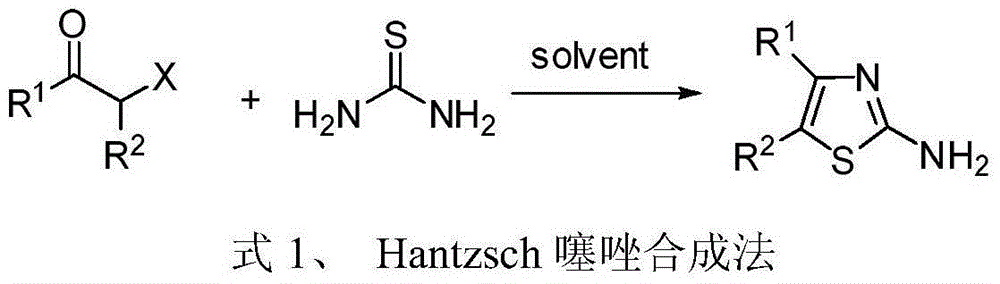
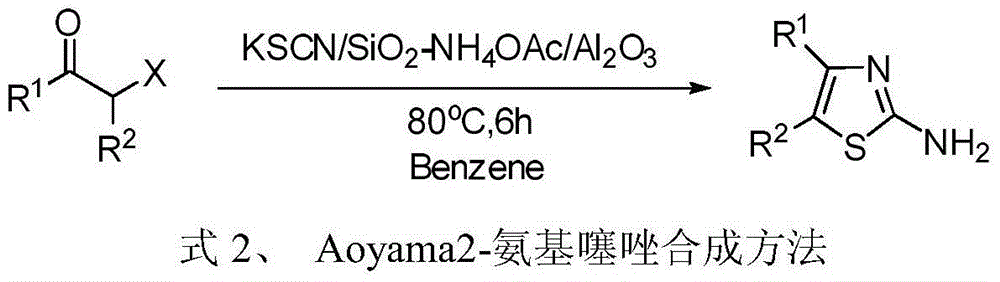
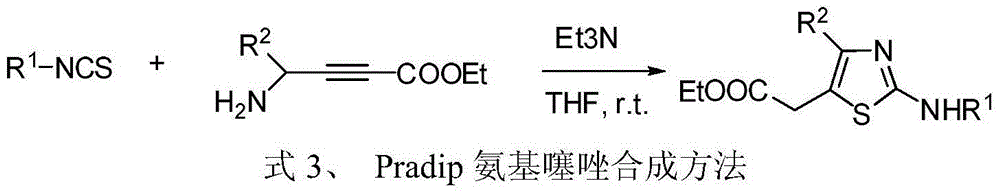
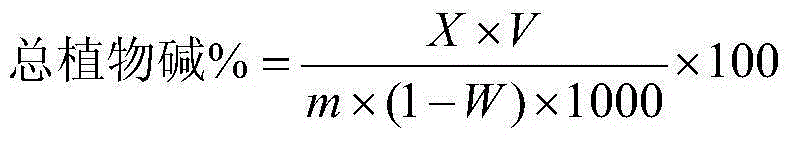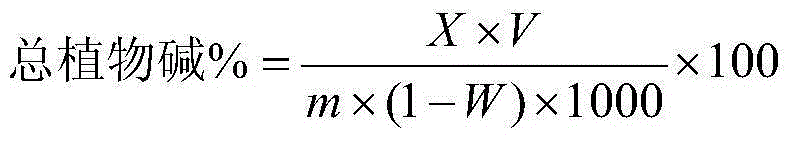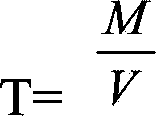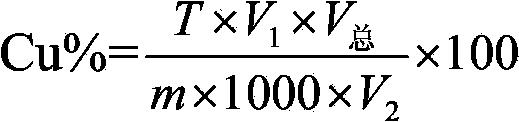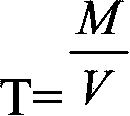Patents
Literature
275 results about "Potassium thiocyanate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Potassium thiocyanate is the chemical compound with the molecular formula KSCN. It is an important salt of the thiocyanate anion, one of the pseudohalides. The compound has a low melting point relative to most other inorganic salts.
4, 5-disubstituted-2-aminothiazole compound and preparation method thereof
InactiveCN104151262ALow priceReduce pollutionOrganic chemistryAntineoplastic agentsRotary evaporatorPotassium thiocyanate
The invention discloses a 4, 5-disubstituted-2-aminothiazole compound. The structural formula is shown in the specification, wherein R1 is 4-tolyl, 4-chlorophenyl, 4-methoxyphenyl, 4-nitrobenzene or propoxy, and R2 is phenyl, 4-tolyl, 4-fluorophenyl, 4-methoxyphenyl, 2-furyl, isopropyl, 4-nitrophenyl or n-propyl. The invention simultaneously provides a preparation method of the 4, 5-disubstituted-2-aminothiazole compound. The preparation method comprises the following steps: enabling an olefin azide type compound and potassium thiocyanate to react at the temperature of 75-85 DEG C in the presence of a solvent and a metal catalyst, concentrating an obtained reaction solution, then extracting with water and ethyl acetate, washing an obtained organic layer, then drying and concentrating by a rotary evaporator; performing silica gel column chromatography on an obtained concentrate to obtain the 4, 5-disubstituted-2-aminothiazole compound.
Owner:ZHEJIANG UNIV
Deplating liquid for NiCuNi plating on surface of sintered NdFeB and deplating process thereof
InactiveCN102787321AFast stripping speedImprove deplating efficiencySodium sulfocyanatePotassium thiocyanate
The invention discloses a deplating liquid for a NiCuNi plating on the surface of sintered NdFeB and a deplating process thereof. The deplating liquid for the NiCuNi plating on the surface of sintered NdFeB adopts deionized water or clean tap water as the liquid solvent, and all components and component contents are as follows: 60-90g / L reserve salt S, 120-150ml / L ethylene diamine, 20-40g / L anti-corrosion complexing agent and 50-100ml / L mineral acid. The solute further comprises a surface active agent of 0.5-1g / L. The anti-corrosion complexing agent is sodium citrate or triammonium citrate; the mineral acid is nitric acid or ammonium nitrate; and the surface active agent is potassium thiocyanate, or sodium sulfocyanate or ammonium thiocyanate. The deplating process comprises the following steps: preparing a deplating bath; disposing a deplating liquid; executing the deplating; and removing the deplating liquid on a product. The deplating liquid has the advantages of stable property, good deplating effect and low cost; and the deplating operation is simple and convenient, pollution is prevented, body harm of operators is relieved, waste is changed into valuable and the benefits are remarkable.
Owner:牛凯
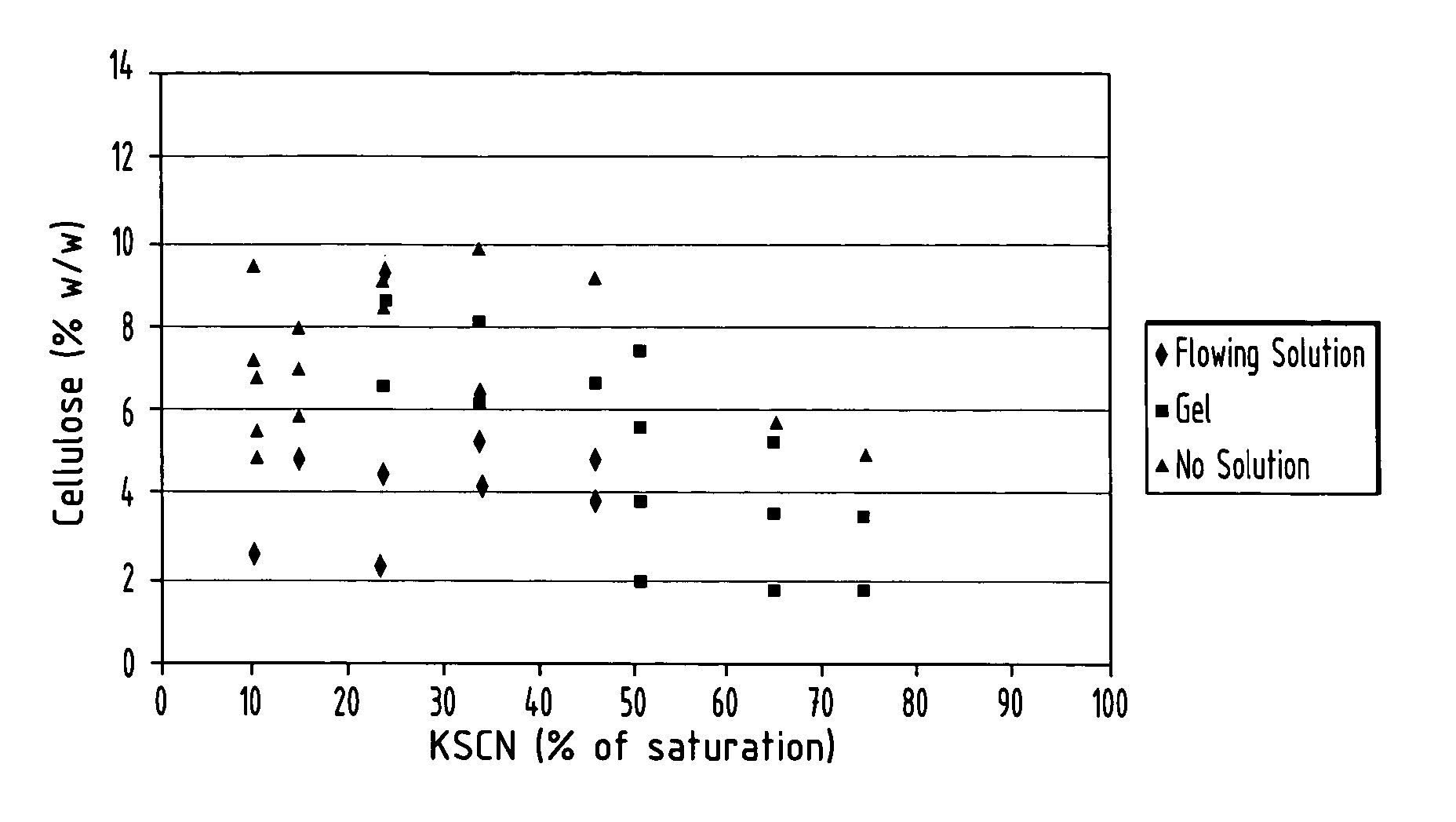
Cellulose solution in novel solvent and electrospinning thereof
InactiveUS20050247236A1Artificial filaments from cellulose solutionsElectric discharge heatingEthylenediamineElectrospinning
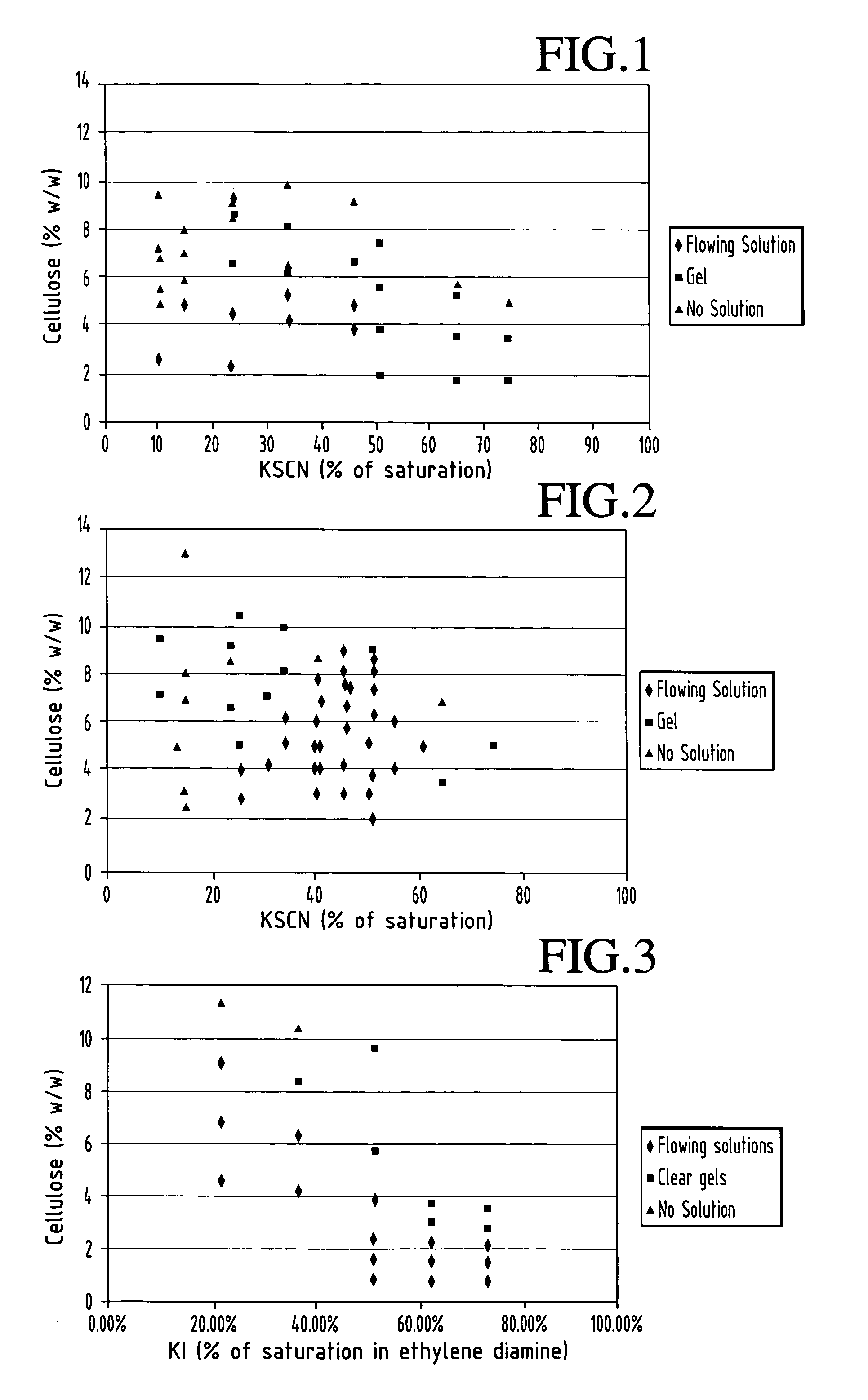
Nanoscale diameter cellulose fibers are produced by dissolving cellulose at a level of 3 to 25% (w / w) in a solvent comprising ethylene diamine and salt selected from the group consisting of potassium thiocyanate, potassium iodide and mixtures thereof, the salt present in an amount of 10 to 75% of its saturation point.
Owner:CORNELL RES FOUNDATION INC
Method for preparaing dephosphorized and ferrum-carried activated carbon adsorbent
InactiveCN102614854AImprove adsorption capacityImprove processing efficiencyOther chemical processesPotassium rhodanidePotassium thiocyanate
The invention belongs to the technical field of sewage treatment, in particular to a method for preparaing dephosphorized and ferrum-carried activated carbon adsorbent, which comprises the following steps of: soaking powdered activated carbon through hydrochloric acid, washing through water, and drying at the temperature of 100-150 DEG C; injecting the obtained activated carbon into strong oxidizing acid solution so as to be soaked, rinsing, and drying a rinsed sample; and finally, mixing the obtained activated carbon and inorganic ferric salt solution, stirring, re-drying, cooling to room temperature, removing surface layer coverings, washing unloaded ferric oxide through water until rinsing to be without obvious color and without red color through test by potassium rhodanide solution, and drying continuously to obtain the high-efficient dephosphorized activated carbon adsorbent rich in iron oxide compound. Compared with the prior art, the method has the advantages of simplicity, moderate preparing condition, easiness in implementation, strong operability, high processing efficacy for waste water containing phosphorus, and purification cost reduction.
Owner:FUDAN UNIV
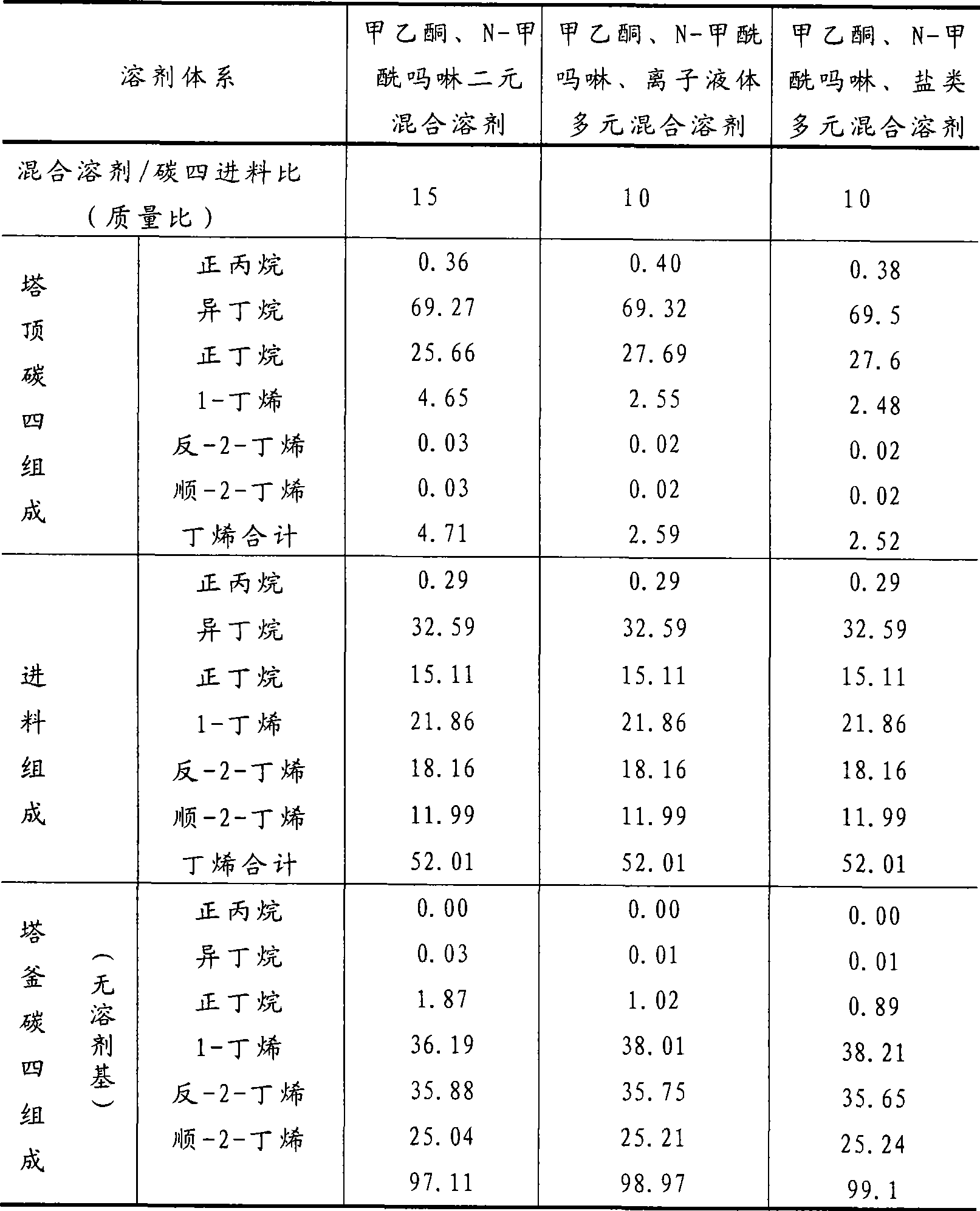
Method for separating butane and butene by using multiple mixed solvent
InactiveCN101417913ARetain solubilityImprove solubilityDistillation purification/separationBulk chemical productionSodium sulfocyanateTetrafluoroborate
The invention relates to a method for spearing butane from butylene with the multicomponent compound of ionic liquid, saline, ethyl methyl ketone and N-formylmorpholine. The content of ionic liquid in the multicomponent compound is 1.0 to 95 percent; the content of the saline in the multicomponent compound is 0.5 to 20 percent; the electropositive ion of the ionic liquid is iminazole electropositive ion, alkyl imidazole electropositive ion or alkyl quaternary ammonium ion, or the compound thereof; the electronegative ion is tetrafluoroborate electronegative ion, hexafluorophosphoric acid electronegative ion, nitrate ion, tetrachloro aluminic acid iron, heptachlor bi aluminic acid iron, chloride ion, bromine electronegative ion or the mixture; and the ionic liquid can be dimethylformamide potassium thiocyanate compound salt, dimethylformamide sodium sulfocyanate compound salt, or the compound; the saline is potassium thiocyanate, sodium sulfocyanate, ammonium thiocyanate, sodium nitrate, potassium nitrate, sodium iodide, potassium iodide, zinc chloride, copper chloride, zinc chloride, potassium bromide, or the compound thereof.
Owner:YANTAI UNIV
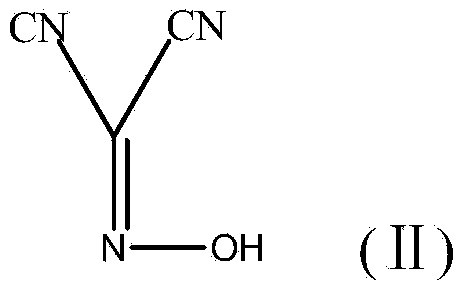
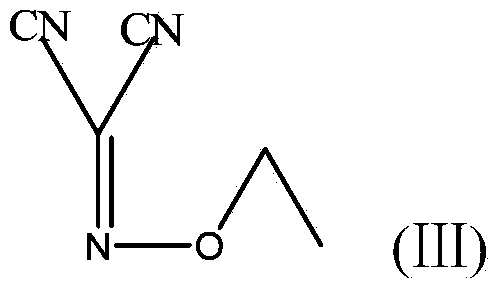
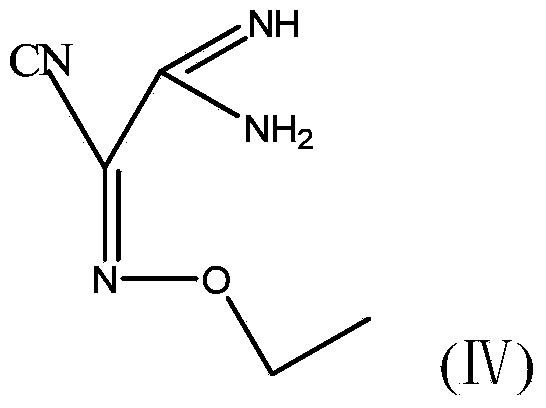
Method for preparing (Z)-5-amino-alpha-(ethoxy imino group)-1, 2, 4-thiadiazole-3-acetic acid
ActiveCN103804321AShort synthetic routeFew synthetic stepsOrganic chemistryAcetic acidPotassium thiocyanate
The invention discloses a method for preparing (Z)-5-amino-alpha-(ethoxy imino group)-1, 2, 4-thiadiazole-3-acetic acid. The method comprises steps of firstly, carrying out oximation reaction on malononitrile and sodium nitrite under the action of acetic acid so as to obtain a compound A, then carrying out Williamson synthesis on the compound A and bromoethane or diethyl sulfate under alkaline condition, so as to obtain a compound B, then carrying out amidine reaction on the compound B and ammonia water, so as to obtain a compound C, carrying out cyclization reaction on the compound C and potassium rhodanide for ring closing so as to obtain a compound D, and finally, hydrolyzing the compound D under the action of a strong base, so as to obtain (Z)-5-amino-alpha-(ethoxy imino group)-1, 2, 4-thiadiazole-3-acetic acid. The method has few synthesis steps, has low cost, uses the materials which are cheap and easy to obtain, is beneficial to industrial production, and has light contamination; the purity of the product can reach 99%, so that synthesis of high-purity ceftaroline fosamil in the subsequent step is guaranteed.
Owner:山西海泰电子材料有限公司
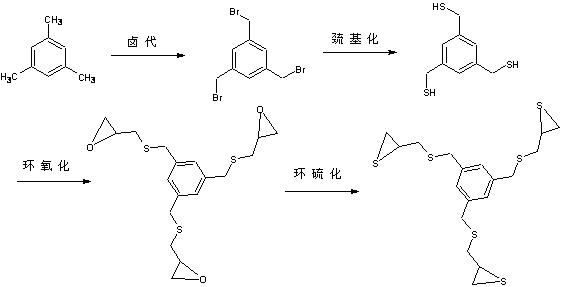
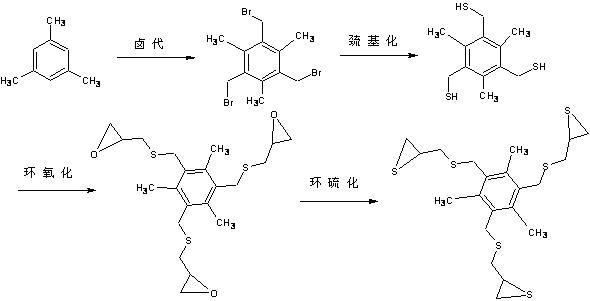
Optical resin monomer with high refractive index and preparation method thereof
ActiveCN102329298AImprove heat resistanceOrganic chemistryOptical elementsBenzoyl peroxidePotassium thiocyanate
The invention provides an optical resin monomer with high refractive index. The monomer has the structural formula shown in the specification, wherein in the structural formula, R is hydrogen, alkyl, alcoxyl, halogen and aryl; Ph is a benzene ring; m and n are integers; m is not less than 1 and not more than 6; and n ranges from 0 to (6-m). A preparation method comprises the following steps: taking the following raw materials: aromatic hydrocarbon, NBS (N-bromosuccinimide), BPO (benzoyl peroxide), thiourea, sodium hydroxide, hydrochloric acid, epichlorohydrin and potassium thiocyanate, wherein the mole ratio of aromatic hydrocarbon to NBS to thiourea to sodium hydroxide to hydrochloric acid to epichlorohydrin to potassium thiocyanate is 1:(3-5):0.1:(3-5):(9-12):(6-10):(3-5):(10-15); step 1. carrying out halogenation; step 2. carrying out halogen methylated thiolation: adopting a thiourea hydrocarbylation hydrolysis method or thiocyanate direct substitution method; 3. carrying out epoxidation: using a universal epichlorohydrin preparation method; and 4. preparing cyclic sulfide products: adopting a universal cyclic sulfide preparation method.
Owner:JIANGSU JUNSHI OPTICS CO LTD
Nano sheet self-assembled MoS2 nano hollow material and preparation and application of MoS2 nano hollow material serving as lithium storage electrode material
InactiveCN102938461AImprove lithium storage performanceGood lookingCell electrodesLithiumPotassium thiocyanate
The invention discloses a nano sheet self-assembled MoS2 nano hollow material and preparation and application of the MoS2 nano hollow material serving as a lithium storage electrode material. The nano sheet self-assembled MoS2 nano hollow material is obtained by mixing raw materials including molybdenum oxide, sodium fluoride and potassium thiocyanate according to the mass ratio of 0.8-0.9:0.5-0.6:1.5-2.9; adding into water-ethanol mixed solvent; placing into a reaction kettle to be sealed; reacting at 140-220 DEG C for 12-30h; and performing washing, separating and drying to obtain products. The method is simple and easy in required condition, cheap and facile in raw materials and controllable in shape and height and easily achieves industrial production. Lithium storage electrodes prepared by adopting the prepared nano sheet self-assembled MoS2 nano hollow material not only have high electro-chemical lithium storage reversible capacity and excellent cycling performance, but also have good rate capability.
Owner:SHANDONG UNIV
Continuous flow method for measuring total alkaloid in tobacco or tobacco products
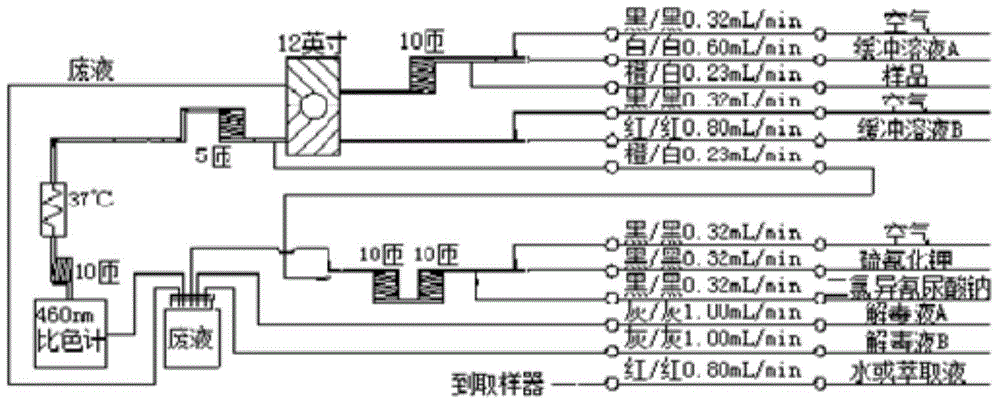
ActiveCN104132937AGuaranteed pHWill not be affected by acid and alkali environmentMaterial analysis by observing effect on chemical indicatorPreparing sample for investigationPotassium thiocyanateContinuous flow
The invention provides a continuous flow method for measuring total alkaloid in tobacco or tobacco products. In the method, potassium thiocyanate and sodium dichloro isocyanurate are adopted to carry out reactions to generate cyanogen chloride on line. The cyanogen chloride can carry out reactions with total alkaloid (calculated in the form of nicotine) in tobacco or tobacco products to break off the pyridine rings of nicotine, and then further carries out reactions with p-aminobenzene sulfonic acid. The reaction products are measured by a chromometer at 460 nm. The method uses a buffer system to control the pH value of the reaction system in a range of 6.0 to 7.5, and the buffer system is composed of a buffer solution A and a buffer solution B. The buffer solution A is prepared by the following steps: weighing disodium hydrogen phosphate and trisodium phosphate, placing the weighed substances in a beaker, dissolving the substances with water, transferring the solution to a volumetric flask (1L), and adding water into the volumetric flask until the water reaches the scale. The buffer solution B is prepared by the following steps: weighing p-aminobenzene sulfonic acid, disodium hydrogen phosphate, sodium dihydrogen phosphate, and sodium citrate, placing the weighed substances into a beaker, dissolving the substances with water, transferring the solution to a volumetric flask (1L), and then adding water into the volumetric flask until the water reaches the scale.
Owner:CHINA NAT TOBACCO QUALITY SUPERVISION & TEST CENT
Preparation method of active meson-phase charcoal micro-balloon with high-ratio surface area and high mesoporosity
The present invention relates to active carbon preparing technology, and is especially preparation process of active carbon microsphere with high specific surface area and high medium pore rate and serving as an intermediate phase. The preparation process includes the following steps: adding ferrocene powder in 1-5 wt% into petroleum asphalt or coal pitch; heating and maintaining, dissolving the product in light component of coal tar, filtering, extracting and washing; heating to carbonize intermediate carbon microsphere; mixing the carbonized intermediate carbon microsphere with activating agent, drying and setting the paste into an activating furnace; cooling, washing the product with dilute hydrochloric acid solution to eliminate alkaline compound and iron oxide, and inspecting iron ion content with potassium thiocyanate solution; and regulating pH value with deionized water to 6-7.
Owner:TIANJIN UNIV +1
Method for measuring copper content in tin-silver-copper solder through iodometry
InactiveCN103776820AAccurate measurementReduce distractionsMaterial analysis by observing effect on chemical indicatorPreparing sample for investigationPotassium thiocyanateDissolution
Provided is a method for measuring copper content in tin-silver-copper solder through iodometry. The method comprises the following steps: a copper standard solution is prepared and the titer of the copper standard solution is measured. A sample to be measured is weighed and added into an Erlenmeyer flask. Concentrated sulfuric acid is added and the mixture is heated for dissolution. The above solution is cooled to the room temperature, perchloric acid is added, after the sample is dissolved fully, heating is carried out until white smoke is generated, concentrated hydrochloric acid is dropwise added into the above solution in batches to remove the tin element in the solution, and the solution is subjected to concentration. The Erlenmeyer flask is taken down and cooled to the room temperature, deionized water is added, the constant volume is 100mL and the above solution is shaken up. Then an ammonium hydroxide solution is added and copper ammonia complex ions are formed. Then ammonium bifluoride is added into the solution and stirred until the blue color disappears. The above solution is cooled to the room temperature by utilization of running water. The solution is placed for half a minute, then potassium iodide is added, and immediately the solution is subjected to titration by a sodium hyposulfite standard solution until a shallow yellow color appears. Then a potassium thiocyanate solution and a starch solution are added, and the solution is subjected to titration by the sodium hyposulfite standard solution until a blue color disappears. The volume of the consumed sodium hyposulfite solution is recorded, and the content of copper in the sample is calculated.
Owner:BEIJING INST OF NONFERROUS METALS & RARE EARTH
Method for measuring chlorine ion in copper-containing zinc electrolyte sample
InactiveCN102706875ARapid determinationAccurate measurementMaterial analysis by observing effect on chemical indicatorIron sulfateElectrolytic agent
The invention relates to a method for measuring chlorine ion in a copper-containing zinc electrolyte sample, which comprises the steps as follows: taking 20-50 mL of a zinc electrolyte sample, adding 5mL of 50% sulfuric acid, adding 0.5-1.0 g of zinc powder with purity of 99.999% or 0.2-0.5 g of aluminum powder with purity of 99.999%, stirring until reaction is finished, and diluting to 100 mL with a volumetric flask; carrying out dry filtering, adding 20-50 mL of filtrate to a 300 mL beaker, adding 5-6 mL of nitric acid, adding known amount of excessive silver nitrate (0.05 mol / L) until chlorine ion is precipitated completely, heating for boiling to precipitate and flocculate silver chloride, filtering, washing precipitate, adjusting the volume to 80-100 mL, adding 1 mL of saturated ferric sulfate solution acidified with nitric acid, titrating with 0.01 mol / L potassium thiocyanate solution until the stable red color appears in the solution, namely the end point of titration. According to the invention, the measuring method can be used for rapidly and accurately measuring chlorine ion content in the zinc electrolysis electrolyte without use of an instrument; and high-purity zinc powder or aluminum powder are adopted to remove interference metal ions, and the use amount of the reagents and the operation steps are strictly controlled, therefore, the method can be used for accurately measuring in situ and medium and small-sized laboratories.
Owner:NORTHWEST RES INST OF MINING & METALLURGY INST
Colorimetric method for detecting lead ions
InactiveCN104655578AHigh sensitivityGood choiceColor/spectral properties measurementsCysteine thiolateNanoparticle
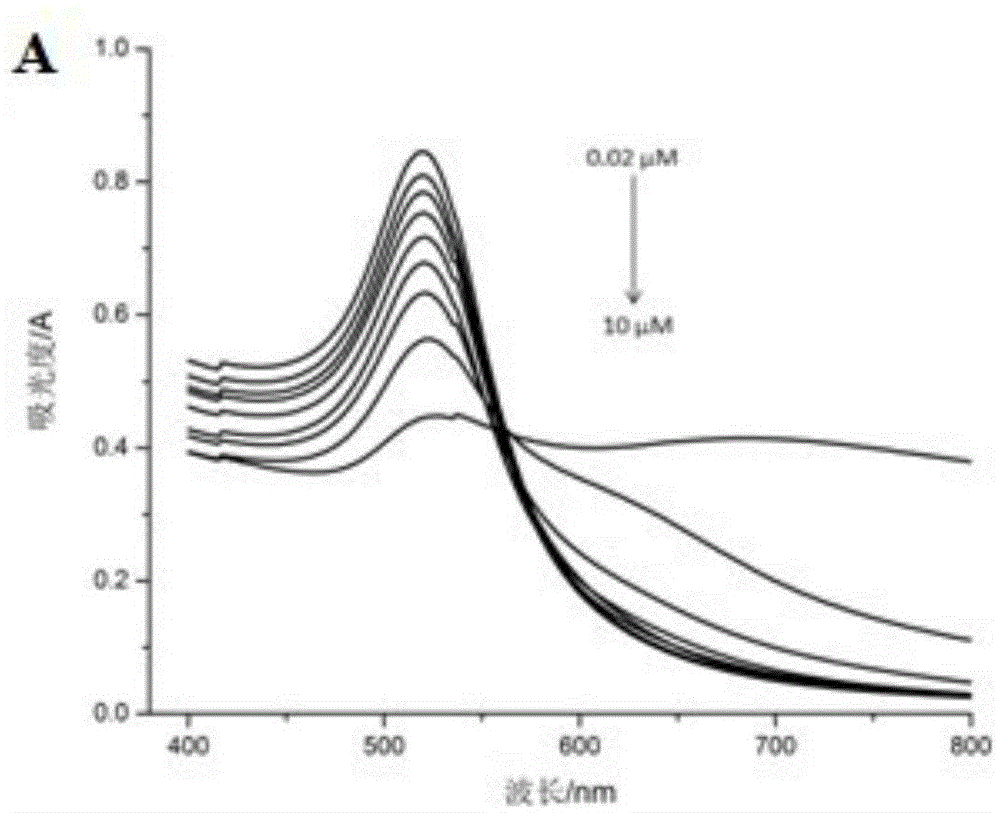
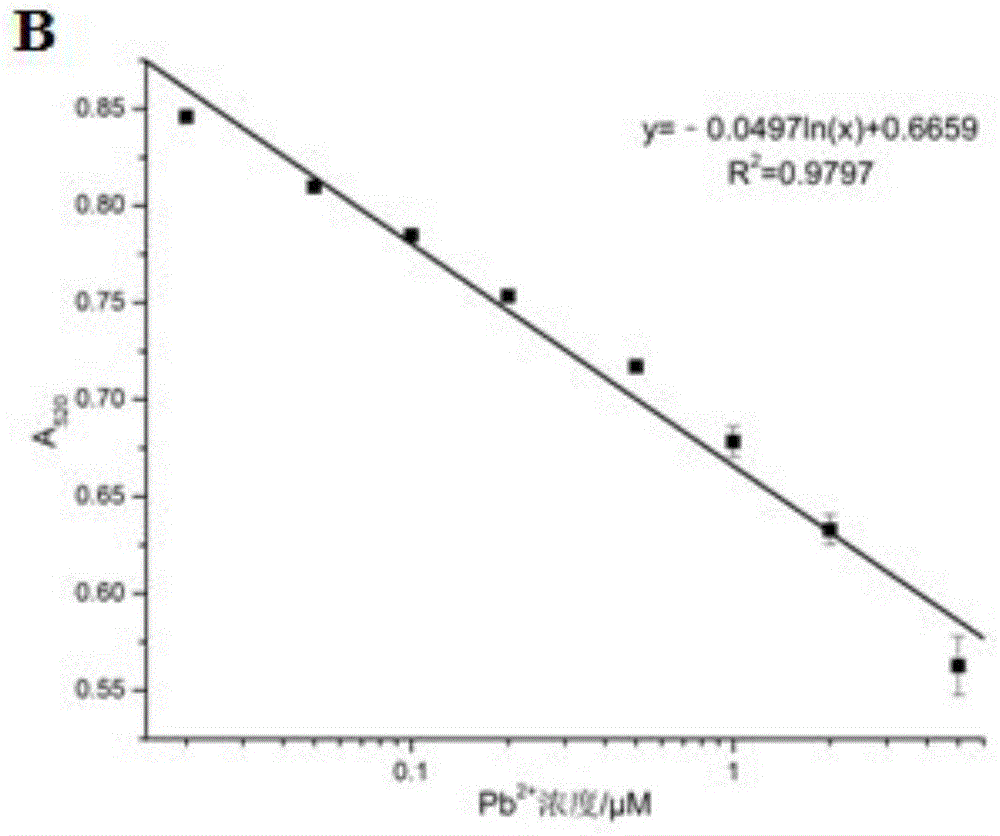
The invention discloses a colorimetric method for detecting lead ions, and specifically relates to a method for detecting lead ions by taking gold nanoparticles as a colorimetric probe. AuNPs(Cys-AuNPs) is modified by l-cysteine, and Cys can capture Pb<2+> by the binding function of a chelating ligand under the induction of Pb<2+>, so that Cys-AuNPs is aggregated, and thus the color of a solution changes, and the change in position and intensity of an AuNPs characteristic peak is caused. The colorimetric method has a detection range of 0.02- 5 microns, and detection limit of 0.02 microns, has the advantages of simplicity, economy, good selectivity (potassium thiocyanate as a screening agent) and the like, and can be used for detecting Pb<2+>.
Owner:王利兵
Method for measuring content of silver in high-bismuth material
ActiveCN103926372AEasy to operateHigh precisionMaterial analysis by observing effect on chemical indicatorChemical analysis using titrationPotassium rhodanidePotassium thiocyanate
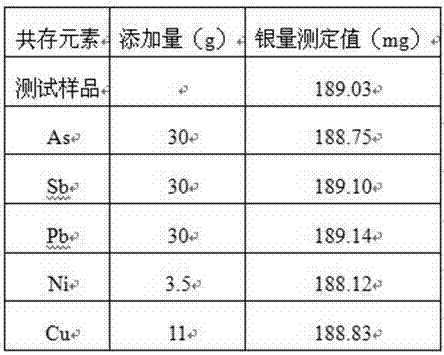
The invention discloses a method for measuring the content of silver in a high-bismuth material. The measuring method is characterized by comprising the following steps: quantitatively trapping precious metal in a sample with bismuth oxide as a trapping agent of precious metal; smelting the sample at the temperature of 900-1100 DEG C for 30-60 min to obtain a bismuth buckle; dissolving the bismuth buckle by nitric acid and tartaric acid under a heating condition; and titrating with a potassium rhodanide standard solution to measure the content of silver in the sample. The measuring method is simple to operate; the accuracy and recycling rate of the measuring method are high and meet the requirement of an analysis method; the impurity interference is small and the problem that the accuracy of measurement on the content of silver is low due to relatively high impurity content in a high-bismuth material sample is solved.
Owner:HUNAN SHUI KOU SHAN NONFERROUS METALS GRP
Method for preparing high-quality sodium thiocyanate from desulfurized and decyanated waste liquid of coke oven gas
The invention discloses a method for preparing high-quality sodium thiocyanate from a desulfurized and decyanated waste liquid of coke oven gas, which comprises the following steps: decolorizing and filtering the desulfurized and decyanated waste liquid through activated carbon, and adding an assistant A into the waste liquid while stirring, wherein the specific quantity of the assistant A is 50-200% of the molal quantity of sodium thiosulfate in the waste liquid; removing sodium thiosulfate ions in the waste liquid system, filtering to remove insoluble substances, and then adding a small quantity of assistant C into the filtrate to regulate the pH value to 5-10; distilling and concentrating to remove the moisture, and centrifuging to separate out inorganic salt impurities based on the difference of various substances in solubility under different temperatures and concentrations; and decolorizing through activated carbon, and further concentrating and crystallizing to produce the sodium thiocyanate finished product. According to the invention, the process is simple, the cost is low, and the weight content of the produced potassium thiocyanate is more than 99%.
Owner:江苏燎原环保科技股份有限公司
Hexafluoropropylene dimmer production method
InactiveCN1876611AHigh selectivitySimple structureHalogenated hydrocarbon preparationPotassium thiocyanateHexafluoropropylene
The invention relates the preparing method of hexafluoropropylene dipolymer, using hexafluoropropylene as raw material, acetonitrile as solvent, and kalium rhodanatum as accelerating agent. The invention provides the reaction device of preparing hexafluoropropylene dipolymer. The device is driven by crank connecting rod to alternating motion.
Owner:浙江莹光化工有限公司
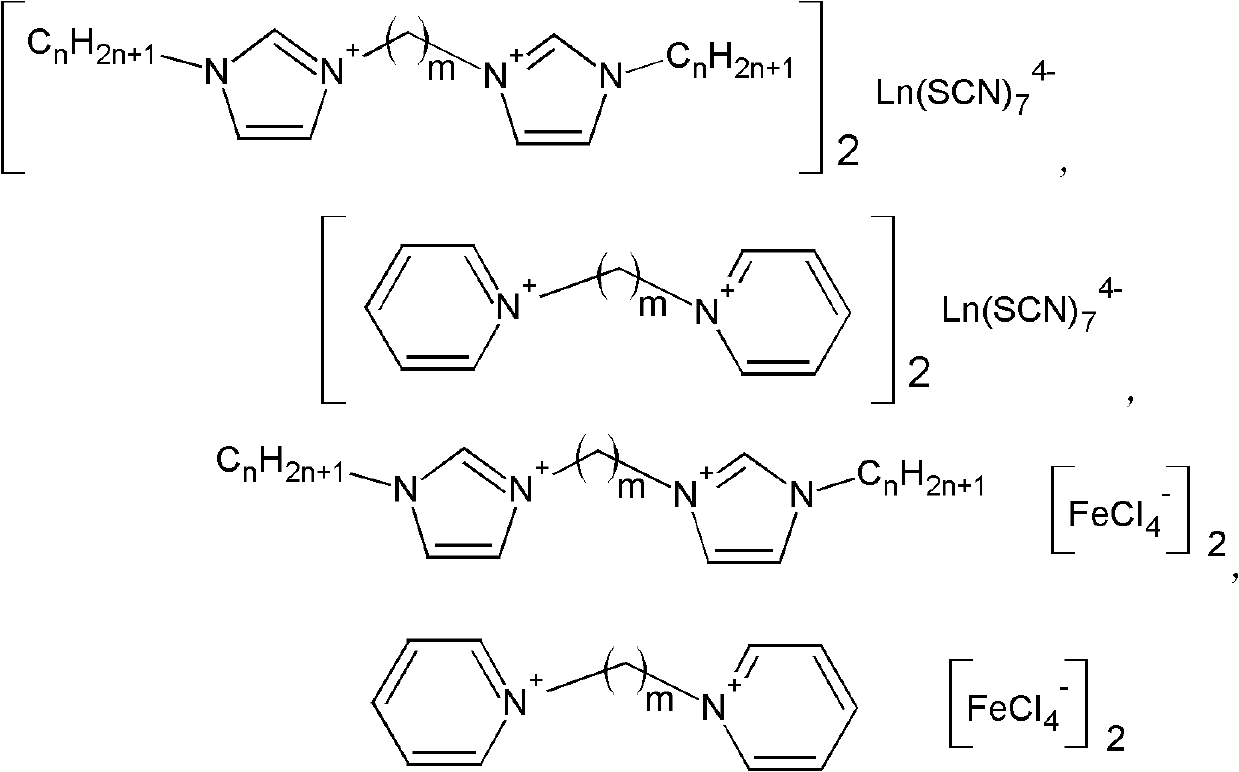
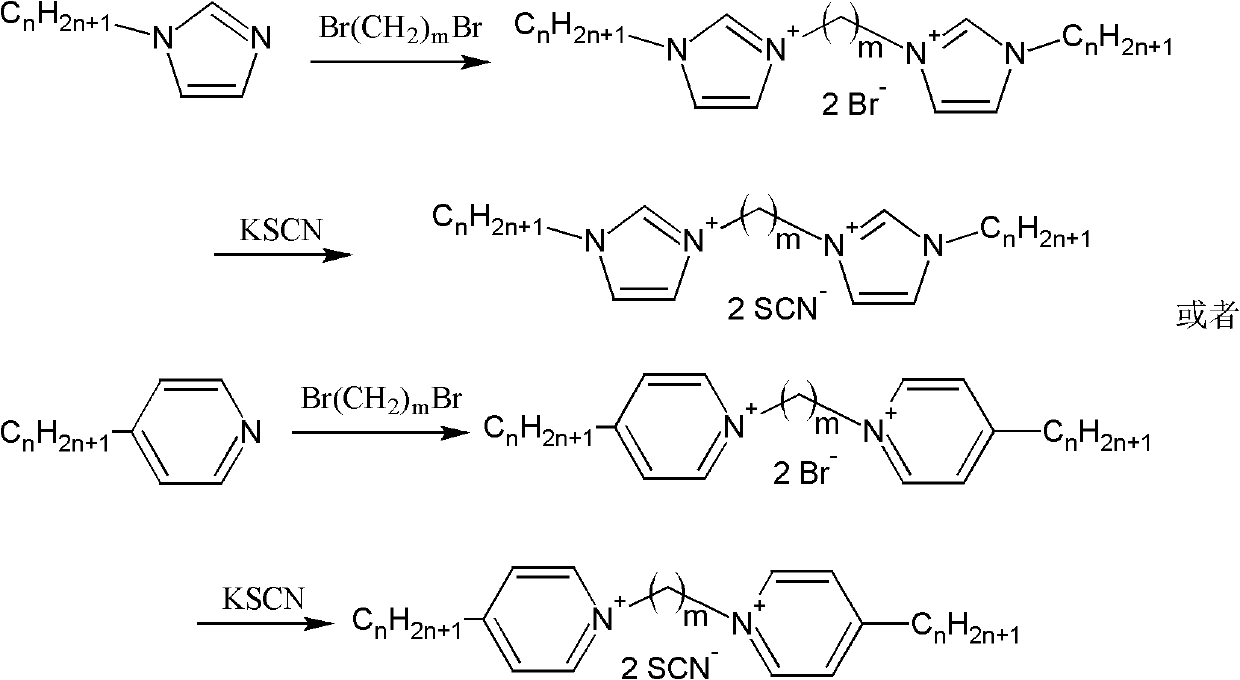
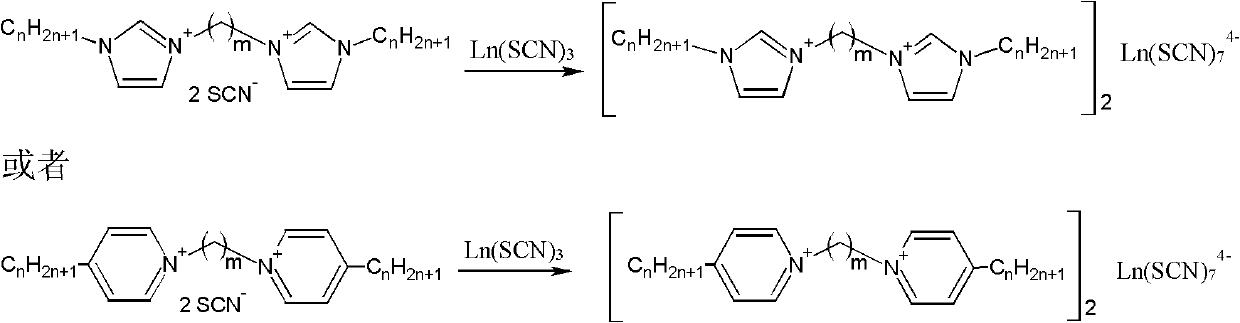
Bivalent cation magnetic ionic liquids and preparation method thereof
ActiveCN102167683AOvercome the disadvantage of uncontrollable magnetismSolve the problem of less magneticOrganic chemistryMagnetic liquidsPotassium rhodanidePotassium thiocyanate
The invention relates to bivalent cation magnetic ionic liquids and a preparation method thereof, and belongs to the technical fields of novel chemical materials and preparation. N-alkyl imidazole, 1,m-dibromoalkane, 1,m-dicholoroalkane, ferric trichloride, lanthanide metal oxide, potassium rhodanide and aqueous solution of perchloric acid are taken as raw materials to be prepared into the bivalent cation magnetic ionic liquids with different anion structures; and compared with the conventional magnetic ionic liquid, the magnetic ionic liquids have high magnetism, magnetic controllability and higher thermal stability. The invention realizes magnetic designability of the magnetic ionic liquids for the first time, and promotes the application of the magnetic ionic liquids in the fields such as aviation, medicines, biology and the like.
Owner:INST OF PROCESS ENG CHINESE ACAD OF SCI
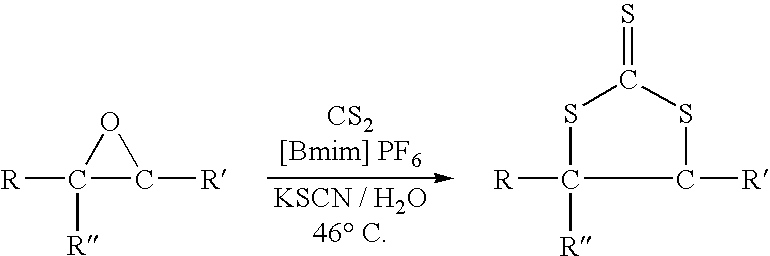
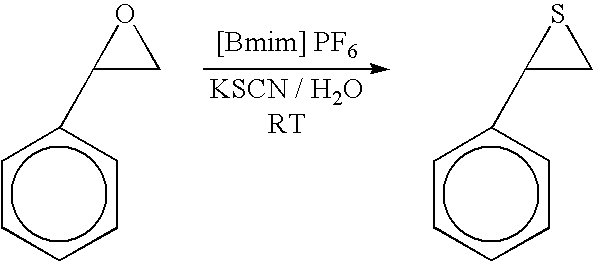
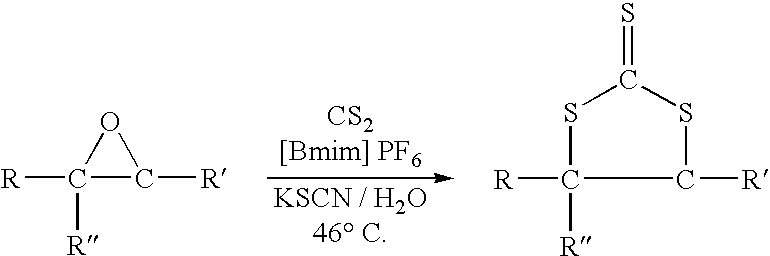
Synthesis of cyclic trithiocarbonates from epoxides
This invention provides a low cost technique for synthesizing cyclic trithiocarbonates by a simple one step process from epoxides that can be conducted under atmospheric pressure. This synthesis can be depicted as follows: wherein R represents a moiety selected from the group consisting of alkyl groups and aryl groups, wherein R′ represents a moiety selected from the group consisting of alkyl groups, aryl groups, and hydrogen atoms, and wherein R″ represents a moiety selected from the group consisting of alkyl groups, aryl groups, and hydrogen atoms, wherein the R moiety and the R′ moiety can be bonded together to form a cyclic structure, with the proviso that R″ represents a hydrogen atom if R′ represents an alkyl group or an aryl group. In this process carbon disulfide and a thiocyanate salt, such as potassium thiocyanate, are reacted with the epoxide in an ionic liquid, such as 1-butyl-3-methylimidazolium hexafluorophosphate ([Bmim] PF6) in the presence of water to produce the cyclic trithiocarbonate.
Owner:THE GOODYEAR TIRE & RUBBER CO
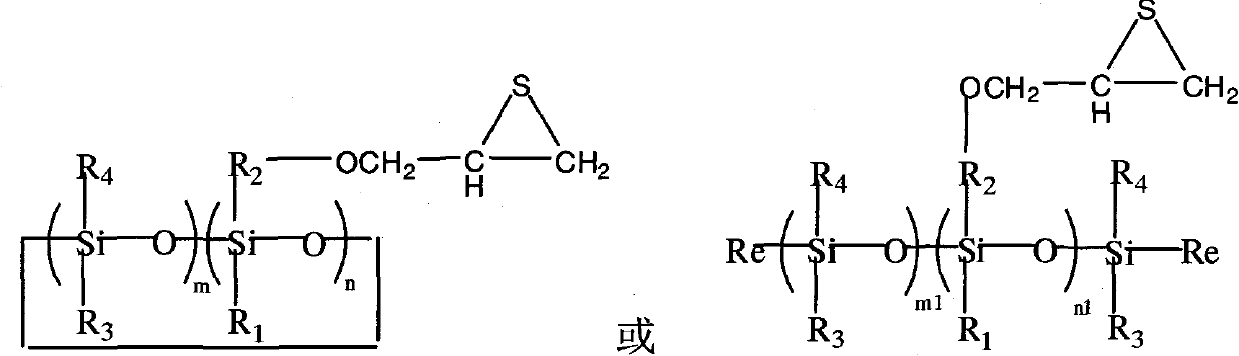
Organic silicon episulfide resin and preparation method thereof
The invention relates to an organic silicon episulfide resin and a preparation method thereof. The preparation method comprises the steps of: mixing organic silicon epoxy resin with deionized water, ethanol and potassium thiocyanate or thiocarbamide, and reacting the mixture at temperature of 15-45 DEG C for 12-36 hours; and after reaction is finished, adding organic solvent so as to divide the reacted mixture into two layers, separating out and washing an organic phase to be neutral, adding anhydrous sodium sulfate into the organic phase, and drying for 5-15 hours, filtering to remove sodiumsulfate, and removing the organic solvent at 2-10mmHg / 45-60 DEG C, thus obtaining organic silicon episulfide resin with the following structure. The organic silicon episulfide resin has the advantages of lower viscosity, high purity, high cure reaction activity, high refractive index, small heat release amount during condensate reaction, high glass transition temperature, low water absorption rate, and the like, thus being expected to be applied in the fields coating, adhesives, electronic device packaging, composite material matrix resin and the like.
Owner:INST OF CHEM CHINESE ACAD OF SCI
Measuring method of iron
ActiveCN102590202AAccurate and reliable measurement resultsEasy to operateMaterial analysis by observing effect on chemical indicatorLithiumPhosphate
The invention discloses a measuring method of iron. The measuring method comprises the following steps of: preparing multiple Fe<3+> standard solutions with different concentrations; respectively adding a first nitric acid solution and a first potassium thiocyanate solution into the multiple Fe<3+> standard solutions so as to carry out a color reaction; preparing a solution to be tested by taking a ferric phosphate lithium sample, and adding the second nitric acid solution and the second potassium thiocyanate solution into the solution to be tested so as to carry out the color reaction; and carrying out color comparison on the solution to be tested after the color reaction and the Fe<3+> standard solutions after multiple color reactions, thus obtaining iron content in the solution to be tested according to a color comparison result. Compared with the ration measuring method in the prior art, the measuring method provided by the invention utilizes a semi-quantitative colorimetric method to measure the iron content in the solution to be tested, the measured result of the method is exact and reliable, the operation is simple, the application is broad, and the cost is lower.
Owner:TIANJIN ENERGIES
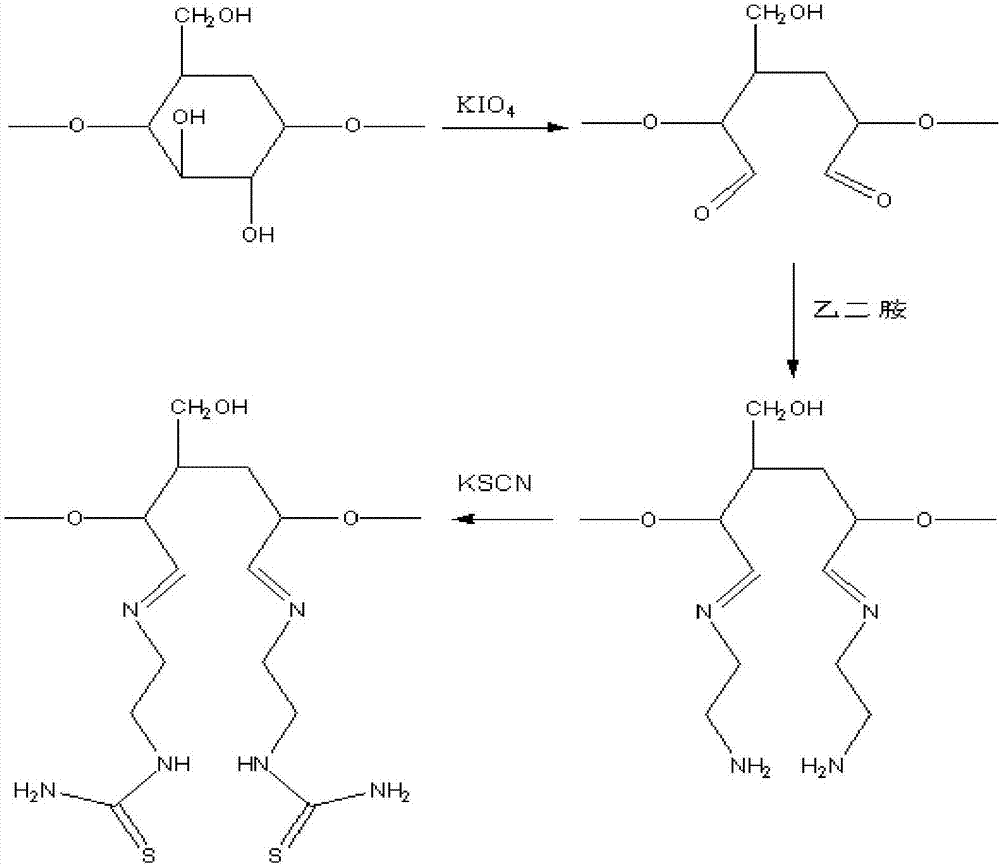
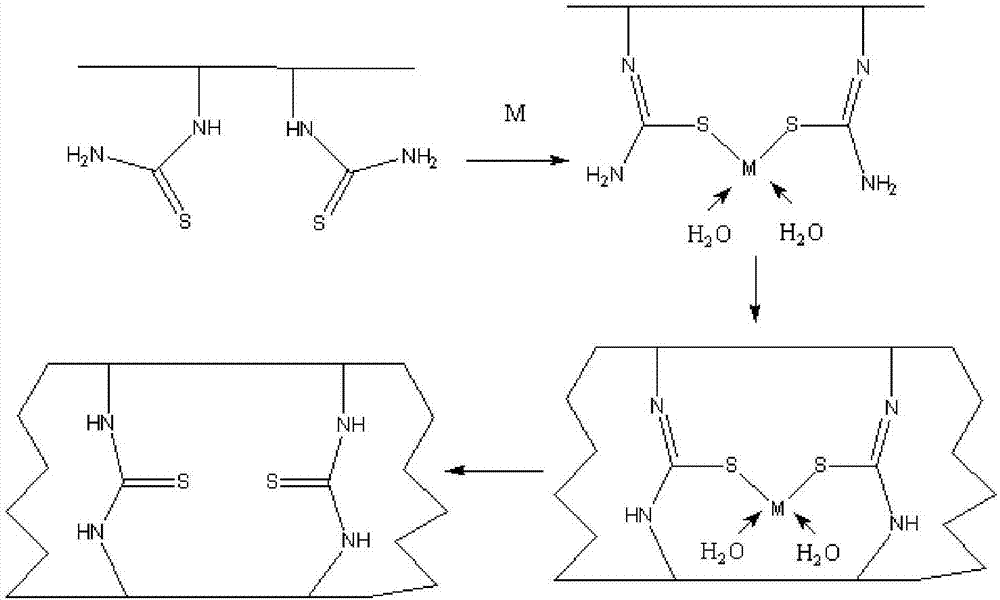
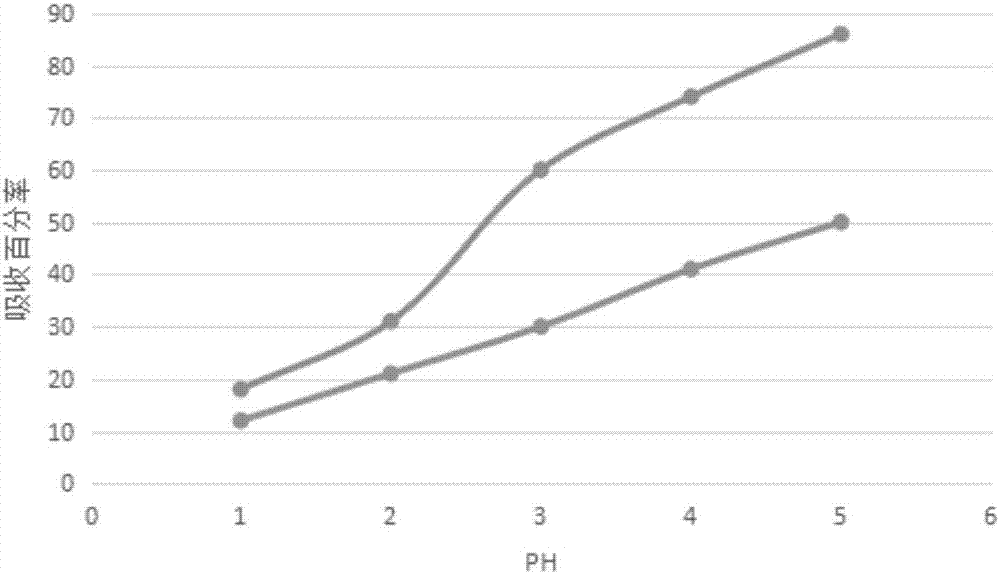
Ion-imprinted nanocellulose adsorbing material and preparation method and application thereof
InactiveCN107138135AImprove adsorption capacityHigh selectivityOther chemical processesAlkali metal oxides/hydroxidesCross-linkEthylenediamine
The invention belongs to the technical field of adsorbing materials, and discloses an ion-imprinted nanocellulose adsorbing material and a preparation method and application thereof. The preparation method comprises the following steps of conducting oxidation modification with potassium periodate, amination modification with ethylenediamine, and thiocarbamide modification with potassium thiocyanate on nanocellulose successively, adding an imprinting metal ion solution and a formaldehyde solution, conducting a cross-linking polymerization reaction with stirring, immersing the polymerization products into an acid solution to remove imprinting metal ions, and obtaining the ion-imprinted nanocellulose adsorbing material. The ion-imprinted nanocellulose adsorbing material has high adsorbability and selectivity for metal ions, and can be used for removing and recycling metal ions, such as mercury ions, lead ions and copper ions.
Owner:SOUTH CHINA UNIV OF TECH
Hydrogen-free precursor synthesized carbon nitride photocatalyst
ActiveCN104399509AEnhance photocatalytic hydrogen production activitySimple production processPhysical/chemical process catalystsHydrogen productionLithium chloridePtru catalyst
The invention discloses a hydrogen-free precursor synthesized carbon nitride photocatalyst, as well as a preparation method and application thereof, and belongs to the technical field of material preparation and photocatalysis. According to the preparation method, cyanuric chloride, potassium thiocyanate and lithium chloride are adopted as the precursor to synthesize the carbon nitride photocatalyst. The prepared carbon nitride photocatalyst is narrow in band gap and high in quantum efficiency; by adopting platinum as a catalyst promoter and triethanol amine as a sacrifice agent, the photocatalytic activity of the carbon nitride photocatalyst for producing hydrogen exceeds that of the commercial titanium dioxide photocatalyst P25 under the lighting condition of greater than 300 nm; the preparation method is simple in technology and low in cost, conforms to the actual production demand, and has great application potential.
Owner:FUZHOU UNIV
Preparation method for MoS2-BiPO4 composite photocatalyst
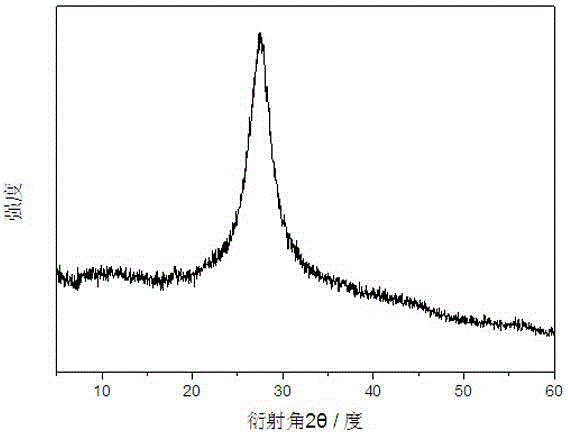
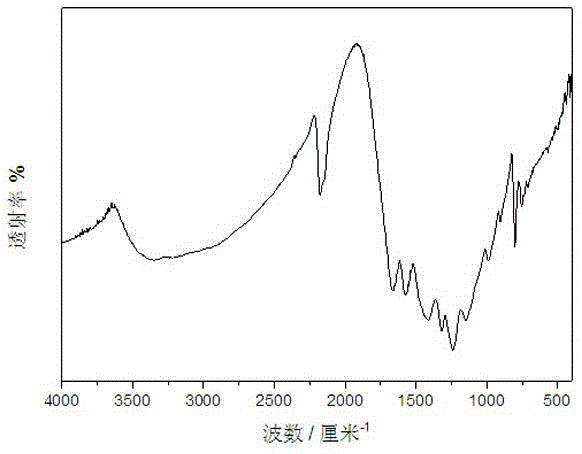
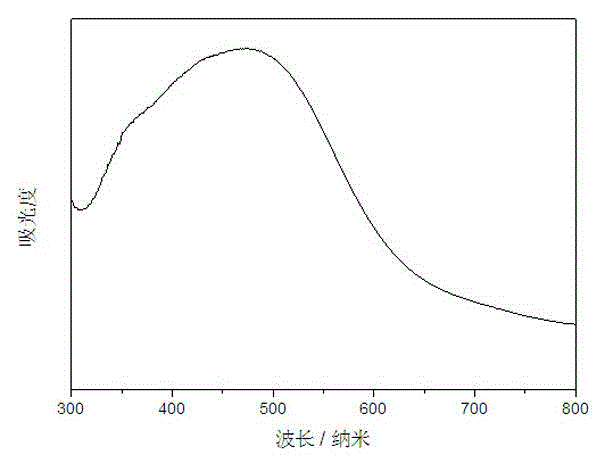
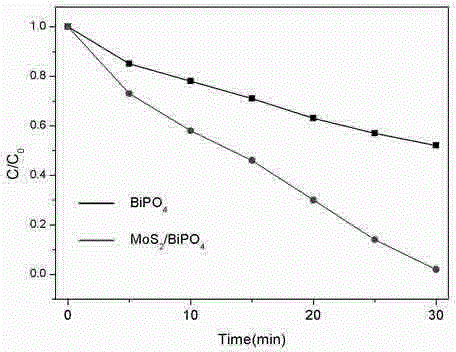
InactiveCN104148099AImprove photocatalytic activityImprove stabilityPhysical/chemical process catalystsPotassium thiocyanateControllability
The invention discloses a preparation method for a MoS2-BiPO4 composite photocatalyst. The preparation method comprises the following steps: (1) dissolving 1mmol of molybdenum trioxide and 2.5mmol of potassium thiocyanate in deionized water to form a mixed solution A, transferring the mixed solution A into a hydrothermal reaction kettle for reacting for 12 to 32 hours at 160 to 200 DEG C, cooling to room temperature, washing, and drying to obtain graphene-like MoS2; (2) ultrasonically dispersing 3 to 9mg of the prepared graphene-like MoS2 into ethylene glycol to obtain a MoS2 dispersion liquid; and (3) adding 1mmol of bismuth nitrate into prepared MoS2 dispersion liquid, magnetically stirring until the bismuth nitrate is completely dissolved, adding 1mmol of sodium dihydrogen phosphate, mixing uniformly to obtain a mixed solution II, then transferring the solution II into the hydrothermal reaction kettle for performing microwave hydrothermal treatment for 30 to 120 minutes at 160 to 200 DEG C, cooling, washing and drying to obtain the MoS2-BiPO4 composite photocatalyst. According to the preparation method, the reaction time is short, the heating rate is high, reaction conditions are mild, a process is simple, the controllability is high, and the large-scale production is easy to realize.
Owner:HENAN NORMAL UNIV
Method for preparing macrolides half-synthesized antibiotics telithromycin
InactiveCN1800198AReduce manufacturing costImprove securityAntibacterial agentsOrganic active ingredientsPotassium thiocyanateHexamethylenetetramine
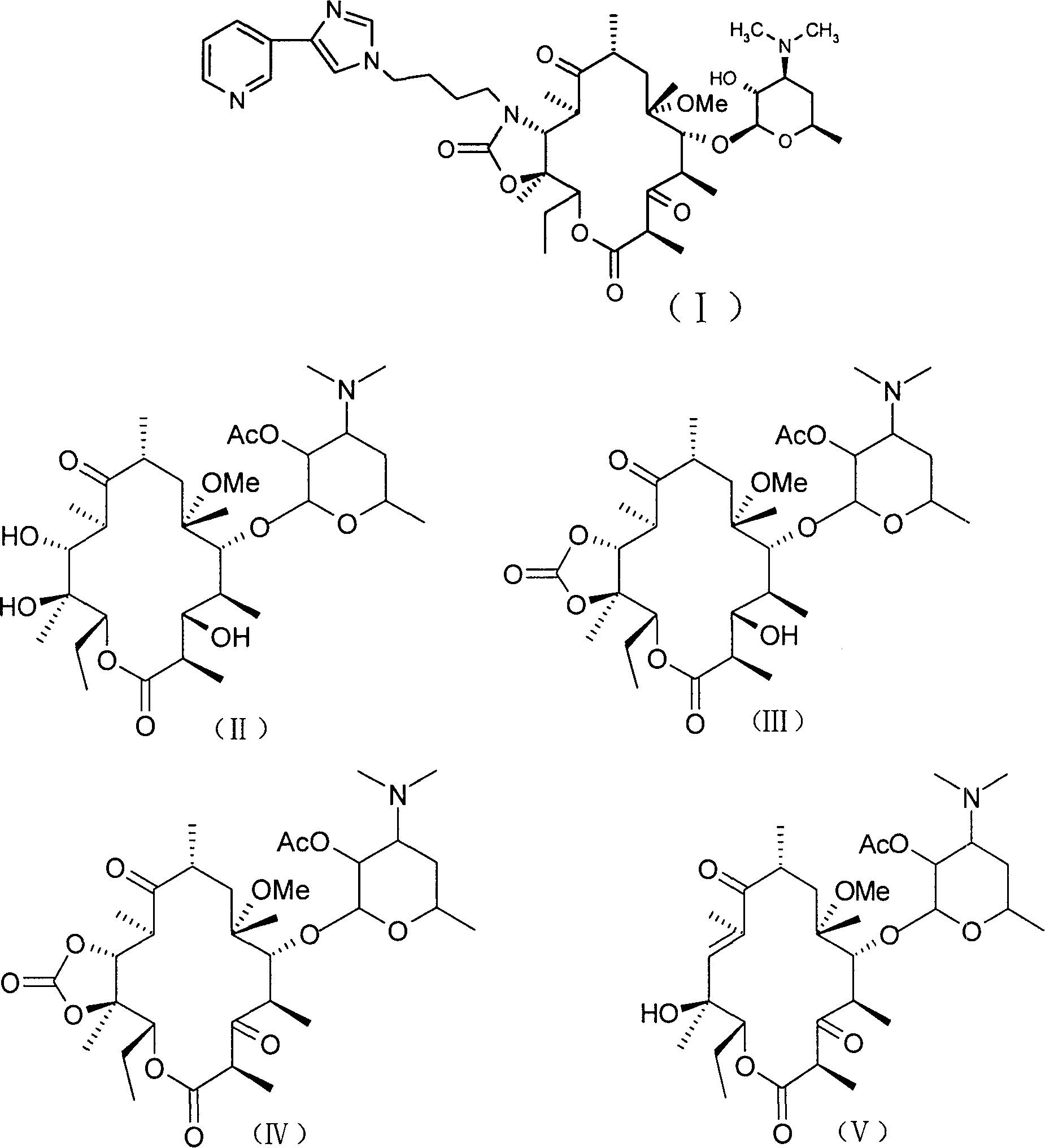
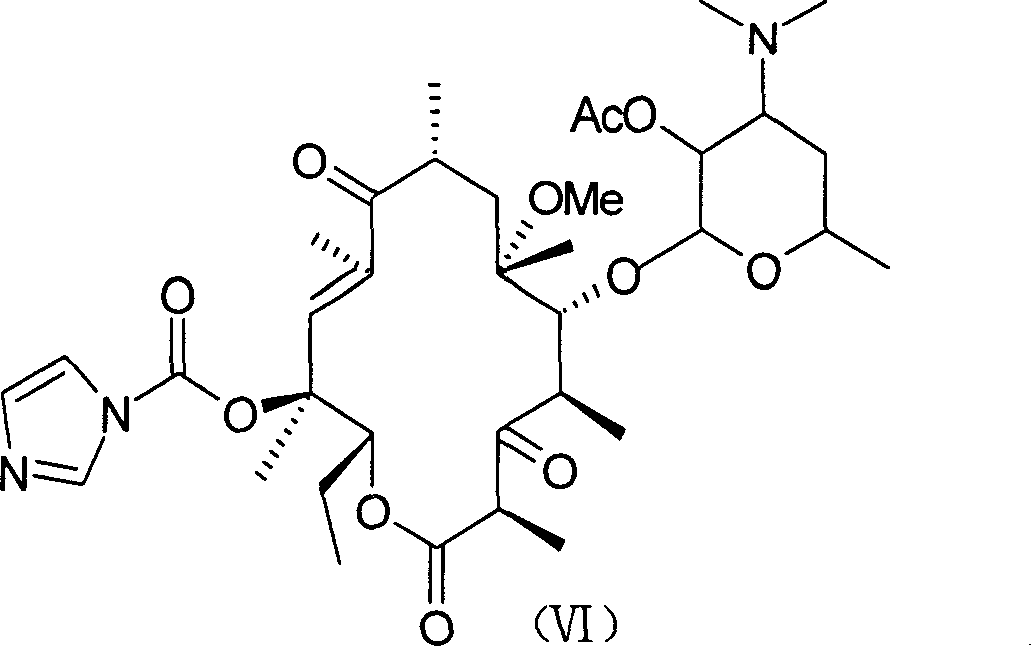
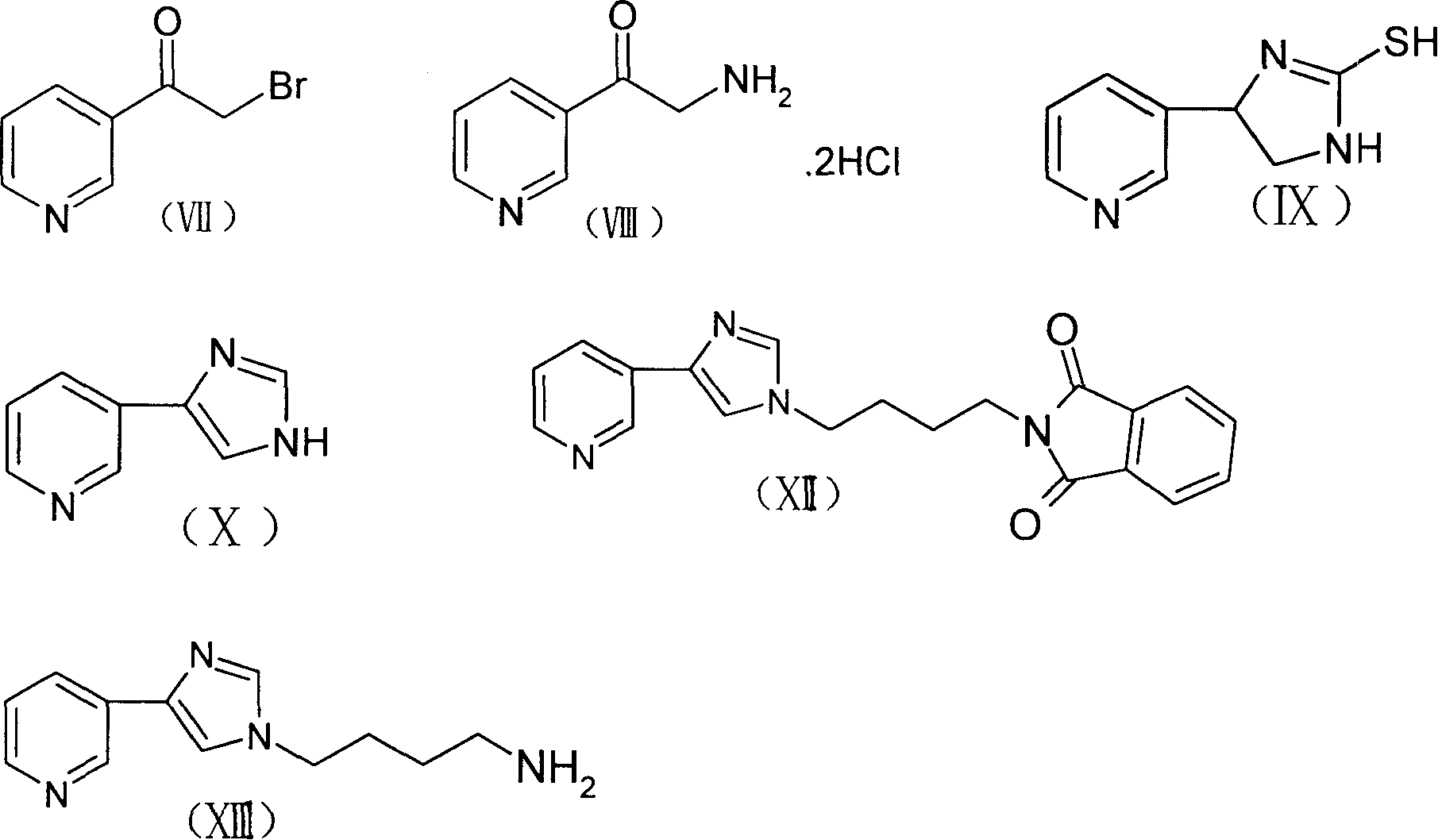
The invention discloses a new method for preparing macrolides semi-synthesizing antibiotic tallysomycin. The mother core part uses 6-methoxy bristacin as raw material, which is dewatered on the effect of the base after dewatering, acylating, re-etherification and oxidant, and then it reacts with the carbonyl diimidazole to obtain the intermediate (VI); the lateral chain part uses 3-acetyl pyridine as raw material and is hydrolyzed after bromide and reacting with the hexamine, then it ringed with the potassium sulfocyanide and reacts with the N-(4-bromide butyl)-phthalimide on base condition after the effect of diluted nitric acid and then it is hydrazinolysized to obtain the lateral chain (XIII); the intermediate (VI) reacts with the lateral chain (XIII) to synthesize the tallysomycin.
Owner:CHINA PHARM UNIV
Plating solution for carrier copper foil stripping layer and preparation method of stripping layer
The invention relates to a plating solution for a carrier copper foil stripping layer and a preparation method of the stripping layer. The plating solution is prepared from components as follows: potassium bitartrate with the concentration of 20-60 g / L, zinc sulfate with the concentration of 8-16 g / L and an additive A with the concentration of 5-20 g / L and the pH of 3.0-5.0, wherein the additive Ais a mixture formed by mixing at least one of 3-(2,3-epoxy propoxy)propyl trimethoxy silane, 3-(2,3-epoxy propoxy)propyltriethoxysilane, 3-(2,3-epoxy propoxy)propyl methoxy dimethyldiethoxylsilane or3-(methylacryloyl oxy)propyl trimethoxy silane with at least one of potassium thiocyanate, monopotassium phosphate, sodium acetate or ammonium sulfate. The carrier copper foil stripping layer obtained with the adoption of the plating solution for the carrier copper foil stripping layer and the preparation method of the stripping layer is a novel nano composite zinc plating layer, is very thin anduniform and can completely and stably strip carrier foil off the pressed very thin copper foil.
Owner:胡旭日
Process for synthesizing diethylaminoethyl mercaptide
The invention discloses a process for synthesizing diethylaminoethyl mercaptide. The process comprises the following steps: pretreating raw materials, preparing thiirane through the reaction of potassium sulfocyanide and ethylene carbonate, and finally synthesizing the thiirane and diethylamine into the product of diethylaminoethyl mercaptide. In the synthesis process, pretreatment is firstly carried out on the raw materials, a sectional heating mode is adopted during synthesis reaction so as to enable the reactants to be reacted completely, no solvents or catalysts are added in the whole process, pressurizing is not required, the yield and purity of the product are high, three wastes are not generated, and the process is environment-friendly.
Owner:张丽学
Thiouracil derivative, preparation method and application thereof
InactiveCN104876881ABroaden your optionsOvercoming the drawbacks of drug resistanceAntibacterial agentsOrganic active ingredientsThioureaPotassium thiocyanate
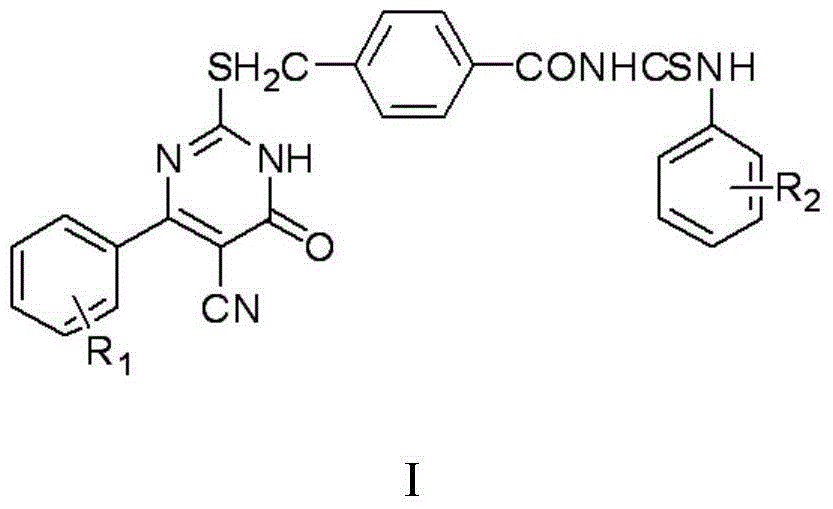
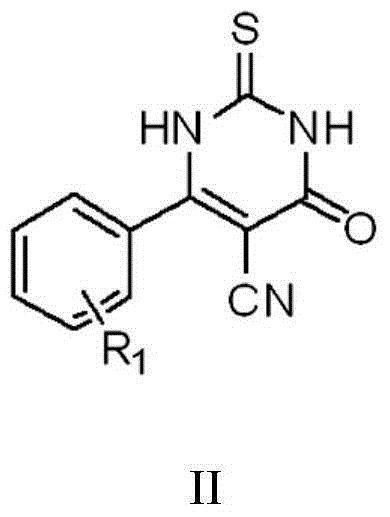
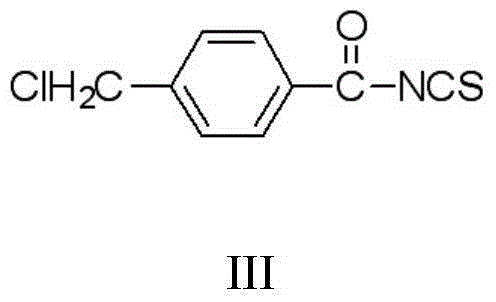
The invention discloses a thiouracil derivative. The chemical formula is shown in the formula I, wherein R1 is hydrogen, halogen or aromatic group, R2 is hydrogen, halogen or alkyl group from C1 to C6. Simultaneously, the invention also discloses a method for synthesizing the derivative. The method comprises the following steps : adopting aromatic aldehyde or an aromatic amine compound as a starting material, and reacting the aromatic aldehyde, ethyl cyanoacetate and thiourea under the catalysis of piperidine to obtain a compound II; reacting chloromethyl-benzoyl chloride and potassium thiocyanate in a methylbenzene / water / TBAB system to obtain a compound III; reacting the compound III and aromatic amine in an acetonitrile solvent to obtain a compound IV, reacting the compound II and the compound IV with the equal mole in the acetonitrile solvent under the catalytic action of potassium carbonate to obtain a target product, i.e., a compound I. The method adopted is simple, the operation is easy, and the large-scale production is easy; and verified by experiment, the prepared compound I has stronger bacteriostatic activity and can be widely applied in bacteriostatic medicine preparations.
Owner:HEBEI UNIVERSITY
A perovskite solar cell doped with potassium thiocyanate and a preparation method thereof
InactiveCN109244251AInhibit migrationImprove efficiencySolid-state devicesSemiconductor/solid-state device manufacturingMetallic electrodePotassium thiocyanate
The invention relates to the perovskite solar cell field, which provides a perovskite solar cell doped with potassium thiocyanate and a preparation method thereof. A perovskite battery is composed ofFTO conductive glass, an electron transport layer, a perovskite light absorbing layer, a hole transport layer and a metal electrode from bottom to top. The invention aims at solving the problems of poor crystallinity of perovskite and large lag of perovskite battery. potassium thiocyanate is doped in the perovskite light absorbing layer, which can change the crystallization kinetics of perovskite,promote the grain growth, passivate the internal defect state, stabilize the phase structure of perovskite, restrain the ion migration, thus effectively improve the crystallization quality of perovskite, improve the photoelectric efficiency and stability of perovskite battery, and eliminate the hysteresis effect.
Owner:UNIV OF SCI & TECH BEIJING +1
After-finishing process of knitted fabric
InactiveCN107326654AExcellent flame retardantGood antibacterialBiochemical fibre treatmentHeat resistant fibresPotassium thiocyanatePolyethylene glycol
The invention discloses an after-finishing process of knitted fabric. The process comprises steps as follows: 1-5 parts by mass of montmorillonite, 2-5 parts by mass of glycerol monolaurate, 0.5-3 parts by mass of zinc oxide and 2-6 parts by mass of polyethylene glycol monostearate are added to 40-60 parts by mass of deionized water and mixed and stirred uniformly; then, 2-6 parts by mass of fatty acid methyl ester sulfonate, 4-10 parts by mass of 1,4-butylene glycol, 2-8 parts by mass of citronella essential oil and 1-5 parts by mass of potassium thiocyanate are slowly added and continuously stirred until the mixture is sufficiently and uniformly mixed, and a finishing agent is obtained; then, antibacterial treatment is performed. According to the after-finishing process of the knitted fabric, the finished fabric has excellent flame retardance, good antibacterial, crease-resistant and antistatic properties and high washability, the antibacterial performance of the fabric can be improved, the antibacterial effect is good, and the service life is long.
Owner:NANTONG SANJIANG TEXTILE CO LTD
Method for preparing 1H-imidazole-4-formic acid
InactiveCN102643237AQuality improvementEasy to storeOrganic chemistryPtru catalystPotassium thiocyanate
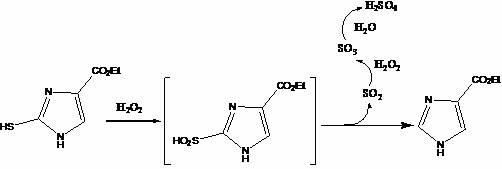
The invention relates to a method for preparing 1H-imidazole-4-formic acid. The method comprises the following steps of: performing cyclization on ethyl acetamidoacetate serving as a raw material and potassium thiocyanate to obtain 2-sulfydryl-4-imidazole ethyl formate; and performing catalytic oxidation by using a catalyst to remove sulfydryl to obtain sulfydryl removed imidazole-4-ethyl formate, hydrolyzing, and thus obtaining a target compound, namely 1H-imidazole-4-formic acid. The method has the advantages of high selectivity, improvement on yield, wide raw material source, low price and reasonable and feasible process design.
Owner:WUHAN UNIV OF TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com