Hydrogen-free precursor synthesized carbon nitride photocatalyst
A catalyst and precursor technology, which is applied in the field of carbon nitride photocatalyst and its preparation, can solve the problems of incomplete polymerization of carbon nitride photocatalyst, inability to fully utilize sunlight, and excessive amino residues, etc. The effect of promotion, high photocatalytic hydrogen production activity, and low energy consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] First, weigh cyanuric chloride, potassium thiocyanate and lithium chloride with a molar ratio of 1:3:4.32, and grind and mix them evenly under a nitrogen protective atmosphere. The uniformly ground and mixed solid powder was placed in an alumina crucible with a lid, and calcined in a nitrogen atmosphere at a temperature of 500° C. for 4 hours. After natural cooling, the sample was taken out and ground into powder to obtain a salt-containing carbon nitride photocatalyst.
Embodiment 2
[0022] First, weigh cyanuric chloride, potassium thiocyanate and lithium chloride with a molar ratio of 1:3:4.32, and grind and mix them evenly under a nitrogen protective atmosphere. The uniformly ground and mixed solid powder was placed in an alumina crucible with a cover, and calcined in a nitrogen atmosphere at a temperature of 500° C. for 4 hours. After natural cooling, the sample was taken out and ground into powder, boiled with deionized water, filtered with suction and dried to obtain the carbon nitride photocatalyst with the salt removed.
Embodiment 3
[0024] First, weigh cyanuric chloride, potassium thiocyanate and lithium chloride with a molar ratio of 1:3:4.32, and grind and mix them evenly under a nitrogen protective atmosphere. The uniformly ground and mixed solid powder was placed in an alumina crucible with a lid, and calcined in a nitrogen atmosphere at a temperature of 550° C. for 4 hours. After natural cooling, the sample was taken out and ground into powder to obtain a salt-containing carbon nitride photocatalyst.
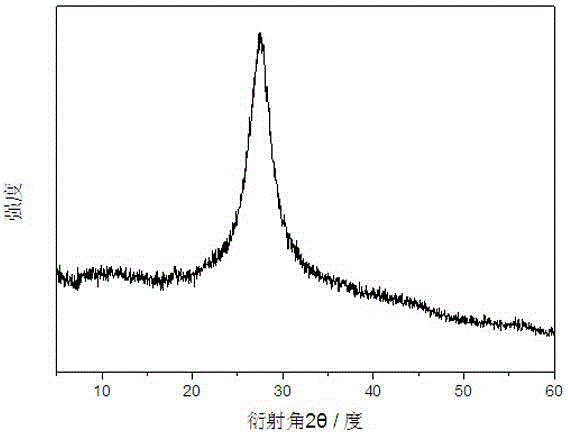
[0025] figure 1 It is the powder X-ray diffraction spectrogram of the carbon nitride photocatalyst obtained in Example 2. It can be found from the figure that the prepared carbon nitride photocatalyst is an amorphous substance.
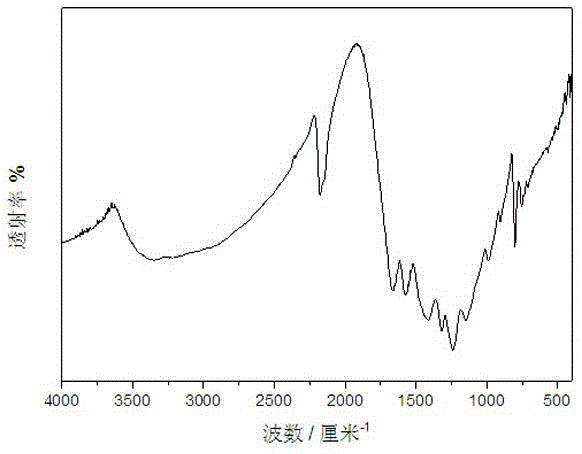
[0026] figure 2 It is the Fourier transform infrared spectrogram of the carbon nitride photocatalyst obtained in Example 2. This figure demonstrates the successful synthesis of carbon nitride photocatalysts. 800 cm in the picture -1 and 1200~1600 cm -1 The signals in t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com