Optical resin monomer with high refractive index and preparation method thereof
A technology of refractive index and optical resin, which is applied in the field of high refractive index optical resin monomer and its preparation, can solve the problems of poor impact strength and heat resistance, and achieve good heat resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
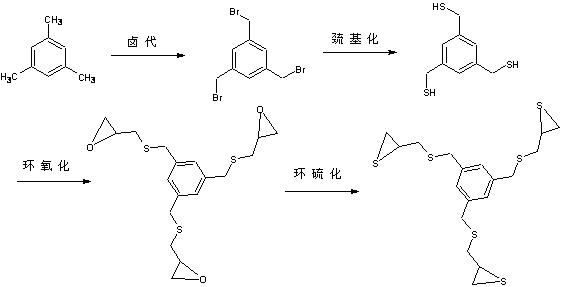
[0083] Example 1: Using NBS as a halogenated reagent to prepare a high refractive index optical resin monomer: (n=0, m=3)
[0084] The steps are as follows: (such as figure 1 shown)
[0085] Raw materials are aromatic hydrocarbons, NBS, BPO, thiourea, sodium hydroxide, hydrochloric acid, epichlorohydrin, potassium thiocyanate; of which aromatic hydrocarbons: NBS: BPO: thiourea: sodium hydroxide: hydrochloric acid: epichlorohydrin: thiocyanate The molar percentage of potassium acid potassium is 1:3~5:0.2~0.4:3~5:9~12:6~10:3~5:10~15;
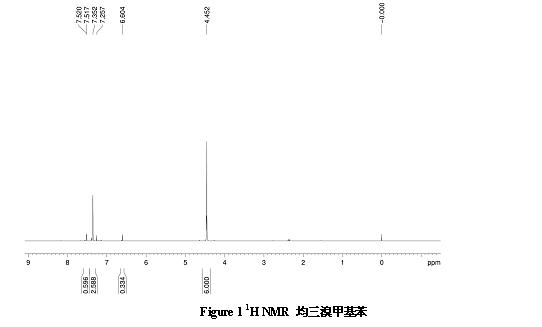
[0086] Step 1: Synthesis of mesitylene: Add 5g of mesitylene, 25.5g of NBS, and 0.1g of BPO as an initiator in a single-port bottle, and then add CCl 4As a solvent, magnetically stirred, heated to reflux, and reacted for 24h. After the reaction was cooled, a large amount of white solids precipitated out. The solids were removed by filtration, and the solvent was removed by rotary evaporation to obtain a light yellow viscous liquid, which was ...
Embodiment 2
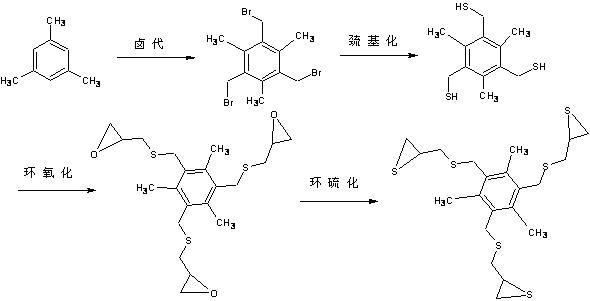
[0093] Example 2: Preparation of high refractive index optical resin monomer by direct halomethylation method: (n=3, m=3)
[0094] The steps are as follows (such as figure 2 ):
[0095] The raw materials are aromatic hydrocarbons, paraformaldehyde, glacial acetic acid, acetic acid solution of hydrogen bromide, thiourea, sodium hydroxide, hydrochloric acid, epichlorohydrin, potassium thiocyanate; among them, aromatic hydrocarbons, paraformaldehyde, glacial acetic acid, hydrogen bromide The molar percentage of acetic acid solution, thiourea, sodium hydroxide, hydrochloric acid, epichlorohydrin and potassium thiocyanate is 1:3~4:15~20:12~20:3~5:9~12:6~ 10: 3-5: 10-15.
[0096] Step 1: Synthesis of 1,3,5-tribromomethyl-2,4,6-trimethylbenzene (A1): Add 12.00 g (0.10 mol) of mesitylene and 10.00 paraformaldehyde in a 250 ml three-necked flask g (0.33 mol), 100 ml of glacial acetic acid, 70 ml of hydrogen bromide in acetic acid solution (31%), react at 120°C for 11 h. During the...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| glass transition temperature | aaaaa | aaaaa |
| hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com