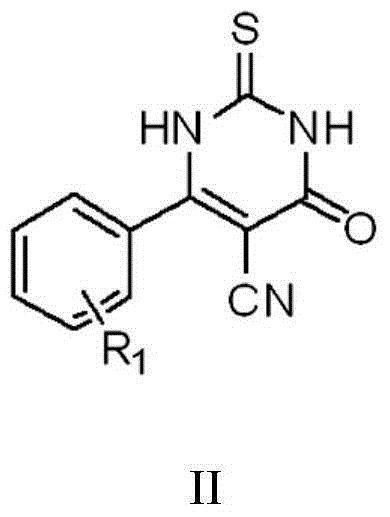
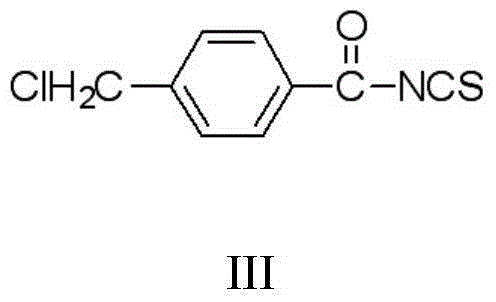
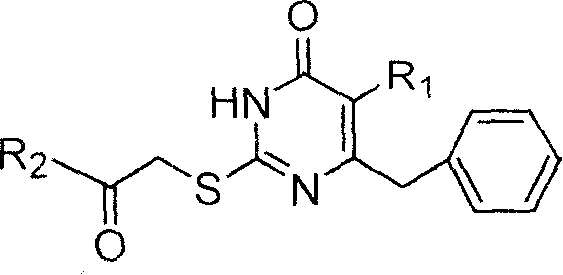
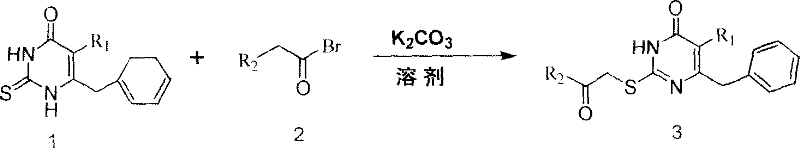
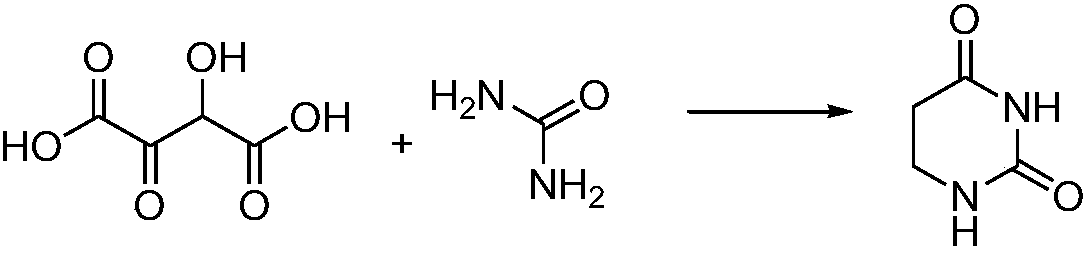
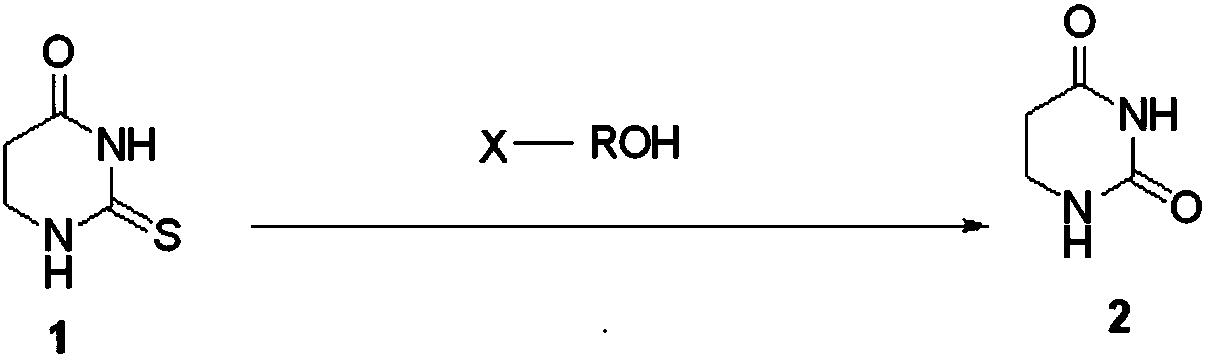
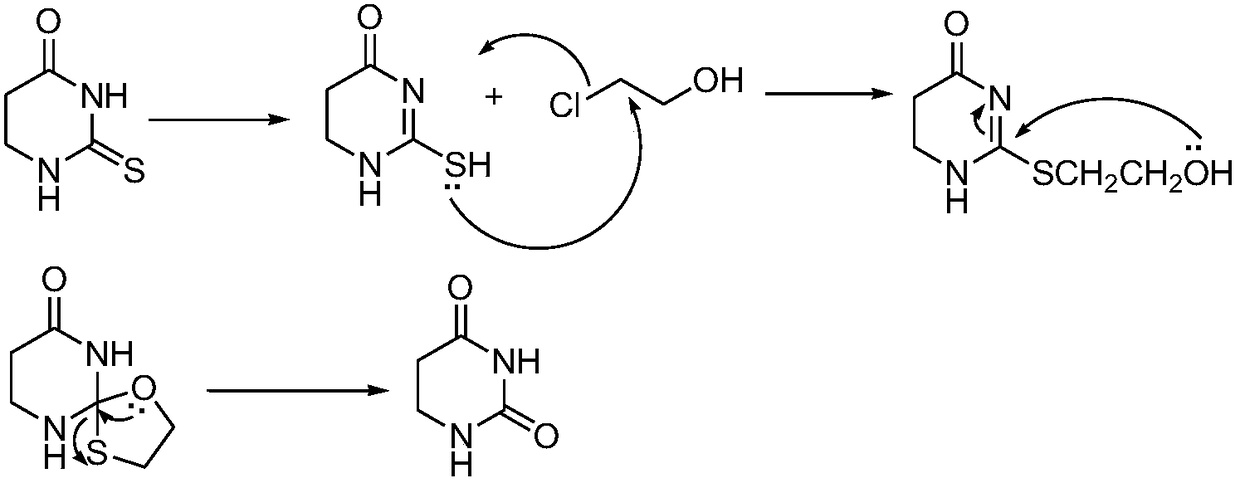
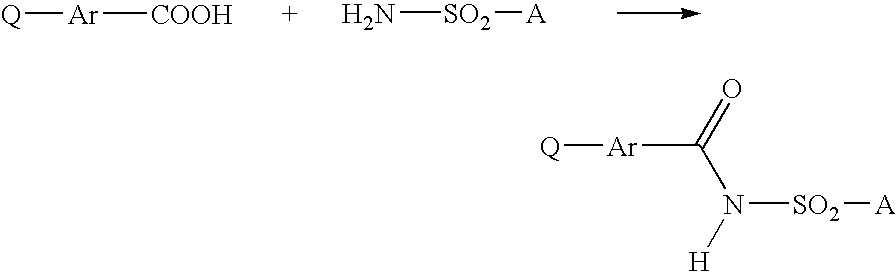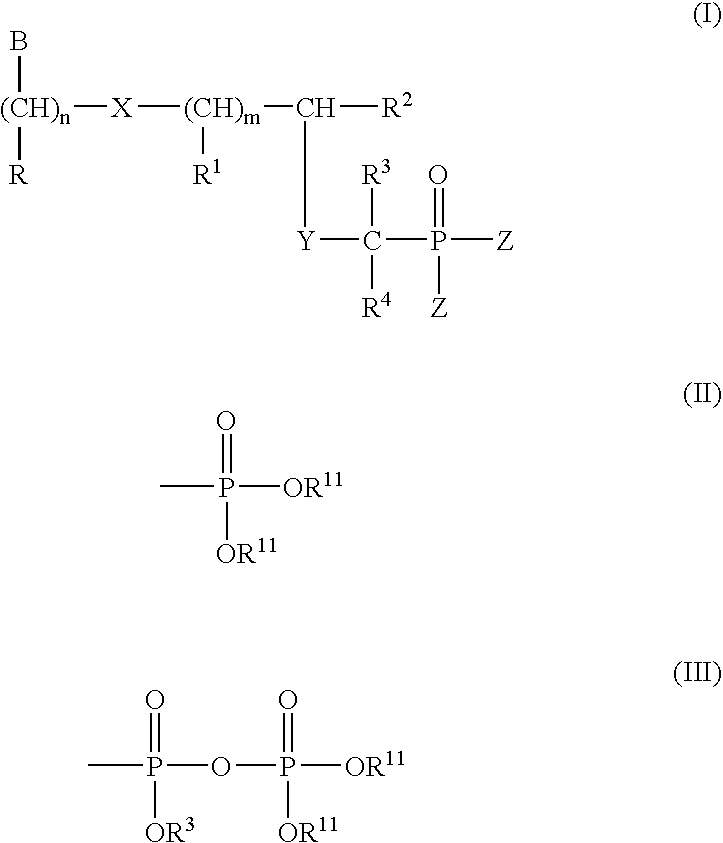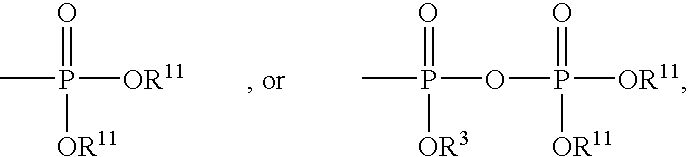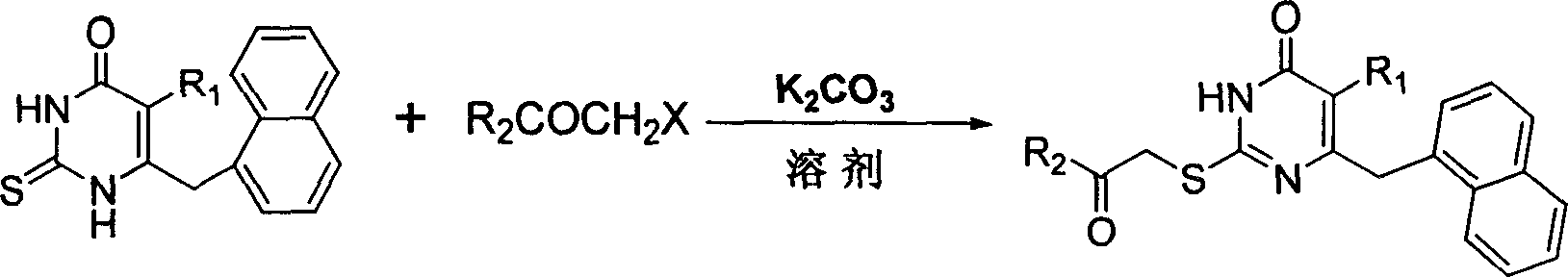Patents
Literature
39 results about "Thiouracil" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Thiouracil refers both to a specific molecule consisting of a sulfated uracil, and a family of molecules based upon that structure.
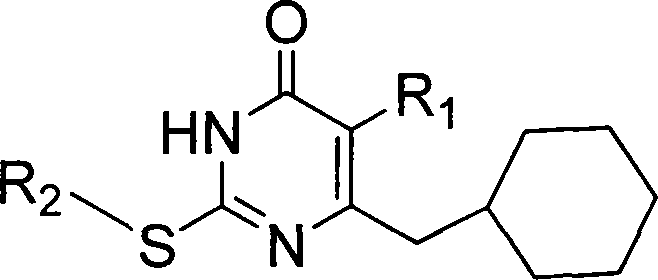
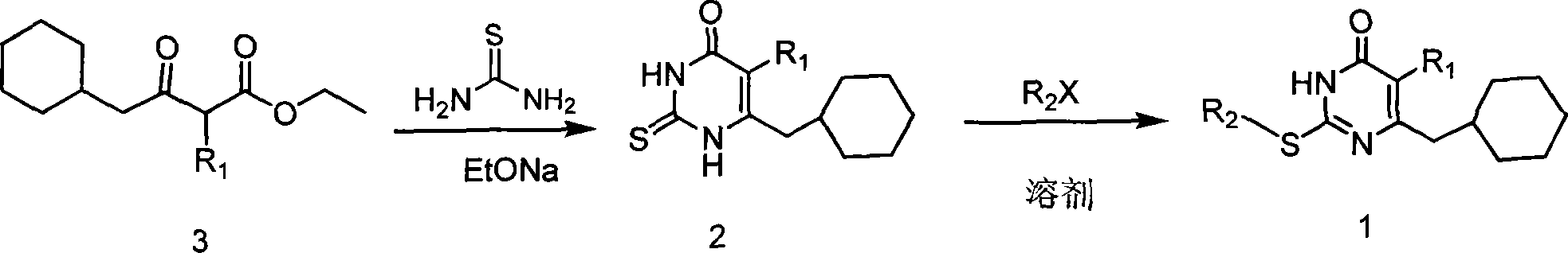
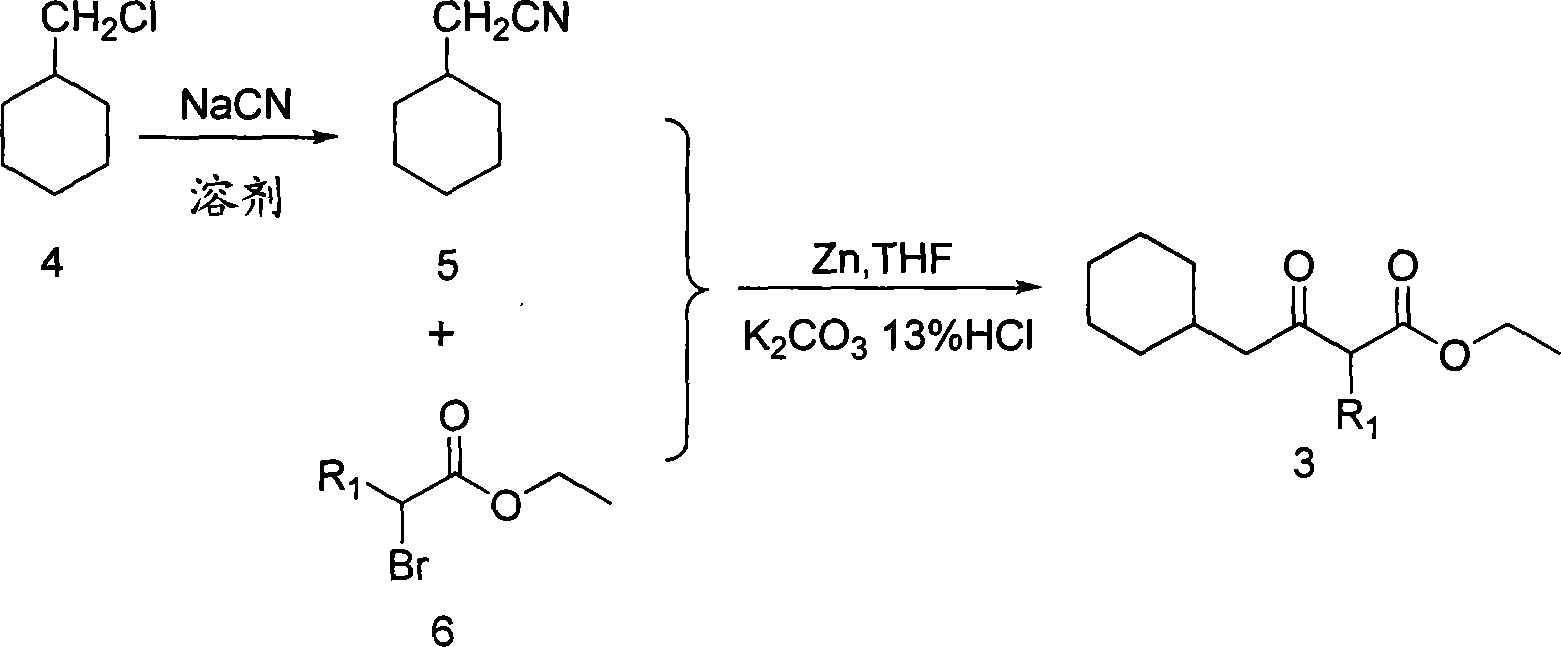
6-cyclohexyl methyl substituted s-DABO compound, method for synthesizing same and uses thereof
InactiveCN101177413AEasy to synthesizeEnhanced inhibitory effectOrganic active ingredientsOrganic chemistryThioureaSide chain
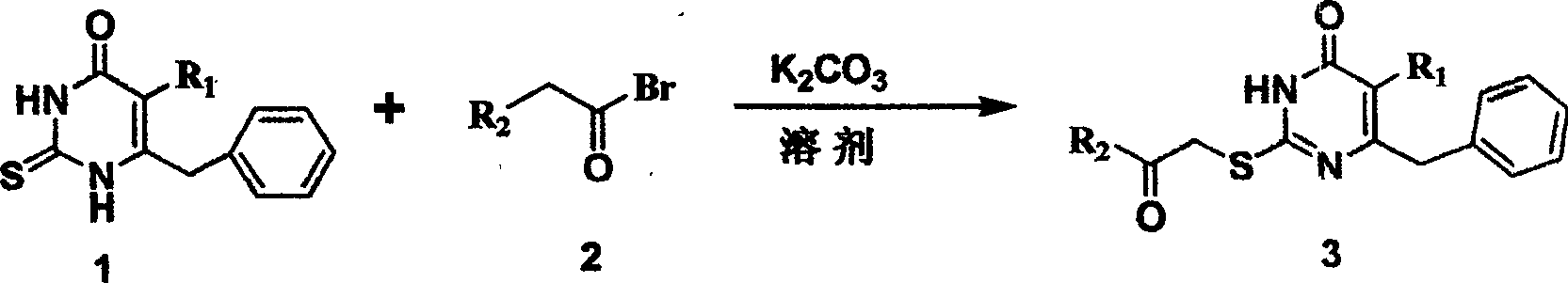
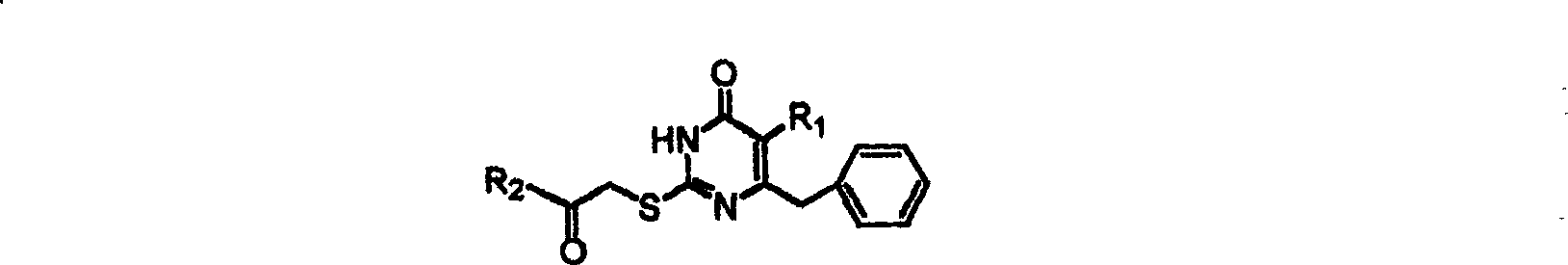
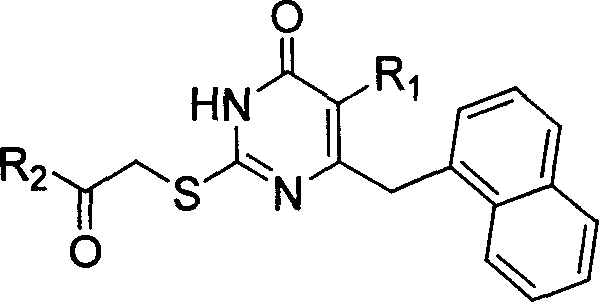
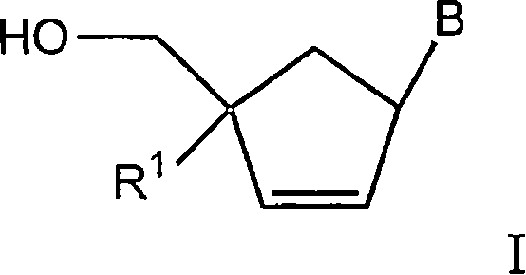
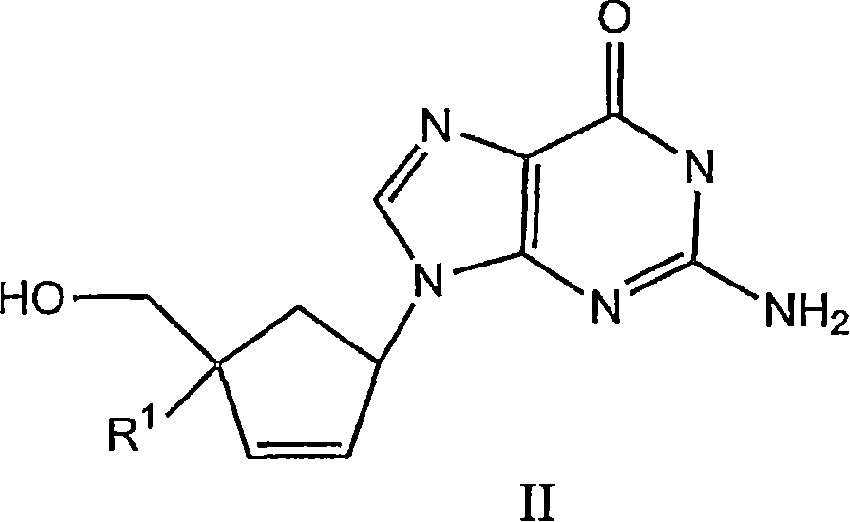
The invention discloses 6-cyclohexyl methyl substituted S-DABO type compound, the synthesis method and the application, belonging to the medical technical field. The invention relates to a 5-alkyl-6-cyclohexyl methyl-2-(alkyl, naphthenic base, naphthenic base methyl, substituted phenylethanone) thiouracil type compound, which has general formula as shown in (I): wherein, R1 is alkyl of C1-3; alkyl, naphthenic base and naphthenic base methyl of R2=C1-8 are as shown in (II); wherein, X=OCH3, H, OH, halogen. Chloromethyl cyclohexane or cyclohexyl acetic acid is respectively used as raw material to prepare Beta- keto ester which is made into a key intermediate 5-alkyl-6-cyclohexyl methyl thiouracil together with thiourea through close loop condensation under the catalyzing of sodium alkoxide; target molecule is prepared through S-alkylation guiding C2-side chain. The invention has the advantages of simple and easy synthesis method, obvious anti-HIV virus activity and anti-resistance for the drugs and ability to be used as an alternative for anti-HIV drug.
Owner:YUNNAN UNIV +1
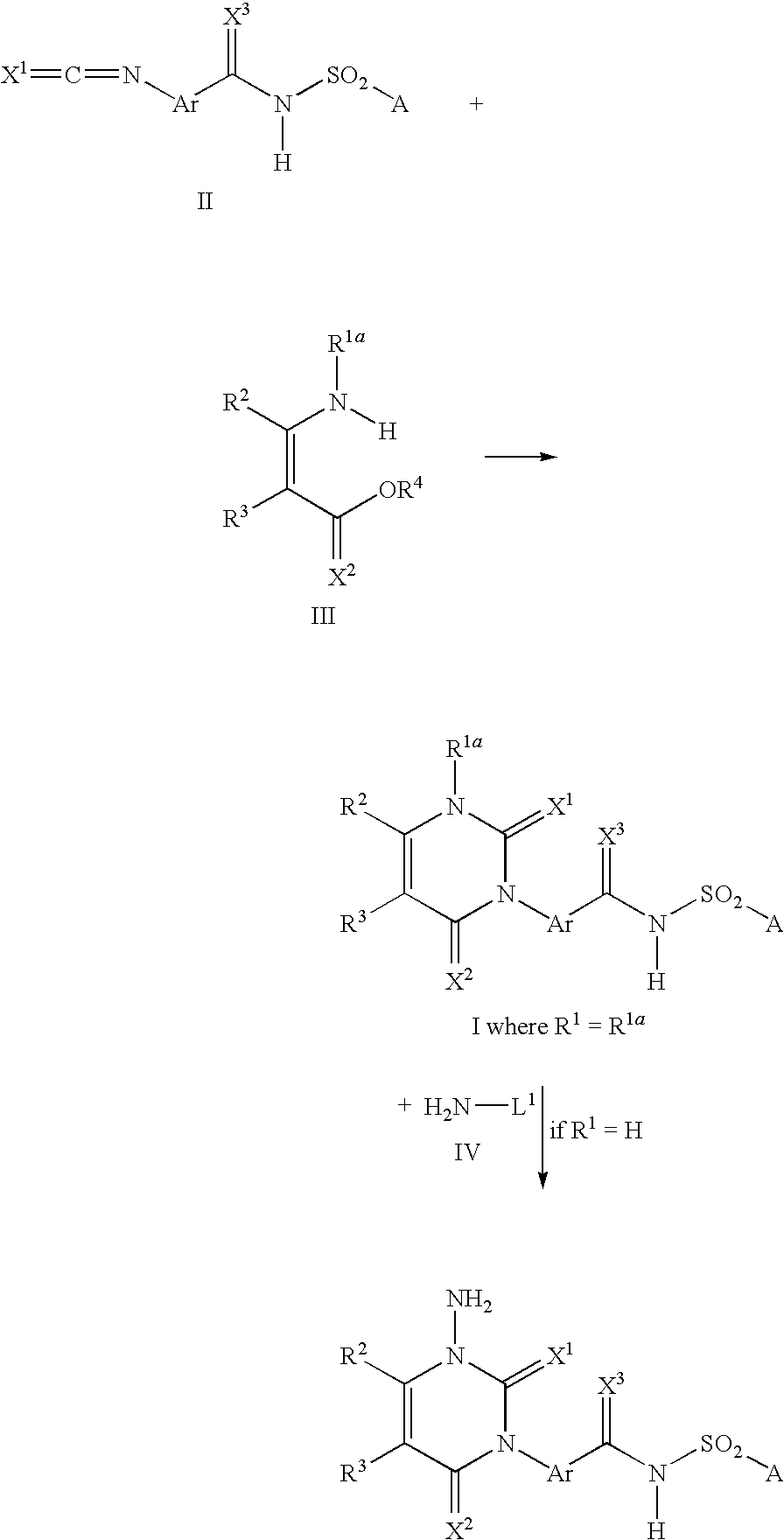
Method for producing 3-phenyl(thio)uracils and 3-phenyldithiouracils
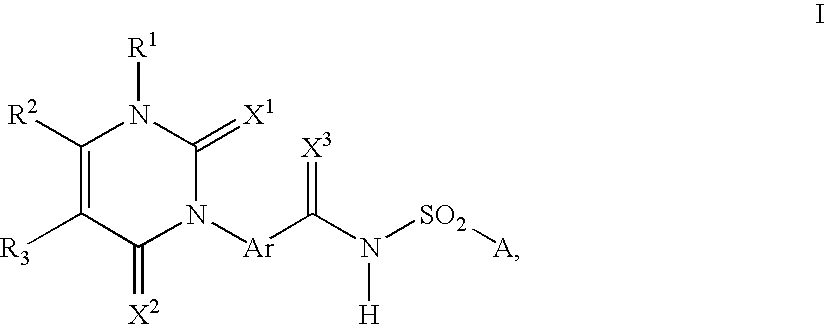
A process is described for preparing 3-phenyl(thio)uracils or 3-phenyldithiouracils of the formula I, by reacting a phenyl iso(thio)cyanate of the formula II with an enamine of the formula III and, if appropriate, in a further step, the resulting 3-phenyl(thio)uracil or 3-phenyldithiouracil of the formula I where R1=R1a, when R1=hydrogen, is reacted with an aminating agent of the formula IV to give 3-phenyl(thio)uracils or 3-phenyldithiouracils of the formula I where R1=aminowhere the variables R1, R1a, R2, R3, R4, X1, X2, X3, Ar, A and L1 are each as defined in claim 1.
Owner:BASF AG
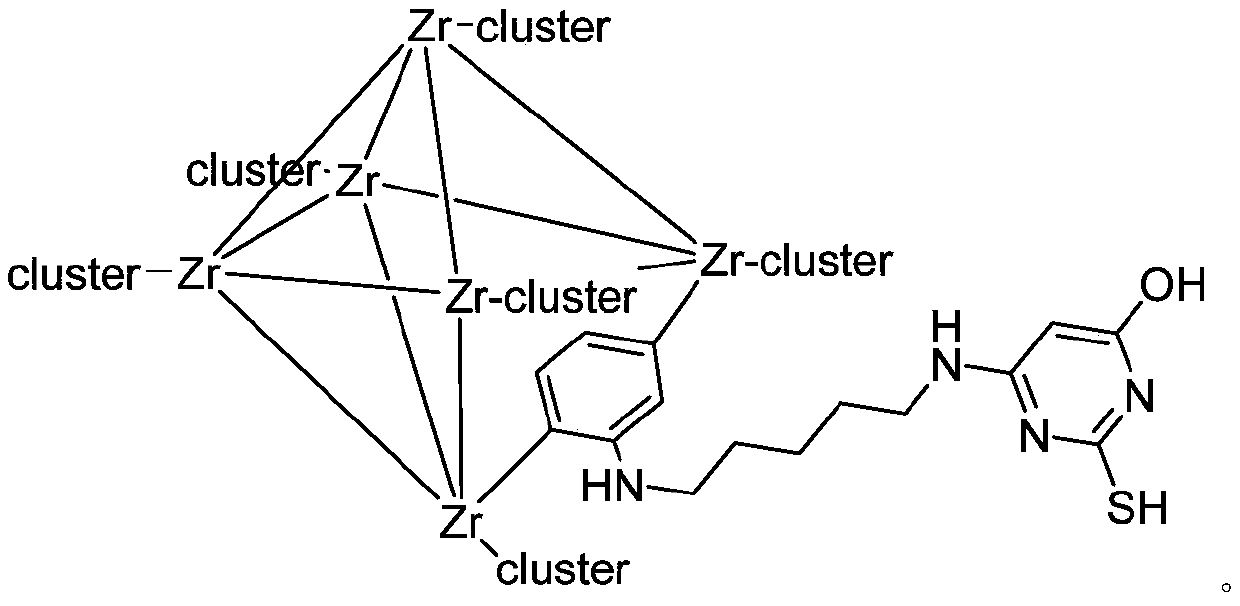
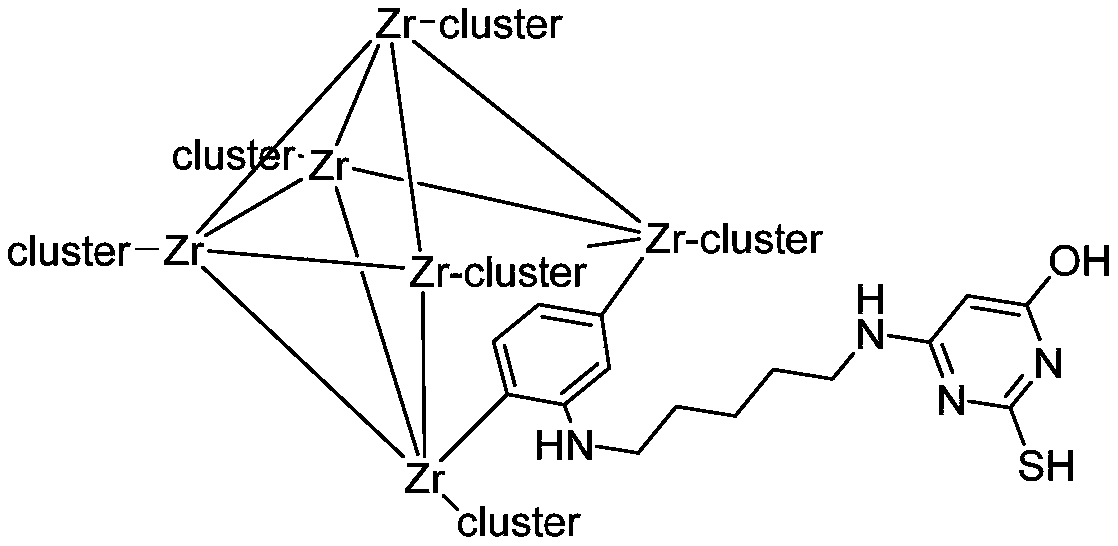
Modified zirconium-based organic metal framework adsorbent for adsorbing lead ions as well as preparation method and application thereof
ActiveCN110813244AImprove adsorption capacityGood selective removal rateOther chemical processesWater contaminantsSorbentMetal-organic framework
The invention discloses a modified zirconium-based organic metal framework adsorbent for adsorbing lead ions as well as a preparation method and application of the modified zirconium-based organic metal framework adsorbent. The method comprises the following steps: reacting 2-aminoterephthalic acid, zirconium tetrachloride and hydrochloric acid in N, N-dimethylformamide to obtain a product UiO-66-NH<2>, then reacting the product UiO-66-NH<2> with glutaraldehyde to obtain a product UiO-66-GD, and reacting UiO-66-GD with 6-amino-2-thiouracil to obtain the modified zirconium-based organic metal framework adsorbent UiO-66-ATA. The method is simple to operate, the preparation method is simple, raw materials and reagents are cheap and easy to obtain, the obtained metal organic framework can be compatible with various functional groups, has ultrahigh specific surface area and permanent porosity, has high selectivity and high flux adsorption effect on lead ions, a novel efficient adsorption material is provided for lead-containing sewage treatment, and a new method is provided for design and synthesis of the novel adsorption material.
Owner:SUN YAT SEN UNIV
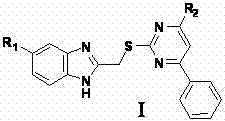
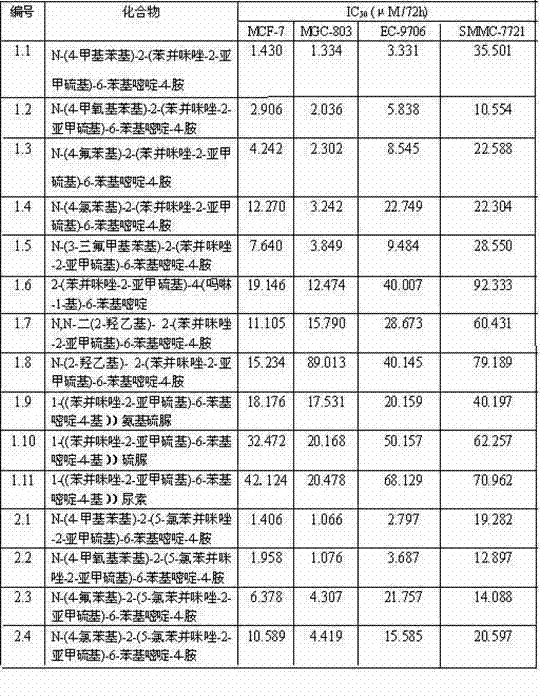
Pyrimidine derivatives with benzimidazole structural units as well as preparation method and application thereof
ActiveCN103497179AReasonable synthetic designMild reaction conditionsOrganic active ingredientsOrganic chemistryChemical synthesisThiourea
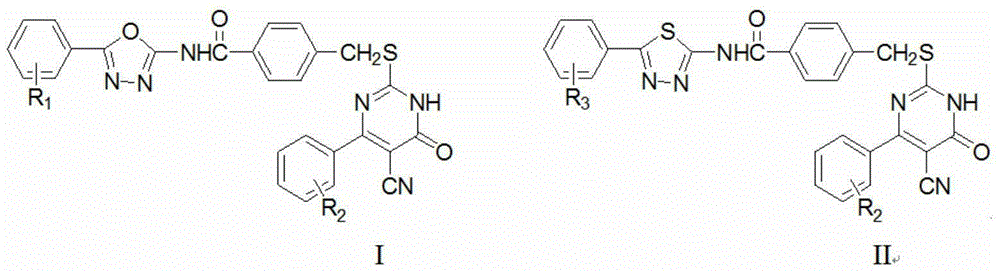
The invention belongs to the technical field of chemical synthesis of drugs and discloses a 2-(5H / Cl-benzimidazole-2-dimehtylthio)-4-substituted-6-phenyl pyrimidine derivatives with antitumor activity as well as a synthesis method and application thereof. A series of 6-phenyl-2-thiouracil derivatives with benzimidazole structural units are prepared through reactions such as cyclization, substitution, chlorination, aminolysis and the like by taking ethyl benzoylacetate as the raw material. The compounds provided by the invention have the general formula as shown in a figure I. In-vitro antitumor activity experiments prove that the compounds have remarkable inhibiting and killing effects for various tumor cells.
Owner:ZHENGZHOU UNIV
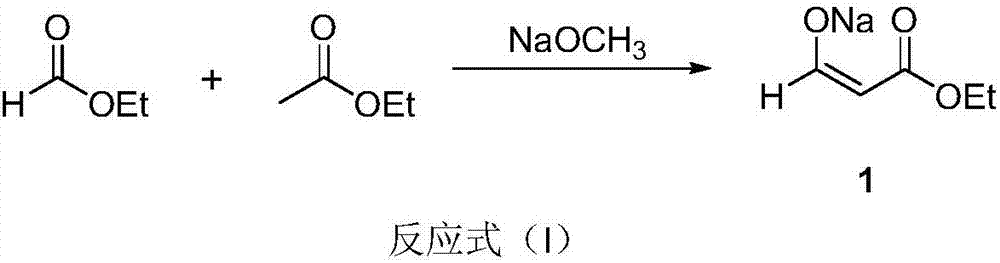
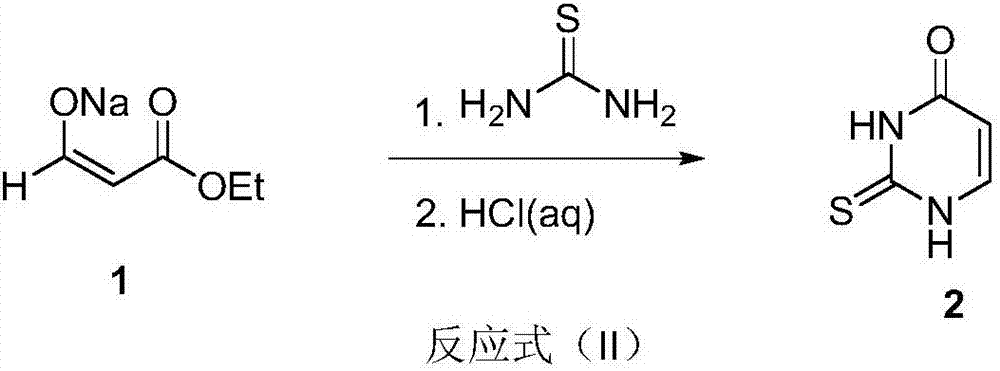
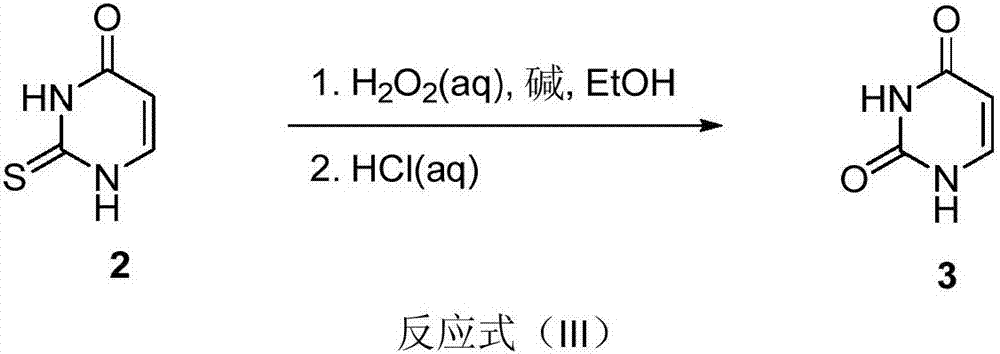
Preparation method of uracil
The invention discloses a preparation method of uracil. The method comprises the following steps: (a) enabling ethyl formate, ethyl acetate and sodium methylate to react so as to prepare sodium (E)-3-ethoxy-3-oxoprop-1-en-1-olate; (b) adding thiourea and ethyl acetate into the sodium (E)-3-ethoxy-3-oxoprop-1-en-1-olate, heating for carrying out a reaction, and acidizing by using hydrochloric acid to obtain thiouracil; (c) adding alkali and ethanol into the thiouracil, cooling and then dropwise adding a hydrogen peroxide water solution into the cooled mixture; after that, heating for carrying out a reaction to obtain the uracil. According to the technology for preparing the uracil by using the thiourea as a raw material, the used raw materials such as ethyl formate, the ethyl acetate, the thiourea and hydrogen peroxide are chemical products which are common, easy to obtain and low in price, so that the production cost is lower. The preparation method of the uracil is simple, convenient and fast to operate and high in yield, thus being suitable for large-scale industrial production.
Owner:上海旭东海普南通药业有限公司
Thiouracil derivative, preparation method and application thereof
InactiveCN104876881ABroaden your optionsOvercoming the drawbacks of drug resistanceAntibacterial agentsOrganic active ingredientsThioureaPotassium thiocyanate
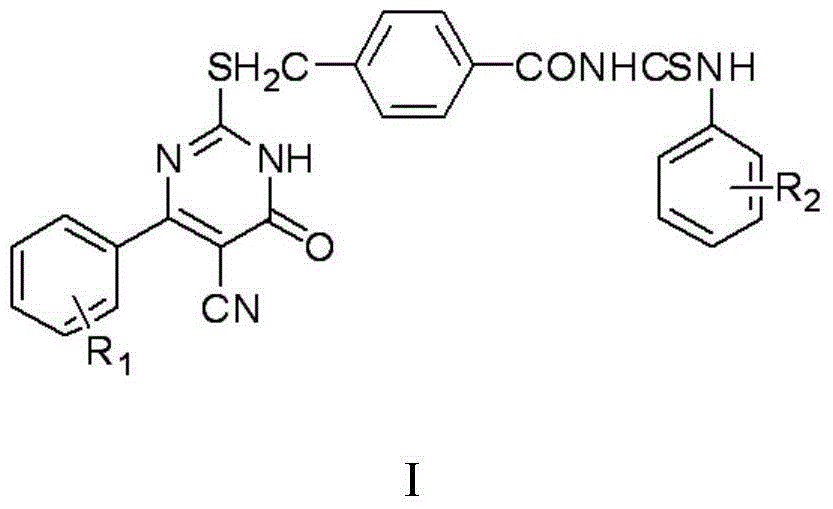
The invention discloses a thiouracil derivative. The chemical formula is shown in the formula I, wherein R1 is hydrogen, halogen or aromatic group, R2 is hydrogen, halogen or alkyl group from C1 to C6. Simultaneously, the invention also discloses a method for synthesizing the derivative. The method comprises the following steps : adopting aromatic aldehyde or an aromatic amine compound as a starting material, and reacting the aromatic aldehyde, ethyl cyanoacetate and thiourea under the catalysis of piperidine to obtain a compound II; reacting chloromethyl-benzoyl chloride and potassium thiocyanate in a methylbenzene / water / TBAB system to obtain a compound III; reacting the compound III and aromatic amine in an acetonitrile solvent to obtain a compound IV, reacting the compound II and the compound IV with the equal mole in the acetonitrile solvent under the catalytic action of potassium carbonate to obtain a target product, i.e., a compound I. The method adopted is simple, the operation is easy, and the large-scale production is easy; and verified by experiment, the prepared compound I has stronger bacteriostatic activity and can be widely applied in bacteriostatic medicine preparations.
Owner:HEBEI UNIVERSITY
S-DABO compound, synthesizing method and usage
InactiveCN101037415AEasy to synthesizeLow cytotoxicityOrganic active ingredientsOrganic chemistryFuranKetone
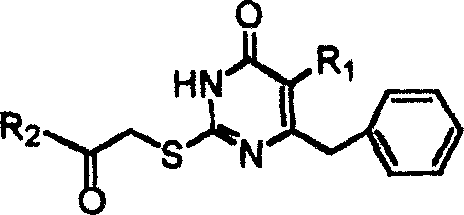
S-DABO composition and its synthesis method and function belong to medicine technical medicine. The invention relates to a 5-alkyl-6-phenyl-2-(substituted arylcarbonylmethylsulfur)uracil composition, having following general formula: wherein, R1 is C1-3 alkyl; R2=C1-6 alkyl, substituted furan ring, thiofuran ring, benzene ring (substituent on the benzene ring is H, OH, Cl3 alkoxy), having the 5-alkyl-6-phenylthiouracil as reagent, reacting with alpha-halogen ketone to get the inventive product which is catalyzed by K2CO3. The synthesis method is easy to operate. The product has an obvious anti-HIV virus activity, a low toxicity, a high selectivity index and can be an anti-HIV medicine candidate.
Owner:YUNNAN UNIV +1
Thiouracil derivatives containing oxadiazole/thiadiazole and preparation method and application of thiouracil derivatives
InactiveCN105153143AEasy to operateEase of mass productionAntibacterial agentsOrganic active ingredientsThioureaThiadiazoles
The invention discloses thiouracil derivatives containing oxadiazole / thiadiazole, and further provides a method for synthesizing the thiouracil derivatives. The general chemical formula of the thiouracil derivatives is shown in figure I or figure II, wherein R1, R2 and R3 are hydrogen or halogen or aryl-. The method includes the steps of making aromatic aldehyde react with ethyl cyanoacetate and thiourea under the catalysis of piperidine to obtain a compound III, making aromatic aldehyde react with semicarbazide to obtain a compound IV, making the compound IV react with bromine to obtain a compound V, making the compound V react with benzoyl chloride to obtain a compound VI, making the compound VI and the compound III have a reflux reaction under the catalysis of potassium carbonate to obtain the general chemical formula I, making aromatic acid react with thiosemicarbazide to obtain a compound VII, making the compound VII react with benzoyl chloride to obtain a compound VIII, and making the compound VIII react with the compound III under the catalysis of potassium carbonate to obtain the general chemical formula II. The adopted method is simple, easy to operate and beneficial to large-scale production; it is verified that the prepared compound has high bacteriostatic activity and can be widely applied in bacteriostatic drug preparations.
Owner:HEBEI UNIVERSITY
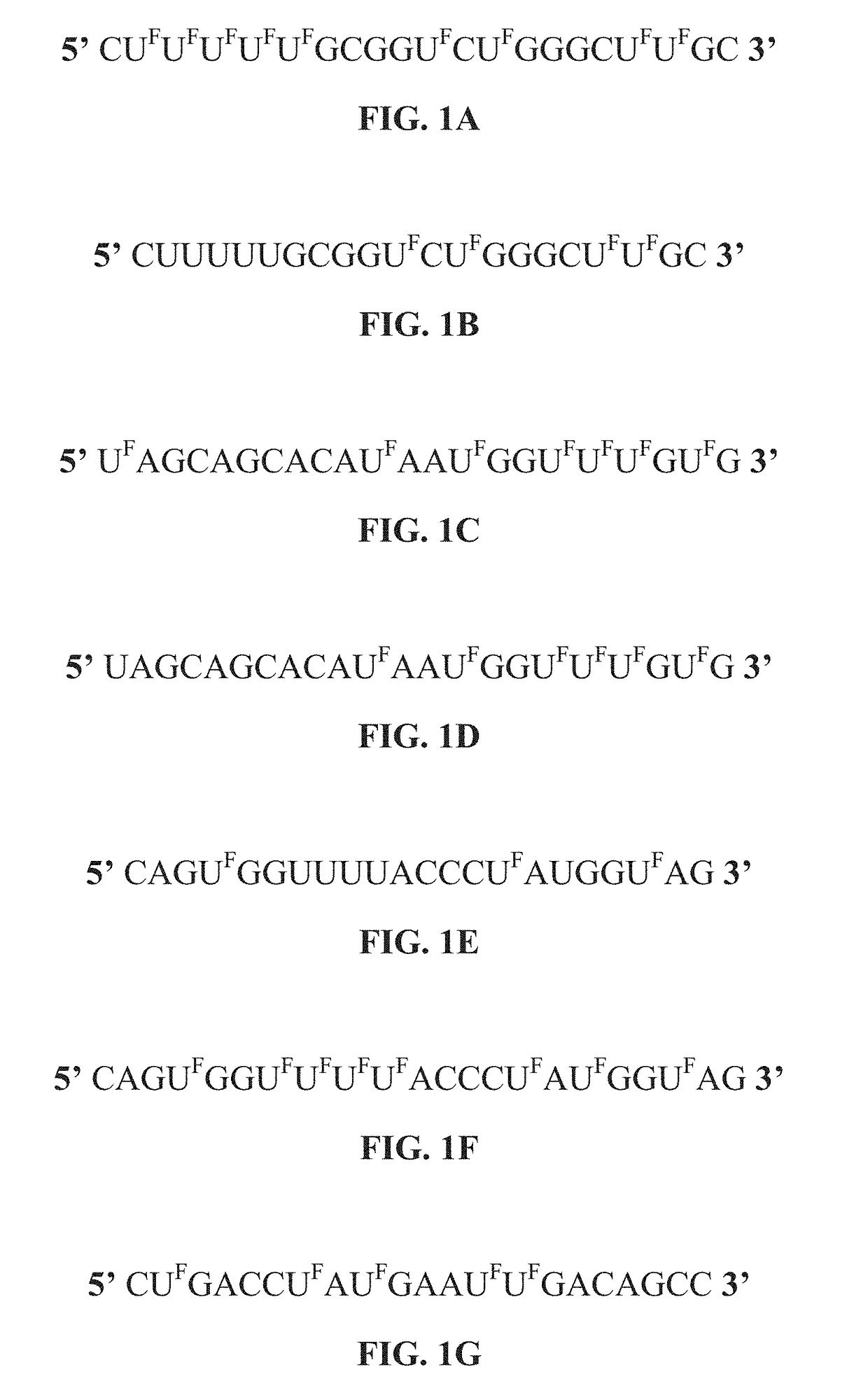
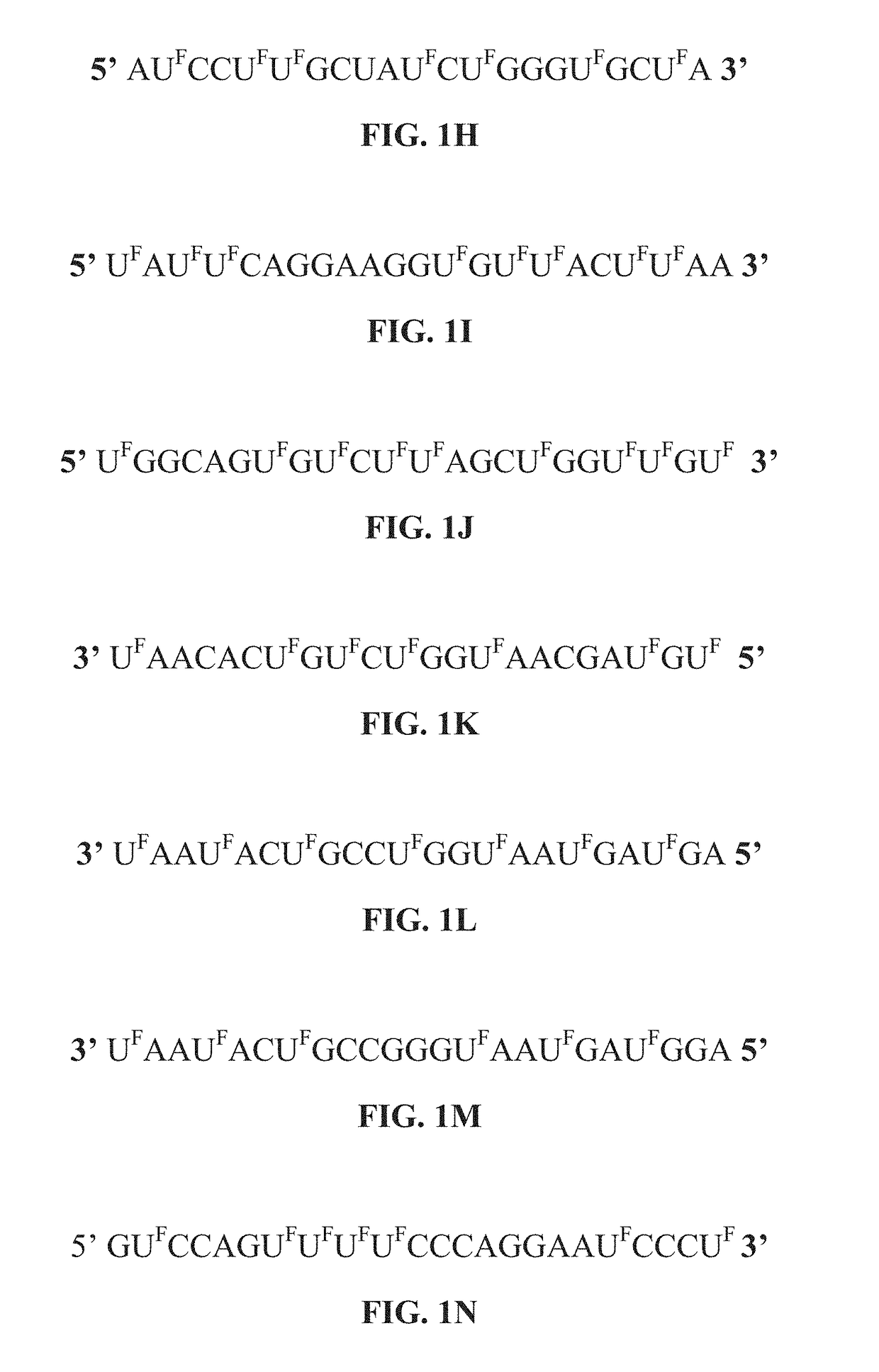
5-halouracil-modified micrornas and their use in the treatment of cancer
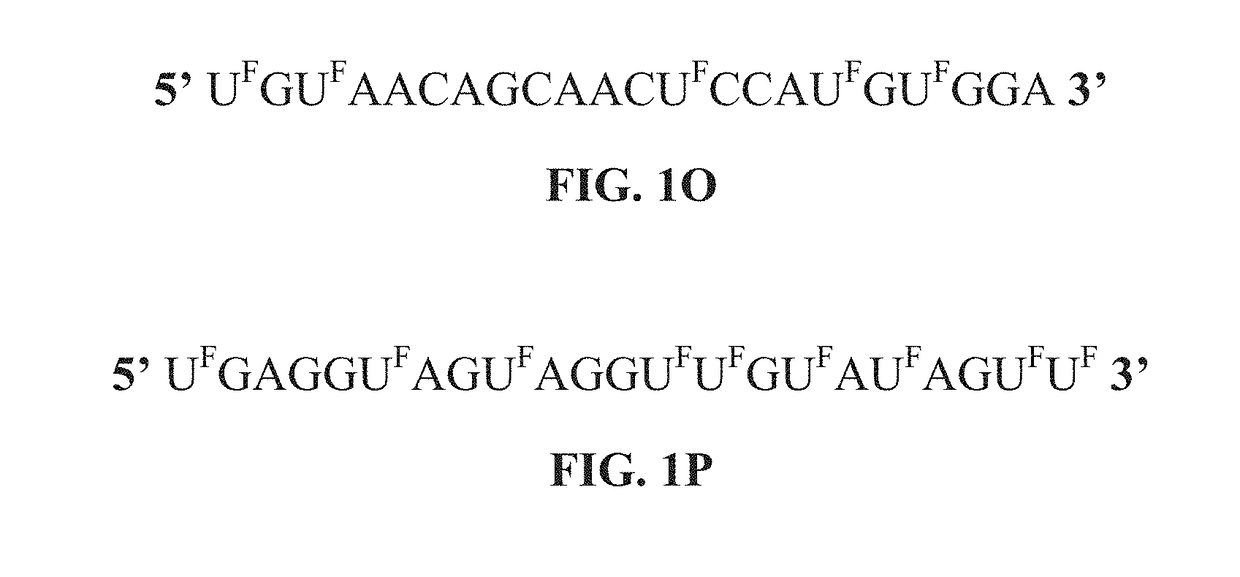
ActiveUS20190062754A1Less side effectsGood curative effectOrganic active ingredientsSugar derivativesNucleic acid sequencingThiouracil
The present disclosure provides nucleic acid compositions that incorporate one or more halouracil molecules. More specifically, the present disclosure reveals that the replacement of uracil nucleotides within a microRNA nucleotide sequence with a 5-halouracil increases the ability of the micro-RNA to inhibit cancer progression and tumorigenesis. As such, the present disclosure provides various nucleic acid (e.g., microRNA) compositions having 5-halouracil molecules incorporated in their nucleic acid sequences and methods for using the same. The present disclosure further provides pharmaceutical compositions comprising the modified nucleic acid compositions, and methods for treating cancers using the same.
Owner:THE RES FOUND OF STATE UNIV OF NEW YORK
Preparation method for lightproof and waterproof polyurethane coating
The invention discloses a preparation method for lightproof and waterproof polyurethane coating. The preparation method for the lightproof and waterproof polyurethane coating comprises the following steps: under the condition that the dibutyltin dilaurate catalyst exists, PTMG and dicyclohexyl methane diisocyanate are mixed; the reaction is conducted for 1-4 hours at a temperature of 60-85 DEC G; methyl thiouracil and drostanolone are added; the reaction time lasts for 1 hour; the reaction temperature is 80 DEC G; a polyurethane prepolymer A is obtained; chain extender, N- methyl pyrrolidone and formamide are added to the polyurethane prepolymer A; the reaction is conducted for 2.5-3.5 hours at 75 -85 DEC G; a mixed liquor A is added; the reaction temperature is 70-95 DEC G; the reaction time is 2-3 hours; a neutralizers is added for a neutralization reaction for 30-50 min; water is added for emulsification; the lightproof and waterproof polyurethane coating is obtained. The prepared lightproof and waterproof polyurethane coating is environment friendly and inexpensive in price. Therefore, the lightproof and water-based polyurethane coating can be extensively applied to surfaces of the wall bodies, furniture and metal wares and served as binding agent of plastics, glass, paper manufacturing and textile.
Owner:HEBEI CHENYANG INDAL & TRADE GROUP CO LTD +1
Licochalcone A thiouracil derivatives with antitumor activity and synthesis method thereof
InactiveCN106674128ATake advantage ofReduce manufacturing costOrganic chemistryAntineoplastic agentsSynthesis methodsThiourea
Owner:SHAANXI UNIV OF SCI & TECH
Tin surface protective agent and preparation method thereof
InactiveCN111118501AReduce thickness requirementsReduce dosageConductive material chemical/electrolytical removalChemical reactionMeth-
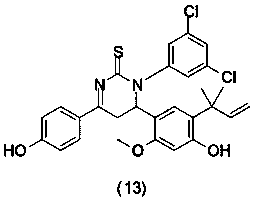
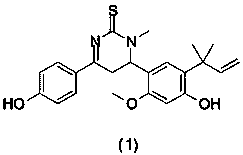
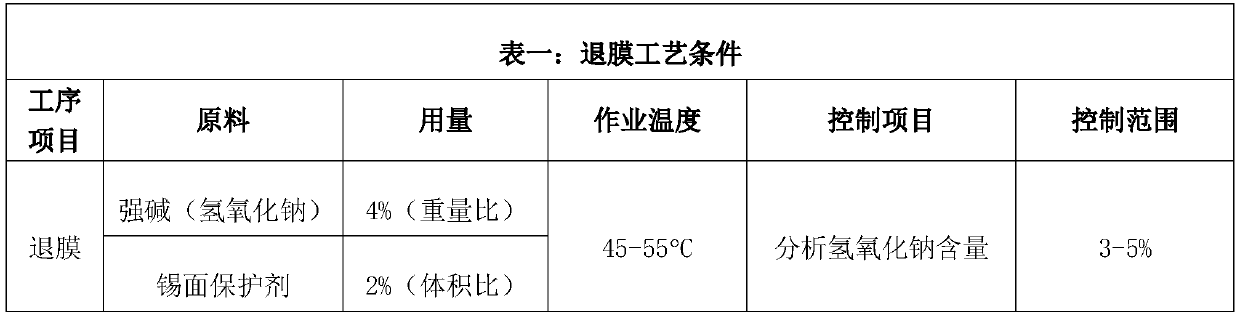
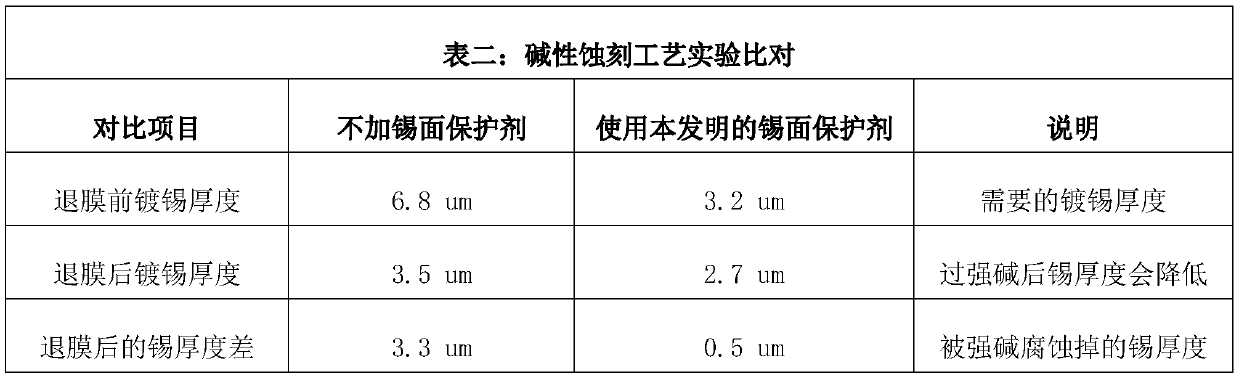
The invention relates to the technical field of printed circuit board process agents, in particular to a tin surface protective agent and a preparation method thereof. The tin surface protective agentcomprises, by weight, 3-8 parts of hexamethylenetetramine, 2-10 parts of urea, 0.7-1.1 parts of polyethylene glycol 300, 0.1-0.3 part of benzotriazole, 0.1-0.3 part of alkylbenzimidazole, 0.1-0.3 part of thiouracil, 0.5-1.0 part of monoethanolamine and 80-94 parts of water. During application of the tin surface protective agent, the redox potential of metal tin can be changed, the chemical reaction between the tin surface and strong alkali is harder, a protection film can be formed on the tin surface, and therefore strong alkali and the tin surface are isolated, and the function of protectingthe tin surface is achieved; and meanwhile, the thickness requirement of the tin surface of a circuit board can be reduced to 2-4 micrometers, the anti-etching requirement can be completely met, andtherefore the use level of metal tin is greatly saved, and conditions are created for reducing the enterprise cost.
Owner:深圳市星扬高新科技有限公司
6-cyclohexyl methyl pyrimidone compounds (s-DACOs) non-nucleoside reverse transcriptase inhibitors (nnrtis) as well as preparation method and use thereof
InactiveCN106866549AStrong inhibitory activityLow toxicityOrganic chemistryAntiviralsChemical synthesisNucleoside Reverse Transcriptase Inhibitor
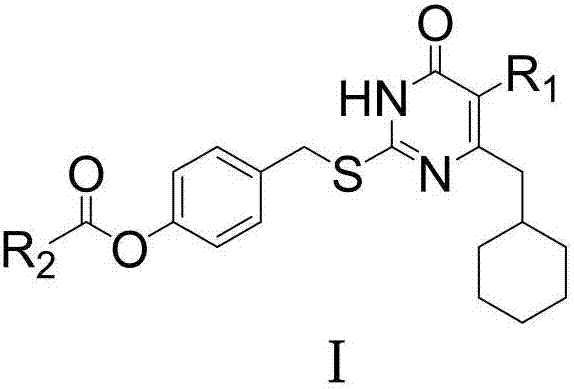
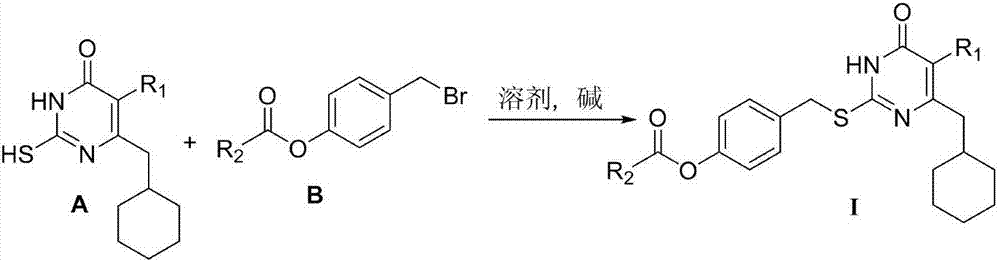
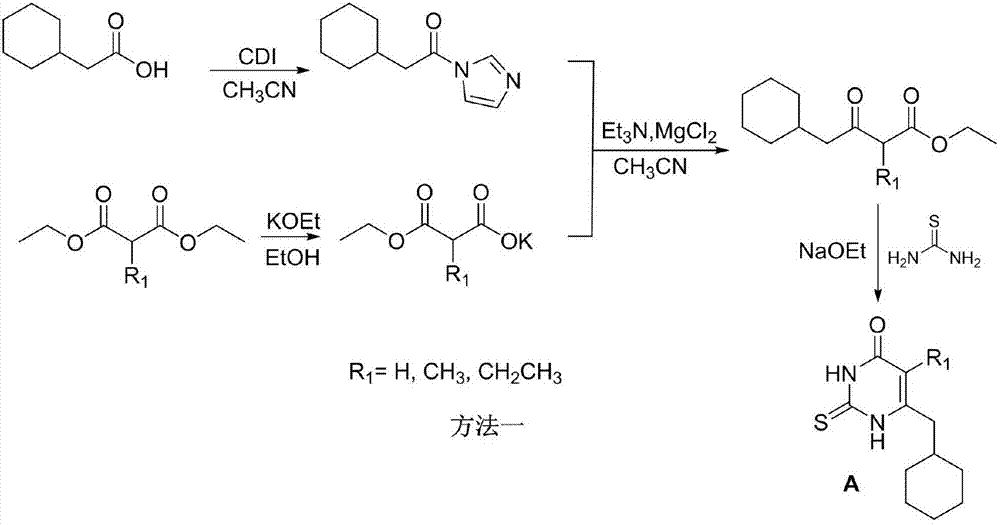
The invention relates to 6-cyclohexyl methyl pyrimidone compounds (S-DACOs) non-nucleoside reverse transcriptase inhibitors (NNRTIs) as well as a preparation method and use thereof, belonging to the technical fields of chemical synthesis and medicine. The compounds are 5-alkyl-6-cyclohexylmethyl-2-(4'-carboxylate benzyl)thio-pyrimidone compounds as shown in a formula I, pharmacologically acceptable salts of the 5-alkyl-6-cyclohexylmethyl-2-(4'-carboxylate benzyl)thio-pyrimidone compounds, or precursors and derivatives having the same biological functions; the formula I is shown in the description, wherein R1 is alkyl or cycloalkyl of C1-C6; R2 is alkyl or cycloalkyl of C1-C8, mono-substituted or poly-substituted phenyl, and the substituent group on a benzene ring of the phenyl is hydrogen, halogen, nitro, amino, cyano, sulfonic group, carboxy, alkyl of C1-C3 or alkoxy of C1-C3; R2 is 1-naphthyl, 2-naphthyl, diphenyl, 2-thienyl or a group shown in the description, and in the group shown in the description, X is hydrogen, halogen, nitro, amino, cyano, sulfonic group, carboxy, alkyl of C1-C3 or alkoxy of C1-C3; the chemical formula of R2 is shown in the description; the NNRTIs are obtained by enabling 5-alkyl- 6-cyclohexylmethyl-thiouracil A, which is taken as a raw material, to react with 4-carboxyester benzyl bromide B respectively under the solvent condition and alkaline condition. The compounds provided by the invention have good human immunodeficiency virus (HIV)-1 inhibition activity and low in toxicity, and can be used for preparing medicines for treating acquired immune deficiency syndrome (AIDS).
Owner:YUNNAN UNIV +1
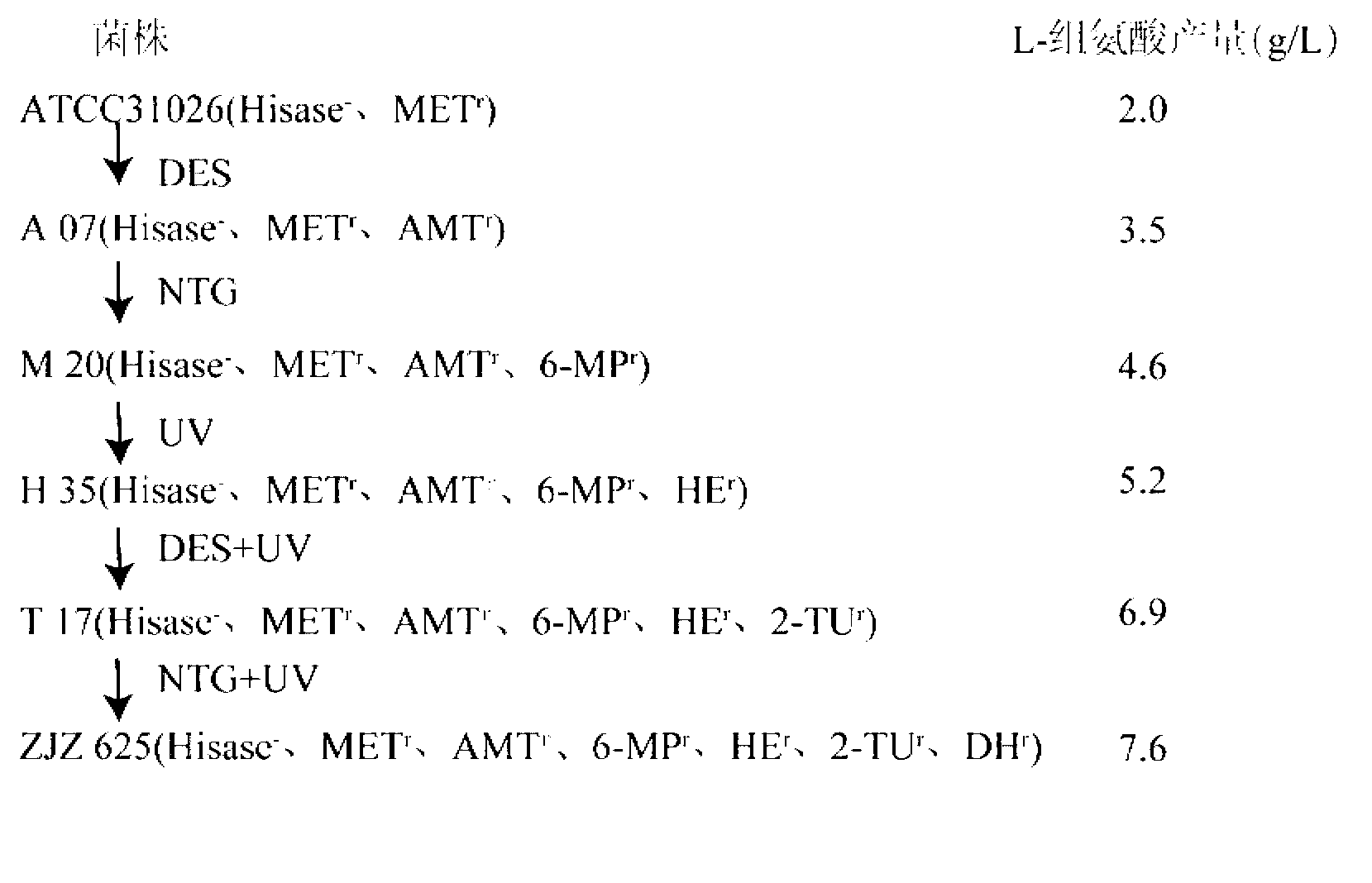
L-histidine high-yielding strain and application thereof
InactiveCN103013876ARelease feedback inhibitionBacteriaMicroorganism based processesCulture mediumsChemistry
The invention provides an L-histidine high-yielding strain and an application thereof, and belongs to the field of a bioengineering technology. The invention provides Serratia marcescens ZJZG25 which is obtained by the following steps of: by taking Serratia marcescens ATCC (American Type Culture Collection) 31026 as a starting strain, carrying out diethyl sulfate (DES), nitrosoguanidine (NTG) and ultraviolet (UV) gradual-grade mutation; and adding analogues of L-histidine including 3-amino-1,2,4-triazole, 6-purinethol, histidine methyl ester, 2-thiouracil, D-histidine and the like into a basic culture medium and screening. The L-histidine is produced by a strain fermentation method; and compared with the starting strain, the capability of the accumulating high-level L-histidine is expressed. The ZJZ 625 is subjected to shake-flask culture for 72 hours and the yield of the L-histidine can reach to 9.7 g / L. The fermentation is a 3-L fermentation tank is carried out for 60 hours and the yield of the L-histidine can reach to 18.1 g / L.
Owner:JIANGNAN UNIV
Quality control material for metabolomics detection and quality control method of quality control material
The invention discloses a quality control material for metabolomics detection and a quality control method of the quality control material. The quality control material comprises mixed standard samples of 4,4'-methylene bis(2-chloroaniline), p-anisidine, L-tyrosine methyl ester, 3-chloroaniline, 2,4-dimethyl quinoline, sulfapyridine, atrazine, sulfadoxine, DL-leucine, N-benzoyl-L-tyrosine ethyl ester, 6-phenyl-2-thiouracil, N-(o-toluoyl) glycine, 2-methyl-5-nitroimidazole-1-ethanol, glycyrrhetinic acid, flavanone, Epsilon-caprolactone and 2-aminopyridine. 17 standard samples in the method comefrom different material categories and are very stable, simple in preparation and small in amount; and the quality control material disclosed by the invention can accurately reflect the state of a chromatograph or a mass spectrometer.
Owner:嘉兴迈维代谢生物科技有限公司
Nucleosides preparation thereof and use as inhibitors of rna viral polymerases
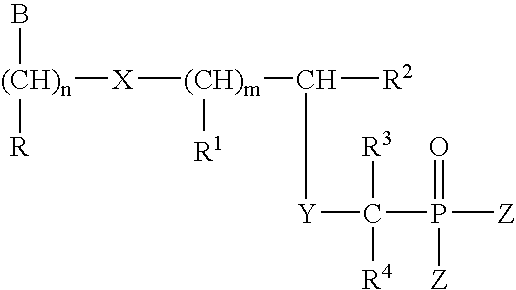
InactiveUS20050033051A1Peptide/protein ingredientsPhosphorous compound active ingredientsPolymerase LPurine
Compounds represented by the formula (I) R is H, OH, alkyl, O-alkyl, CH2—O-alkyl, (CH2)nOH, (CH2)nNH2, (CH2)nCONH2, (CH2)nOOOH; R1 is H, OH, alkyl, O-alkyl, CH2—O-alkyl, C6H11, CH2OH; R2 is H, alkyl, OH, CH2OH, CH2—O-alkyl, CH(OH)-alkyl, CH(OH)CH2OH, CH2-halogen; R3 and R4 independently is H, OH, alkyl; Z is OR5, OR6, or aminoacids and esters thereof R5 and R6 independently is H, alkyl, aryl, pivaloyloxymethyl, C(R7)2OC(O) X (R8)a formula (II), R7 independently is —H, C1-C12 alkyl, C5-C12 aryl, C2-C12 alkenyl, C2-C12 alkynyl, C7-C12 alkenylaryl, C7-C12 alkynylaryl, or C6-C12 alkaryl, any of which is unsubstituted or is substituted with 1 or 2 halo, cyano, azido, nitro, or —OR9; R9 is C1-C12 alkyl, C2-C12 alkenyl, C2-C12 alkynyl or C5-C12 aryl; provided that at least one R8 is not H; and a is 1 when X is CH2, or direct bond, or 1 or 2 when X is N with the proviso that when a is 2 and X is N, (a) two N-linked R groups can be taken together to form a carbocyclic or oxygen containing heterocycle, (b) one N-linked R8 additionally can be —OR9 or (c) both N-linked R8 groups can be —H; R10 is H or C1-C8 alkyl; R11 is selected from H, alkyl, alkenyl, alkynyl, aryl, acyloxyalkyl, and pivaloyloxyalkyl n is 1-5 m is 0 to 5 X is S, N(R8) or direct bond Y is O, S, N (R8), and CHR1 B is selected from the group consisting of adenine, guanine, cytosine, uracil, thymine, modified purines and pyrimidines such as inosin-9-yl, 2-amino-purin-9-yl, 2amino-6-chloro-purin-9-yl, 2-6-diamino-purin-9-yl, 3-carboxamido-1, 2, 4-triazol-1-yl, 3-deaza-adenin-9-yl, 3-deaza-guanin-9-yl, 3-deaza-inosin-9-yl, 3-deaza-2-amino-purin-9-yl, 3-deaza-2-amino-6-chloro-purin-9-yl, 3-deaza-2, 6-diamino-purin-9-yl, 7-deaza-adenin-9-yl, 7-deaza-guanin-9-yl, 7-deaza-inosin-9-yl, 7-deaza-2-amino-purin-9-yl, 7-deaza-2-amino-6-chloro-purin-9-yl, 7-deaza-2-6-diamino-purin-9-yl, 7-deaza-8-aza-adenin-9-yl, 7-deaza-8-aza-guanin-9-yl, 7-deaza-8-aza-inosnin-9-yl, 7-deaza-8-aza-2-amino-purin-9-yl, 7-deaza-8-aza-2-amino-6-chloro-purin-9-yl, 7-deaza-8-aza-2-6-diamino-purin-9-yl, -8-aza-adenin-9-yl,-8-aza-guanin-9-yl, -8-aza-inosnin-9-yl, -8-aza-2-amino-purin-9-yl, -8-aza-2-amino-6-chloro-purin-9-yl, -8-aza-2-6-diamino-purin-9-yl, 5-aza-thymin-1-yl, 5-aza-cytosin-1-yl, 5-aza-uracil-1-yl, 6-aza-thymin-1-yl, 6-aza-cytosin-1-yl, 6-aza-uracil-1-yl, 2-thiouracil-1-yl, 4-thiouracil-1-yl, 2 thiocytosine-1-yl, uracil-5-yl, 2-thiouracil-5-yl, 4-thiouracil-5-yl, substituted pyridine derivatives such as 6-azauracil, and azacyzosine. In general, attachment may be at different positions in the ring at nitrogen or carbon. These B ring systems may be substituted with halo, alkyl, substituted alkyl (F, Cl, Br, I, OH), NH2, N3, aryl, substituted aryl (F, Cl, Br, I, OH, NH2), aralkyl; and pharmaceutically acceptable salts thereof and prodrugs thereof are provided.
Owner:BIOCRYST PHARM INC
Mono-propargylamine modified thiouracil compound, and applications thereof
ActiveCN106946854ANovel structureOrganic active ingredientsNervous disorderDiseaseCholinesterase inhibition
The invention relates to a mono-propargylamine modified thiouracil compound, and applications thereof. The structure of the mono-propargylamine modified thiouracil compound is novel; the mono-propargylamine modified thiouracil compound possesses cholinesterase inhibition activity, monoamine oxidase activity, and metal ion chelating properties, is capable of inhibiting generation of metal ion-induced free radicals, and inhibiting metal ion-induced Abeta aggregation, is low in cytotoxicity, and is high in blood brain barrier penetration rate in vitro experiment. Preparation technology is simple; preparation cost is low; and it is promising to prepare multifunctional drugs used for treating, improving and / or preventing Alzheimer disease from the mono-propargylamine modified thiouracil compound.
Owner:EAST CHINA UNIV OF SCI & TECH
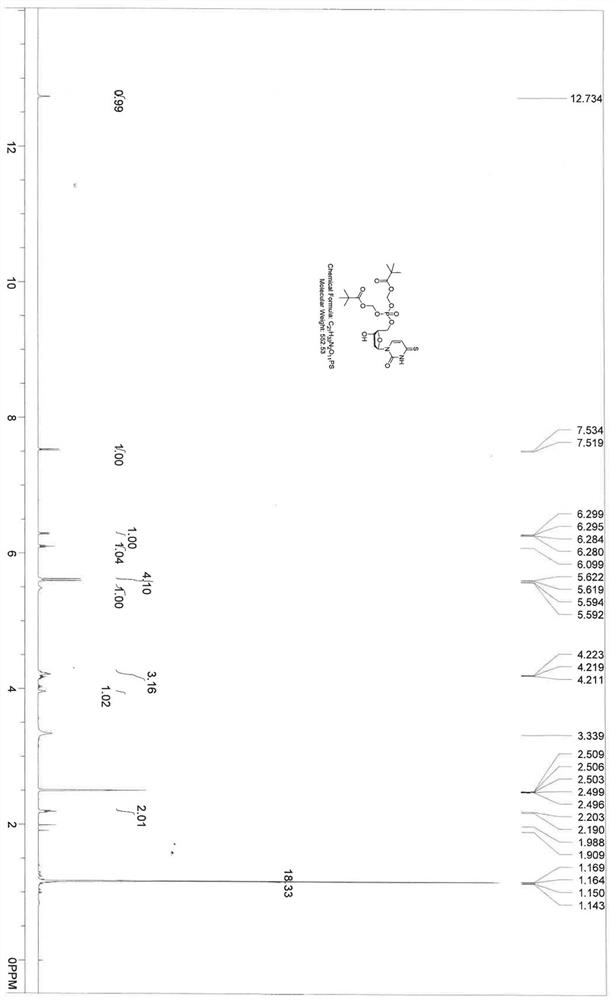
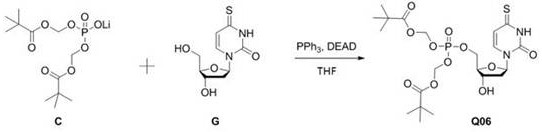
4-thiouracil deoxynucleoside phosphate and application thereof to antiviral drugs
ActiveCN113501853AConvenient treatmentOrganic active ingredientsSugar derivativesAntiviral drugThio-
The invention discloses 4-thiouracil deoxynucleoside phosphate, the structural formula of which is Q06, and a new drug molecule not only has a new choice for hepatitis B treatment drugs, but also has important significance for developing more ideal hepatitis B treatment drugs.
Owner:南京颐媛生物医学研究院有限公司 +2
S-DABO compound, synthesizing method and usage
InactiveCN100519540CEasy to synthesizeLow cytotoxicityOrganic active ingredientsOrganic chemistryFuranKetone
The S-DABO compound, its synthesis method and application belong to the technical field of medicine. The invention relates to a 5-alkyl-6-phenyl-2-(substituted arylcarbonylmethylthio)uracil compound, which has the following general formula: wherein: R1 is a C1-3 alkyl group; R2=C1-6 alkyl, substituted furan ring, thiophene ring, benzene ring (substituents on the aromatic ring are H, OH, C1-3 alkoxy). The product of the present invention is obtained by reacting 5-alkyl-6-phenylthiouracil with a-halogenated ketone under K2CO3 catalysis. The synthesis method is simple and easy, and the product has significant anti-HIV virus activity, low toxicity and high selectivity index, and can be used as a candidate anti-HIV drug.
Owner:YUNNAN UNIV +1
Pypithione biocides enhanced by zinc metal ions and organic amines
InactiveCN100341409CLong-term commercial activityBiocideDead animal preservationZinc metalPyrithione
The invention is composed of 0.5%-30% thiouracil or thiouracil complex, 0.1%-10% zinc source and 30%-80% organic amine. The percentage above is based on weight of concentrate of combination.
Owner:ARCH CHEM INC
Cattail extract compounded corrosion inhibitor as well as preparation method and application thereof
PendingCN113549923AOvercome high pricesOvercoming toxicitySolid solvent extractionEngineeringThiouracil
The invention discloses a cattail extract compounded corrosion inhibitor as well as a preparation method and application thereof. The cattail extract compounded corrosion inhibitor is prepared from, 0.1 g / L-1.0 g / L of cattail extract, 0.1 g / L-0.2 g / L of polyacrylamide, 0.1 g / L-0.5 g / L of coco fatty acid diethanol amide, 0.01 g / L-0.1 g / L of 6-amino-2-thiouracil and the balance pickling solution. The preparation method comprises the step of uniformly mixing and stirring the raw materials according to the formula ratio to obtain the target cattail extract compound corrosion inhibitor; and the cattail extract compounded corrosion inhibitor is applied to preparation of a corrosion inhibitor for steel and iron materials.
Owner:SOUTHWEST FORESTRY UNIVERSITY
Preparation method of 2-thiouracil modified porous magnetic xanthan gum micro-spheres
InactiveCN106902755AGood physical and chemical stabilityHigh mechanical strengthOther chemical processesWater contaminantsFumaryl chlorideSorbent
The invention discloses a preparation method of 2-thiouracil modified porous magnetic xanthan gum micro-spheres. The preparation method is characterized by including the steps: taking silicone oil as an organic phase, taking xanthan gum and nano-Fe3O4 magnetic particles as a water phase, spraying the water phase into the organic phase under the indoor temperature, separating solid and liquid, soaking a solid phase by the aid of deionized water for 24 hours, rapidly freezing soaked solid phase in a plastic container for 4 hours at the temperature of 18 DEG C below zero, placing the freezed solid phase into a freezing and drying oven after taking out, and freezing and drying the solid phase for 24 hours to obtain the porous magnetic xanthan gum micro-spheres; sequentially adding components into a reactor in weight percentage, dissolving 76-82% of N,N-dimethyl amide and 3-6% of 2-thiouracil, adding 10-16% of porous magnetic xanthan gum micro-spheres, stirring mixture, dripping 2.5-5% of Fumaryl chloride, performing reflux reaction for 2-3h at the temperature ranging from 48 DEG C to 52 DEG C, separating solid and liquid, and drying the separated solid to obtain the2-thiouracil modified porous magnetic xanthan gum micro-spheres. An adsorbent has high adsorption capacity for silver, can be repeatedly used and is low in cost, green and environmentally friendly, and the adsorbent has magnetism, so that the adsorbent is easily separated.
Owner:UNIV OF JINAN
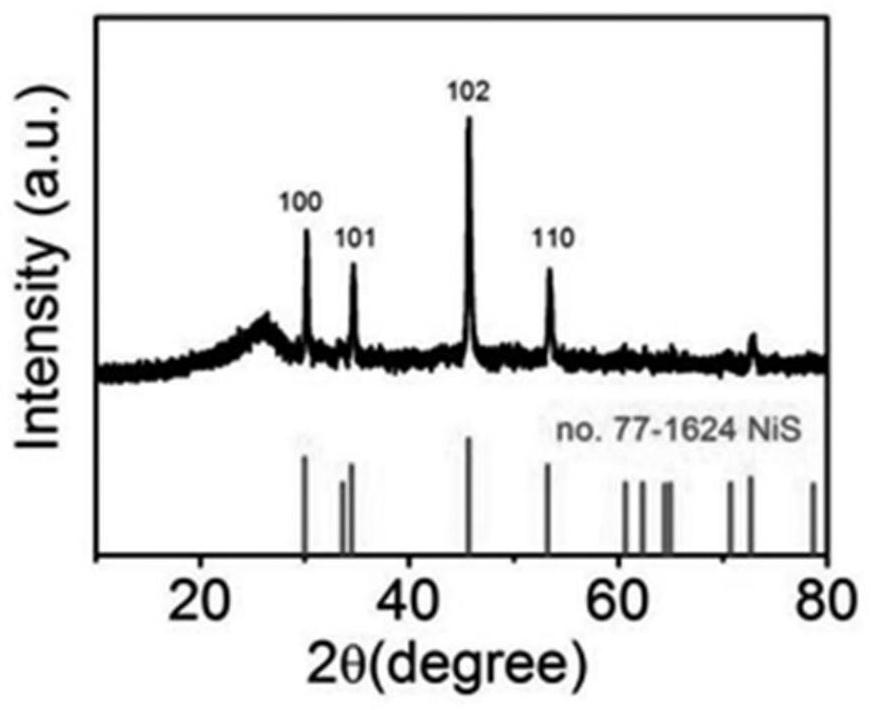
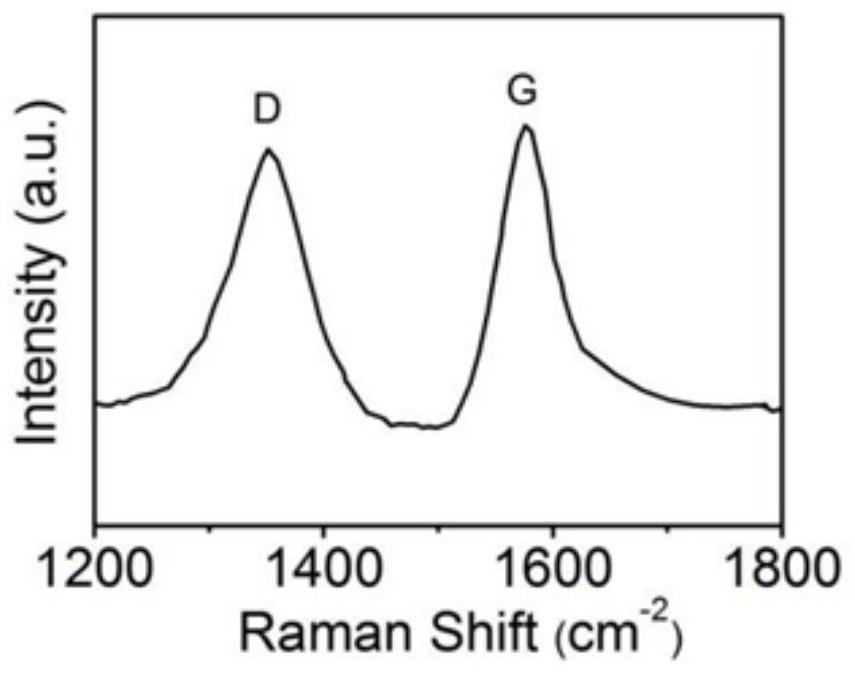
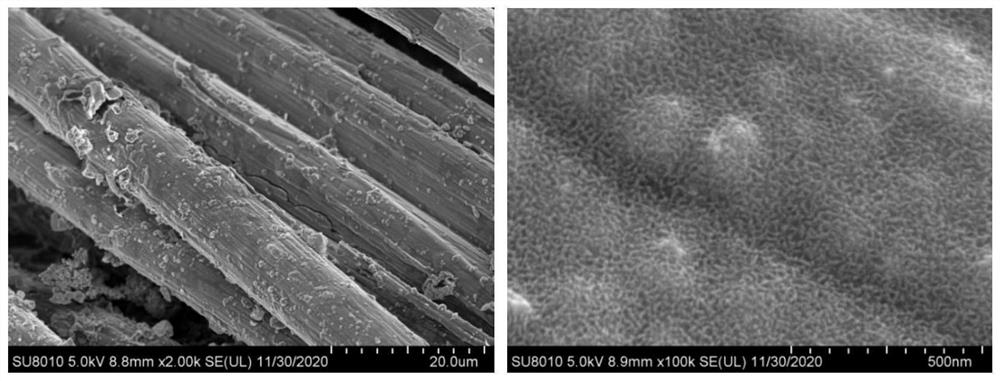
Sulfur vacancy-containing NiS quantum dot/S, N, O co-doped carbon electrode material and preparation method thereof
PendingCN113694952AImprove corrosion resistanceImprove high temperature resistancePhysical/chemical process catalystsWater contaminantsFuraldehydeThiourea
The invention discloses a sulfur vacancy-containing NiS quantum dot / S, N, O co-doped carbon network electrode material and a preparation method thereof. The sulfur vacancy-containing NiS quantum dots are uniformly embedded in an S, N, O co-doped carbon network grown on carbon cloth to form a carbon cloth self-supported composite electrode material. The preparation method comprises the following steps of mixing NiCl2.6H2O, methyl thiouracil and ethylene glycol, heating and stirring to form a homogeneous liquid, uniformly dispensing the homogeneous liquid on the surface of carbon cloth, putting the carbon cloth into a porcelain boat, putting the porcelain boat wrapped by an aluminum foil into a tubular furnace, replacing air with high-purity nitrogen, keeping the temperature at 400-600 DEG C for 0.5-5 hours under a closed condition, and performing one-step pyrolysis, vulcanization and coupling synergistic reaction to obtain the electrode material. The electrode material is used for preparing 2, 5-furandicarboxylic acid through electro-oxidation of 5-hydroxymethylfurfural and preparing hydrogen through electro-reduction of water, and has very high electro-catalytic activity.
Owner:乌海瑞森新能源材料有限公司
Compound of multiple substituted uracil class, preparation method and usage
InactiveCN1245390CThe synthesis method is simpleHas anti-HIV viral activityOrganic active ingredientsOrganic chemistryReverse transcriptaseThiourea
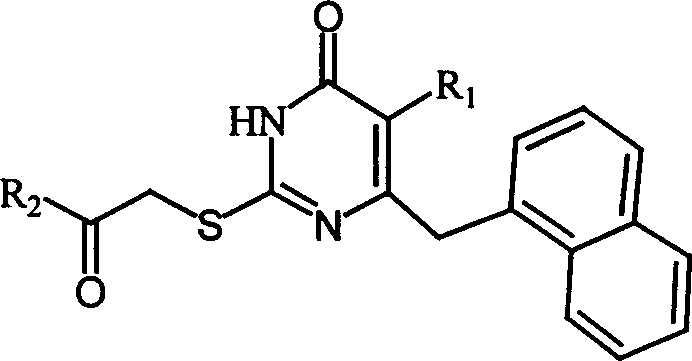
This invention relates to S-DABO type reverse transcriptiveenzyme inhibitor-2-(substituted aryl alkyl or alkoxy carbonyl methyl sulfur)-5-alkyl-6-(1-naphthyl methyl) uracil compound, and its prepn. method, its application of anti-HIV virus, with its formula, where: R1=H, C1-5 alkyl; R2=C1-6 alkyl; C1-6 alkoxy, arylcyclo-R3, aryl heterocycle-R3, C3-6 cycloalkyl-R3, arylcyclo, aryl heterocycle, C3-6 cycloalkyl substituting group R3 being H, 1-3 same or different; C1-3 alkyl, halogen, group R3 being H, 1-3 same or different: C1-3 alkyl, halogen, C1-3 ether group, OH. In this invention, 5-alkyl-6-(1-naphthylmethyl) thiourea uracil is used as reactor reacting with alpha-halogenated ketone or alpha-halogenated acetate, in the presence of catalyst of K2CO3. Said invention products can eliminate HIV-1 virus, HIV-2 SOD virus and HIV-1 (III B) SO561945.
Owner:FUDAN UNIV
A kind of ultra-thin copper foil and preparation method thereof
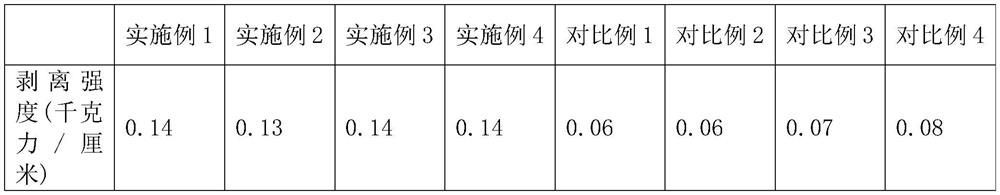
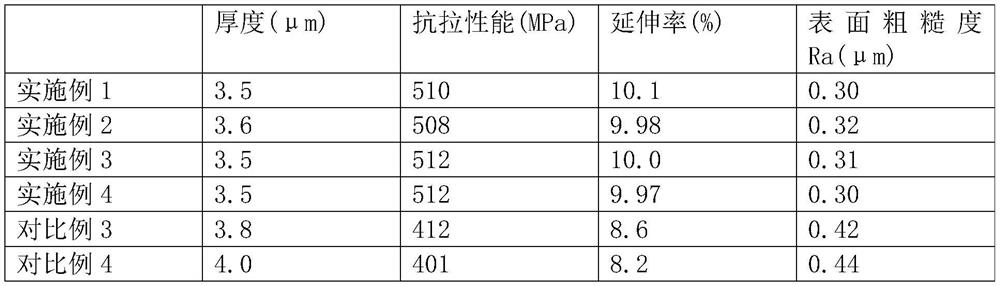
ActiveCN112981481BHigh peel strengthGood stripping potentialElectrode carriers/collectorsRare-earth elementElectrolytic agent
The invention discloses an ultra-thin copper foil and a preparation method thereof, belonging to the technical field of ultra-thin copper foil preparation. The present invention uses cyclohexyl hexaphosphoric acid and 2-thiouracil as an organic layer to adsorb on a metal foil carrier, then deposits a tungsten-nickel alloy containing rare earth elements Pr and Nd on the organic layer, and then deposits copper three times To prepare ultra-thin copper foil, the copper deposition electrolyte of the present invention contains a composite additive of sodium lignosulfonate and chitosan oligosaccharide, and the copper deposition electrolyte realizes ultra-thin copper foil under the action of rare earth elements Pr and Nd in the alloy layer. Lightweight uniform deposition enables ultra-thin copper foil to break through the limit of 4μm to achieve ultra-light weight of 3.5μm, and the tensile performance is as high as 510MPa, and the elongation rate can reach up to 10%, endowing lightweight ultra-thin copper foil with excellent performance.
Owner:GUANGDONG FINE YUAN SCI TECH CO LTD
A kind of electroplating tin solution with good deep plating ability and electroplating method thereof
The invention discloses an electroplating tin solution with good deep plating ability, which is characterized in that it is prepared from the following raw materials: stannous methanesulfonate 100ml / L-300ml / L, methanesulfonic acid 70wt% 75ml / L- 150ml / L, isopropanol 10g / L~30g / L, sodium lauryl sulfate 1g / L~10g / L, p-methoxyphenol 0.5g / L~3g / L, quercetin 0.02g / L ~0.05g / L, 6‑phenyl‑2‑thiouracil 0.1g / L~0.2g / L, malic acid 0.2g / L~0.5g / L. The beneficial effect of the invention is that the deep-plating ability of the electroplating solution is greatly improved, it is very suitable for the plating of long tube-shaped workpieces, and the long-standing difficult technical problems in the prior art are solved.
Owner:粤海中粤(秦皇岛)马口铁工业有限公司
Green production process of uracil
The invention provides a green production process of uracil. The process comprises the following steps that (1) thiouracil is used as raw materials; water is added; a halogenide or a sulfur acid compound is also added; heating for the night, temperature reduction and filtering drying are performed to obtain high-purity uracil; (2) the thiouracil is continuously added into mother liquid obtained through filtering in the step (1); heating is performed; the operations are cycled. The green production process has the following technical effects that the reaction mother liquid and the materials canbe used indiscriminately; the yield and the purity are not influenced; the process belongs to a green and environment-friendly process. The high-purity uracil can be obtained; the HPLC detection purity is 95 percent or higher; even the uracil being 98 percent or higher can be obtained.
Owner:无锡富泽药业有限公司
A kind of quality control product and its quality control method for metabolomics detection
ActiveCN110618215BEasy to prepareReduce dosageComponent separationMethylenebis(chloroaniline)Quinoline
The invention discloses a quality control product for metabolomics detection and a quality control method thereof, which comprises 4,4'-methylenebis(2-chloroaniline), p-anisidine, L-tyrosine methyl ester, 3-Chloroaniline, 2,4-Dimethylquinoline, Sulfapyridine, Atrazine, Sulfadoxine, DL-Leucine, N-Benzoyl-L-Tyrosine Ethyl Ester, 6-Benzene 2-thiouracil, N-(o-toluoyl)glycine, 2-methyl-5-nitroimidazole-1-ethanol, glycyrrhetinic acid, flavanones, ε-caprolactone, 2-aminopyridine The 17 standard samples of this method come from different substance categories, are very stable, and are simple to prepare and use less. The quality control product of the present invention can accurately reflect the instrument status of a chromatograph or a mass spectrometer.
Owner:WUHAN MAIWEI METABOLIC BIOTECHNOLOGY CO LTD
4'-substituted carbovir-and abacavir-derivatives as well as related compounds with HIV and HCV antiviral activity
The application relates to compounds with activity against infectious viruses. Accordingly, in one embodiment the invention provides a compound of the invention which is a compound of Formula I: (I) wherein: B is adenine, guanine cytosine, uracil, thymine, 7-deazaadenine, 7-deazaguanine, 7-deaza-8-azaguanine, 7-deaza-8-azaadenine, inosine, nebularine, nitropyrrole, nitroindole, 2-aminopurine, 2-amino-6-chloropuriine, 2,6-diaminopurine, hypoxanthine, pseudouridine, pseudocytosine, pseudoisocytosine, 5-propynylcytosine, isocytosines, isoguanine, 7-deazaguanine, 2-thiopyrimidine, 6-thioguanine, 4-thiothymine, 4-thiouracil O<6> -methylguanine, N<6> -methyladenine, O<4>-methylthymine, 5,6-dihydrothymine, 5,6-dihydroucacil, 4-methylindole, triazole, or pyrazolo[3,4-d]pyrimidine; and B is optionally substituted with one or more alkyl, alkenyl, alkynyl, cycloalkyl, (cycloalkyl)alkyl, hydroxy, or halo; and R<1> is alkyl, alkenyl, alkynyl, cyano, azido, or fluoromethyl; or a pharmaceutically acceptable salt or solvate thereof.
Owner:GILEAD SCI INC
Preparation of a 2-thiouracil-modified porous magnetic xanthan gum microsphere
InactiveCN106902755BGood physical and chemical stabilityHigh mechanical strengthOther chemical processesWater contaminantsFreeze-dryingSorbent
The invention discloses a preparation method of 2-thiouracil modified porous magnetic xanthan gum micro-spheres. The preparation method is characterized by including the steps: taking silicone oil as an organic phase, taking xanthan gum and nano-Fe3O4 magnetic particles as a water phase, spraying the water phase into the organic phase under the indoor temperature, separating solid and liquid, soaking a solid phase by the aid of deionized water for 24 hours, rapidly freezing soaked solid phase in a plastic container for 4 hours at the temperature of 18 DEG C below zero, placing the freezed solid phase into a freezing and drying oven after taking out, and freezing and drying the solid phase for 24 hours to obtain the porous magnetic xanthan gum micro-spheres; sequentially adding components into a reactor in weight percentage, dissolving 76-82% of N,N-dimethyl amide and 3-6% of 2-thiouracil, adding 10-16% of porous magnetic xanthan gum micro-spheres, stirring mixture, dripping 2.5-5% of Fumaryl chloride, performing reflux reaction for 2-3h at the temperature ranging from 48 DEG C to 52 DEG C, separating solid and liquid, and drying the separated solid to obtain the2-thiouracil modified porous magnetic xanthan gum micro-spheres. An adsorbent has high adsorption capacity for silver, can be repeatedly used and is low in cost, green and environmentally friendly, and the adsorbent has magnetism, so that the adsorbent is easily separated.
Owner:UNIV OF JINAN
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com