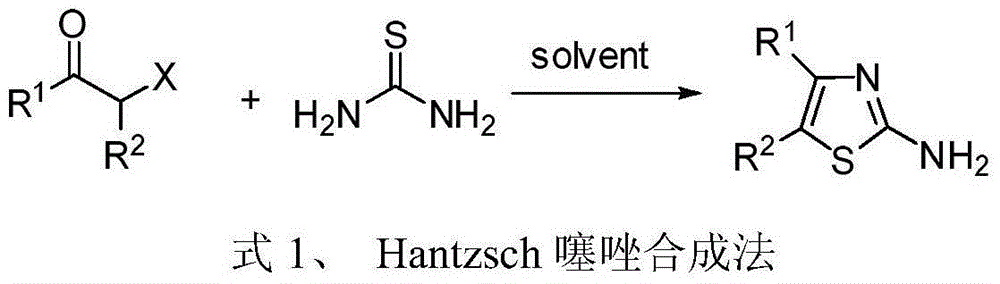
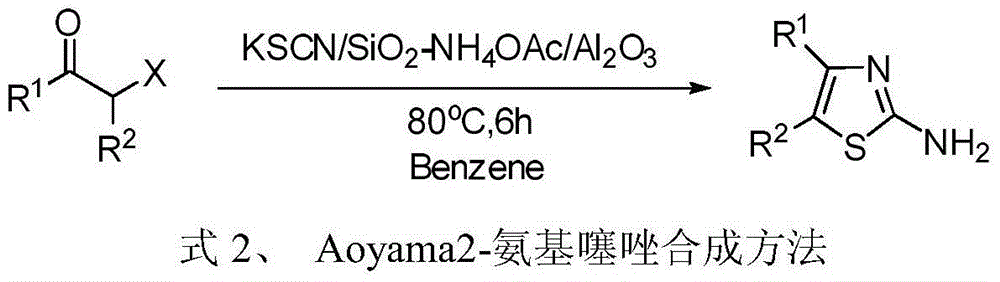
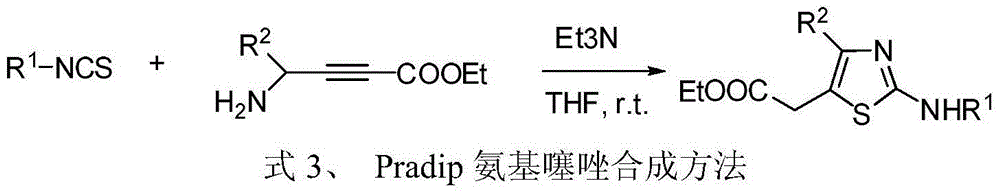
4, 5-disubstituted-2-aminothiazole compound and preparation method thereof
An aminothiazole and compound technology, applied in the field of compound synthesis, can solve the problems of poor applicability of different functional groups, difficult to obtain raw materials, difficult to obtain raw materials, etc., and achieves the effects of less environmental pollution, high yield, and easy availability of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] Embodiment 1, 4-(4-chlorobenzoyl)-5-phenyl-2-aminothiazole (m1)
[0050] Add 141.9 mg (0.5 mmol) of 1-p-chlorophenyl-2-azido-3-phenyl-2-propen-1-one and 97.2 mg (1 mmol) of potassium thiocyanate to the reaction flask, and then add CH 3 CH 2 CH 2 OH (n-propanol) 2.0ml, ferrous sulfate heptahydrate 69.5mg (0.25mmol), after the addition is complete, stir and react at 80°C for 12 hours, TLC detects the reaction (petroleum ether: ethyl acetate = 1:1 volume ratio) .
[0051] Remarks: When TLC detection shows that 1-p-chlorophenyl-2-azido-3-phenyl-2-propen-1-one disappears, it means that the reaction has ended.
[0052] After the reaction was completed, concentrate to remove n-propanol, cool to room temperature, add 60ml of water, and extract the reaction solution three times with 3×20mL ethyl acetate, and wash the organic layer (at the upper layer) with 3×30mL saturated brine three times after merging, and then use Anhydrous sodium sulfate (2.0g) was dried for 30 minutes...
Embodiment 2
[0077] Embodiment 2, 4-benzoyl-5-phenyl-2-aminothiazole (m2)
[0078] Replace 1-p-chlorophenyl-2-azido-3-phenyl-2-propen-1-one with 1-phenyl-2-azido-3-phenyl-2-propen-1-one, mol Quantity is constant, all the other are equal to embodiment 1. 128.8 mg of the product 4-benzoyl-5-phenyl-2-aminothiazole was obtained as a yellow solid, with a yield of 92%.
[0079] Its structural formula is:
[0080]
[0081] Yellow solid; mp:160.4-160.8℃; 1 H NMR (500MHz, DMSO) δ7.84(d, J=7.05, 2H), 7.59(t, J=6.88, 1H), 7.47(d, J=7.31, 2H), 7.35-7.24(m, 7H) ; 13 C NMR (125MHz, DMSO) δ190.76, 166.14, 143.60, 137.12, 133.12, 131.11, 129.66, 128.99, 128.56, 128.39, 128.35, 127.59; HRMS (ESI): m / zcalcd for C 16 h 12 N 2 OS[M+H] + :281.0749,found:281.0748.
Embodiment 3
[0082] Example 3, 4-(4-methylbenzoyl)-5-phenyl-2-aminothiazole (m3)
[0083] Replace 1-p-chlorophenyl-2-azido-3-phenyl-2-propene-1-one with 1-p-methylphenyl-2-azido-3-phenyl-2-propene-1-one Ketone, molar weight is constant, all the other are equal to embodiment 1. 138.2 mg of the product 4-(4-methylbenzoyl)-5-phenyl-2-aminothiazole was obtained as a yellow solid, with a yield of 94%.
[0084] Its structural formula is:
[0085]
[0086] Yellow solid; mp:189.4-189.9℃; 1 H NMR (500MHz, DMSO) δ7.75 (d, J=8.07, 2H), 7.33 (s, 2H), 7.28-7.21 (m, 7H), 2.35 (s, 3H); 13C NMR (125MHz, DMSO) δ190.63, 166.21, 143.88, 143.74, 134.45, 131.18, 129.85, 128.98, 128.60, 128.19, 127.91, 127.47, 21.22; HRMS (ESI): m / z calcd for C 17 h 14 N 2 OS[M+H] + :295.0905,found:295.0907.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com