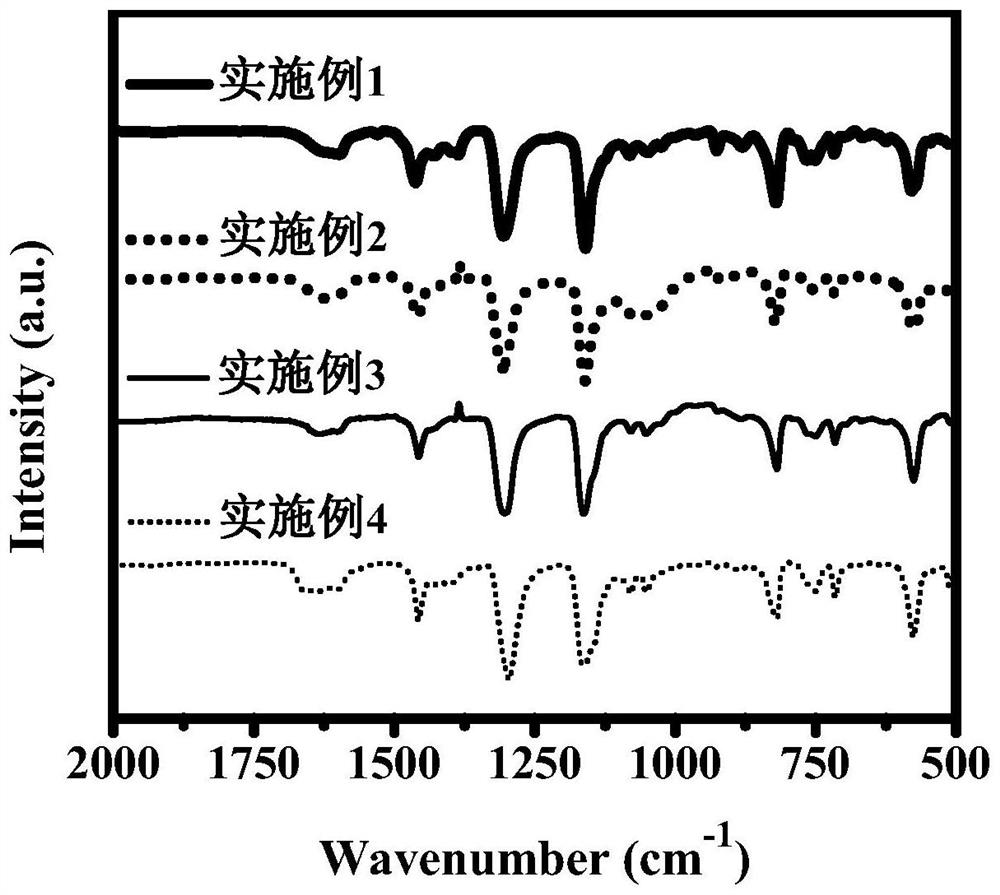
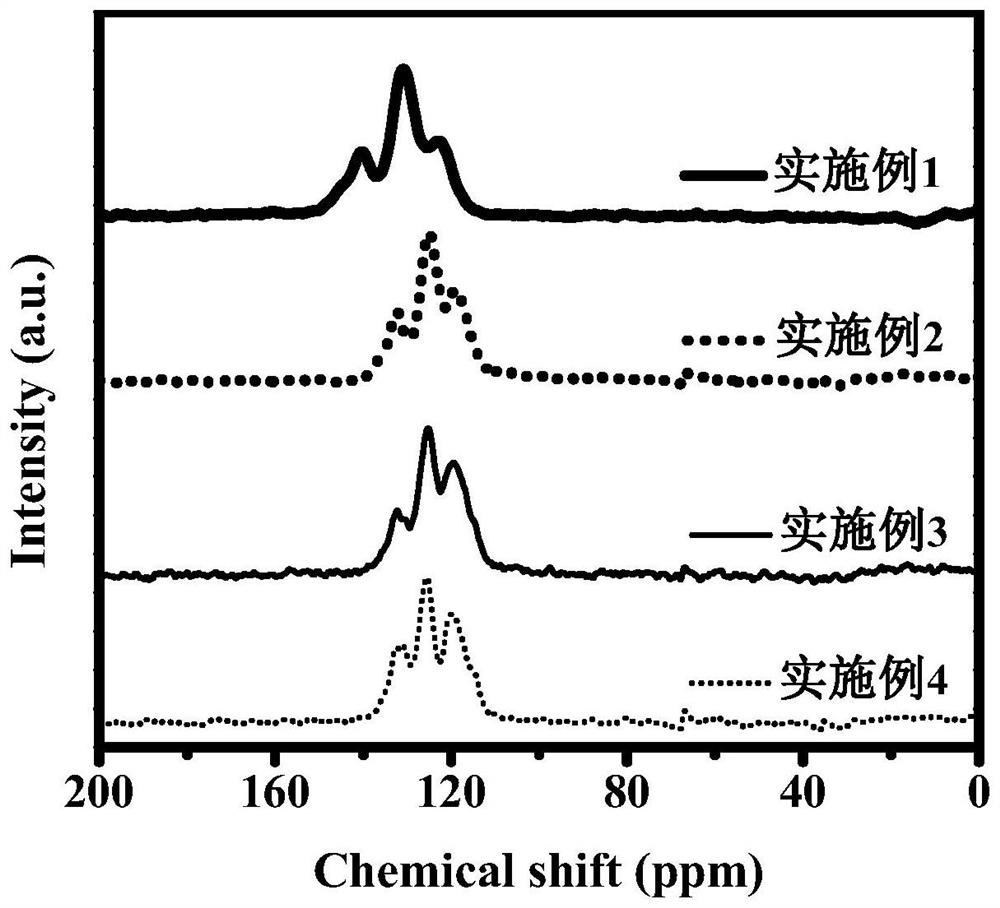
Dibenzo fused dinaphthyl polymer photocatalyst for hydrogen production by photocatalytic decomposition of water and preparation method thereof
A benzo-fused dinaphthyl, photocatalyst technology, applied in the direction of organic compound/hydride/coordination complex catalysts, physical/chemical process catalysts, chemical instruments and methods, etc., can solve the limitation of the development of organic polymer photocatalysts and other problems, to achieve excellent photocatalytic performance, environmental protection, and the effect of improving coplanarity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Under nitrogen protection, 20 mL of N,N-dimethylformamide and 4 mL of 2mol / L potassium carbonate aqueous solution were added to a solution containing 322.0 mg (0.5 mmol) of 2,7,10,15-tetrabromodibenzo-fused dinaphthalene. , 468.2 mg (1.0 mmol) of 3,7-bis(4,4,5,5-tetramethyl-1,3,2-dioxaboran-2-yl)dibenzothiophene sulfone and 20.0 mg (17.3 μmol) in the reaction flask of tetrakis (triphenylphosphine) palladium, heated to 150 ° C for reflux reaction for 48 hours, cooled to room temperature after the reaction, washed with dichloromethane, methanol and water for many times, and dried at 100 ° C under vacuum conditions After 24 hours, a yellow-green solid powder DBC-BTDO was obtained.
Embodiment 2
[0030] Under nitrogen protection, 20 mL of N,N-dimethylformamide and 2 mL of 2mol / L potassium carbonate aqueous solution were added to a solution containing 128.8 mg (0.2 mmol) of 2,7,10,15-tetrabromodibenzo-fused dinaphthalene. , 234.1 mg (0.5 mmol) 3,7-bis(4,4,5,5-tetramethyl-1,3,2-dioxaboran-2-yl) dibenzothiophene sulfone, 37.4 mg (0.1 mmol) 3,7-dibromodibenzothiophene sulfone and 20.0 mg (17.3 μmol) tetrakis(triphenylphosphine) palladium in a reaction flask, heated to 150° C. for reflux reaction for 48 hours, cooled to room temperature after the reaction was completed, and used Dichloromethane, methanol and water were washed for several times, and dried under vacuum at 100 °C for 24 hours to obtain a yellow-green powder DBC-BTDOs-1, and the dibenzo-fused dinaphthalene unit and dibenzothiophene in DBC-BTDOs-1 were obtained. The molar ratio of sulfone units was 1:3.
Embodiment 3
[0032] Under nitrogen protection, 20 mL of N,N-dimethylformamide and 2 mL of 2mol / L potassium carbonate aqueous solution were added to a solution containing 64.4 mg (0.1 mmol) of 2,7,10,15-tetrabromodibenzo-fused dinaphthalene. , 187.3 mg (0.4 mmol) 3,7-bis(4,4,5,5-tetramethyl-1,3,2-dioxaboran-2-yl) dibenzothiophene sulfone, 74.8 mg (0.2 mmol) 3,7-dibromodibenzothiophene sulfone and 20.0 mg (17.3 μmol) tetrakis(triphenylphosphine) palladium in a reaction flask, heated to 150° C. for reflux reaction for 48 hours, cooled to room temperature after the reaction was completed, and used Dichloromethane, methanol and water were washed for several times, and dried at 100 °C under vacuum for 24 hours to obtain a yellow-green powder DBC-BTDOs-2, and the dibenzo-fused dinaphthalene units in DBC-BTDOs-2 were combined with dibenzothiophene. The molar ratio of sulfone units was 1:6.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com