of 28% ammonia water was added thereto, followed by stirring at room temperature for 24 hours. After completion of the reaction, the reaction solution was concentrated under a reduced pressure, and the residue was separated into 600 ml of ethyl acetate and 200 ml of water. The organic layer was separated, and then washed with 200 ml of 2 N hydrochloric acid, 100 ml of water, 100 ml of a saturated sodium bicarbonate aqueous solution, and 100 ml of a saturated sodium chloride solution. It was then dried over anhydrous magnesium sulfate. After removing the drying agent by filtration, the filtrate was concentrated under a reduced press...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Mass | aaaaa | aaaaa |
| Mass | aaaaa | aaaaa |
| Mass | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com