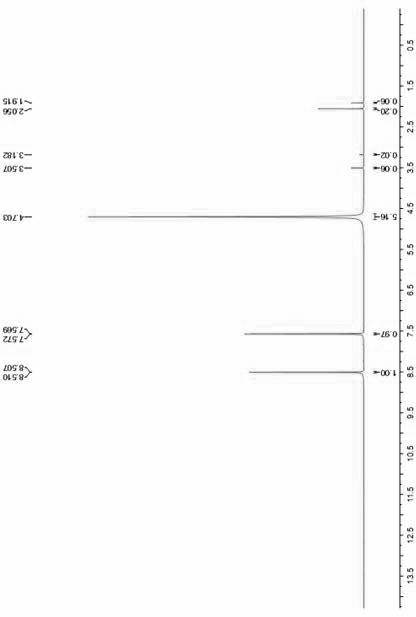
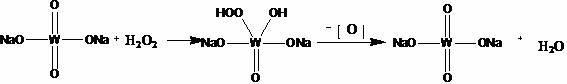
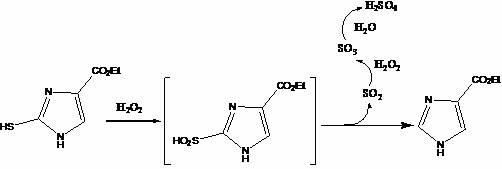
Method for preparing 1H-imidazole-4-formic acid
A technology of imidazole ethyl formate and imidazole, which is applied in the field of preparation of 1H-imidazole-4-carboxylic acid raw materials, can solve the problems of toxicity of catalyst RaneyNi, increase industrial production cost, catalyst poisoning, etc., and achieves reasonable and feasible synthetic route design. The effect of reducing the amount of use and reducing by-products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] 1), the preparation of ethyl 2-mercapto-4-imidazole carboxylate:
[0045] In a 1-liter three-neck reaction flask equipped with a thermometer, a reflux condenser, and a stirrer, add 14.5 grams of ethyl acetylglycinate and 50 milliliters of ethanol to dissolve, add 37 grams of potassium tert-butoxide and 150 milliliters of ethyl formate, and store at 40 ° C. Stir the reaction until it is thick. After standing still, remove the solvent by rotary evaporation to obtain a light yellow viscous liquid. Dissolve the above liquid in water, add 22 grams of potassium thiocyanate under ice bath to dissolve, slowly add 160 milliliters of concentrated hydrochloric acid, and dissolve at 40 The reaction was stirred at ℃, and the progress of the reaction was monitored by TLC. After the reaction was completed, the solvent was removed by rotary evaporation to obtain 14.67 g of a yellow powdery solid, namely ethyl 2-mercapto-4-imidazole carboxylate, with a yield of 85.3%.
[0046] 2), the ...
Embodiment 2
[0052] 1), the preparation of ethyl 2-mercapto-4-imidazole carboxylate:
[0053] In a 500 ml three-neck reaction flask equipped with a thermometer, a reflux condenser, and a stirrer, add 7 g of ethyl acetylglycinate and 22 ml of acetone to dissolve, add 23 g of sodium ethoxide and 120 ml of ethyl formate, and stir the reaction at 40°C After standing still, the solvent was removed by rotary evaporation to obtain a light yellow viscous liquid. Dissolve the above liquid in water, add 12 grams of potassium thiocyanate under ice cooling to dissolve, slowly add 75 milliliters of concentrated hydrochloric acid, at 40 ° C The reaction was stirred, and the progress of the reaction was monitored by TLC. After the reaction was completed, the solvent was removed by rotary evaporation to obtain 7.3 g of a yellow powdery solid, namely ethyl 2-mercapto-4-imidazole carboxylate, with a yield of 88.0%.
[0054] 2), the preparation of ethyl imidazole-4-carboxylate:
[0055] In a 150 ml three-n...
Embodiment 3
[0059] 1), the preparation of ethyl 2-mercapto-4-imidazole carboxylate:
[0060] In a 500 ml three-neck reaction flask equipped with a thermometer, a reflux condenser, and a stirrer, add 10.5 g of ethyl acetylglycine and 35 ml of isopropyl ether to dissolve, add 30 g of sodium methoxide and 115 ml of ethyl formate, and store at 45 ° C. Stir the reaction until thick. After standing still, remove the solvent by rotary evaporation to obtain a light yellow viscous liquid. Dissolve the above liquid in water, add 18 grams of potassium thiocyanate under ice cooling to dissolve, slowly add 86 milliliters of concentrated hydrochloric acid, and dissolve at 45 The reaction was stirred at ℃, and the progress of the reaction was monitored by TLC. After the reaction was completed, the solvent was removed by rotary evaporation to obtain 11.2 g of a yellow powdery solid, namely ethyl 2-mercapto-4-imidazole carboxylate, with a yield of 89.5%.
[0061] 2), the preparation of ethyl imidazole-4-...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Yield | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com