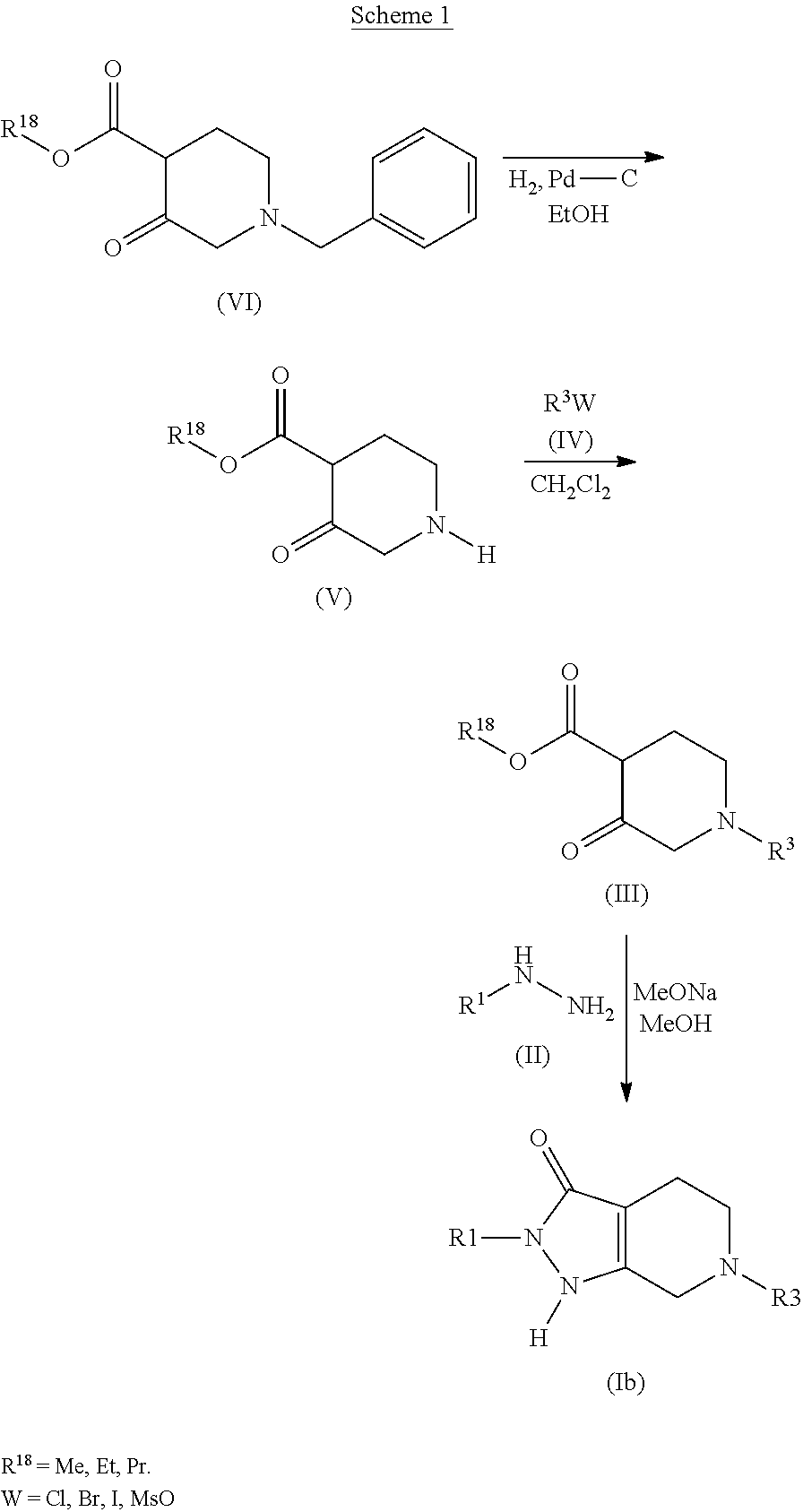
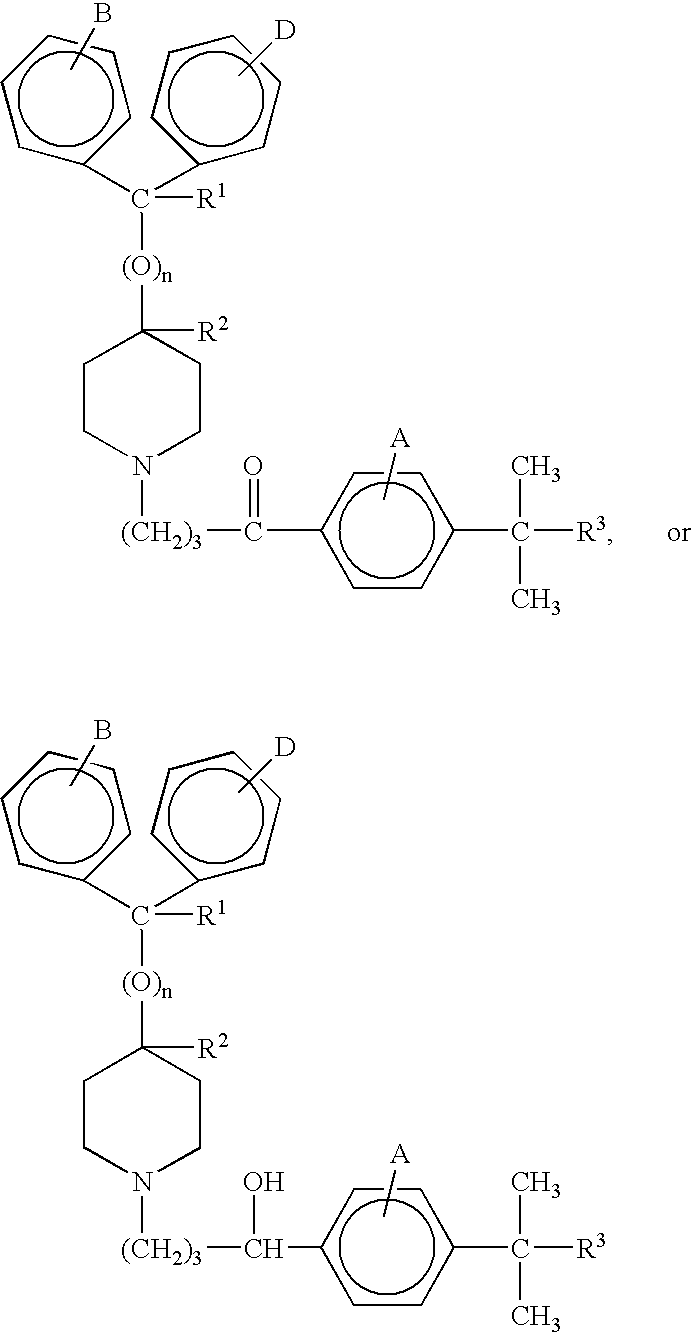
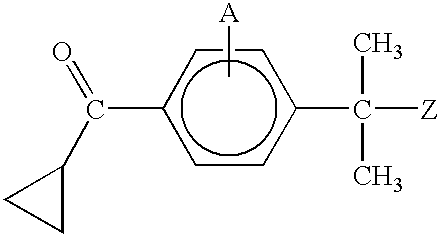
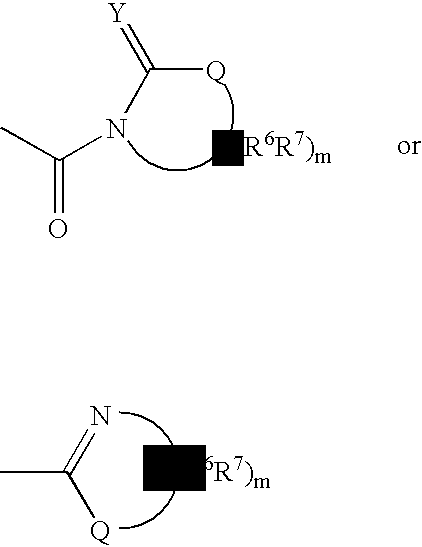
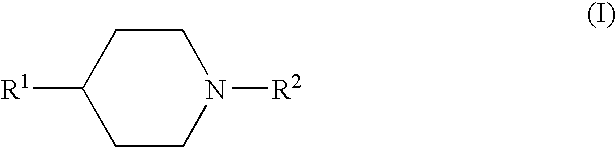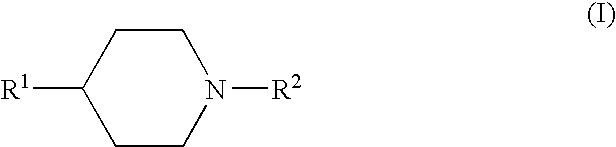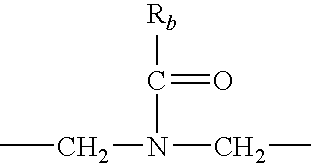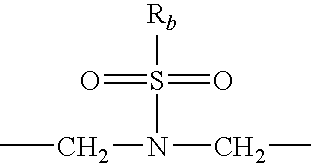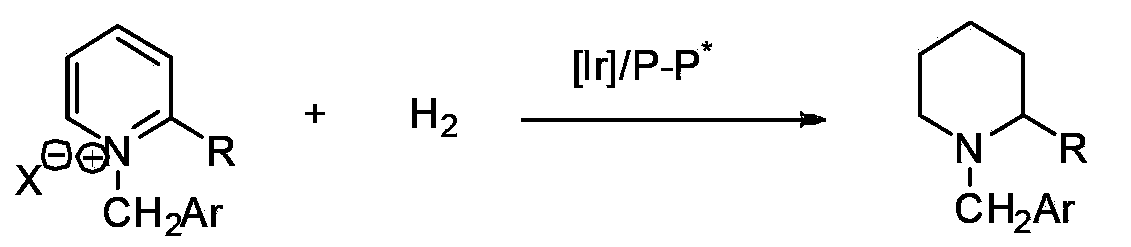Patents
Literature
155 results about "Lepidine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Lepidine, or 4-methylquinoline, is a heterocyclic aromatic organic compound. Its methyl group is fairly acidic, allowing for condensations to occur at this position, especially when the nitrogen is quaternized. It is used in the preparation of certain dyes.
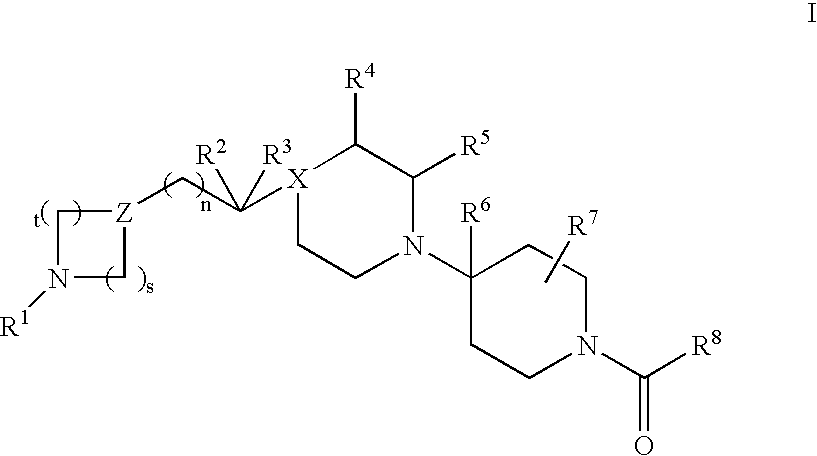
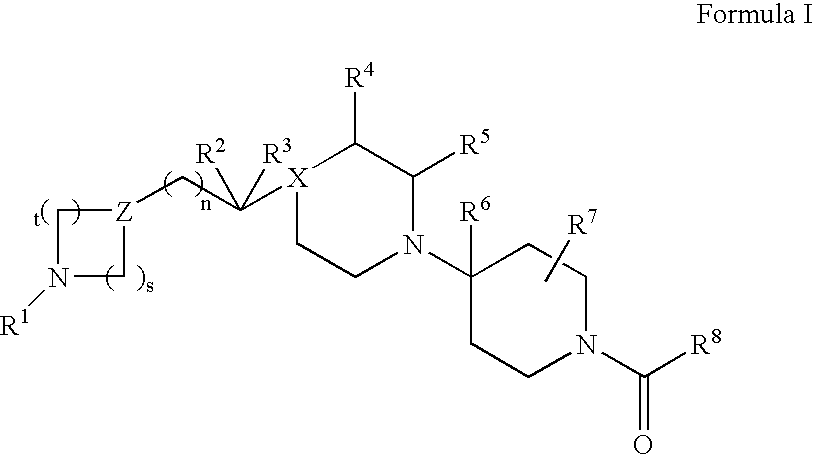
Indole derivative having piperidine ring
InactiveUS20050256103A1High affinityEnhanced inhibitory effectBiocideNervous disorderHydrogen atomSerotonin 1A Receptor
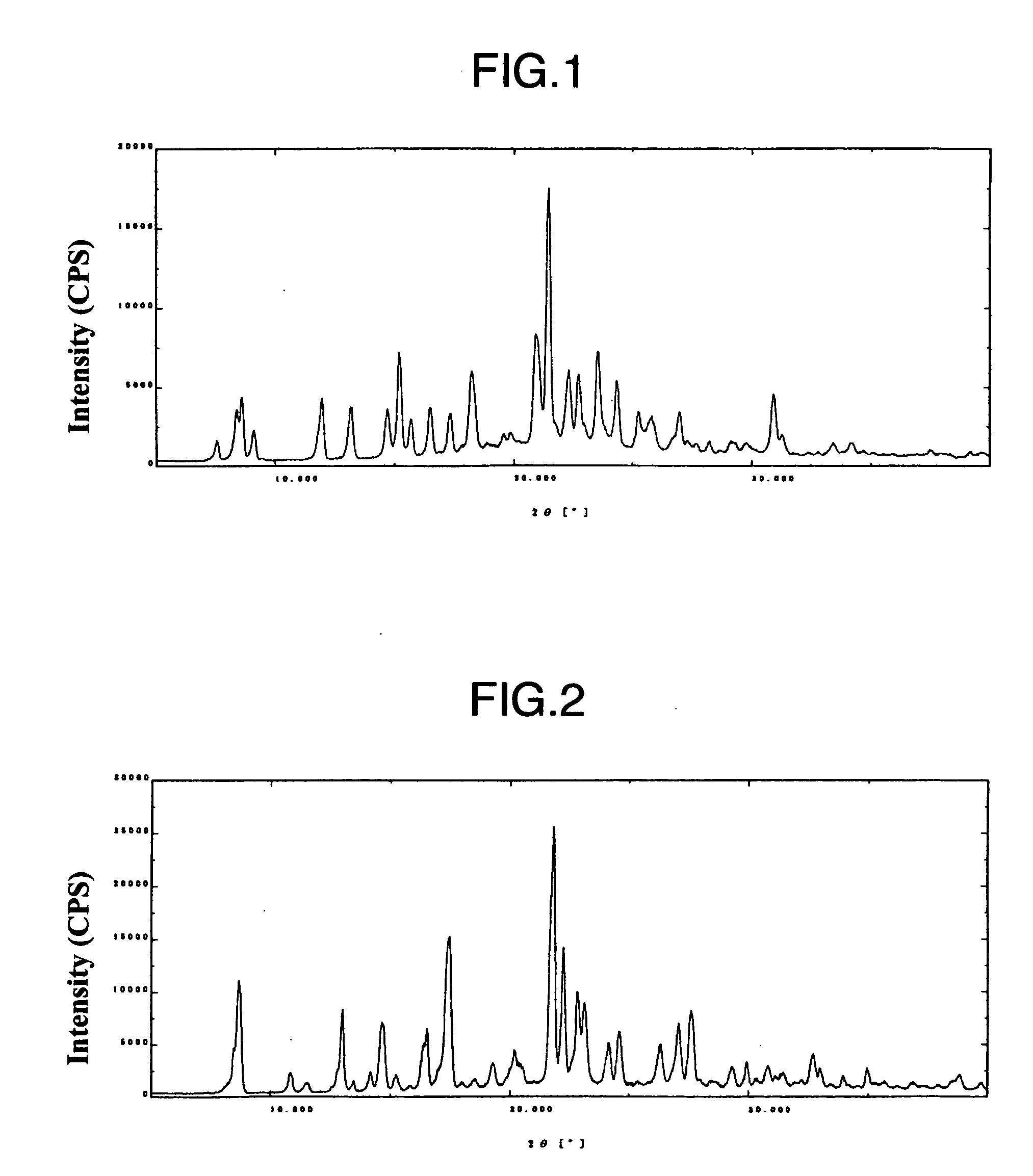
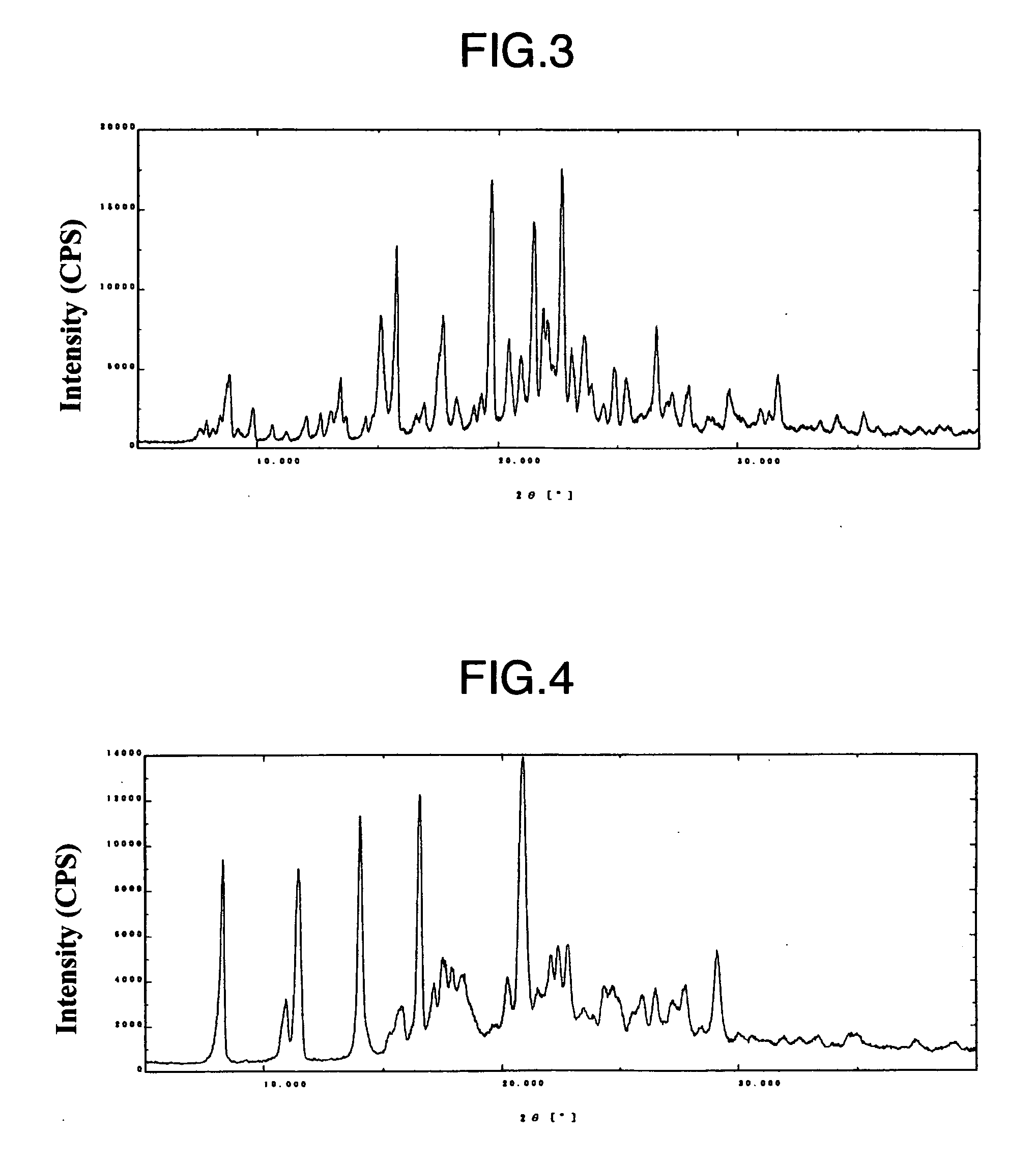
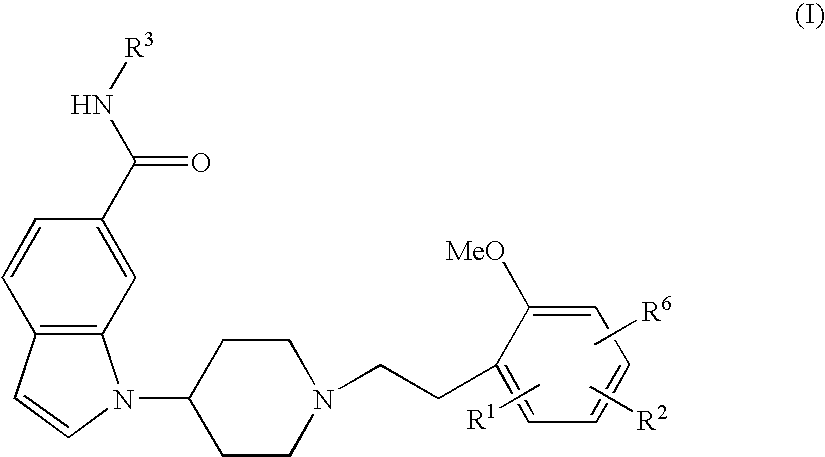
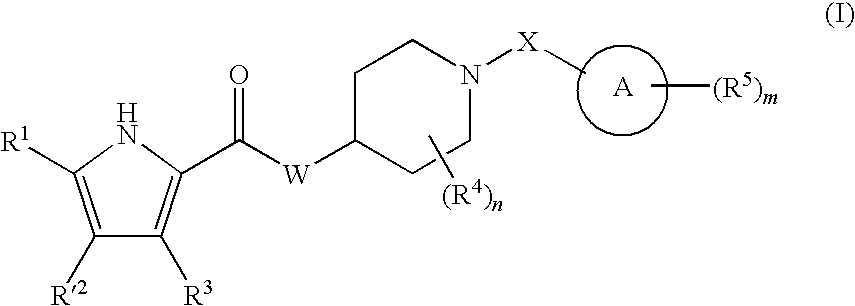
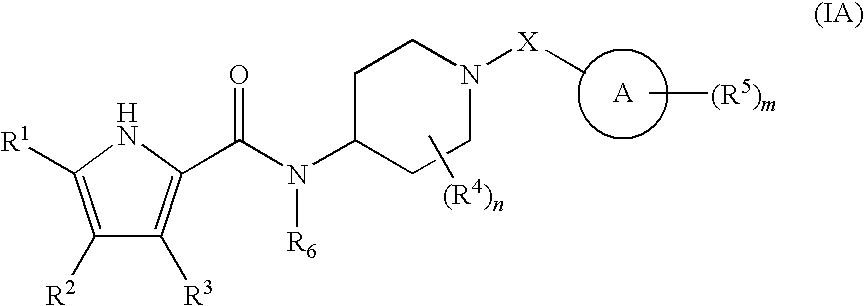
The present invention relates to a compound represented by the following formula, a pharmacologically acceptable salt thereof, or a use thereof as a pharmaceutical: wherein R1 and R2 are substituents adjacent to each other, and together with two carbon atoms to each of which they attach, form a 5- to 7-membered non-aromatic carbocyclic group or the like, which may be substituted by 1 to 4 substituents selected from (1) an oxo group, (2) a hydroxyl group, and the like; R3 represents a hydrogen atom or the like; and R6 represents a hydrogen atom or the like. It is an object of the present invention to discover an agent for treating or preventing lower urinary tract symptoms, and particularly symptoms regarding urinary storage, which has a superior strength of binding to a 5-HT1A receptor and an antagonism to the receptor.
Owner:EISIA R&D MANAGEMENT CO LTD
Fluorides of 4-substituted piperidine derivatives
The present invention provides a novel compound having an excellent acetylcholinesterase inhibitory effect. That is, it provides a 4-substituted piperidine compound fluoride represented by the following formula, a pharmaceutically acceptable salt thereof or hydrates thereof (provided that 1-benzyl-4-[(5,6-dimethoxy-2-fluoro-1-indanon)-2-yl]methylpiperidine, a pharmaceutically acceptable salt thereof and hydrates thereof are excluded).wherein R<1 >and R<2 >represent substituents.
Owner:EISIA R&D MANAGEMENT CO LTD
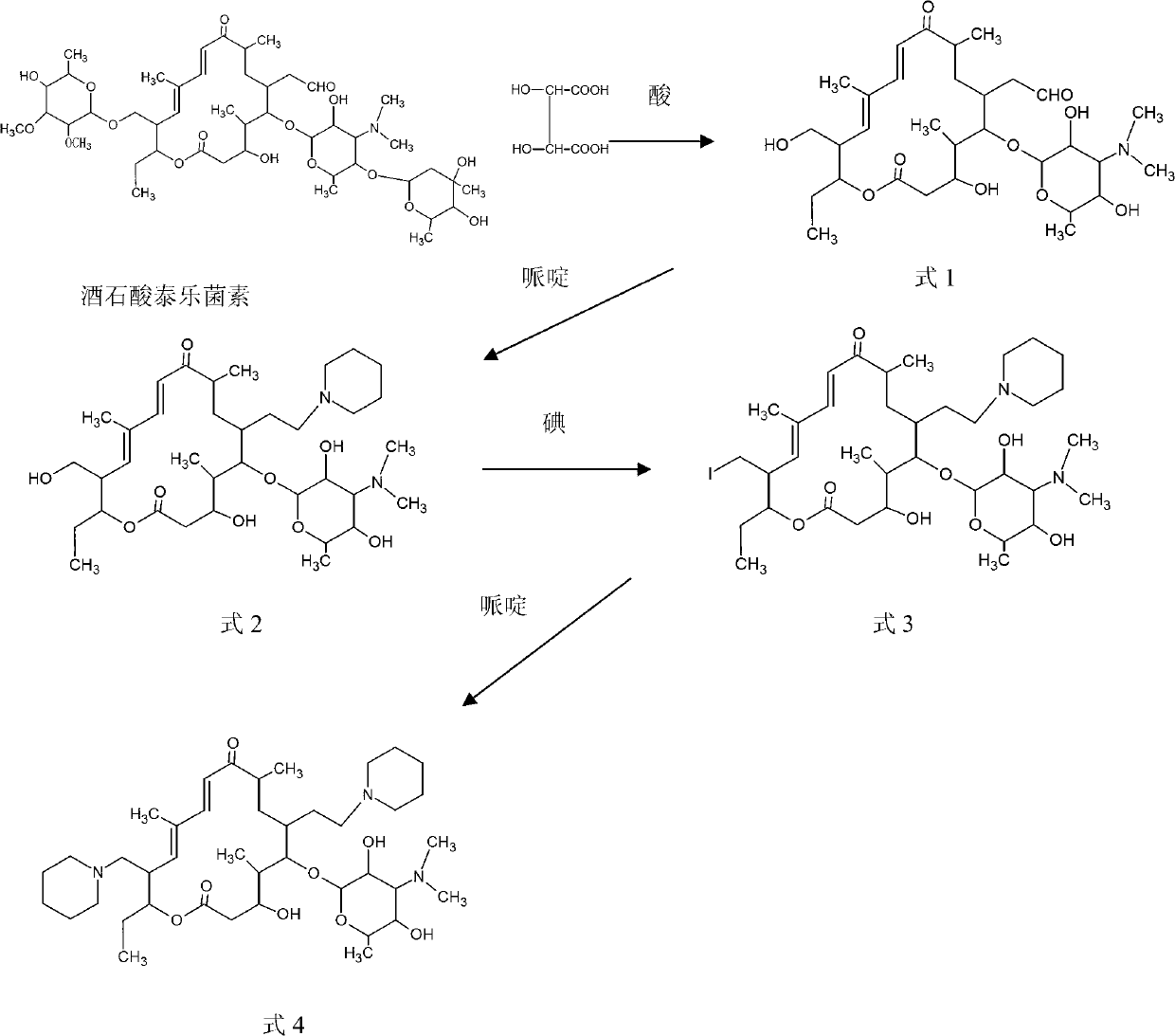
Process for preparing 20,23-bi-piperidyl-5-O-carbon mould amine glycosyl-tylosin lactone
ActiveCN102863487AOvercoming rare and expensive shortcomingsReduce manufacturing costSugar derivativesSugar derivatives preparationOrganic solventIodine
The invention relates to a process for preparing 20,23-bi-piperidyl-5-O-carbon mould amine glycosyl-tylosin lactone. Tylosin tartrate serves as a raw material, an intermediate product of 23-oxhydryl-5-O-carbon mould amine glycosyl-tylosin lactone is obtained through hydrolyzation, an organic phase is extracted through phase inversion, and an intermediate product of 20-piperidyl-23-oxhydryl-5-O-carbon mould amine glycosyl-tylosin lactone is produced through combination with piperidine under the effect of methanoic acid. A final product of the 20,23-bi-piperidyl-5-O-carbon mould amine glycosyl-tylosin lactone is formed by ammonization with the piperidine through iodination. By the aid of the process, the production technology is simplified, the dosage of auxiliary raw materials such as the piperidine, iodine and an organic solvent is greatly reduced, the purity of the obtained product is higher than 98%, the yield of the final product of the 20,23-bi-piperidyl-5-O-carbon mould amine glycosyl-tylosin lactone reaches 58.7%, and the process has good industrial application prospects.
Owner:QILU ANIMAL HEALTH PROD
Antibacterial piperdine derivatives
Owner:ASTRAZENECA AB
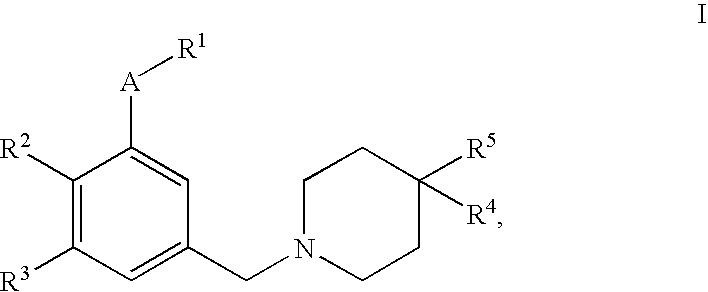
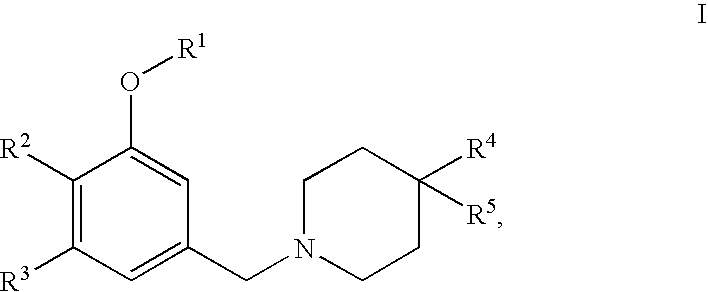
4,4-disubstituted piperidine derivatives
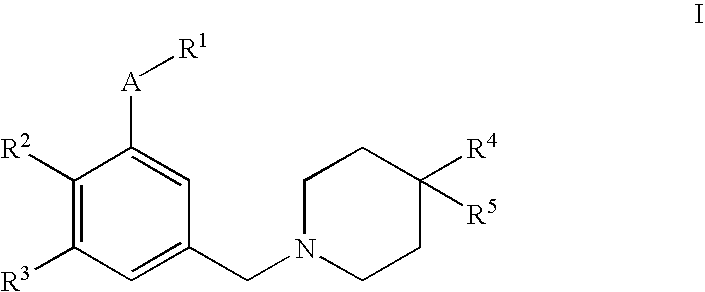
This invention relates to 4,4-disubstituted piperidine derivatives of the formulawherein A and R1 to R5 are as defined in the specification, and pharmaceutically acceptable salts thereof. The invention further relates to pharmaceutical compositions containing such compounds, to a process for their preparation and to their use for the treatment and / or prevention of diseases which are associated with the modulation of SST receptors subtype 5.
Owner:F HOFFMANN LA ROCHE & CO AG
Polymorphs of a c-MET/HGFR inhibitor
This invention relates to polymorphs of (R)-3-[1-(2,6-Dichloro-3-fluoro-phenyl)-ethoxy]-5-(1-piperidin-4-yl-1H-pyrazol-4-yl)-pyridin-2-ylamine that are useful in the treatment of abnormal cell growth, such as cancer, in mammals. This invention also relates to compositions including such salts and polymorphs, and to methods of using such compositions in the treatment of abnormal cell growth in mammals, especially humans.
Owner:PFIZER INC
Use of chiral oxazoline
InactiveCN101099936AOrganic-compounds/hydrides/coordination-complexes catalystsPreparation by hydrogen cyanide additionEthyl groupChirality
Owner:HEFEI UNIV OF TECH
Method for preparing disubstituted piperidine and intermediates
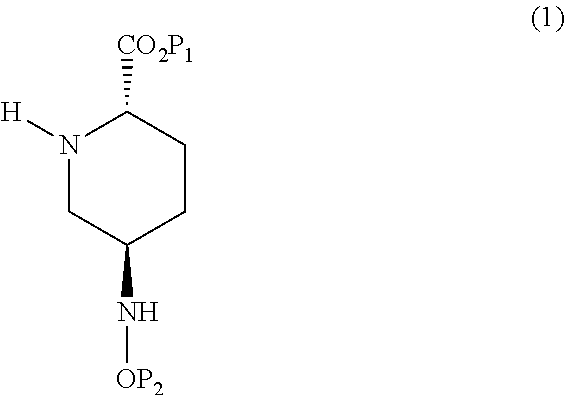
The disclosure relates to a method for preparing a compound of formula (I),wherein P1 and P2 are groups protecting the carboxylic acid and oxyamine functions, starting from pyroglutamic acid (S). The disclosure also relates to novel intermediates.
Owner:FOREST LAB HLDG LTD
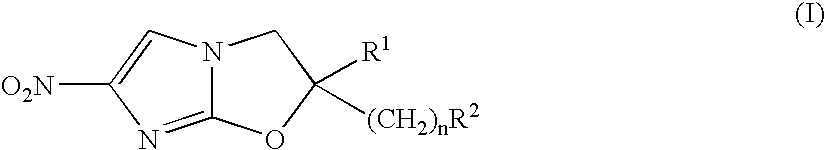
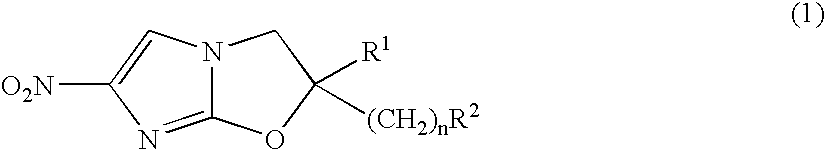
2,3-Dihydro-6-Nitroimidazo (2,1-b) Oxazole Compounds for the Treatment of Tuberculosis
InactiveUS20080119478A1Improve the bactericidal effectAntibacterial agentsBiocideNitroimidazoleHydrogen atom
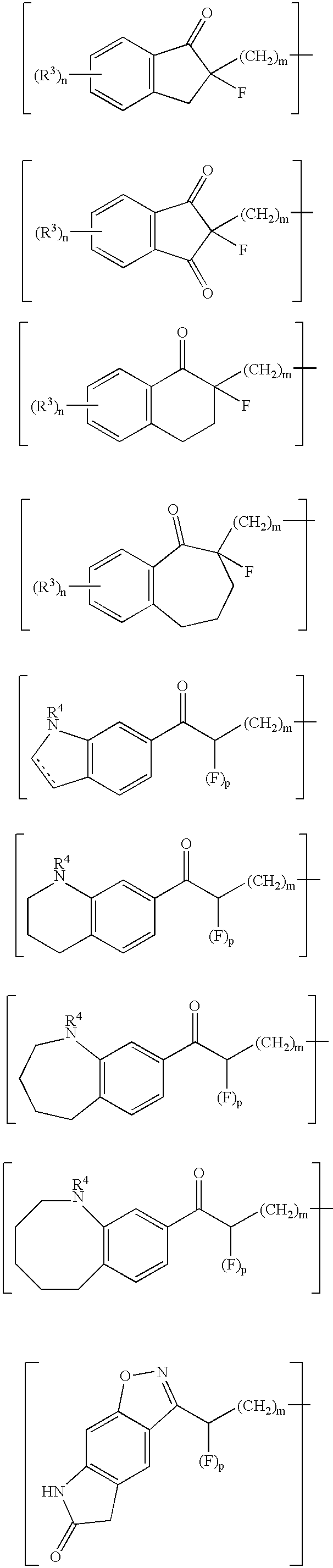
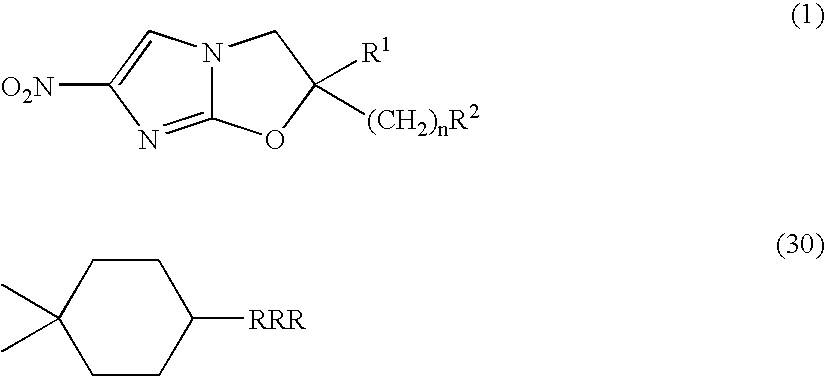
The present invention provides a 2,3-dihydro-6-nitroimidazo[2,1-b]oxazole compound represented by the following general formula: (1) in the above formula (1), R1 represents a hydrogen atom or C1-C6 alkyl group, n represents an integer of 0 to 6, R1 and —(CH2)nR2 may form a spiro ring represented by the formula (30) below, together with the adjacent carbon atom (in the formula below, RRR represents a piperidyl group which may have substituents on the piperidine ring), (30) and R2 represents a benzothiazolyloxy group, quinolyloxy group, pyridyloxy group or the like. The present compound has an excellent bactericidal action against Mycobacterium tuberculosis, multi-drug-resistant Mycobacterium tuberculosis, and atypical acid-fast bacteria.
Owner:OTSUKA PHARM CO LTD
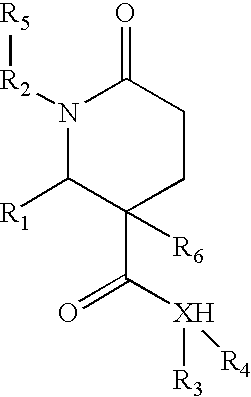
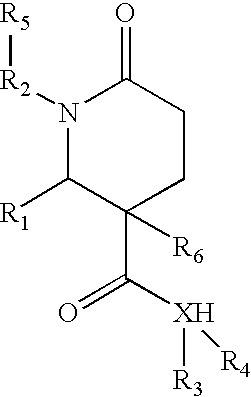
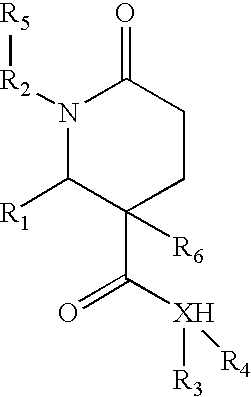
1,2-disubstituted-6-oxo-3-phenyl-piperidine-3-carboxamides and combinatorial libraries thereof
InactiveUS20030171588A1Organic chemistry methodsOrganic compound librariesFormamideCombinatorial chemistry
The invention relates to combinatorial libraries containing two or more novel piperidine-3-carboxamide derivative compounds, methods of preparing the piperidine-3-carboxamide derivative compounds and piperidine-3-carboxamide derivative compounds bound to a resin
Owner:LION BIOSCIENCE AG
Method for preparing piperidine and piperidine derivative
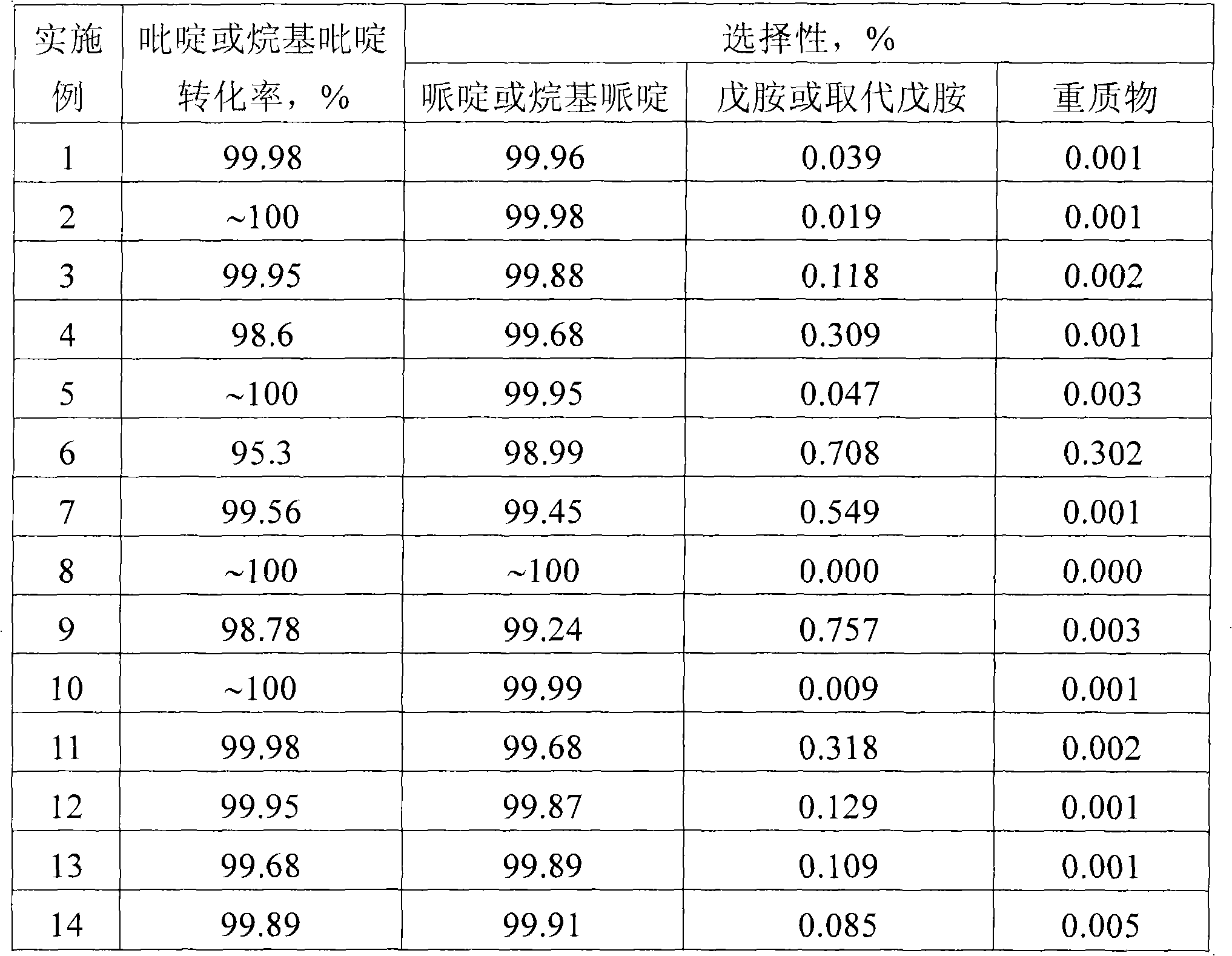
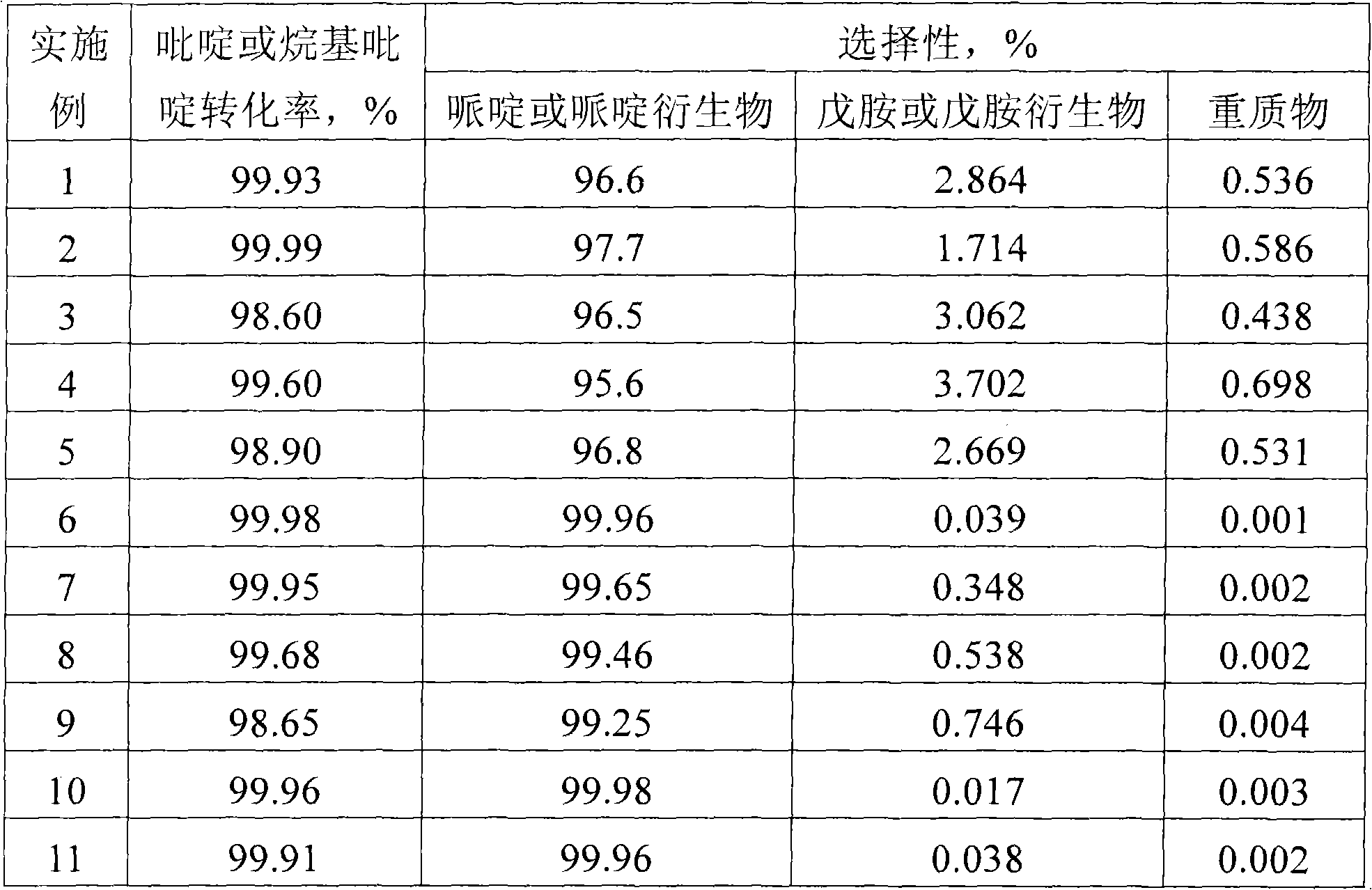
The invention discloses a method for preparing piperidine and a piperidine derivative. A reaction system consists of the piperidine, or the piperidine derivative, hydrogen and a catalyst; the reaction temperature is between 100 DEG C and 200 DEG C; the reaction pressure is between 1.0 MPa and 10.0 MPa; the liquid space velocity of the piperidine or the piperidine derivative is between 0.05 h<-1> and 2.5 h<-1>; the molar ratio of H2 to the piperidine or the piperidine derivative is 100-250; the catalyst takes Al2O3, active carbon, ZrO2 or SiO2 as a carrier; a load active component can be one or more of Pd, Ni, Cu, Fe, Co, Ru or / and TiO2; and the piperidine or the piperidine derivative can be transformed into piperidine and alkyl piperidine products respectively at high activity and high selectivity under the action of a catalyst.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
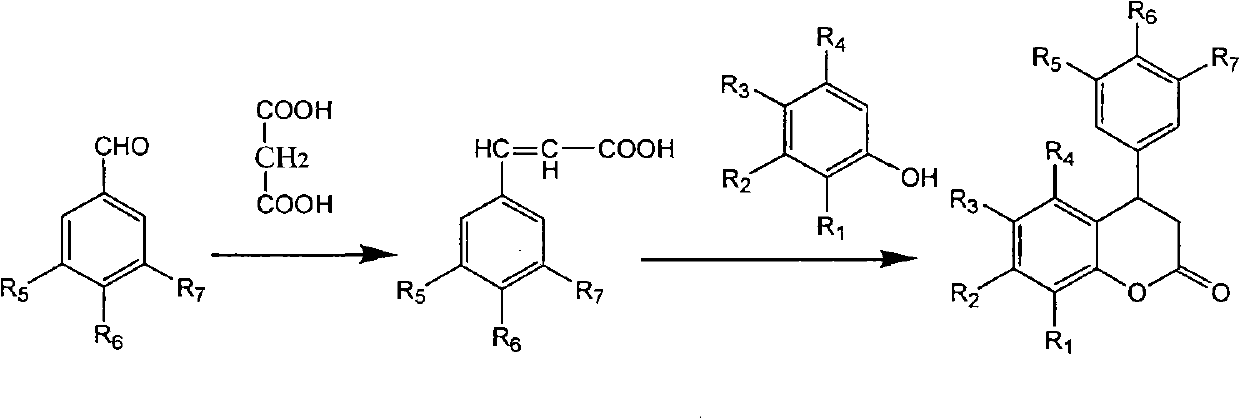
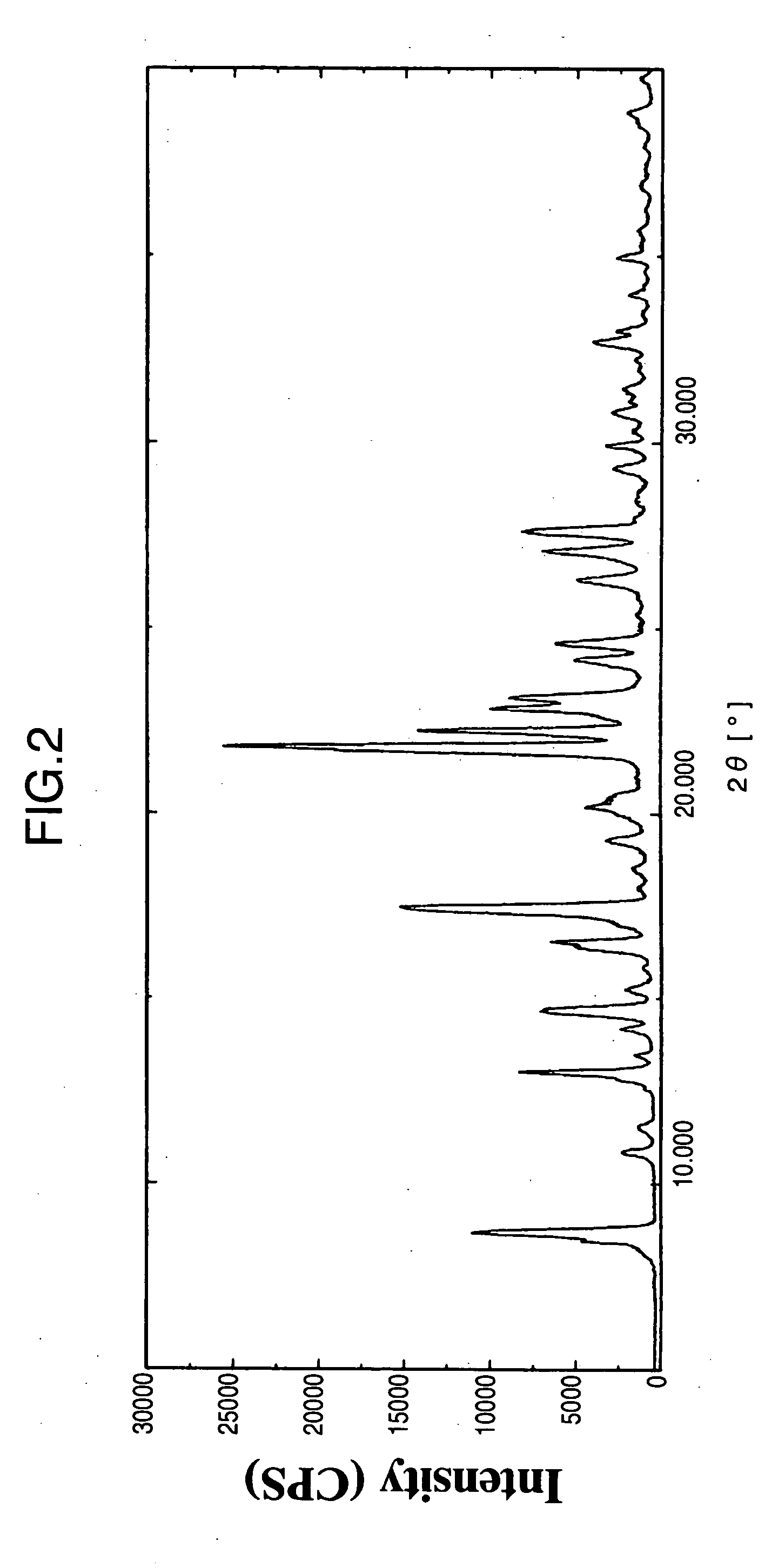
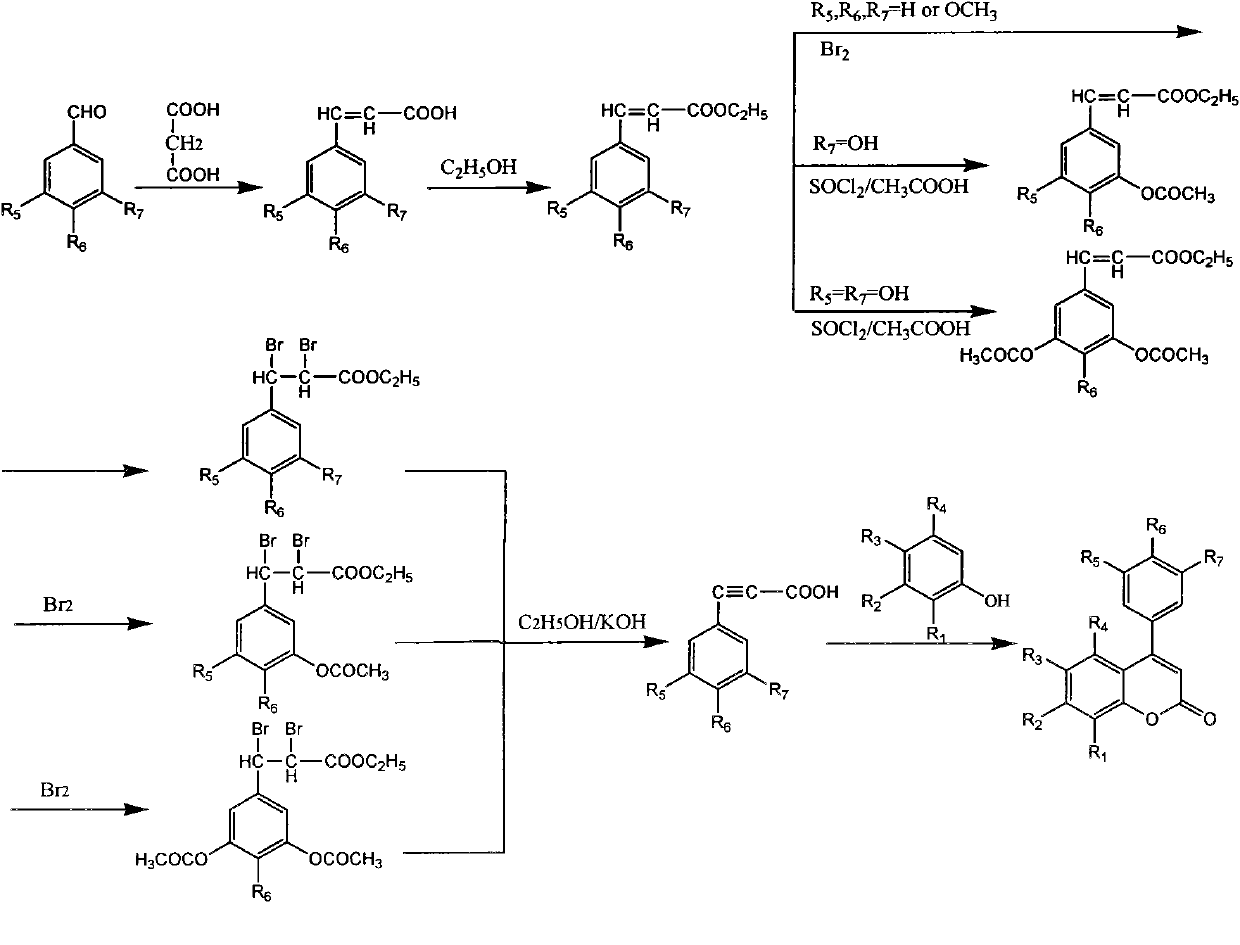
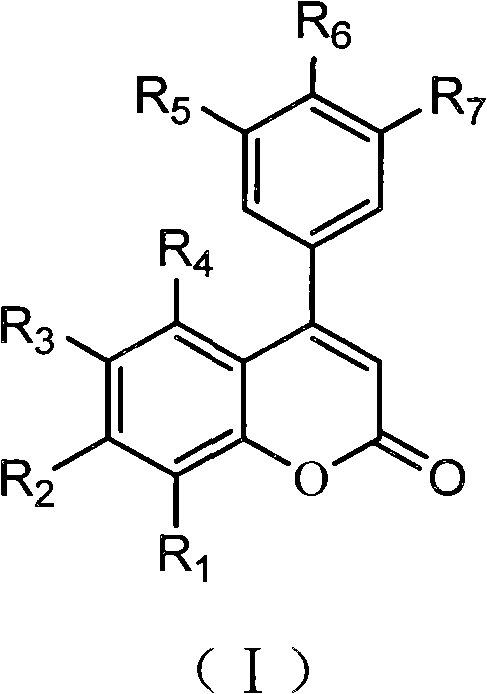
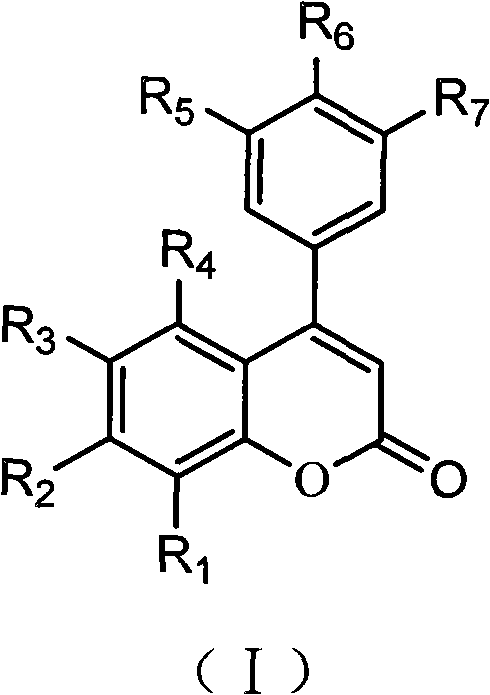
3,4-dihydro-4-aryl coumarin compounds as well as preparation method and application thereof
InactiveCN101906090AHas a chemical structureBiologically activeAntibacterial agentsOrganic chemistryVascular proliferationBenzaldehyde
The invention discloses 3,4-dihydro-4-aryl coumarin derivatives as well as a preparation method and application thereof. The preparation method comprises the following steps of: with substituted benzaldehyde and malonic acid as raw materials, heating in the presence of pyridine and piperidine and generating a Perkin reaction and a decarboxylic reaction to obtain substituted phenylacrylic acid derivatives; and then subjecting the substituted phenylacrylic acid derivatives and phenol compounds to a reaction in the presence of the catalysis of boron trifluoride diethyl ether and phosphorus oxychloride to obtain the 3,4-dihydro-4-aryl coumarin compounds. The compounds can be used for preparing medicaments for resisting tumors, abnormal vascular proliferation, bacterial and oxidation.
Owner:GUANGZHOU CHEM CO LTD CHINESE ACADEMY OF SCI
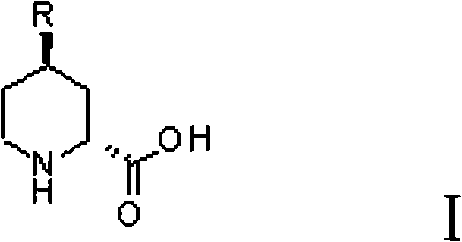
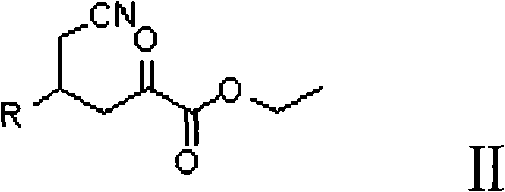
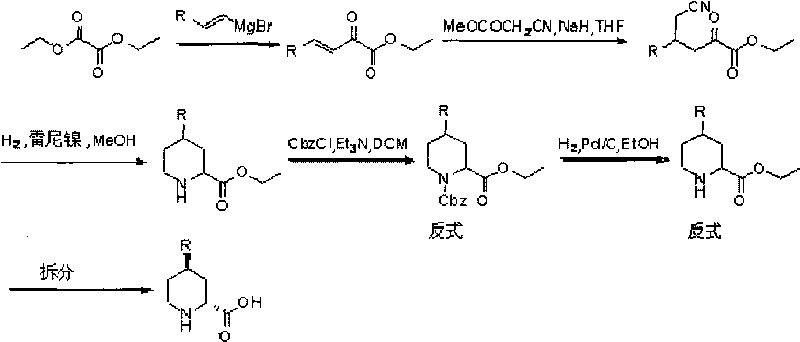
Preparation method for (2R, 4R)-4-substituted-2-piperidine carboxylic acid compound and intermediate thereof
InactiveCN101712645ARaw materials are easy to getSimple processAsymmetric synthesesSynthesis methodsOrganic synthesis
The invention relates to a synthesis method for preparing a (2R,4R)-4-methyl-2 -piperidine carboxylic acid compound taking diethyl oxalate as starting materials and an intermediate thereof, and belongs to the field of organic synthesis. The synthesis method comprises the following steps of: taking the diethyl oxalate and 1-bromo-substituted-propylene as the starting materials, performing a Grignard reaction and an addition reaction on the diethyl oxalate and 1-bromo-3-substituted-propylene to obtain intermediate 2-carbonyl-4-substituted-5 cyan ethyl valerate; and then performing a cyclization reaction, a benzyl ester protection reaction and a deprotection reaction on the intermediate 2-carbonyl-4-substituted-5 cyan ethyl valerate to obtain trans-4-substituted-2-piperidine ethyl formate; and finally, splitting the trans-4-substituted-2-piperidine carboxylic acid ethyl ester to obtain a chiral target product (2R,4R)-4-methyl-2-piperidine formic acid compound. The preparation method has the advantages of readily available raw materials, simple process, and mild reaction condition.
Owner:CHONGQING WORLD HAORUI PHARM CHEM
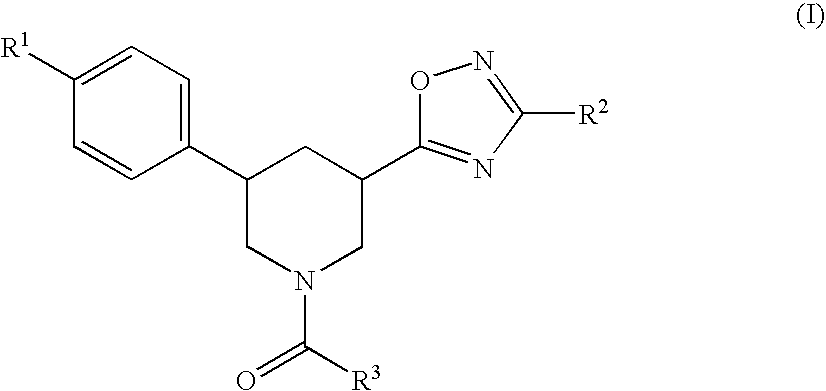
Heterocyclic-substituted piperidine compounds and the uses thereof
The invention relates to Heterocyclic-Substituted Piperidine Compounds, compositions comprising an effective amount of a Heterocyclic-Substituted Piperidine Compound and methods to treat or prevent a condition, such as pain, comprising administering to an animal in need thereof an effective amount of a Heterocyclic-Substituted Piperidine Compound.
Owner:PURDUE PHARMA LP
Indole Derivative Having Piperidine Ring
InactiveUS20070219179A1Enhanced inhibitory effectIncrease frequencyBiocideNervous disorderHydrogen atomSerotonin 1A Receptor
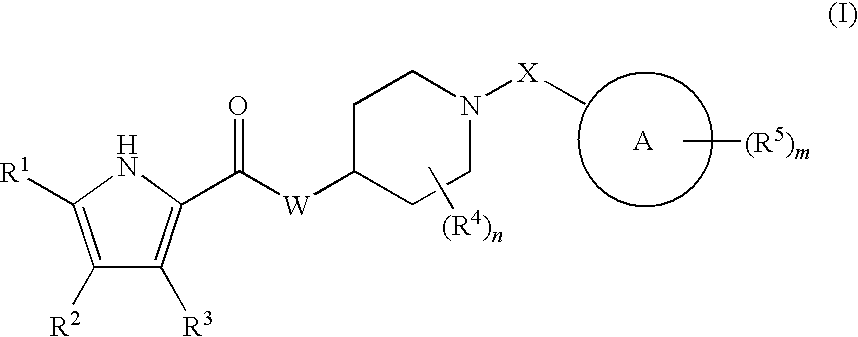
The present invention relates to a compound represented by the following formula, a pharmacologically acceptable salt thereof, or a use thereof as a pharmaceutical: wherein R1 and R2 are substituents adjacent to each other, and together with two carbon atoms to each of which they attach, form a 5- to 7-membered non-aromatic carbocyclic group or the like, which may be substituted by 1 to 4 substituents selected from (1) an oxo group, (2) a hydroxyl group, and the like; R3 represents a hydrogen atom or the like; and R6 represents a hydrogen atom or the like. This compound has a superior strength of binding to a 5-HT1A receptor and an antagonism to the receptor, and is useful as an agent for treating or preventing lower urinary tract symptoms, and particularly symptoms regarding urinary storage.
Owner:EISIA R&D MANAGEMENT CO LTD
4-aryl coumarin compound and preparation method and application thereof
InactiveCN101967135AHas a chemical structureBiologically activeAntibacterial agentsOrganic chemistryBenzaldehydeElimination reaction
The invention discloses a 4-aryl coumarin compound and a preparation method and application thereof. The 4-aryl coumarin compound has a structure shown in a formula (I). The preparation method comprises the following steps: taking substituted benzaldehyde and propandioic acid as raw materials, heating under condition of existing pyridine and piperidine, and generating Perkin reaction and decarboxylic reaction to obtain a series of substituted phenylacrylic acid compounds; carrying out bromination and elimination reaction on the substituted phenylacrylic acid compounds or acetylation to protect a phenolic hydroxyl group, and then carrying out bromination and elimination reaction to obtain substituted phenylpropiolic acid compounds; and enabling the substituted phenyl propargylic acid compounds and phenolic compounds to react under the catalysis of boron trifluoride etherate and phosphorus oxychloride or trifluoroacetic acid to obtain a series of 4-aryl coumarin compounds. The compounds can be used for preparing antineoplastic medicine, anti-abnormal-angiogenesis medicine, antimicrobial medicine, antioxidation medicine and antimalarial medicine.
Owner:GUANGZHOU CHEM CO LTD CHINESE ACADEMY OF SCI
Process and intermediates for resolving piperidyl acetamide steroisomers
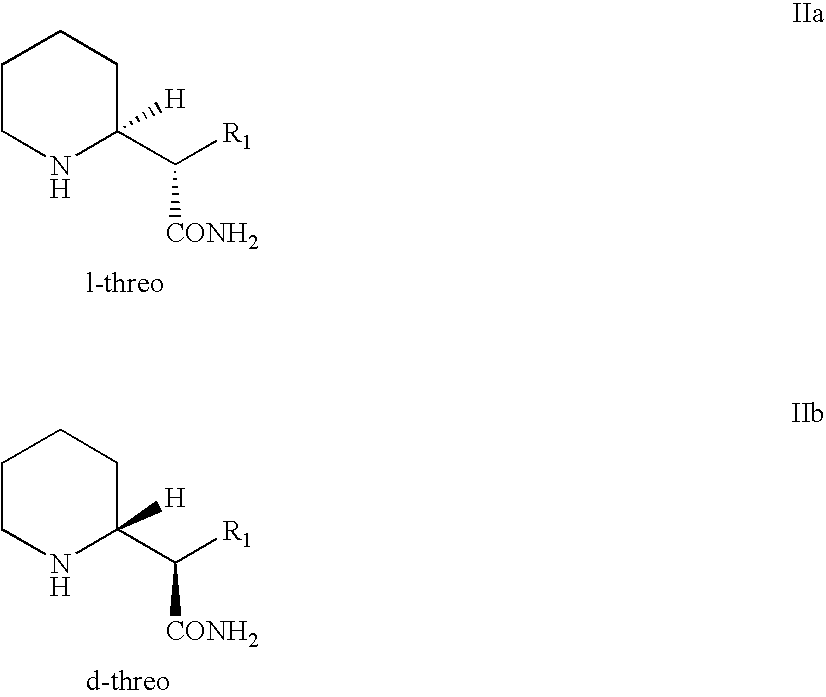
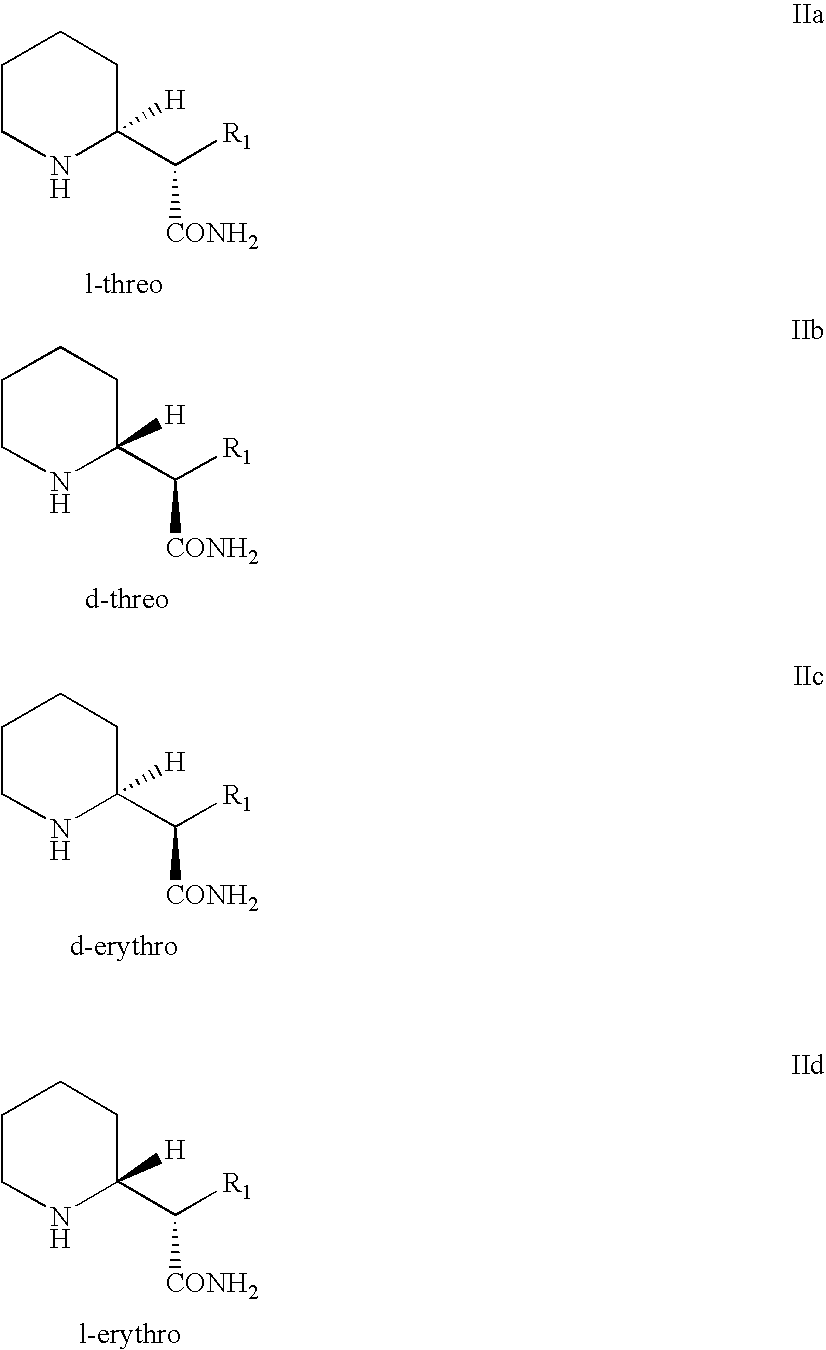
Processes and intermediates for preparing 2-substituted piperidines such as 2-substituted d-threo piperidines are provided, including processes and intermediates for resolution of piperidyl acetamide stereoisomers.
Owner:CELGENE CORP
Substituted piperidines
Owner:BAYER INTELLECTUAL PROPERTY GMBH
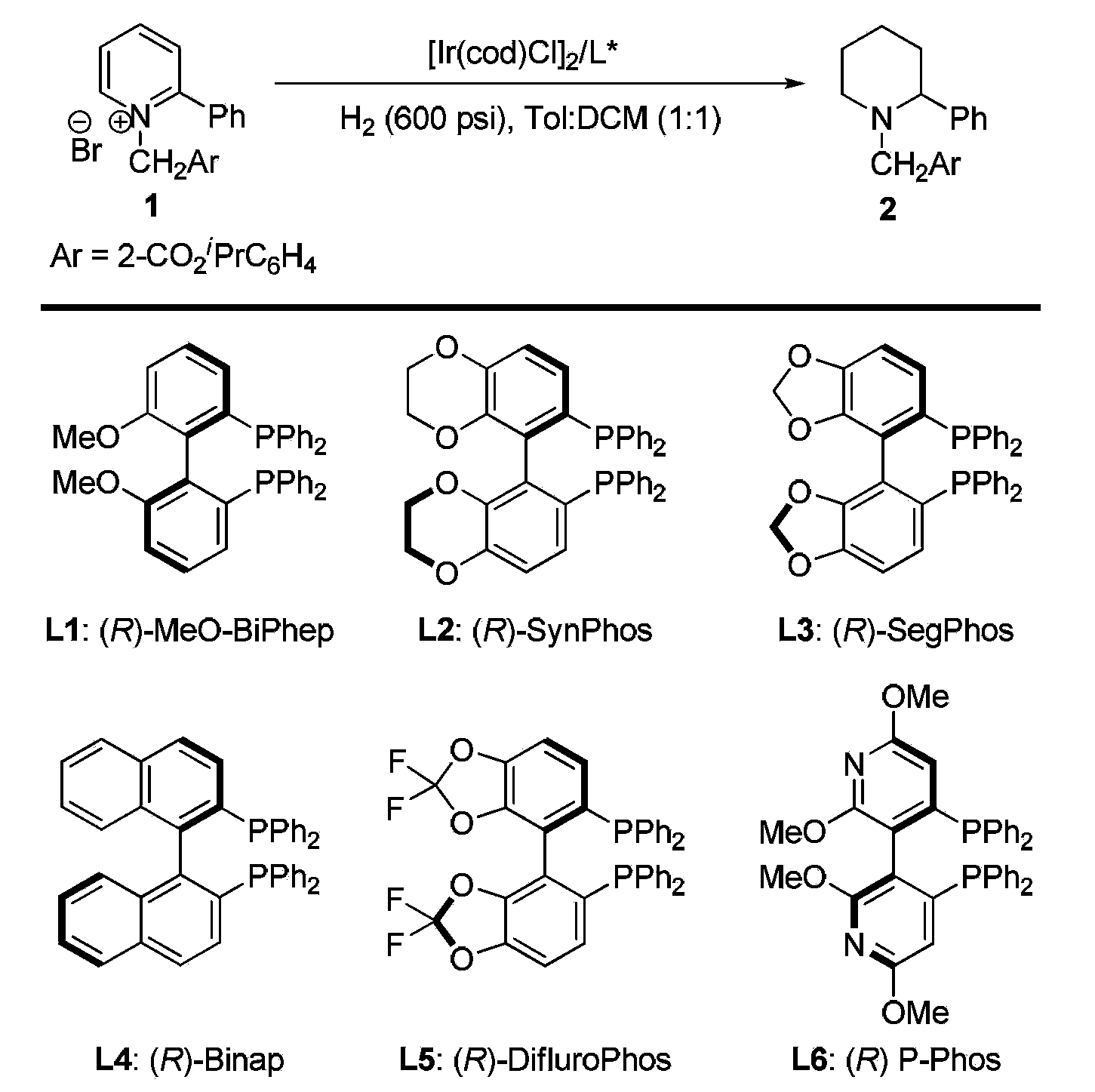
Method for synthesis of chiral piperidine derivative through iridium-catalyzed asymmetric hydrogenation of pyridine
ActiveCN103387533AHigh reactivityHigh enantioselectivityAsymmetric synthesesDiphosphinesIridium chloride
A method for synthesis of chiral piperidine derivatives through iridium-catalyzed asymmetric hydrogenation of pyridine employs a catalytic system of chiral diphosphine complex of iridium. The reaction can be carried out under the following conditions: temperature of 25-60 DEG C; a mixed solvent of toluene / dichloromethane (V / V=1:1); pressure of 13-50 barometric pressure; a ratio of substrate and catalyst of 50 / 1; and a catalyst of a complex of (1,5-cyclo-octadiene) iridium chloride dimer and diphosphine ligand. Hydrogenation of pyridine salt can obtain a corresponding chiral 2-substituted piperidine derivative, whose enantiomeric excess can reach 93%. The invention has advantages of simple and practical operation, easily available raw materials, high enantioselectivity, and good yield; in addition, the reaction has green atom economy and is friendly to environment.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Reagent for synthesis of para-methoxbenzyl (PMB) ethers and associated methods
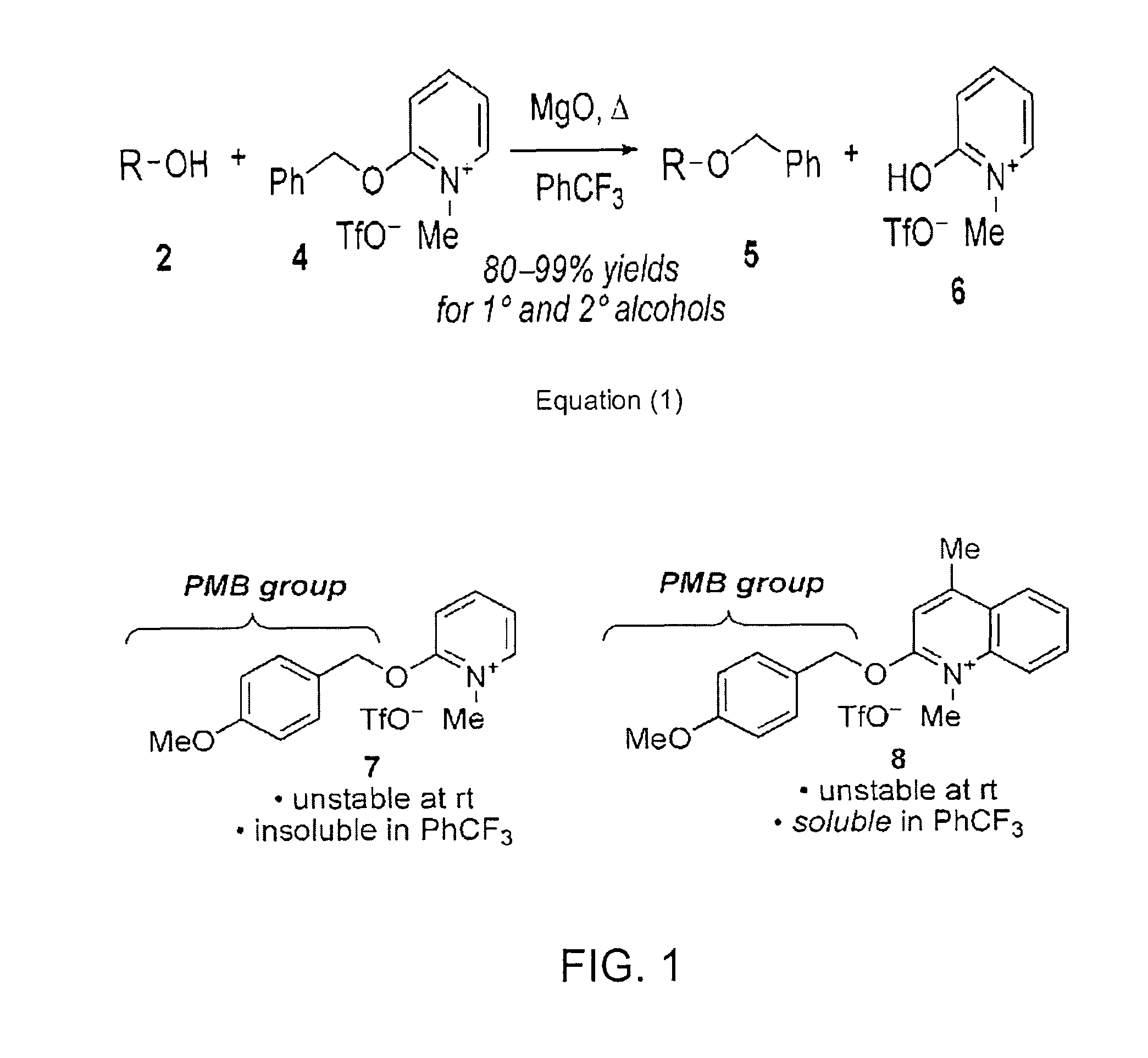
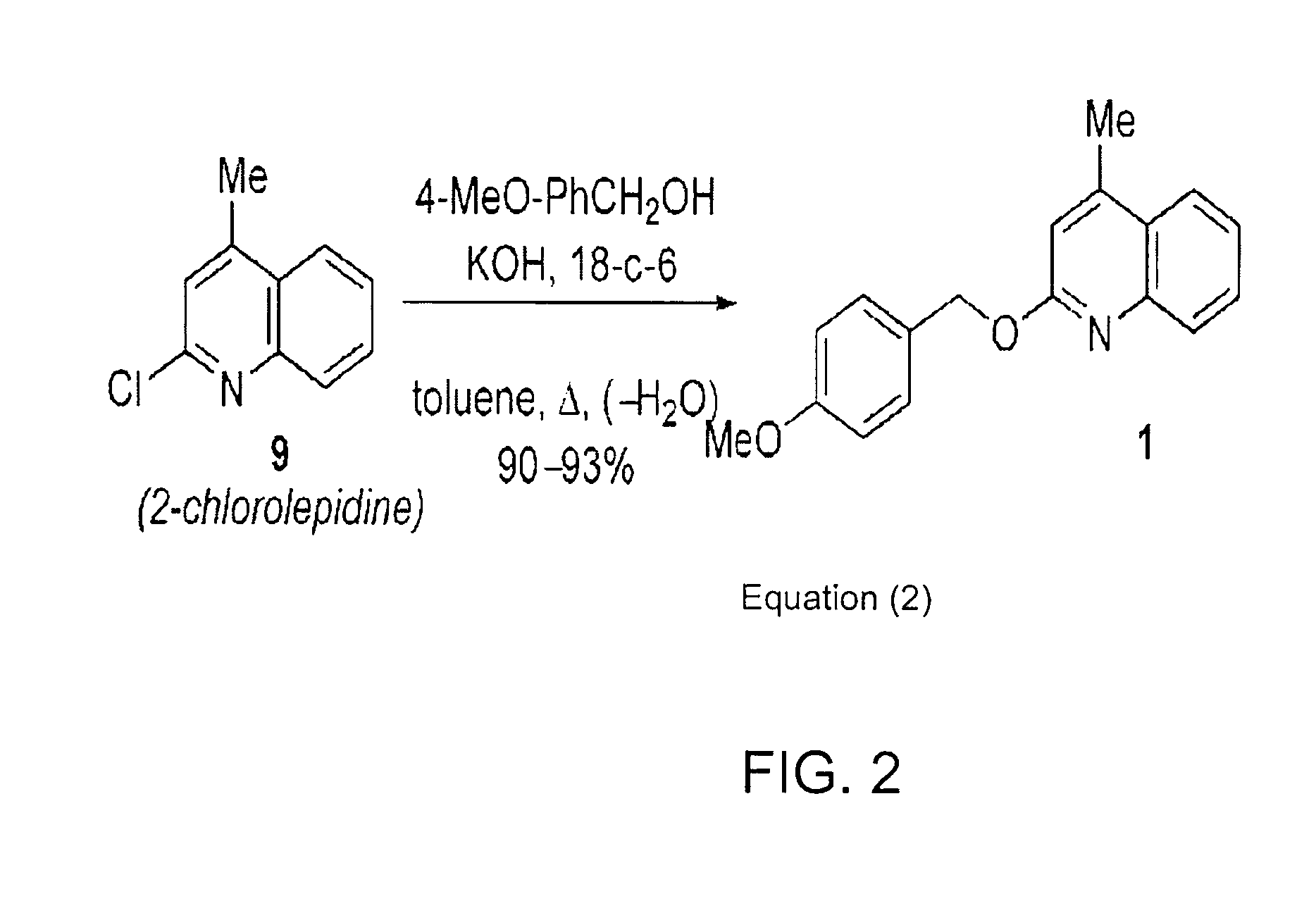
A newly synthesized compound designated lepidine ether 2-(4-Methoxybenzyloxy)-4-methylquinoline reacts with methyl triflate in the presence of alcohols to generate a neutral organic salt that transfers the para-methoxybenzyl (PMB) protecting group onto alcohols in high yield and under mild conditions.
Owner:FLORIDA STATE UNIV RES FOUND INC
Catalyst for use in preparation of piperidine and piperidine derivatives
InactiveCN102091638AHigh selectivityIncrease production capacityOrganic chemistryMetal/metal-oxides/metal-hydroxide catalystsHydrogen pressureFluidized bed
The invention discloses a catalyst for use in the preparation of piperidine and piperidine derivatives by hydrogenation of pyridine and pyridine derivatives. The catalyst consists of an active component, an assistant, and a carrier. The active component is one or several of transitional metals Pd, Rh and Ni. The carrier is one of Al2O3, active carbon, ZrO2 or SiO2. In fixed fluidized bed reactor, the pyridine and pyridine derivatives can be converted into the piperidine and piperidine derivative products with high activity and high selectivity at certain temperature and hydrogen pressure and under the action of the catalyst.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
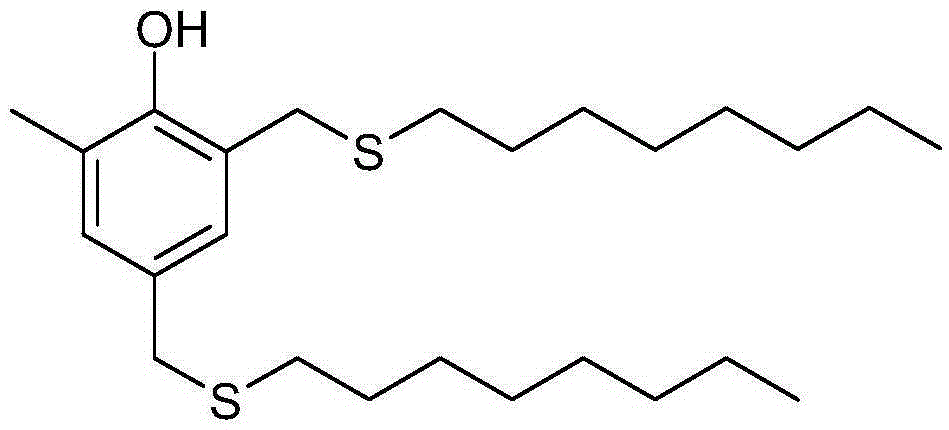
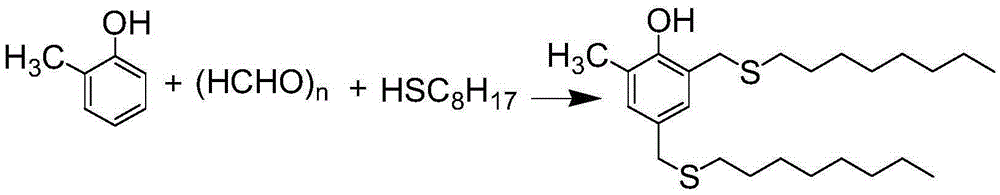
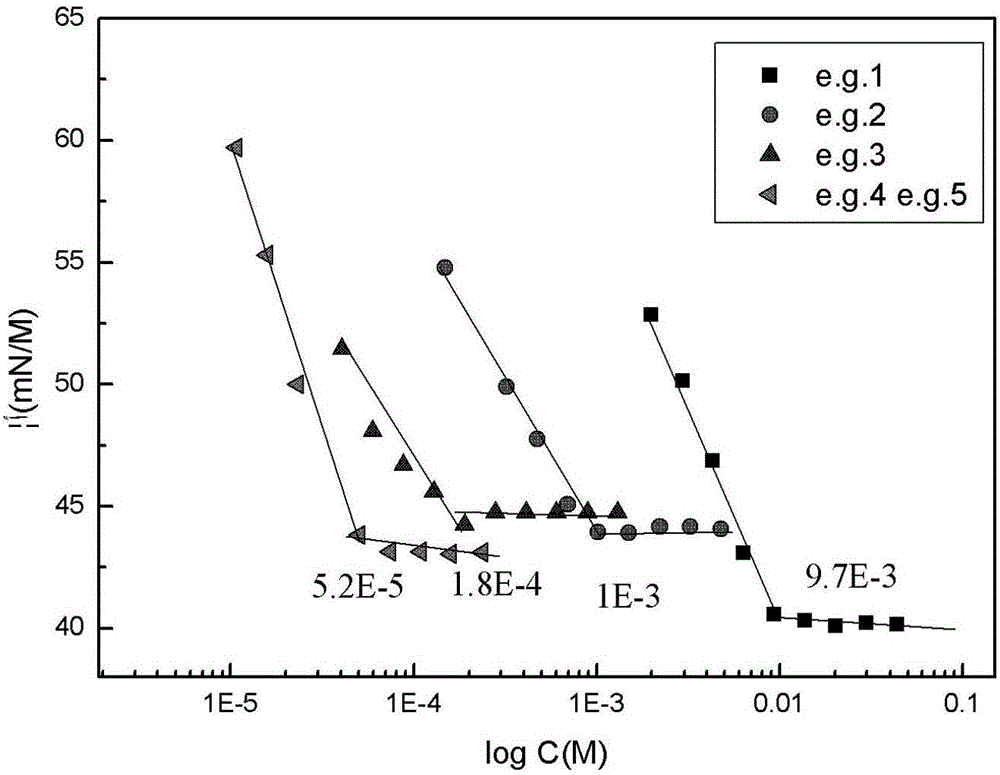
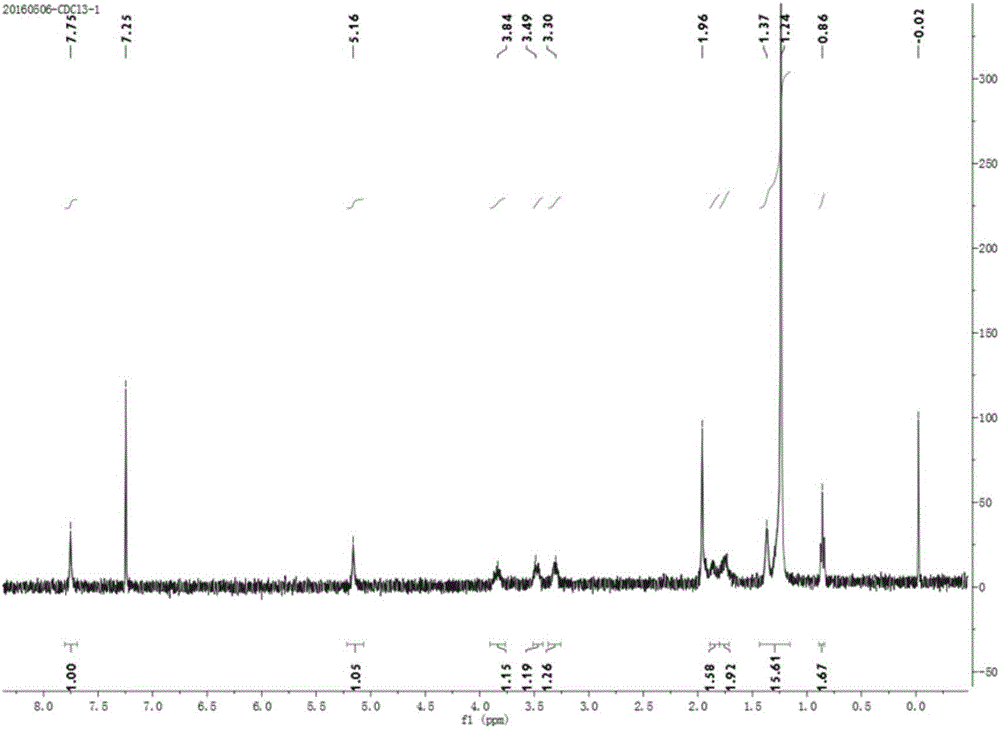
Preparation method for antioxidant 2,4-bi(n-octyl sulfur methylene)-6-methylphenol
The invention relates to a novel preparation method for antioxidant 2,4-bi(n-octyl sulfur methylene)-6-methylphenol. Under piperidine catalysis, raw materials, namely, o-methylphenol, paraformaldehyde and octyl mercaptan react to generate antioxidant 2,4-bi(n-octyl sulfur methylene)-6-methylphenol; after the reaction, a solvent, water generated through the reaction, a catalyst and excessive unreacted raw materials are removed, and a crude product is obtained; the crude product is refined through a crystallization or cooling washing method. The product obtained through the method is colorless and is free of the disgusting smell of octyl mercaptan.
Owner:CHANGZHOU UNIV
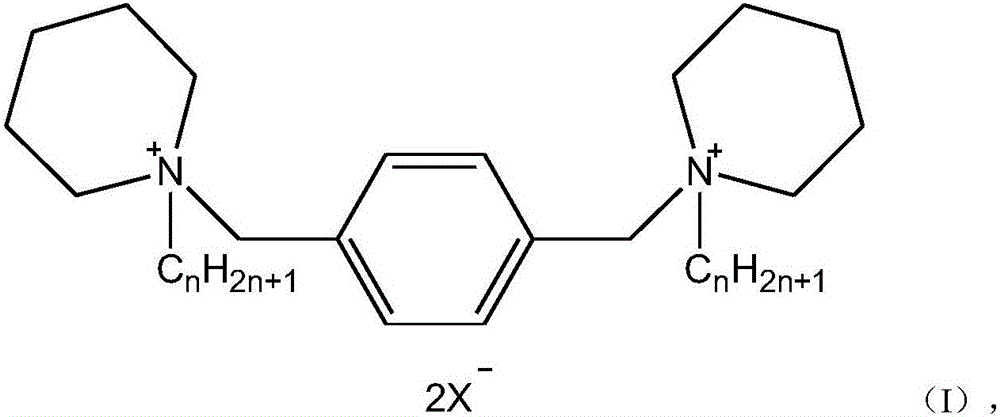
Cyclic quaternary ammonium salt gemini surfactant and preparation method thereof
ActiveCN106512848AHigh yieldHigh purityOrganic chemistryTransportation and packagingSubstitution reactionSURFACTANT BLEND
The invention discloses a cyclic quaternary ammonium salt gemini surfactant. The preparation method comprises: carrying out a substitution reaction by using 1-bromoalkane and piperidine as raw materials to obtain alkyl substituted piperidine; and carrying out a quaternization reaction on benzyl dihalide and the alkyl substituted piperidine to obtain the heterocyclic quaternary ammonium salt gemini surfactant. According to the present invention, the cyclic head group and the rigid linking chain group are concurrently introduced, such that the space volume is much larger than the linear chain alkyl chain structure, the maximum single molecule occupation area of the surfactant is significantly improved, and the surface adsorption function and the efficiency of the surfactant are improved; and the method has advantages of easily-available raw materials, simple preparation process, low pollution, high product yield, high product purity, and important practical application significance.
Owner:WUHAN OXIRAN SPECIALTY CHEM CO
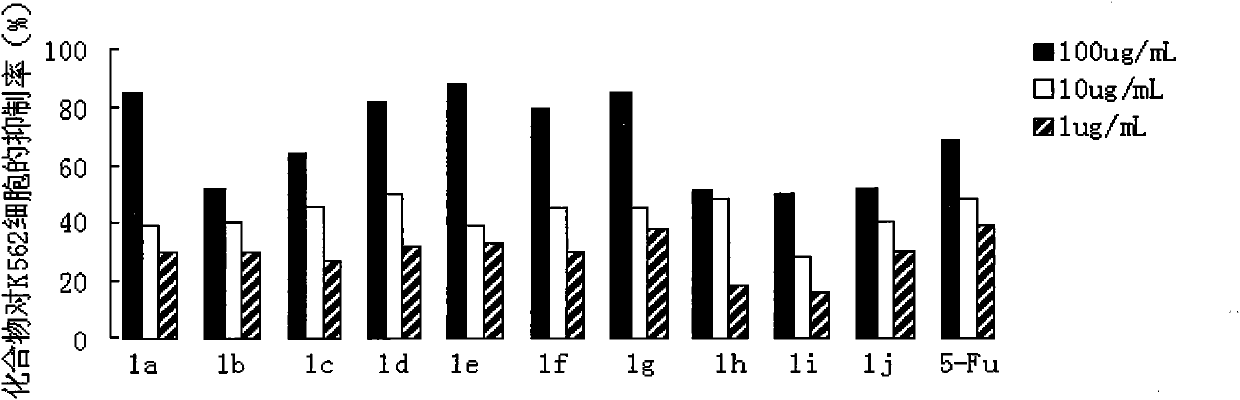
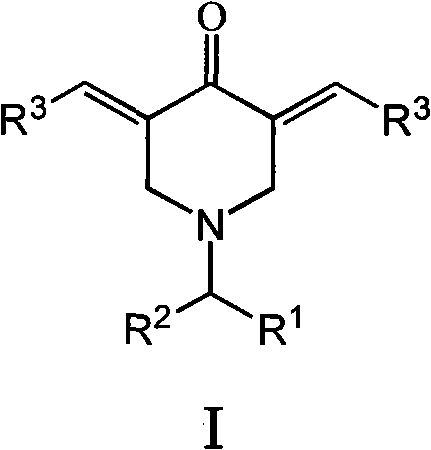
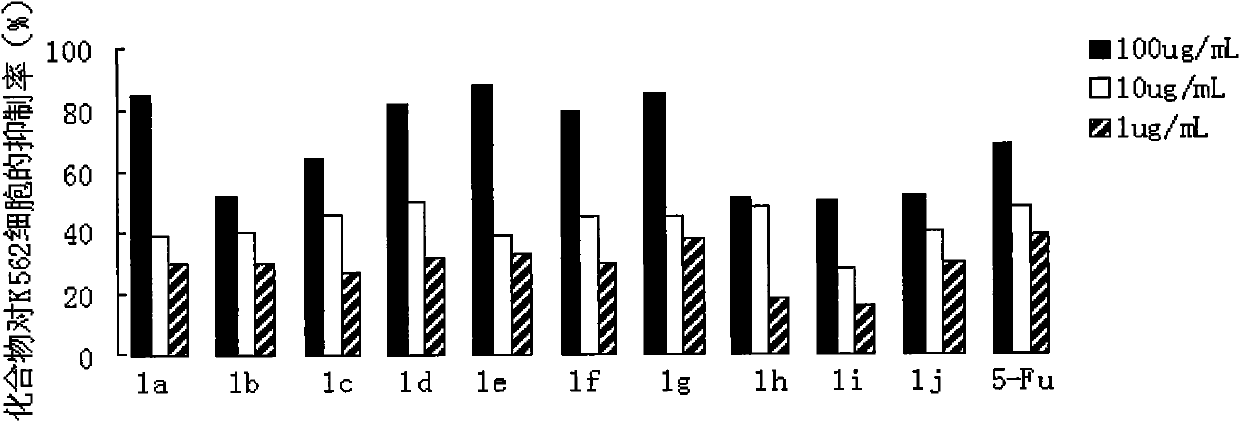
Preparation method and application of N-substituted-3,5-dibenzal piperidine-4-one
InactiveCN101973935APracticalStrong inhibitory activityOrganic chemistryAntineoplastic agentsKetoneHydrolysis
The invention belongs to a lead compound of a novel drug for preventing leukaemia K562 cell proliferation and relates to a preparation method and application of N-substituted-3,5-dibenzal piperidine-4-one which can effectively inhibit leukaemia K562 cell proliferation. The preparation method comprises the following steps of: performing Michael addition reaction for substituted amine and methyl acrylate to prepare a yellow oily object N,N-di(beta-methyl propionate) substituted amine; performing Dieckmann condensation under effect of sodium alkoxide and performing hydrolysis and decarboxylationunder effect of acid to obtain a yellow oily object N-substituted piperidine-4-one; and dehydrating the product obtained to obtain the N-substituted-3,5-dibenzal piperidine-4-one with the general formula (I). The product of the invention has higher inhibition activity to eukaemia K562 cell proliferation and the method has the advantages of simple process and easy production.
Owner:SHANGHAI NORMAL UNIVERSITY
Piperidine derivatives useful as CCR5 antagonists
The present invention provides a compound of the formula or a pharmaceutically acceptable salt or solvate thereof, wherein the various moieties are as defined in the specification. The present invention also provides pharmaceutical compositions containing the compound of this invention, and methods of treatment using the compound of this invention. The invention also relates to the use of a combination of a compound of this invention and one or more antiviral or other agents useful in the treatment of Human Immunodeficiency Virus (HIV). The invention further relates to the use of a compound of this invention, alone or in combination with another agent, in the treatment of solid organ transplant rejection, graft v. host disease, arthritis, rheumatoid arthritis, inflammatory bowel disease, atopic dermatitis, psoriasis, asthma, allergies or multiple sclerosis.
Owner:SCHERING CORP
High-yield high-purity DAST source powder synthetic process
The invention discloses a high-yield high-purity DAST source powder synthetic process. The synthetic process comprises the following two steps: 1, reacting 4-methylpyridine with methyl p-toluenesulfonate in the presence of absolute ethyl alcohol serving as a solvent to obtain an absolute ethyl alcohol solution of 4-methyl-N-methylpyridine tosilate; and 2, reacting 4-methyl-N-methylpyridine tosilate with p-dimethylaminobenzaldehyde in the presence of absolute ethyl alcohol serving as a solvent and under the catalytic action of di-n-butylamine or piperidine, so as to obtain high-yield (85-95%) high-quality (90-95%) DAST source powder. According to the synthetic process, absolute ethyl alcohol is used as a reaction solvent, harm of poisonous and harmful solvents such as methylbenzene and methyl alcohol to the body of an operator can be avoided and the pollution of waste liquid to the environment can be avoided. The research success of the high-yield high-purity DAST source powder synthetic process is beneficial to culture of high-quality large-sized DAST crystal, thereby laying a good material and theoretical basis for research on the DAST crystal and related products.
Owner:CHINA ELECTRONICS TECH GRP NO 46 RES INST
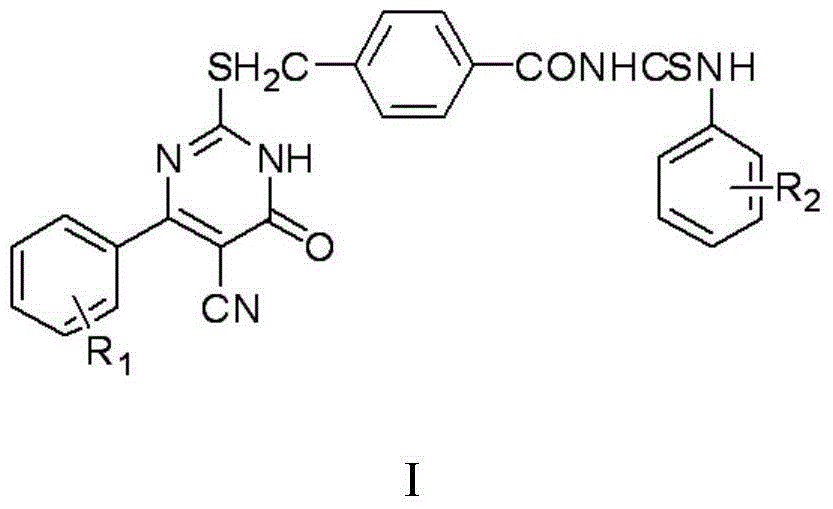
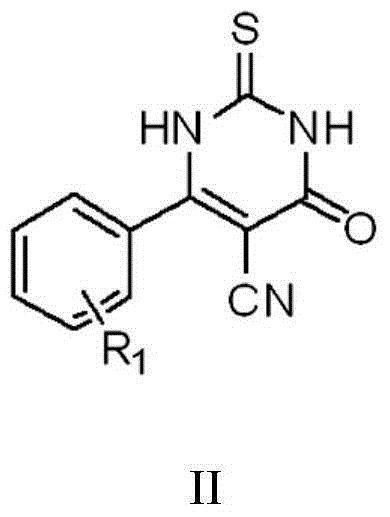
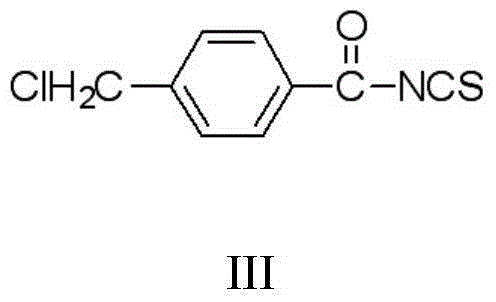
Thiouracil derivative, preparation method and application thereof
InactiveCN104876881ABroaden your optionsOvercoming the drawbacks of drug resistanceAntibacterial agentsOrganic active ingredientsThioureaPotassium thiocyanate
The invention discloses a thiouracil derivative. The chemical formula is shown in the formula I, wherein R1 is hydrogen, halogen or aromatic group, R2 is hydrogen, halogen or alkyl group from C1 to C6. Simultaneously, the invention also discloses a method for synthesizing the derivative. The method comprises the following steps : adopting aromatic aldehyde or an aromatic amine compound as a starting material, and reacting the aromatic aldehyde, ethyl cyanoacetate and thiourea under the catalysis of piperidine to obtain a compound II; reacting chloromethyl-benzoyl chloride and potassium thiocyanate in a methylbenzene / water / TBAB system to obtain a compound III; reacting the compound III and aromatic amine in an acetonitrile solvent to obtain a compound IV, reacting the compound II and the compound IV with the equal mole in the acetonitrile solvent under the catalytic action of potassium carbonate to obtain a target product, i.e., a compound I. The method adopted is simple, the operation is easy, and the large-scale production is easy; and verified by experiment, the prepared compound I has stronger bacteriostatic activity and can be widely applied in bacteriostatic medicine preparations.
Owner:HEBEI UNIVERSITY
Pyrazolo piperidine derivatives as NADPH oxidase inhibitors
The present invention is related to pyrazolo piperidine derivatives of Formula (I), pharmaceutical composition thereof and to their use for the treatment and / or prophylaxis of disorders or conditions related to Nicotinamide adenine dinucleotide phosphate oxidase (NADPH Oxidase).
Owner:GENKYOTEX SA
Process for production of piperidine derivatives
Owner:ALBANY MOLECULAR RESEARCH INC
Method for preparing Niraparib of PARP (poly-ADP-ribose polymerase) inhibitor
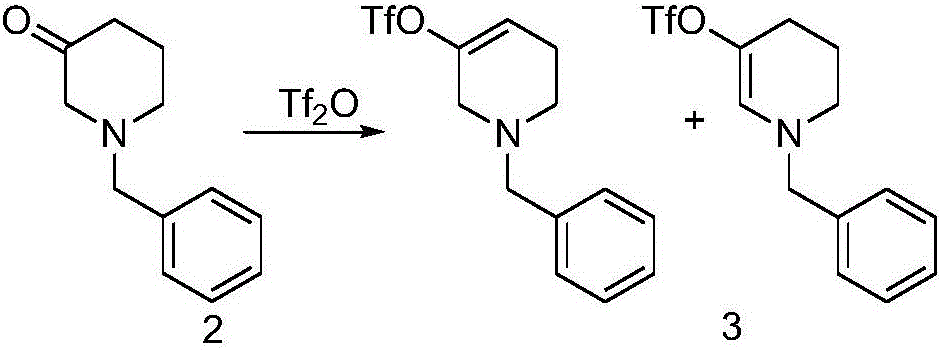
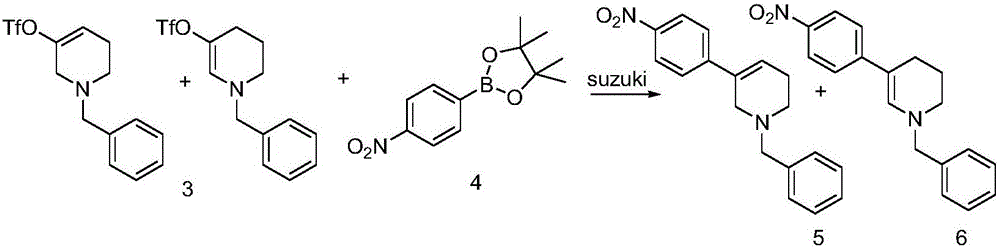
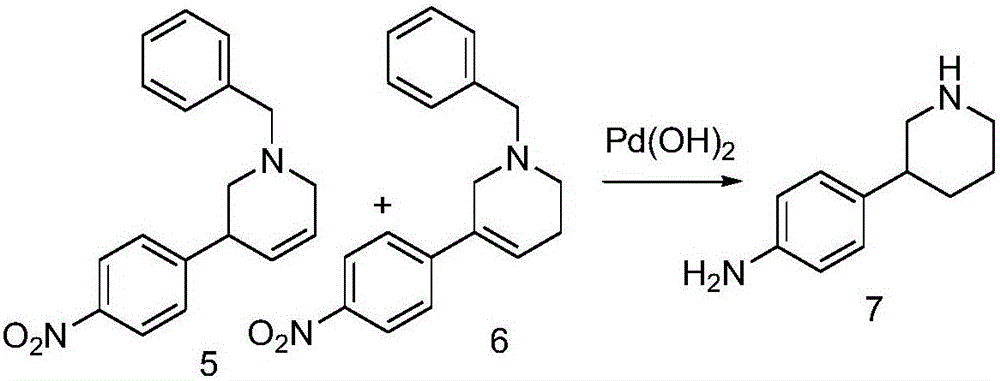
The invention discloses a method for preparing 2-(4-((3S)-3-piperidyl) phenyl)-2H-indazole-7-formamide of a compound. Benzyl protected-piperidone is generated into piperidone of 3-triflic anhydride under the action of triflic anhydride, the piperidone and nitrobenzoic acid are subjected to Suzuki reaction to obtain a coupled product, 3-(4-aminophenyl) piperidine is obtained under the action of a palladium reagent, (S)-3-(4-halogenated phenyl) piperidine is prepared with a chirality resolution reagent, the (S)-3-(4-halogenated phenyl) piperidine is condensed with 3-formyl-2-methyl nitrobenzoate to form pyrazolone ring under the action of sodium azide, and the Niraparid (with the molecular entity of 2-(4-((3S)-3-piperidyl) phenyl)-2H-indazole-7-formamide) is prepared via aminolysis.
Owner:NANJING CORE TECH CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com